Abstract
Background
The Women’s Health Initiative (WHI) low fat (20% kcal) diet modification (DM) trial (1993–2005) demonstrated a non-significant reduction in breast cancer, a nominally significant reduction in ovarian cancer and no effect on other cancers (mean 8.3 years intervention). Consent to non-intervention follow-up was 83% (n=37,858). This analysis was designed to assess post-intervention cancer risk in women randomized to the low-fat diet (40%) versus usual diet comparison (60%).
Methods
Randomized, controlled low fat diet intervention for prevention of breast and colorectal cancers conducted in 48,835 postmenopausal U.S. women, aged 50–79 years at 40 U.S. sites. Outcomes included total invasive cancer, breast and colorectal cancer, cancer-specific and overall mortality.
Results
There were no intervention effects on invasive breast 1.08 (0.94, 1.24) or colorectal cancer, other cancers, cancer-specific or overall mortality during the post-intervention period or the combined intervention and follow-up periods. For invasive breast cancer, the HRs were 0.92 (0.84, 1.01) during intervention, during the post-intervention period, and 0.97 (0.89, 1.05) during cumulative follow up. A reduced risk for estrogen receptor positive/progesterone receptor negative tumors was demonstrated during follow-up. Women with higher baseline fat intake (quartile), point estimates of breast cancer risk were HR-0.76; 0.62, 0.92 during intervention versus HR-1.11; 0.84, 1.4 during post-intervention follow-up (p-diff=.03).
Conclusions
Dietary fat intake rose post-intervention in intervention women; no long-term reduction in cancer risk or mortality was shown in the WHI DM trial.
Impact
Dietary advisement to reduce fat for cancer prevention after menopause generally was not supported by the WHI DM trial.
Keywords: breast cancer, colorectal cancer, mortality, low-fat diet, randomized controlled trial
INTRODUCTION
During the 1990’s, preclinical, early ecological and observational studies historically reported that dietary fat intake was positively associated with breast (1) and colorectal cancer (2) risk. A sizeable randomized, controlled trial with adequate statistical power was designed to test this hypothesis. The Women’s Health Initiative (WHI) randomized low-fat dietary modification (DM) trial (3) was initiated with incidence of (invasive) breast cancer and colorectal cancer as primary outcomes, to test the hypothesis that an intervention involving reduced total fat (<20% energy) would reduce incidence of these outcomes over a 9 year period in 48,000 free-living postmenopausal women (4–6). Intervention dietary goals focused on lowering total dietary fat intake and increasing fruit, vegetable and grain consumption (3).
The DM trial intervention period ended in 2005 with simultaneous reports on breast cancer (7), colorectal cancer (8) and cardiovascular disease (9). Subsequent reports were published for the DM trial on other cancers, including ovarian cancer (10). There was no overall intervention effect on colorectal or breast cancer, although there were fewer invasive breast cancers in the low-fat diet group than the comparison group with a hazard ratio (HR) of 0.91; 95% confidence interval (CI): 0.83, 1.01 and a significant interaction (P=0.04) between the baseline percentage of energy from fat as measured by 4 day food records. Women who reported relatively higher percentage of energy from dietary fat at baseline made larger reductions in fat intake and demonstrated a nominally significant reduction in breast cancer risk (HR 0.78; 95% CI, 0.64–0.95) (7).
This paper updates the DM trial analyses by including an additional 5.2 years of post-intervention follow-up, through September, 2010. The specific objectives for this follow-up analysis were to evaluate the longer-term effects of 8.3 years on average of diet intervention on incident breast, colorectal, other and total cancers as well as cancer-specific and overall mortality over the 12.3 year combined intervention and post-intervention follow-up period.
MATERIALS AND METHODS
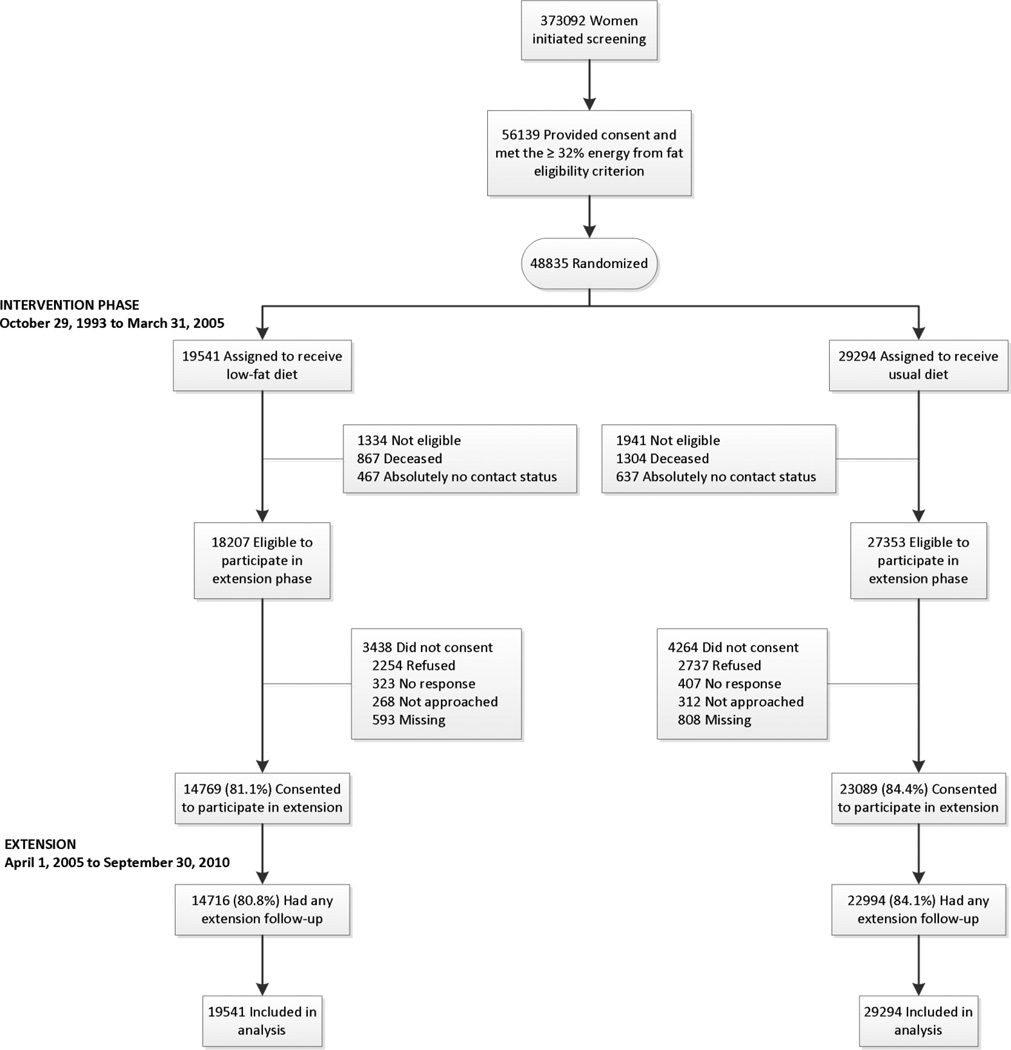
Details of the WHI Dietary Modification (DM) Trial have been provided (11–13). Briefly, postmenopausal women, aged 50–79 y, were recruited at 40 clinical centers across the U.S. between 1993 and 1998. Women entering the WHI DM trial also could participate in the WHI hormone therapy trial (14) or the calcium plus vitamin D supplementation trial (15). Exclusions for participation in the DM trial included prior breast cancer or colorectal cancer, other cancer within the past ten years, predicted survival less than three years, adherence or retention concerns (e.g., alcoholism, dementia), or baseline fat intake < 32% of total calories as estimated by a food frequency questionnaire (FFQ) (16). No exclusions for body mass index (BMI) were applied. A mammogram, not suspicious for cancer, was required for study entry and subsequently at 2 year intervals, or annually for women concurrently enrolled in the hormone trial; colorectal cancer screening was not protocol-mandated but self-reported information regarding screening was collected. Women were randomized 40%/60% into the intervention and control arms of the trial; this analysis includes all DM participants.
Between 1993 and 1998, 48,835 women were randomized (40:60) to intervention (n=19,541) or comparison (n=29,294) groups. Demographic characteristics and medical history were collected by self-report using standardized questionnaires. Anthropometric measurements including height, weight, and waist circumference at the umbilicus were collected at baseline and annually throughout the trial by trained personnel in the local WHI clinics using standardized protocols (10); no post-intervention period follow-up anthropometric measurements were taken. Body Mass Index (BMI) was calculated as weight in kg/height in meters-squared.
Diet was assessed by a validated food frequency questionnaire (FFQ) during the intervention period as previously described (16). “Four-day food records were collected on all DM trial women at baseline as a component of eligibility. The stored records were analyzed early on in a 4.6% sample and later for cases for use in breast cancer case-only analysis. Diet also was assessed by a validated food frequency questionnaire (FFQ) for all DM women at baseline, year 1 and on a rotating basis for one third of women each year during the intervention period as previously described. Assessment of dietary intake after intervention period participation was collected on a sub-sample and consisted of a single 24 hour dietary recall for 1311 DM trial participants who re-consented for continued assessment between the years of 2005–2010 as part of their post-intervention period participation. Recalls were collected by telephone using trained staff and applying the USDA multi-pass method. No post-randomization 4-day food records were collected. Assessment of dietary intake after intervention period participation was collected on a sub-sample and consisted of a single 24 hour dietary recall for 1311 DM trial participants who re-consented for continued assessment between the years of 2005–2010 as part of their post-intervention period participation. Recalls were collected by telephone using trained staff and applying the USDA multi-pass method (17).
The principal goal of the DM low-fat diet intervention was to reduce fat intake to 20% of total energy (13). Women were instructed and supported through behavioral modification strategies to increase vegetable, fruit and whole grain intake daily. Total energy intake was not restricted nor was weight loss advocated. The intervention was largely implemented using 18 group meetings in the first year and quarterly thereafter. Comparison group women received printed health-related materials only.
Clinical outcomes were collected through annual clinic visits during the trial and semi-annual mailed questionnaires during the trial and follow-up period. Outcomes for cancer were verified, initially by trained physician adjudicators at the local clinical centers by medical record and pathology review, followed by final central blinded adjudication (18). Vital status of all participants were cross-checked against The National Death Index at 2–3 year intervals.
At the protocol-specified termination date of March 31, 2005 for the intervention period, representing a mean 8.1 years of trial participation, vital status was known for 96% of participants with 4.9% deceased. Subsequently, DM participants were contacted by mail for consent to participate in an additional post-intervention period for collection of clinical outcomes (including cancers). The characteristics of the sample of WHI-DM women who re-consented for the extended follow-up (81.1%, n=14769 intervention versus 84.4%, n=23089, comparison, p<0.001) are provided in Supplementary Table 1.
Outcomes
The two primary study outcomes were invasive breast or colorectal cancers. Other cancer outcomes were assigned based upon immunohistochemistry tests described in the pathology reports obtained from medical records and adjudicated by WHI-trained physician adjudicators. The intervention period sample size calculation was based on an anticipated 13% lower invasive breast cancer incidence in the dietary intervention group which would be achieved approximately linearly during the intervention period. An external data Safety and Monitoring Board (DSMB) monitored the trial.
Statistical Analyses
The analytic plan for the combined intervention and post-intervention follow-up periods was similar to the approach taken in prior reports of outcomes during post-intervention follow up in the WHI hormone therapy (HT) trials (19). All participants were included in analyses by randomized group assignment until they last provided follow-up information. Baseline characteristics for women who provided additional consent were compared by randomization group using chi-squared and t tests.
Annualized rates of clinical events were estimated for the intervention period, the post-intervention period, and the entire follow-up period by dividing the number of events by the corresponding person-time in each period. Cumulative incidence curves were generated for the entire active study participation period (low-fat diet intervention and comparison control) and separately during the post-intervention period. Hazard ratios (HR), 95% confidence intervals (CI) and p-values were computed from Cox proportional hazards models that were stratified by age, prior disease (if appropriate), and randomization status in the WHI Hormone Trials.
Models were constructed for each clinical endpoint where women contributed follow-up time until the end of the interval, the date of their first relevant clinical event, or the date of death or withdrawal from the study, whichever came first. For breast cancer subgroups, analysis by tumor subtype was also explored. Formal tests of the differences between HR in the intervention vs. post-intervention period were calculated by inclusion of a binary term for period (intervention vs. post-intervention) as a time-dependent variable. All statistical tests were two-sided. Nominal P-values are reported without adjustment for multiple outcomes or sequential looks during the clinical trial follow-up period. Permutation tests were performed post hoc to help interpret the invasive breast cancer results by determining whether the cumulative data were more consistent with (i) an overall null hypothesis or (ii) with an alternative hypothesis in which there was a real, but not quite significant, risk reduction during the intervention period, followed by no effect during the post-intervention period. In both scenarios 10,000 permutations were generated.
To investigate potential imbalances due to differing consent rates, entry baseline characteristics for women who re-consented and re-consent rates were compared by randomization group and by age, race/ethnicity, and other pertinent demographics (Supplementary Table 1). Furthermore, a re-consent model was constructed and the inverse of the estimated probabilities of re-consent were used as weights in Cox regression models.
Subgroup analyses based on baseline percentage of energy from fat were conducted also. Instead of FFQ data, baseline 4-day diet records data were analyzed using NDS-R® due to the fat intake by FFQ was applied for eligibility screening, an approach that resulted in a truncated baseline FFQ fat intake and consequently upwardly biased assessment. The 4.6% sample was used only to provide estimates of baseline intake quartiles for the DM trial cohort as a whole, for use in the case-only HR analyses. Statistical software SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina) and R, version 2.15 (R Foundation for Statistical Computing, http://www.r-project. org/) were used for these analyses.
RESULTS
Baseline Characteristics and Intervention Effects on Percent Energy from Fat
The DM participant flow including screening, consent, randomization, eligibility and follow up during intervention and post-intervention periods is detailed in Figure 1. At entry, baseline characteristics were similar in the two randomization groups (7–9). The percentages of DM women, who consented to follow-up, by treatment arm and participant characteristics, are described in Supplementary Table 1; comparison group participants were somewhat more likely to consent to follow-up. The characteristics of the women who consented to, and provided data during, extended follow-up are described in Table 1. The majority of the women were overweight or obese at baseline with mean (SD) BMI of 28.9 (5.8) kg/m2 and, from FFQs, mean daily dietary intake of percent calories from total fat, saturated and trans- fat, polyunsaturated fat of 37.6%, 17.5%, and 7.7 %, respectively and mean daily fiber intake of 15.5 grams. Mean servings per day of fruit and vegetables were 1.6 and 2.0, respectively.
Figure 1.
CONSORT Diagram: Women’s Health Initiative randomized trial of dietary modification through extended follow-up.
Table 1.
Baseline Characteristics Among WHI-Diet Modification Participants that Consented to Extended Follow-Up1
| Intervention (n-14716) | Comparison (n=22994) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P-Value | |
| Age group at screening | 0.77 | ||||
| 50–59 | 5598 | 38.0 | 8789 | 38.2 | |
| 60–69 | 7009 | 47.6 | 10869 | 47.3 | |
| 70–79 | 2109 | 14.3 | 3336 | 14.5 | |
| Race/ethnicity | 0.23 | ||||
| White | 12264 | 83.3 | 19282 | 83.9 | |
| Black | 1461 | 9.9 | 2135 | 9.3 | |
| Hispanic | 445 | 3.0 | 713 | 3.1 | |
| American Indian | 59 | 0.4 | 73 | 0.3 | |
| Asian/Pacific Islander | 309 | 2.1 | 514 | 2.2 | |
| Unknown | 178 | 1.2 | 277 | 1.2 | |
| Education | 0.33 | ||||
| ≤ High school/GED or less | 3033 | 20.7 | 4847 | 21.2 | |
| School after high school | 5681 | 38.8 | 8935 | 39.1 | |
| College degree or higher | 5914 | 40.4 | 9080 | 39.7 | |
| Body-mass index (kg/m2), baseline | 0.57 | ||||
| <25 | 3985 | 27.2 | 6194 | 27.1 | |
| 25 – <30 | 5262 | 35.9 | 8338 | 36.4 | |
| ≥30 | 5406 | 36.9 | 8353 | 36.5 | |
| Alcohol use | 0.69 | ||||
| Non Drinker | 5856 | 40.0 | 9243 | 40.3 | |
| ≤ 1 drink/day | 7342 | 50.1 | 11375 | 49.6 | |
| > 1 drink/day | 1459 | 10.0 | 2296 | 10.0 | |
| Bilateral oophorectomy | 2901 | 20.1 | 4681 | 20.8 | 0.15 |
| Aspirin use ≥80 mg | 2591 | 17.6 | 4215 | 18.3 | 0.07 |
| Multivitamin use > 5 yrs | 2424 | 16.5 | 3762 | 16.4 | 0.77 |
| Mean | SD | Mean | SD | P-Value | |
| Age at screening | 61.9 | 6.7 | 61.9 | 6.7 | 0.90 |
| Body-mass index (kg/m2), baseline | 28.9 | 5.8 | 28.9 | 5.8 | 0.26 |
| Total expenditure/wk from phys act, MET-hours | 10.3 | 11.8 | 10.3 | 12.1 | 0.60 |
| Daily Nutrient Consumption Estimates | |||||
| Energy (kcal) | 1794.2 | 688.4 | 1791.6 | 683.4 | 0.71 |
| Percent Calories from Fat | 37.6 | 5.0 | 37.6 | 5.0 | 0.79 |
| Percent Calories from Polyunsaturated Fat | 7.7 | 1.9 | 7.7 | 1.9 | 0.21 |
| Percent Calories from Saturated Fat | 12.7 | 2.5 | 12.6 | 2.5 | 0.42 |
| Percent Calories from Monounsaturated Fat | 14.4 | 2.3 | 14.4 | 2.2 | 0.70 |
| Fiber (g) | 15.5 | 6.4 | 15.5 | 6.3 | 0.58 |
| Potassium (mg) | 2599.6 | 936.3 | 2593.1 | 924.9 | 0.51 |
| Servings of Fruit | 1.6 | 1.0 | 1.6 | 1.0 | 0.78 |
| Servings of Vegetables | 2.0 | 1.1 | 2.0 | 1.1 | 0.50 |
| Animal Protein (g) | 52.8 | 24.4 | 52.6 | 23.9 | 0.40 |
| Vegetable Protein (g) | 20.7 | 8.8 | 20.7 | 8.7 | 0.70 |
Participants from the Women’s Health Initiative who consented to extended follow-up after enrollment in the Dietary Modification Trial, and had any follow-up during the extension phase.
Calculation based on available carbohydrates.
Dietary fat intake by DM group assignment during the intervention period has been previously described (7). Longitudinal plots of mean (95% CI) percentage of energy as fat during follow-up and subsequently the extension period, stratified by quartiles of percentage energy as fat at baseline, suggest that this diet exposure is somewhat preserved by randomization arm over time (Supplementary Figure 1). During the post-intervention period, the intervention group reported a somewhat smaller percent energy from fat than the comparison group with mean (95%CI) differences of −2.8 (−4.9, −0.7), −4.6 (−6.6, −2.5), −3.5 (−5.4, −1.6), and −3.6 (−5.5, −1.7) for increasing quartiles of baseline fat, respectively, based on 4-day diet records at baseline and year 1 followed by 24 hour dietary recalls during the remainder of follow-up (n= 1311 with post-intervention data; 2.7% of total sample).
Cancer Event Rates Overall and Comparing Intervention and Post-Intervention Periods
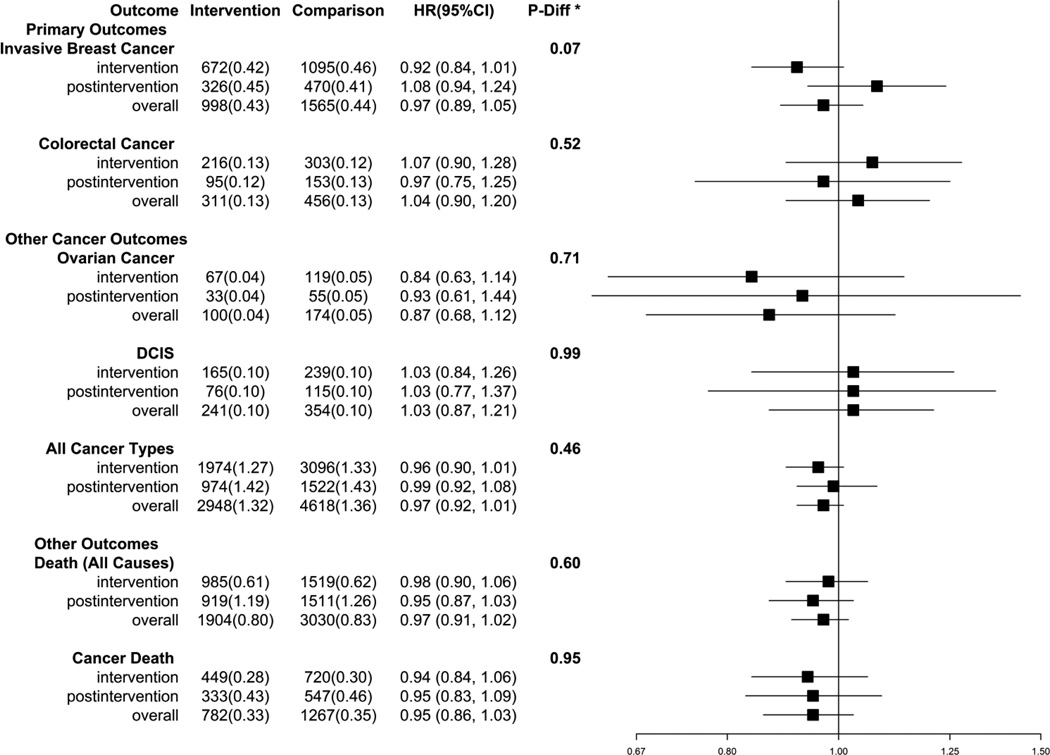
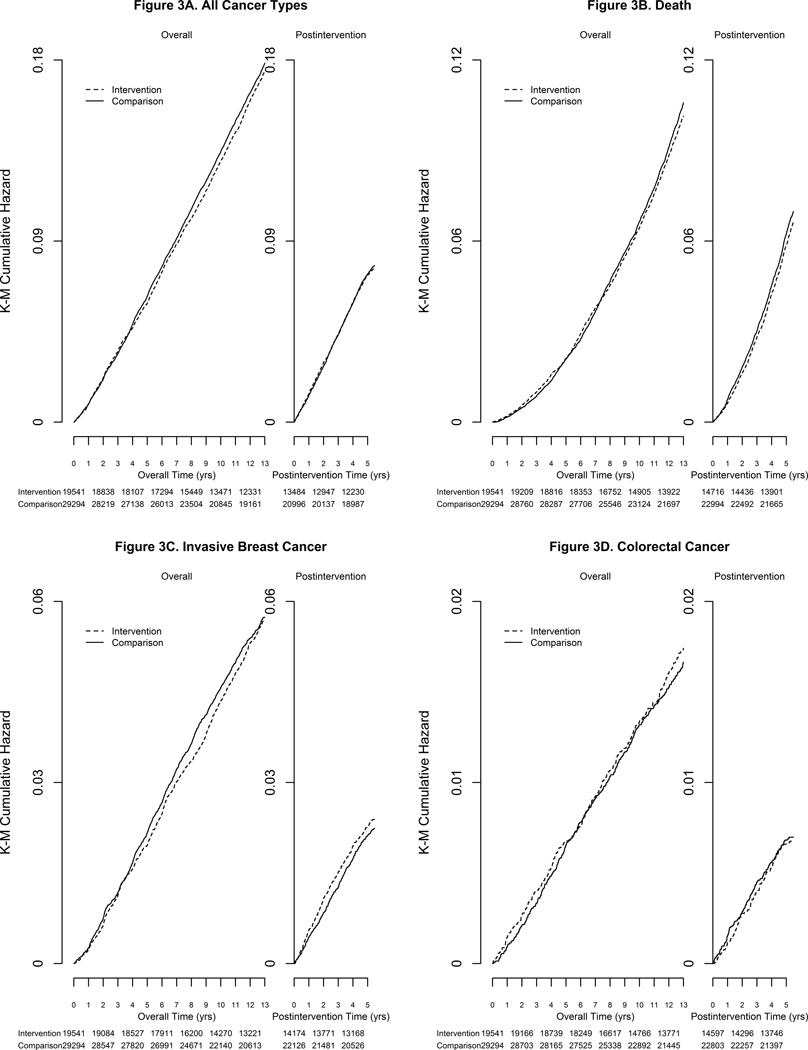
The influence of the low fat intervention on cancer and mortality outcomes over the combined intervention and post-intervention periods (12.3 [3.4] years) as well as for each period separately (intervention period mean [SD] of 8.3 [1.7] years; post-intervention period 5.2 [0.8] years) are summarized in Figure 2 and Supplementary Table 2. There was no indication that the low fat diet assignment during the combined periods was associated with lower risk for total cancer or total mortality (Figure 2 and Figure 3C, 3D). For total mortality, the cumulative HR associated with the low fat diet was 0.97 (95% CI: 0.91, 1.02, 0.80% [n=1904] vs. 0.83% [n=3030]).
Figure 2.
Number of events (annualized %) and hazard ratios (95%CI) for outcomes in the WHI Dietary Modification Trial during the intervention period, post-intervention period, and overall. The p-value corresponds to a test of whether intervention and post-intervention hazard ratios differ.
Figure 3.
Kaplan-Meier estimates for cumulative hazards of invasive breast cancer, colorectal cancer, total cancer and total mortality.
The annual incidence of invasive breast cancer was not significantly lower in the low-fat diet intervention women compared to the comparison women (annual percent 0.43 vs 0.44%, HR − 0.97; 95% CI: 0.89, 1.05) over the combined intervention and follow-up period. During the intervention period, mammogram frequency was closely comparable between randomization groups and remained similar during post-intervention follow-up period (93% and 92%, in low fat versus comparison, respectively). Log-rank tests suggested a possible benefit of a low-fat diet in relation to invasive breast cancer risk during the intervention period (HR − 0.92; 95% CI: 0.84, 1.01), that was not sustained in the post-intervention period (HR − 1.08; 95% CI: 0.94, 1.24; p-diff = 0.07; Figure 2, Supplementary Table 2 and Figure 3A). A permutation test confirmed the validity of p-diff under an overall null hypothesis (p-value of permutation test = 0.07) and suggested the alternative hypothesis of a reduced HR in the intervention period, followed by an HR of one in the post-intervention period was somewhat more plausible (p-value of permutation test = 0.17).
Colorectal cancer risk did not differ by group assignment overall (HR – 1.04; 95% CI: 0.90, 1.20) either during the intervention period or during the post-intervention period (Figure 2 and Figure 3B). For ovarian cancer, the point estimate remained < 1.0 in the intervention period and post-intervention periods, but the overall HR was not significant (HR − 0.87; 95% CI: 0.68, 1.12; Figure 2). Similarly, a non-significant HR was observed for all other cancer types combined (Figure 3C).
Subgroup Analyses by Baseline Percent Energy from Fat
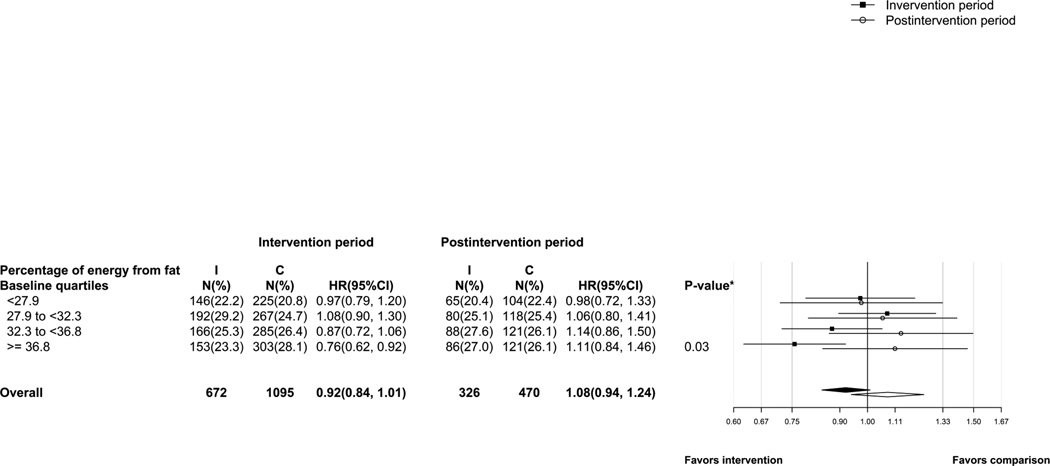
Figure 4 provides the HR for invasive breast cancer for the intervention and post-intervention periods by baseline percentage energy from fat by quartiles as assessed by 4-day food records. A change in HR from the intervention to the post-intervention period was evident for women who reported baseline percentage of energy from fat within the highest quartile [(HR − 0.76; 95%CI: 0.62, 0.92) during the intervention period versus (HR − 1.11; 95% CI: 0.84, 1.46) during the post-intervention period (P- diff = 0.03)].
Figure 4.
Number of events (proportion of cases) and hazard ratios (95%CI) for invasive breast cancer by quartiles of percentage energy from fat at baseline. The p-value corresponds to a test of whether intervention and post-intervention hazard ratios differ among the highest quartile of fat consumption
Analyses of Tumor Characteristics for Breast Cancer Events
Given observational evidence that the relationship between diet and breast cancer may vary by tumor subtypes (20, 21), although not consistently (22), the association between the low fat diet and incidence of breast tumors by subtype also was evaluated. The results supported a possible association between diet assignment and breast cancer for the ER+/PR-tumor subtype over the entire period (HR − 0.70; 95% CI: 0.56, 0.88) (Table 2), with the suggested protective associations during the intervention period (HR − 0.69; 95% CI: 0.53, 0.89) slightly attenuated during the post-intervention period (HR − 0.76; 95% CI: 0.48, 1.20). No association were shown among the remaining ER/PR subtypes (p-het=0.03). Similarly, reclassification of the ER+/PR-tumor subtype as luminal B tumors showed a non-significant reduced point estimate during both periods. A test of heterogeneity during the entire follow-up suggested a differential effect of the dietary intervention for PR− tumors overall (p-het=0.03; HR – 0.83; 95% CI: 0.71, 0.97). In analyses by baseline BMI, we did not find effect modification of DM randomization by BMI overall or BMI × hormone therapy (HT) for any of the tumor subtypes (data not shown).
Table 2.
Effects of Randomization Assignment to Dietary Modification Intervention vs. Comparison on Clinical Outcomes Before and After Termination of the Intervention in the Women’s Health Initiative Dietary Modification Trial
| Events on or Before March 31, 2005 (Trial Phase), N=48835 |
Events April 1, 2005–September 30, 2010 (Post-trial Phase1), N=37710 |
Overall3 Data through September 30, 2010 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention (n=19541) |
Comparison (n=29294) |
Hazard Ratio4 (95% CI) |
Intervention (n=14716) |
Comparison (n=22994) |
Hazard Ratio5 (95% CI) |
P- diff2 |
Intervention (n=19541) |
Comparison (n=29294) |
Hazard Ratio4 (95% CI) |
P- het6 |
|
|
Follow-up time (month) |
99.6 | 99.9 | 62.7 | 62.6 | 146.9 | 149.1 | |||||
| Outcomes | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||||
| Invasive Breast Cancer | 672 (0.42) | 1095 (0.46) | 0.92 (0.84, 1.01) | 326 (0.44) | 470 (0.41) | 1.08 (0.94, 1.24) | 0.07 | 998 (0.43) | 1565 (0.44) | 0.97 (0.89, 1.05) | |
| ER/PR Status | 0.03 | ||||||||||
| ER+/PR+ | 429 (0.27) | 651 (0.27) | 0.99 (0.87, 1.12) | 236 (0.32) | 338 (0.30) | 1.08 (0.92, 1.28) | 0.40 | 665 (0.29) | 989 (0.28) | 1.02 (0.92, 1.13) | |
| ER+/PR− | 85 (0.053) | 185 (0.077) | 0.69 (0.53, 0.89) | 28 (0.038) | 58 (0.051) | 0.76 (0.48, 1.20) | 0.69 | 113 (0.049) | 243 (0.069) | 0.70 (0.56, 0.88) | |
| ER−/PR+ | 10 (0.006) | 18 (0.008) | 0.84 (0.39, 1.82) | 6 (0.008) | 4 (0.004) | 2.36 (0.67, 8.38) | 0.16 | 16 (0.007) | 22 (0.006) | 1.11 (0.58, 2.12) | |
| ER−/PR− | 87 (0.055) | 142 (0.059) | 0.92 (0.70, 1.20) | 42 (0.057) | 58 (0.051) | 1.14 (0.76, 1.69) | 0.39 | 129 (0.055) | 200 (0.057) | 0.98 (0.79, 1.22) | |
| ER/PR/HER2 | 0.50 | ||||||||||
| Luminal A | 254 (0.16) | 419 (0.17) | 0.91 (0.78, 1.07) | 191 (0.26) | 289 (0.25) | 1.02 (0.85, 1.23) | 0.36 | 445 (0.19) | 708 (0.20) | 0.96 (0.85, 1.08) | |
| Luminal B | 124 (0.078) | 177 (0.074) | 1.05 (0.83, 1.32) | 64 (0.087) | 82 (0.072) | 1.23 (0.88, 1.70) | 0.45 | 188 (0.081) | 259 (0.073) | 1.10 (0.92, 1.33) | |
| Triple negative | 39 (0.024) | 71 (0.030) | 0.82 (0.55, 1.21) | 24 (0.033) | 36 (0.032) | 1.05 (0.63, 1.76) | 0.46 | 63 (0.027) | 107 (0.030) | 0.90 (0.66, 1.23) | |
| Her 2 overexpressing | 19 (0.012) | 31 (0.013) | 0.92 (0.52, 1.62) | 14 (0.019) | 13 (0.011) | 1.70 (0.80, 3.61) | 0.21 | 33 (0.014) | 44 (0.012) | 1.14 (0.73, 1.79) | |
Post-trial phase includes WHI extension data (data after March 31, 2005). 14716(80.8.%) of 18207 eligible participants randomized to Intervention, and (84.1%) of 27353 eligible participants randomized to Control, consented for the extension and had extension follow-up.
From a proportional hazards model stratified by age group (50–54, 55–59, 60–69, 70–79), hormone trial and randomization arm, and trial phase (time-dependent). Time to event equals zero on date of randomization. Tests whether the hazard ratio for the trial phase equals the hazard ratio for the post-trial phase.
Data as of September 17,2012; Events through September30, 2010.
From a proportional hazards model stratified by age group (50–54, 55–59, 60–69, 70–79), hormone trial and randomization arm. Time to event equals zero on date of randomization.
From a proportional hazards model stratified by age group (50–54, 55–59, 60–69, 70–79), hormone trial and randomization arm. Time to event equals zero on April 1, 2005.
P-het from a competing risks analysis that tests whether hazard ratios differ between tumor types.
Sensitivity Analyses
In general, estimates of hazard ratios obtained by accounting for censoring due to lack of consent for post-intervention follow-up via inverse probability weighting were similar to those presented above. For example, the post-intervention HR for invasive breast cancer changed from 1.08 to 1.07.
DISCUSSION
The WHI Dietary Modification trial was the first long-term, randomized controlled low-fat dietary intervention study to test the hypothesis that adoption of a low fat eating pattern after menopause would yield a reduction in invasive breast cancer and/or colorectal cancer incidence (11). Here we present the longer term cancer outcomes for the WHI DM trial participants. These analyses show that risk reduction for invasive breast was not evident during the additional 5.2 years of post-intervention follow-up period, despite some suggestion of a risk reduction during the intervention period for a subgroup of women who entered the trial with relatively high self-reported dietary fat intake.
At the time the WHI DM trial was designed, efforts to protect the statistical power included setting an exclusionary criterion for women who reported dietary intake below 32% of total energy as fat with the expectation that the greatest benefit would occur among women with habitual diets comparatively higher in fat and for whom a greater overall reduction in fat intake would be required to achieve study dietary goals. At the end of the intervention period women who reported the highest dietary fat intake at baseline (≥ 36.8% of energy from fat) and were assigned to the low-fat diet arm demonstrated reduced risk for invasive breast cancer that was nominally significant compared to women randomized to the comparison group (HR 0.78 95% CI 0.64–0.95), but this was not evident during the post-intervention period.
The non-significant post-intervention breast cancer HR of 1.08 suggests no influence of assignment to the low-fat diet on breast cancer risk during the post-intervention period. However, the cumulative data are evidently less consistent with an overall null hypothesis and more consistent with an alternative hypothesis in which there is a real (but not quite significant) reduction during the intervention period, followed by no effect during the post-intervention period. Speculatively, this may suggest that any protective effect of a low-fat intervention does not carry forward after stopping the intervention, when dietary fat intake tended to increase. The data may be most consistent with the possibility that the intervention induced a delayed onset of clinically detectable breast cancer, with catch-up occurring during the post-intervention period.
Overall dietary fat, although not reduced on average to the goal of 20% of total energy intake, was significantly lower for women in the intervention versus comparison diet group during the trial. Diet measures in a subgroup of DM women who continued to be observed during the extension period (n =1311) suggest that dietary fat intake remained somewhat lower in the women randomized to the low-fat diet group, although fat intake appeared to increase in the intervention group with time. Intervention women who reported fat intake being in the highest quartile of energy intake as fat at baseline may have experienced a risk reduction that reflected reduced energy exposure given the higher caloric value of dietary fat versus other macronutrients (23). Earlier analysis of calibrated energy intake in DM women supports an increased breast cancer risk in relation to energy intake (24).
In the subgroup of women wherein risk differed during the intervention period versus post-intervention period, one might argue that any liberalization of dietary fat (and potentially energy) intake after the intervention period may have contributed to an increase in body weight and as a consequence, mediating factors such as circulating cytokines, insulin or estrogen levels could have promoted breast cancer events. In fact, there was an overall reduction in body weight and body fat in women randomized to the low-fat diet versus comparison arms of WHI DM during the early (12 months) period of intervention (25, 26) partly supporting this hypothesis. But the mean difference in BMI between the two randomization groups was not significant after 7 years of follow-up. What is unknown is if the weight loss was associated with a lowering of inflammatory, metabolic and/or estrogen levels such that modification of risk for additional cancer events could be realized in such a short follow-up period. Evidence from WHI did show a lower estradiol level in women randomized to the low fat intervention versus comparison arms, but while significant, the difference between the randomization groups may not have been pronounced enough to lead to a differential risk for breast cancer. Importantly, adjustment for change in body weight during the trial period did not appreciably change the hazard ratios for the remaining cancer outcomes (data not shown). The potential modulating effects of body weight, and related biological exposures, could be evaluated in future analysis of WHI data.
In WHI the evidence for breast cancer risk reduction with the low-fat diet intervention was restricted to ER+/PR− tumor subtypes (HR − 0.69; 95% CI: 0.53–0.89 during intervention period; HR − 0.76; 95% CI: 0.48–1.20 during the post-intervention period). Importantly, these tumors express higher proliferation markers and thus could be fueled by insulin and inflammation as well as estrogen, metabolic exposures also associated with obesity. Only one other intervention trial evaluated dietary fat in relation to breast cancer risk by tumor subtypes. In a trial of 4690 conducted in Canada, women with high breast density, many of whom were pre-menopausal, were randomized to a diet of 15% total energy as fat. A total of 118 invasive breast cancer occurred over an average follow up period of 10 years. No overall intervention effect was demonstrated. Elevated risk for ER+ breast cancer was described for women who reported lower carbohydrate at baseline and during active trial participation (27). Further, results of observational studies have reported that dietary fat, and in particular animal fat, may be associated with greater risk across all common tumor subtypes (22).
Earlier analysis suggested a potential reduction in ovarian cancer risk related to the low-fat diet assignment, but only after 4 years of intervention (10). Results from this analysis are generally equivalent when evaluating ovarian cancers occurring after 4 years on intervention period through the end of this extended observation period for events (HR(95% CI) = 0.79(0.59–1.06). Although not significant, these results suggest that the role of low fat diet in ovarian cancer risk warrants further study. A lack of significant effect of the low fat eating plan on colorectal and total cancers and on cancer-specific and overall mortality was observed throughout the intervention and post-intervention periods.
The current report providing data on the longer term, post-intervention period of cancer outcomes in the WHI dietary modification trial has limitations. First, while the assessment of clinical outcomes over time followed the same protocol used during the intervention period, information on dietary intake is limited to a small subset of women providing serial 24 hour dietary recalls and may not reflect dietary changes in the overall post-intervention sample. Further, it is unknown whether other lifestyle changes such as changes in body weight, alcohol consumption, physical activity or even health screening behavior could have influenced clinical outcomes during the post-intervention period as re-assessment of these variables were not collected during the post-intervention follow-up period. Finally, measurement error in self-reported dietary intake, including dietary fat intake, a factor used to select women for trial participation, and energy intake and/or expenditure continues to be of concern, particularly given the lack of a well-validated biomarker of dietary fat intake, with related uncertainty in the magnitude of dietary difference between randomization groups and with possibly reduced overall statistical power for the trial (28, 29). Further, dietary differences between the intervention and comparison groups are uncertain, as there may be differential reporting by randomization assignment. In the case of energy consumption, for which a reliable biomarker (DLW) exists, there appeared to be greater underreporting in the intervention versus comparison group, a difference of about 100 kcal/d when evaluated near the end of the intervention period (30). Future studies are intended that will apply blood and urine metabolomics for objective assessment of this difference during the intervention period.
Summary
The WHI DM low-fat diet intervention did not result in a significantly lower risk for breast, colorectal, ovarian or other cancers or cancer-specific or total mortality over the combined intervention and post-intervention period. Whether other diet interventions, integrated within a comprehensive lifestyle approach that considers physical activity and weight control will show efficacy in primary prevention of cancer is yet to be determined. Our observational analysis from WHI support this hypothesis (31).
Supplementary Material
ACKNOWLEDGMENTS
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Harvard School of Public Health, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis H.Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Acknowledgments also go to Nicole Bergier, University of Arizona who supported the formatting and citation reporting for this work.
Funding:
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C as well as Arizona Comprehensive Cancer Center Support grant funded by NIH-NCI grant # CCSG-CA023074. (Cynthia A. Thomson, Linda Van Horn, Bette J. Caan, Aaron K. Aragaki, Rowan T. Chlebowski, JoAnn E. Manson, Thomas E. Rohan, Lesley F. Tinker, Lewis H. Kuller, Lifang Hou, Dorothy S. Lane, Karen C. Johnson, Mara Z. Vitolins, Ross Prentice).
REFERENCES
- 1.IARC Handbooks on Cancer Prevention. World Health Organization Press; 2003. [Google Scholar]
- 2.Carroll KK, Khor HT. Dietary fat in relation to tumorigenesis. Prog Biochem Pharmacol. 1975;10:308–353. [PubMed] [Google Scholar]
- 3.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 4.Insull W, Jr, Henderson MM, Prentice RL, Thompson DJ, Clifford C, Goldman S, et al. Results of a randomized feasibility study of a low-fat diet. Arch Intern Med. 1990;150:421–427. [PubMed] [Google Scholar]
- 5.Henderson MM, Kushi LH, Thompson DJ, Gorbach SL, Clifford CK, Insull W, Jr, et al. Feasibility of a randomized trial of a low-fat diet for the prevention of breast cancer: dietary compliance in the Women's Health Trial Vanguard Study. Prev Med. 1990;19:115–133. doi: 10.1016/0091-7435(90)90014-b. [DOI] [PubMed] [Google Scholar]
- 6.Freedman L, Anderson G, Kipnis V, Prentice R, Wang CY, Rossouw J, et al. Approached to monitoring the results of long-term disease prevention trials: examples from the Women's Health Initiative. Control Clin Trials. 1996;17:509–525. doi: 10.1016/s0197-2456(96)00016-5. [DOI] [PubMed] [Google Scholar]
- 7.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 8.Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 9.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 10.Prentice RL, Thomson CA, Caan B, Hubbell FA, Anderson GL, Beresford SA, et al. Low-fat dietary pattern and cancer incidence in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2007;99:1534–1543. doi: 10.1093/jnci/djm159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritenbaugh C, Patterson RE, Chlebowski RT, Caan B, Fels-Tinker L, Howard B, et al. The Women's Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 12.Patterson RE, Kristal A, Rodabough R, Caan B, Lillington L, Mossavar-Rahmani Y, et al. Changes in food sources of dietary fat in response to an intensive low-fat dietary intervention: early results from the Women's Health Initiative. J Am Diet Assoc. 2003;103:454–460. doi: 10.1053/jada.2003.50068. [DOI] [PubMed] [Google Scholar]
- 13.Tinker LFBE, Henry H, Patterson R, Rupp L, Van Horn L. The Women's Health Initiative: overview of the nutrition components. Gaithersburg MD: ASPEN publishers; 1996. [Google Scholar]
- 14.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Brunner RL, Cochrane B, Jackson RD, Larson J, Lewis C, Limacher M, et al. Calcium, vitamin D supplementation, and physical function in the Women's Health Initiative. J Am Diet Assoc. 2008;108:1472–1479. doi: 10.1016/j.jada.2008.06.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 17.Moshfegh AJ, Raper N, Ingwersen L, Cleveland L, Goldman J, Lacomb R. An improved approach to 24-hour dietary recall methodology. Ann Nutr Metab. 2001;45(suppl):156. (abstr). [Google Scholar]
- 18.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roswall N, Olsen A, Christensen J, Dragsted LO, Overvad K, Tjonneland A. Micronutrient intake and breast cancer characteristics among postmenopausal women. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2010;19:360–365. doi: 10.1097/cej.0b013e32833ade68. [DOI] [PubMed] [Google Scholar]
- 21.Zhang CX, Ho SC, Cheng SZ, Chen YM, Fu JH, Lin FY. Effect of dietary fiber intake on breast cancer risk according to estrogen and progesterone receptor status. European journal of clinical nutrition. 2011;65:929–936. doi: 10.1038/ejcn.2011.57. [DOI] [PubMed] [Google Scholar]
- 22.Bao PP, Shu XO, Zheng Y, Cai H, Ruan ZX, Gu K, et al. Fruit, vegetable, and animal food intake and breast cancer risk by hormone receptor status. Nutrition and cancer. 2012;64:806–819. doi: 10.1080/01635581.2012.707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prentice RL, Neuhouser ML, Tinker LF, Pettinger M, Thomson CA, Mossavar-Rahmani Y, et al. An Exploratory Study of Respiratory Quotient Calibration and Association with Postmenopausal Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-13-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentice RL, Shaw PA, Bingham SA, Beresford SA, Caan B, Neuhouser ML, et al. Biomarker-calibrated energy and protein consumption and increased cancer risk among postmenopausal women. American journal of epidemiology. 2009;169:977–989. doi: 10.1093/aje/kwp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carty CL, Kooperberg C, Neuhouser ML, Tinker L, Howard B, Wactawski-Wende J, et al. Low-fat dietary pattern and change in body-composition traits in the Women's Health Initiative Dietary Modification Trial. The American journal of clinical nutrition. 2011;93:516–524. doi: 10.3945/ajcn.110.006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, et al. Low-fat dietary pattern and weight change over 7 years: the Women's Health Initiative Dietary Modification Trial. JAMA. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 27.Martin LJ, Li Q, Melnichouk O, Greenberg C, Minkin S, Hislop G, et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011;71:123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- 28.Prentice RL, Pettinger M, Tinker LF, Huang Y, Thomson CA, Johnson KC, et al. Regression calibration in nutritional epidemiology: example of fat density and total energy in relationship to postmenopausal breast cancer. American journal of epidemiology. 2013;178:1663–1672. doi: 10.1093/aje/kwt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuhouser ML, Di C, Tinker LF, Thomson C, Sternfeld B, Mossavar-Rahmani Y, et al. Physical activity assessment: biomarkers and self-report of activity-related energy expenditure in the WHI. American journal of epidemiology. 2013;177:576–585. doi: 10.1093/aje/kws269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuhouser ML, Tinker L, Shaw PA, Schoeller D, Bingham SA, Horn LV, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. American journal of epidemiology. 2008;167:1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 31.Thomson CA, McCullough ML, Wertheim BC, Chlebowski RT, Martinez ME, Stefanick ML, et al. Nutrition and physical activity cancer prevention guidelines, cancer risk, and mortality in the women's health initiative. Cancer prevention research. 2014;7:42–53. doi: 10.1158/1940-6207.CAPR-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.