Abstract
Addition of sucrose to a solution bathing an excised developing soybean cotyledon causes a transient depolarization of the membrane potential, as measured using standard electrophysiological techniques. The magnitude of the depolarization is dependent on the concentration of both sucrose and protons in a manner which suggests carrier mediation; this process has an apparent Km for sucrose of about 10 millimolar. Agents interfering with the generation or maintenance of a proton electrochemical gradient eliminate these depolarizations. Electrogenic sugar transport is sensitive to sulfhydryl-modifying reagents; their effect appears to be through a direct interaction with the carrier protein and/or with the process establishing the proton electrochemical gradient across the plasma membrane. p-Chloromercuribenzene sulfonate appears to be a selective inhibitor of the carrier-mediated process itself.
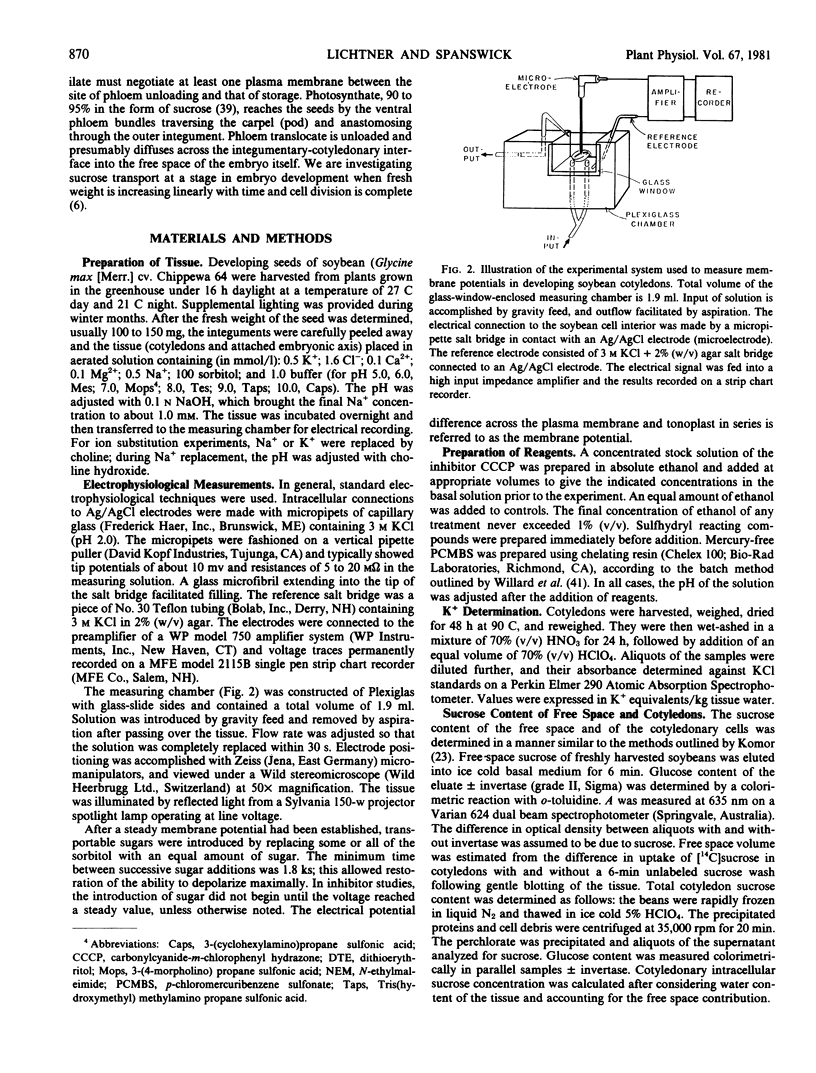
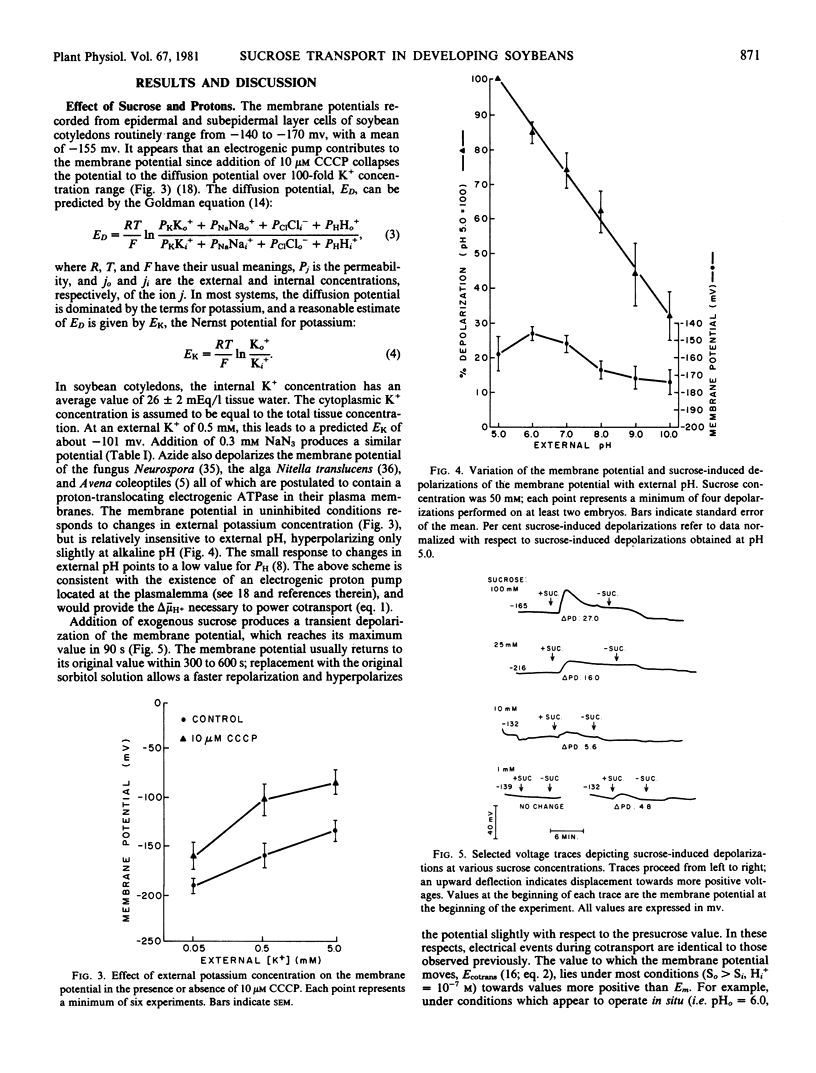
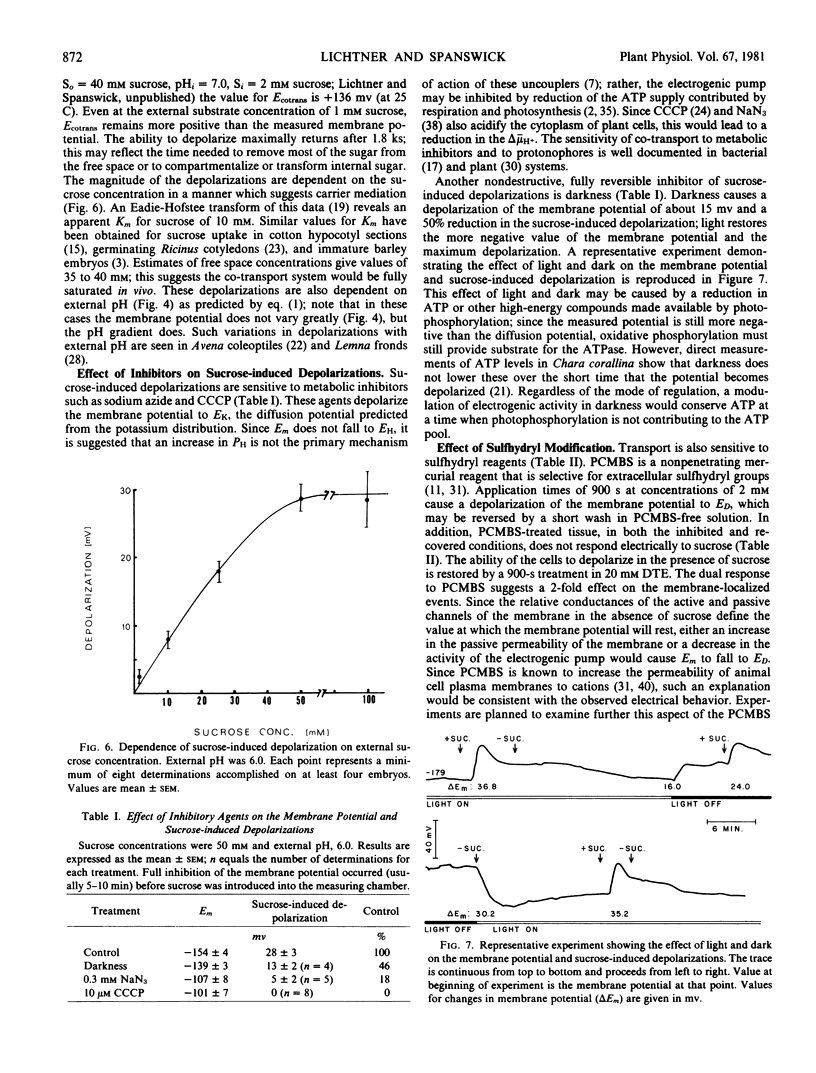
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Crane R. K. The gradient hypothesis and other models of carrier-mediated active transport. Rev Physiol Biochem Pharmacol. 1977;78:99–159. doi: 10.1007/BFb0027722. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T. Phloem loading of sucrose: involvement of membrane ATPase and proton transport. Plant Physiol. 1979 Apr;63(4):744–748. doi: 10.1104/pp.63.4.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hampson S. E., Loomis R. S., Rains D. W. Characteristics of sugar uptake in hypocotyls of cotton. Plant Physiol. 1978 Dec;62(6):846–850. doi: 10.1104/pp.62.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Patel L. The role of functional sulfhydryl groups in active transport in Escherichia coli membrane vesicles. Biochemistry. 1978 May 2;17(9):1640–1646. doi: 10.1021/bi00602a010. [DOI] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor E., Schwab W. G., Tanner W. The effect of intracellular pH on the rate of hexose uptake in Chlorella. Biochim Biophys Acta. 1979 Aug 23;555(3):524–530. doi: 10.1016/0005-2736(79)90406-1. [DOI] [PubMed] [Google Scholar]
- Komor E., Weber H., Tanner W. Essential Sulfhydryl Group in the Transport-catalyzing Protein of the Hexose-Proton Cotransport System of Chlorella. Plant Physiol. 1978 May;61(5):785–786. doi: 10.1104/pp.61.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Cell potentials, cell resistance, and proton fluxes in corn root tissue: effects of dithioerythritol. Plant Physiol. 1976 Sep;58(3):276–282. doi: 10.1104/pp.58.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The driving force for proton(s) metabolites cotransport in bacterial cells. FEBS Lett. 1976 Jul 15;66(2):159–163. doi: 10.1016/0014-5793(76)80493-0. [DOI] [PubMed] [Google Scholar]
- Sacher J. A. The regulation of sugar uptake and accumulation in bean pod tissue. Plant Physiol. 1966 Jan;41(1):181–189. doi: 10.1104/pp.41.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough G. A. Properties of Neurospora crassa plasma membrane ATPase. Arch Biochem Biophys. 1977 Apr 30;180(2):384–393. doi: 10.1016/0003-9861(77)90052-2. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M., Miller A. G. Measurement of the Cytoplasmic pH in Nitella translucens: Comparison of Values Obtained by Microelectrode and Weak Acid Methods. Plant Physiol. 1977 Apr;59(4):664–666. doi: 10.1104/pp.59.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERNON L. P., ARONOFF S. Metabolism of soybean leaves. IV. Translocation from soybean leaves. Arch Biochem Biophys. 1952 Apr;36(2):383–398. doi: 10.1016/0003-9861(52)90424-4. [DOI] [PubMed] [Google Scholar]
- Will P. C., Hopfer U. Apparent inhibition of active non-electrolyte transport by an increased sodium permeability of the plasma membrane. Mechanism of action of p-chloromercuribenzene sulfonate. J Biol Chem. 1979 May 25;254(10):3806–3811. [PubMed] [Google Scholar]
- Willard J. M., Davis J. J., Wood H. G. Phosphoenolpyruvate carboxytransphosphorylase. IV. Requirement for metal cations. Biochemistry. 1969 Aug;8(8):3137–3144. doi: 10.1021/bi00836a002. [DOI] [PubMed] [Google Scholar]


