Abstract
Background
Uveal melanoma is the most common primary intraocular tumor in adults and up to 50% of patients will develop liver metastases. Complete surgical resection of these metastases can improve 5-year survival, but only a few patients are eligible for radical surgical treatment. The aim of this study was to introduce a near-infrared (NIR) fluorescence laparoscope during minimally-invasive surgery for intraoperative identification of uveal melanoma hepatic metastases and to use it to provide guidance during resection.
Methods
Three patients diagnosed with one solitary liver metastasis from uveal melanoma are presented. Patients received 10 mg indocyanine green (ICG) intravenously 24 h before surgery. A NIR fluorescence laparoscope was used to detect malignant liver lesions.
Results
In all 3 patients, laparoscopic NIR fluorescence imaging using ICG successfully identified uveal melanoma metastases. In 2 patients, multiple additional lesions were identified by inspection and NIR fluorescence imaging, which were not identified by preoperative conventional imaging. In one patient, one additional lesion, not identified by computed tomography, magnetic resonance imaging, laparoscopic ultrasonography and inspection, was observed with NIR fluorescence imaging only.. Importantly, NIR fluorescence imaging provided guidance during resection of these metastases.
Conclusions
We describe the successful use of laparoscopic identification and resection of uveal melanoma liver metastases using NIR fluorescence imaging and ICG. This procedure is minimally-invasive, and should be used as complementary to conventional techniques for the detection and resection of liver metastases.
INTRODUCTION
Uveal melanoma is the most common primary intraocular tumor in adults and up to 50% of patients will ultimately develop distant metastases [1]. In 90–95% of these patients, the metastases will involve the liver [2,3]. Complete resection of these metastases can prolong survival, however only few patients are eligible for radical surgical treatment [4–6]. Therefore, it is of great importance to select and treat these patients carefully, to prevent unnecessary laparotomies and to fully resect sites of metastasis. Minimally-invasive procedures have become important in daily clinical practice, are increasingly used for liver surgery, and might help to optimize the resection of malignant disease.
Near-infrared (NIR) fluorescence imaging using indocyanine green (ICG) is a promising technique to assist in the intraoperative identification of liver metastases in real time [7–10]. ICG is excreted exclusively into the bile after intravenous injection and it has been hypothesized that colorectal liver metastases can be visualized due to passive accumulation of ICG caused by hampered biliary excretion, which results in a fluorescent rim around metastases [7,11].
Several clinical studies describe the use of NIR fluorescence imaging to visualise primary hepatocellular carcinomas[8], as well as liver metastases from colorectal[12], and pancreatic cancers[9] after intravenous injection of ICG, 1 to 14 days prior to surgery. The dose and interval between ICG administration and surgery are key determinants of the remaining background fluorescence signal in the liver and the fluorescent rim surrounding the tumor. A dose finding study using an open intraoperative imaging system (Mini-FLARE[13]) in patients with colorectal liver metastases was recently performed in our center[12], and 10 mg ICG administered 24 h before surgery was found to be the most favorable dosage and time point of ICG administration. Moreover, in 12.5% of patients additional small and superficially located lesions were detected using NIR fluorescence, which were otherwise undetectable.
Published cases to date for intraoperative detection of liver metastases using NIR fluorescence and ICG were performed as open procedures. A laparoscopic operation is preferable for patients with liver metastases from uveal melanoma, due to the high risk of multiple small metastases. When multiple metastases are identified during surgery, patients would not benefit from liver resection, and can be saved an unnecessary laparotomy by performing this minimally-invasive technique. This technical report is the first to describe the use of ICG and laparoscopic NIR fluorescence imaging to identify and resect melanoma liver metastasis during laparoscopic liver surgery.
METHODS
Intraoperative Laparoscopic Near-Infrared Imaging System
Intraoperative NIR fluorescence imaging of the liver was performed using a newly developed laparoscopic high definition (HD) fluorescence imaging system by Karl Storz, Germany, which included a plasma light guide and a 30-degree angle, 10-mm laparoscope, applicable for white light (WL), autofluorescence, and ICG-imaging. The system was used for intraoperative conventional imaging (WL mode) and real-time fluorescence imaging (760-nm light, ICG mode) and allowed easy switching between WL mode and ICG mode by using a foot pedal. No overlay of conventional and fluorescent images was possible yet, but anatomical orientation could be maintained due to the easy switching between light modes, the anatomical translucence during ICG mode and the stable position of the laparoscope. Images were recorded using a charge-coupled device camera.
Preparation and Administration of Indocyanine Green
ICG (25 mg vials) was purchased from Pulsion Medical Systems (Munich, Germany) and resuspended in 10 cc of sterile water for injection to yield a 2.5-mg/ml (3.2 mM) stock solution. Of this stock solution 4 mL, corresponding to a dose of 10 mg, was administered intravenously 24 h before surgery. This optimal timing was based on previous studies in patients with colorectal liver metastases and the Mini-FLARE open quantitative imaging system.
Ex vivo Imaging and Fluorescence Microscopy of Resected Lesion
After slicing of the resected lesion at the Pathology department, fluorescent imaging was performed with the FLARE imaging system, which was previously described [14]. Fluorescence microscopy images were obtained with the Odyssey Infrared Imaging System (LI-COR, USA).
Preoperative Imaging Modalities
Patients received standard-of-care preoperative imaging modalities including ultrasound (US), computed tomography (CT, Toshiba Aquilion 16), magnetic resonance imaging (MRI, Philips Intera 1.5T) and/or positron emission tomography (PET, Philips Gemini 64 PET/CT) scans. For PET scan imaging, the radiopharmaceutical agent 18F-FDG was used.
Clinical Trial
The study was approved by the Local Medical Ethics Committee of the Leiden University Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975. The study was registered in the Netherlands Trial Register as NTR3869.
RESULTS
We present three patients; all diagnosed with a solitary liver metastasis by preoperative US or CT. One patient was referred from another hospital, diagnosed with uveal melanoma and a solitary liver metastasis. Two patients were previously treated for uveal melanoma.
The first patient was a 75-year old woman, referred from another hospital, diagnosed with uveal melanoma of the left eye. By US, CT and PET scans, a suspected 28 mm metastasis was found in the right liver lobe. She was planned for simultaneous laparoscopic metastasectomy and enucleation of the left eye. 10 mg ICG was administered intravenously 24 h before surgery. During surgery, multiple lesions of 1 to 10 mm were identified on the surface of the left and right liver lobe by inspection and by a clear fluorescent rim around the tumors (Figure 1). One of the suspected lesions proved to be malignant, and no further liver resection was performed. Enucleation of the left eye was performed, and patient was offered systemic therapy. Patient experienced progressive disease under Dacarbazine treatment and subsequent Ipilimumab treatment during 10 months of follow-up.
Figure 1. Identification of liver metastases.
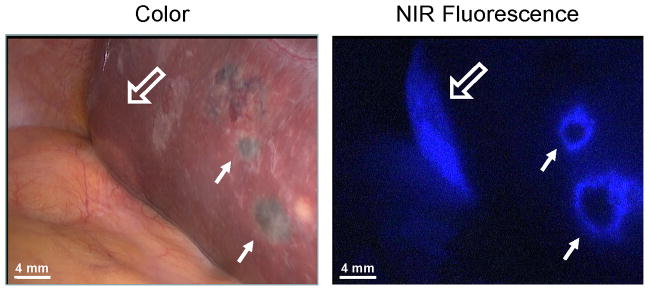
Multiple uveal liver metastases, identified by inspection and NIR fluorescence as a typical fluorescent rim (arrows) around each lesion, 24 h after intravenous injection of 10 mg ICG. Open arrows indicate the preoperative known metastasis.
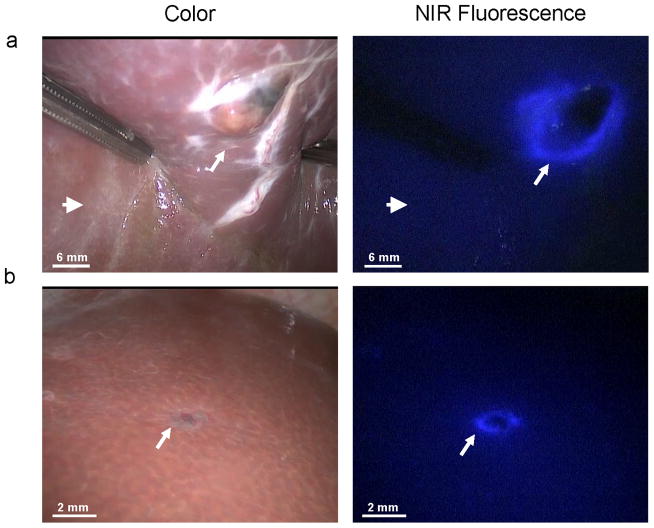
The second patient was a 66-year-old woman treated 22 months earlier by enucleation of the right eye because of uveal melanoma. During 20 months of follow-up using US, CT, MRI and PET scans, no liver metastases were seen. Now she was diagnosed with a 12 mm liver metastasis in liver segment III on both CT and MRI scans. There were no signs of other hepatic or extrahepatic metastatic lesions. She was planned for laparoscopic metastasectomy. 10 mg ICG was administered intravenously 24 h before surgery. The previously diagnosed lesion was detected by inspection, laparoscopic ultrasonography (LUS), and NIR fluorescence, and was removed under fluorescent guidance (Figures 2a and 3; supplementary video’s). Using NIR fluorescence, an additional suspicious lesion in segment III was identified, which showed a typical 1.5 mm fluorescent rim (Figure 2b). This lesion was characterized as a hemangioma by inspection, and was not identified with LUS. It was resected under fluorescent guidance. Both removed lesions proved to be malignant by histological assessment and were radically removed (Figure 4). No other lesions were identified by inspection, LUS or NIR fluorescence imaging. Patient recovered well from surgery, and no recurrent disease was seen during 15 months of follow-up.
Figure 2. NIR fluorescence imaging of uveal liver metastases.
A. An uveal liver metastasis (arrow) is clearly identified by a rim around the tumor in vivo. Normal liver tissue (arrowhead) shows minimal background fluorescence. B. A small superficial metastasis (arrow) was identified by NIR fluorescence imaging, but was characterized as a hemangioma by visual inspection and was not visible by LUS.
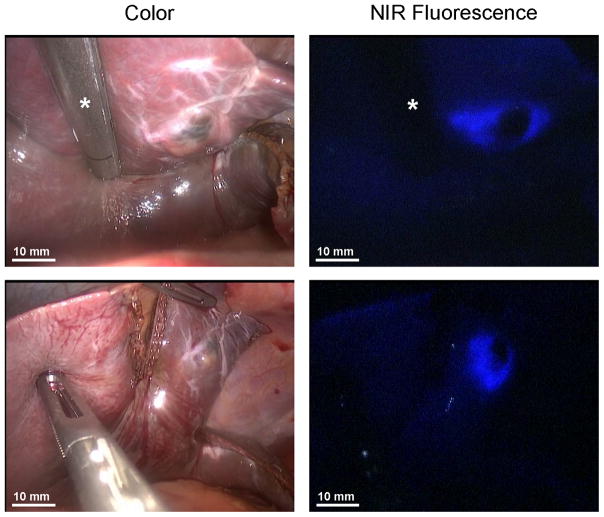
Figure 3. Fluorescent guidance during resection.
NIR fluorescence imaging was used as guidance during resection of uveal liver metastases. Resection was performed using an Endo GIA™ 45 reload stapler (Covidien) (asterisk).
Figure 4. Ex vivo imaging and fluorescence microscopy of resected lesion.
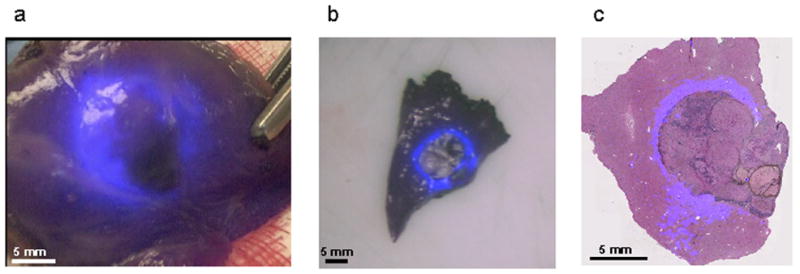
A. Ex vivo imaging of resected lesion using the laparoscope. B. Ex vivo imaging after slicing under FLARE imaging system guidance. C. Microscopic overlay of Hematoxylin and Eosin staining of the resected lesion and fluorescent signal (Odyssey Infrared Imaging System, LI-COR). A clear fluorescent rim was observed around the malignant lesion.
The third patient was a 65-year-old woman treated 6 months earlier with ruthenium for uveal melanoma. She was now diagnosed with a 17 mm liver metastasis in segment IV on US and CT. There were no signs of other liver lesions or extrahepatic disease and she was planned for a laparoscopic metastasectomy. 10 mg ICG was administered intravenously 24 h before surgery. During surgery, multiple lesions from 1 to 10 mm were identified on the surface of the left and right liver lobe by inspection and a clear fluorescent rim around the tumor using the ICG mode of the laparoscope. Uveal melanoma metastases were confirmed by frozen section of two lesions in segments III and IVb, respectively. No further resections were performed and the patient was offered systemic therapy. Patient experienced stable disease for 16 months under protein kinase C inhibitor treatment, whereafter new progression was seen.
No adverse reactions associated with the use of ICG or fluorescent imaging were observed in any patient.
DISCUSSION
Because only 3% [15,16] of patients with uveal melanoma are candidates for curative resection of hepatic metastases, a precise and minimally invasive method for selecting appropriate candidates is desperately needed. To the best of our knowledge, this is the first paper describing the use of laparoscopic NIR fluorescence imaging with ICG for the detection and removal of liver metastasis in this disease. Due to hampered visibility and the inability to palpate the liver surface during laparoscopy, NIR fluorescence imaging adds value to the procedure itself. In contrast to ultrasonography, which can only show where the resection margins can be kept before resection, NIR fluorescence imaging provides real-time guidance, helps minimize the resection of normal liver tissue, and permits inspection of the area to ensure the completeness of tumor resection.
In all three patients of this study, lesions were found by NIR fluorescence that were not seen on preoperative US and CT scans. Moreover, in one patient, one lesion intraoperatively characterized as hemangioma by visual inspection, and not identified by LUS, was successfully identified as metastasis with NIR fluorescence imaging and could be radically removed under real-time fluorescence guidance.
A major limitation of NIR fluorescence imaging is its limited depth penetration of ≈ 5 mm. In figure 4, a depth penetration of at least 5 mm can be inferred because the NIR fluorescence signal of the lesion was identified at the anterior surface of the liver (supplementary video part 1), while the lesion itself was located at the posterior surface of the liver. More sensitive imaging systems and fluorophores with higher quantum yield could potentially increase depth penetration even further. Preoperative CT scanning and LUS do not have this disadvantage and are consequently more appropriate for deeper lesions. However, small and superficially located occult metastases are known to be difficult to detect using LUS and inspection. Although LUS is a reliable modality to identify liver metastases[17], and still required to identify deep (≥ 5 mm) metastases in the liver, our results suggest that NIR fluorescence imaging is complementary, and helps to find small, superficially located lesions. In addition, as shown in the supplementary video, NIR fluorescence imaging becomes of value in the resection of even deep lesions once they are initially identified.
Further technical developments of laparoscopic NIR imaging systems are in progress and aim to improve the real-time intraoperative display of NIR fluorophores[18,19].
In conclusion, we describe the successful use of a novel NIR fluorescence laparoscopy procedure for the identification and resection of uveal melanoma liver metastasis using 10 mg ICG administered intravenously 24 h prior to surgery. This procedure is minimally-invasive, relatively easy to perform, and is complementary to conventional techniques for the detection and complete resection of liver metastases from uveal melanoma.
Acknowledgments
This study was performed within the framework of CTMM, the Center for Translational Molecular Medicine (MUSIS project, grant 03O-202). This work was supported by the Dutch Cancer Society grant UL2010-4732 and NIH grant R01-CA-115296. We thank Karl Storz for supplying the laparoscopic near-infrared imaging system. Q.R.J.G. Tummers and F.P.R. Verbeek share first authorship.
Footnotes
Oral presentation: This study is accepted for oral presentation during the 21st International Congress of the EAES in Vienna, 19–22 June 2013.
DISCLOSURES
Q.R.J.G. Tummers, F.P.R. Verbeek, H.A.J.M. Prevoo, A.E. Braat, C.I.M. Baeten, C.J.H. van de Velde and A.L. Vahrmeijer have no conflicts of interest or financial ties to disclose. Dr. J.V. Frangioni: FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. It has been licensed to the FLARE™ Foundation, a non-profit organization focused on promoting the dissemination of medical imaging technology for research and clinical use. Dr. Frangioni is the founder and chairman of the FLARE™ Foundation. The Beth Israel Deaconess Medical Center will receive royalties for sale of FLARE™ Technology. Dr. Frangioni has elected to surrender post-market royalties to which he would otherwise be entitled as inventor, and has elected to donate pre-market proceeds to the FLARE™ Foundation.
Contributor Information
Quirijn R.J.G. Tummers, Email: q.r.j.g.tummers@lumc.nl.
Floris P.R. Verbeek, Email: f.p.r.verbeek@lumc.nl.
Hendrica A.J.M. Prevoo, Email: h.a.j.m.prevoo@lumc.nl.
Andries E. Braat, Email: a.e.braat@lumc.nl.
Coen I.M. Baeten, Email: c.i.m.baeten@lumc.nl.
John V. Frangioni, Email: jfrangio@bidmc.harvard.edu.
Cornelis J.H. van de Velde, Email: c.j.h.van_de_velde@lumc.nl.
References
- 1.Bedikian AY. Metastatic uveal melanoma therapy: current options. Int Ophthalmol Clin. 2006;46(1):151–166. doi: 10.1097/01.iio.0000195852.08453.de. [DOI] [PubMed] [Google Scholar]
- 2.Spagnolo F, Caltabiano G, Queirolo P. Uveal melanoma. Cancer Treat Rev. 2012;38(5):549–553. doi: 10.1016/j.ctrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Becker JC, Terheyden P, Kampgen E, Wagner S, Neumann C, Schadendorf D, et al. Treatment of disseminated ocular melanoma with sequential fotemustine, interferon alpha, and interleukin 2. Br J Cancer. 2002;87(8):840–845. doi: 10.1038/sj.bjc.6600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsueh EC, Essner R, Foshag LJ, Ye X, Wang HJ, Morton DL. Prolonged survival after complete resection of metastases from intraocular melanoma. Cancer. 2004;100(1):122–129. doi: 10.1002/cncr.11872. [DOI] [PubMed] [Google Scholar]
- 5.Frenkel S, Nir I, Hendler K, Lotem M, Eid A, Jurim O, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009;93(8):1042–1046. doi: 10.1136/bjo.2008.153684. [DOI] [PubMed] [Google Scholar]
- 6.Vahrmeijer AL, van de Velde CJ, Hartgrink HH, Tollenaar RA. Treatment of melanoma metastases confined to the liver and future perspectives. Dig Surg. 2008;25(6):467–472. doi: 10.1159/000184738. [DOI] [PubMed] [Google Scholar]
- 7.Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115(11):2491–2504. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh K, Yamada T, Ishikawa O, Takahashi H, Eguchi H, Yano M, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol. 2009;100:75–79. doi: 10.1002/jso.21272. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama N, Otani T, Hashidate H, Maeda C, Katada T, Sudo N, et al. Real-time detection of hepatic micrometastases from pancreatic cancer by intraoperative fluorescence imaging: Preliminary results of a prospective study. Cancer. 2011;118:2813–9. doi: 10.1002/cncr.26594. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Takatsuki M, Hidaka M, Hara T, Muraoka I, Soyama A, et al. Is a fluorescence navigation system with indocyanine green effective enough to detect liver malignancies? J Hepatobiliary Pancreat Sci. 2013 doi: 10.1002/jhbp.17. [DOI] [PubMed] [Google Scholar]
- 11.Verbeek FP, van der Vorst JR, Schaafsma BE, Hutteman M, Bonsing BA, van Leeuwen FW, et al. Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J Hepatobiliary Pancreat Sci. 2012;19:626–637. doi: 10.1007/s00534-012-0534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Vorst JR, Schaafsma BE, Hutteman M, Verbeek FP, Liefers GJ, Hartgrink HH, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer. 2013;119(18):3411–3418. doi: 10.1002/cncr.28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mieog JS, Troyan SL, Hutteman M, Donohue KJ, van der Vorst JR, Stockdale A, et al. Towards Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann Surg Oncol. 2011;18:2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16(10):2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman P, Machado MA, Montagnini AL, D’Albuquerque LA, Saad WA, Machado MC. Selected patients with metastatic melanoma may benefit from liver resection. World J Surg. 2007;31(1):171–174. doi: 10.1007/s00268-006-0375-z. [DOI] [PubMed] [Google Scholar]
- 16.Rose DM, Essner R, Hughes TM, Tang PC, Bilchik A, Wanek LA, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg. 2001;136(8):950–955. doi: 10.1001/archsurg.136.8.950. [DOI] [PubMed] [Google Scholar]
- 17.Vigano L, Ferrero A, Amisano M, Russolillo N, Capussotti L. Comparison of laparoscopic and open intraoperative ultrasonography for staging liver tumours. Br J Surg. 2013;100(4):535–542. doi: 10.1002/bjs.9025. [DOI] [PubMed] [Google Scholar]
- 18.Ashitate Y, Stockdale A, Choi HS, Laurence RG, Frangioni JV. Real-time simultaneous near-infrared fluorescence imaging of bile duct and arterial anatomy. J Surg Res. 2011 Jul;176(1):7–13. doi: 10.1016/j.jss.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsui A, Tanaka E, Choi HS, Winer JH, Kianzad V, Gioux S, et al. Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery. 2010;148(1):87–95. doi: 10.1016/j.surg.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]