Abstract
Purpose
There is great potential for real-time investigation of metabolism with MRS and hyperpolarized (HP) 13C agents. Unfortunately, HP technology has high associated costs and efficiency limitations that may constrain in vivo studies involving many animals. To improve the throughput of preclinical investigations, we evaluate the feasibility of performing HP MRS on multiple animals simultaneously.
Methods
Simulations helped assess the viability of a dual-coil strategy for spatially-localized multivolume MRS.A dual-mouse system was assembled and characterized based on bench- and scanner-based experiments. Enzyme phantoms mixed with HP [1-13C] pyruvate emulated real-time metabolism and offered a controlled mechanism for evaluating system performance. Finally, a normal mouse and a mouse bearing a subcutaneous xenograft of colon cancer were simultaneously scanned in vivo using an agent containing HP [1-13C] pyruvate.
Results
Geometric separation/rotation, active decoupling, and use of low input impedance preamplifiers permitted an encode-by-channel approach for spatially-localized MRS. A pre-calibrated shim allowed straightforward metabolite differentiation in enzyme phantom and in vivo experiments at 7 T, with performance similar to conventional acquisitions.
Conclusion
The initial feasibility of multi-animal HP 13C MRS was established. Throughput scales with the number of simultaneously-scanned animals, demonstrating the potential for significant improvements in study efficiency.
Keywords: Throughput, Hyperpolarized, 13C pyruvate, multianimal, multiple-mouse, metabolism, spectroscopy, colon cancer
Introduction
The complexity of cancer requires diverse interrogation for accurate diagnosis, disease staging, and evaluation of therapeutic response. Noninvasive examination of morphology and function are readily performed with MRI and biochemical processes may be evaluated with MRS. Recent advancements in hyperpolarized (HP) technology can overcome the detectability limitations of endogenous 13C by dramatically enhancing signal from exogenous 13C-labeled substrates (1). HP 13C MR shows great promise for real-time evaluation of cancer metabolism in vivo (2,3). Many malignancies exhibit high rates of aerobic glycolysis (4,5), and measuring the conversion of HP [1-13C] pyruvate into HP lactate can be used as a biomarker (6). Successful progress in HP 13C MR will enable quantitative evaluation of morphological, functional, and metabolic characteristics of tumors during one imaging session.
Dynamic nuclear polarization (DNP)methods require expensive consumables including cryogens, the 13C-enriched substrate, and the polarizing radical. Furthermore, achieving maximum enhancement for 13C-labeled metabolites requires lengthy polarization times. Rapid T1 decay to thermal equilibrium requires a newly polarized sample for each imaging session, imposing throughput limitations that are constrained by polarization time. With the commercially available HyperSense™ (Oxford Instruments, Abingdon, Oxfordshire, UK), a fixed 4-mL dissolution volume is required to counterbalance the frozen solid state agent to yield a biocompatible injectable that is at body temperature. Unfortunately, only about 5% (~200 μL) of the final agent volume may be safely injected into a mouse. Methods to improve the HP compound concentration for preclinical imaging are advantageous (7), but a significant fraction of the HP agent remains wasted for small-animal acquisitions.
The cost to acquire HP preclinical data can be prohibitive for experiments requiring a large number of animals, and new methods to reduce cost are desirable (2). Efficiency improvements are being investigated to provide cheaper alternatives for hyperpolarization, such as parahydrogen-based methods (8,9), and to automate and parallelize the polarization process with new polarizers (10) such as the SpinLab™ (GE Healthcare, Waukesha, WI). In this preliminary work, we assess the feasibility of performing HP 13C MRS on multiple animals simultaneously. By utilizing a higher percentage of the HP agent and by performing parallel experiments in vivo, we aim to reduce cost and increase throughput of preclinical HP 13C studies to promote the integration of HP MR into routine biological research.
Methods
Multianimal Approach
Previous multianimal imaging studies have used arrays of volume coils (11-15), arrays of surface coils (16-19), or a combination of the two (20). Volume coils are preferred when larger spatial coverage or improved isolation between imaging volumes with RF shielding are desired over the enhanced local sensitivity and parallel imaging (21) capability offered by arrays of receive coils. For HP 13C MRS, arrays of shielded volume coils require dual-tuning to provide the capability for 1H imaging and 13C detection, which inherently compromises sensitivity compared to equivalent single-tuned coils. Because arrays of surface coils offer enhanced local sensitivity to tumors, a low initial hardware cost, and maintain compatibility with available dual-tuned volume coils, this feasibility study considers an array of receive-only surface coils for dual-mouse MRS.
Spatial Encoding Strategy
Unlike MRI, where encoding gradients provide in-plane spatial localization, slice-selective pulse-acquire sequences for HP MRS cannot directly encode the spatial origin of metabolites. An encode-by-channel strategy (Figure 1) was therefore employed to achieve spatial localization for the HP MRS scans. A single axial slice was prescribed to contain all volumes of interest (VOIs) with distinct surface coils placed over each tumor and connected to separate 13C receive channels. Spatial localization was achieved through unique coil sensitivities alone and therefore mutual inductive coupling and intervolume sensitivity, defined as the ratio of a primary surface coil’s mean sensitivity to the secondary VOI with respect to its mean sensitivity to the primary VOI, were minimized.
Figure 1.
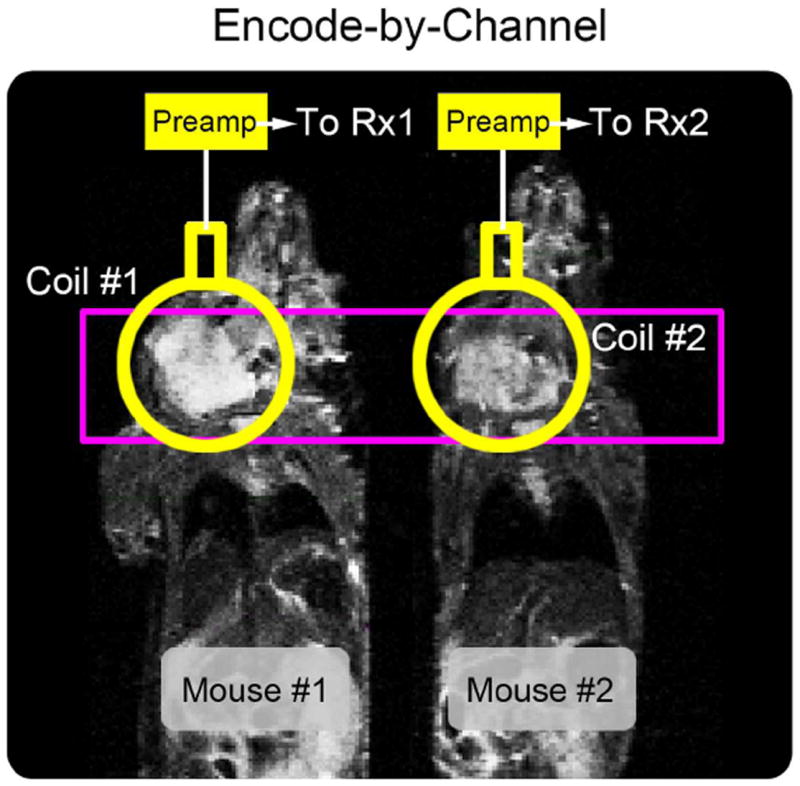
Multivolume spatial encoding strategy. The encode-by-channel method requires independent 13C receive channels, minimization of mutual inductive coupling between RF coils, and low intervolume sensitivities to achieve spatial localization for hyperpolarized MRS acquisitions.
Numerical Simulations
Numerical simulations were performed to gain insight regarding coil coupling and intervolume sensitivity. B1 fields of two virtual 16-mm diameter surface coils were generated using Matlab. Calculations were based on the Biot-Savart law, instead of a full-wave analysis, because coil size is very small compared to the wavelength at resonance for 13C at 7 T. The distance between coils was varied and each coil was rotated along its central axis to determine the rotation angle required for optimal geometric decoupling. Transverse B1 fields were used to form coil sensitivity maps (22), allowing the calculation of intervolume sensitivities to cylindrical phantoms located at each VOI.
Hardware Development
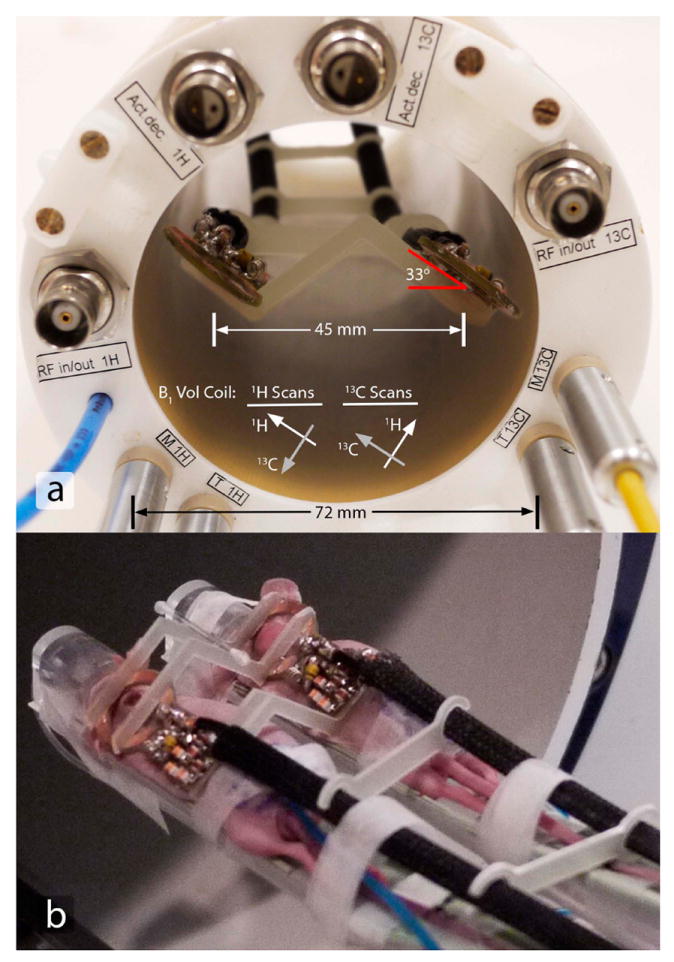
The infrastructure to support simultaneous dual-mouse experiments on a 7-T 30-cm bore BioSpec MRI system with BGA12 gradients (Bruker BioSpin MRI, Ettlingen, Germany) includes RF coils, a broadband multichannel receiver, and a dual-animal sled. An actively-detuned 72-mm inner diameter dual-tuned linear volume coil was used for 1H imaging and 13C transmission, and two 13C surface coils (two-turns, 16-mm diameter) were manufactured for enhanced reception. The coil substrates were fabricated using a circuit board milling machine (LPKF Laser and Electronics AG, Garbsen, Germany) and were populated with components for tuning (75.5 MHz at 7 T), 50 Ω matching, and active coil detuning to reduce interactions with the volume coil during transmit. As shown in Figure 2a, receive coils were each rotated by ~33° and rigidly fixed to geometrically minimize mutual inductive coupling between coils (see Results). To minimize interactions between 13C transmit and receive coils and shading caused by receive coils during 1H imaging, the volume coil was rotated such that the linear B1 fields for the nuclei of interest were parallel to the receive coil loops (Figure 2). The lengths of coaxial cables to low input impedance preamplifiers (Microwave Technology, Inc., Fremont, CA) were adjusted to reduce loop currents. Multichannel 13C acquisitions were supported by a custom 16-channel broadband receiver. A dual-mouse sled with water heating pads and anesthesia lines leading to nose cones was manufactured (Figure 3). Sled offsets could be independently adjusted along the B0 field and the sled mates with a rail system to provide global position adjustments.
Figure 2.
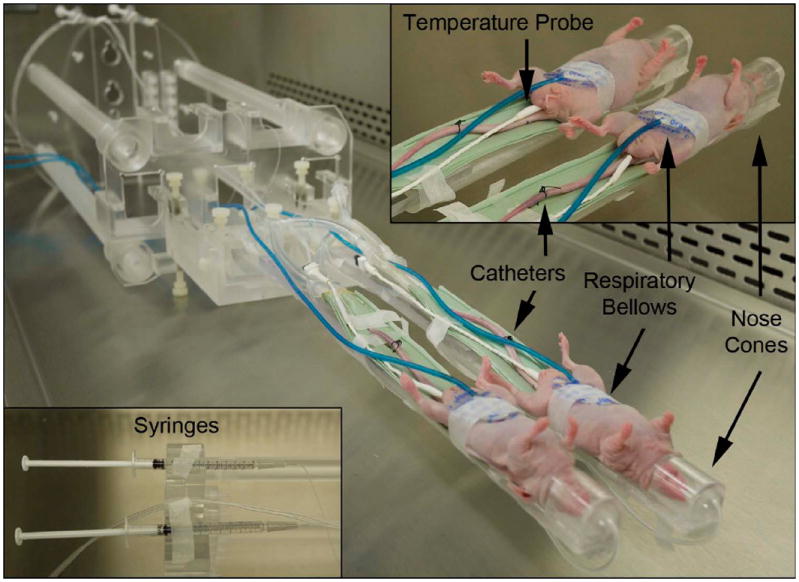
Dual-element coil array. a. A rear view depicts a solution to fix the receive coils at 33° rotations with a 45-mm separation along with the spatial relationship between volume and surface coils. Although the receive coils are in close proximity to the inner diameter of the volume coil, the volume coil was rotated such that interactions with receive coils were minimized for both 13C and 1H acquisitions (see arrows indicating target direction of linear volume coil B1 fields). The rotation angle of the volume coil in this figure does not represent one of the two optimal rotations. b. The coil array placed over the two simultaneously scanned mice.
Figure 3.
Custom-built dual-mouse sled permits global and local spatial offsets along the main field axis. The sled is conducive to anesthesia distribution and physiological monitoring of body temperature and respiratory rate. Careful sled separation permitted imaging within a 72-mm diameter dual-tuned volume coil.
Hardware Characterization
Phantom and laboratory experiments offered a controlled mechanism for characterizing the hardware performance. To measure inductive coupling between RF coils, preamplifiers were removed and S21 between coils was measured using a network analyzer. Current reductions due to active detuning and preamplifier decoupling were measured using a pair of decoupled probes that were loosely coupled to the surface coils. For intervolume sensitivity measurements, the ratios of each coil’s mean sensitivity to primary and secondary VOIs were compared using a ~1-cm diameter cylindrical 8-M 13C-labeled urea phantom. Sensitivity was evaluated based on the integrated real signal derived from a slice-selective pulse-acquire sequence (TR = 4000 ms, 90° flip angle, 2048 readout points, 4.96-kHz bandwidth, an 8-mm slice).To gain insight regarding coil and sample losses, unloaded-to-loaded quality factor (Q) measurements were performed on the bench with and without a loading sample, containing 0.5% percent NaCl in 30 mL of distilled water, in place.
Dual-Volume Shimming
B0 homogeneity for multi-volume acquisitions is perhaps the most limiting factor for acquisitions that are sensitive to low pixel bandwidths and/or off-resonance effects (12,13). For MRS, linewidths must be low enough to distinguish metabolites of interest that are particular to the HP 13C-labeled substrate. For HP [1-13C] pyruvate, the 2.6-ppm separation between pyruvate hydrate and alanine represents the lowest spectral separation (23,24).
Achieved linewidths at each VOI were compared based on two shim settings. The first setting consisted of the mean shim values achieved through optimization over each VOI individually and derived from an automated first-order shim adjustment routine (12, 13, 20). The second shim setting was pre-calibrated based on a 70–mm diameter spherical phantom placed at isocenter. Using each shim setting,13C spectra were acquired with point resolved spectroscopy (PRESS) over 1-cm3 voxels of the urea phantom, corresponding with each VOI location. Measurements were performed with the dual-tuned volume coil in transceive mode. Linewidths were calculated based on the full width at half maximum (FWHM).
Polarization
Polarization of a 13 or 26 mg sample of [1-13C]pyruvic acid, containing 15 mM of OX063 (GE Healthcare) and 1.5 mM gadoteridol, was performed with a HyperSense™ DNP polarizer as previously described (25,26). After approximately 45 minutes of polarization time, the solution was rapidly dissolved, yielding an injectable containing 40 or 80 mM [1-13C] pyruvate for enzyme phantom or mouse experiments, respectively.
Dynamic Enzyme Phantom Experiments
Evaluating the acquisition of dynamic chemical processes is not ideally performed in vivo since biological variations reduce the ability to control experiments. Therefore, trials involving dynamic enzyme phantoms (26) were used to emulate metabolism in multi-volume HP experiments. Separate 200 μL samples of 40 mM HP [1-13C] pyruvate were mixed with enzyme solutions containing β-NADH (Isotech Sigma Aldrich, St. Louis, MO) and either 2 U/mL or 7 U/mL of lactate dehydrogenase (LDH) (Worthington Biochemical Corp., Lakewood, NJ). A pre-calibrated shim was loaded, and a dynamic pulse-acquire sequence (TR = 2000 ms, 15° flip angle, 2048 readout points, 4.96-kHz bandwidth, and 100 repetitions) was acquired before, during, and after the mixtures were injected into separate phantom enclosures located at each receive coil. Data were acquired through two distinct receive channels using the encode-by-channel approach for spatial localization. To compare the performance of dual-volume against single-volume acquisitions, two experiments with corresponding enzyme phantom concentrations were repeated with a surface coil at isocenter. The single-volume datasets were used to investigate the impact of coupling and intervolume sensitivity on dynamic dual-volume experiments. A primary data set (i.e. from the 2 U/mL LDH phantom) was synthetically contaminated by the secondary data set (i.e. from the 7 U/mL LDH phantom) according to the worst-case coupling and intervolume sensitivity measurements. A second synthetic dual-volume data set was similarly generated with the primary and secondary data sets interchanged.
In Vivo Experiments
One month prior to experiment, male athymic nude mice were injected with 5×106 HCT116 cells to generate subcutaneous xenografts of colorectal cancer on the nape. A tumor-bearing mouse and a normal athymic nude mouse were anesthetized, catheterized, and placed prone onto the dual-animal sled. The array was placed such that one coil element was sensitive to the tumor on one mouse and the other element was sensitive to the nape of the normal mouse (Figure 2b). Respiratory rates and body temperature were monitored with a commercially available multianimal monitoring system (Small Animal Instruments Inc., Stony Brook, NY) and 0-2.5% isoflurane in oxygen was administered through nose cones. Proton imaging consisted of positioning and T2-weighted coronal and axial scout acquisitions. A slice-localized 13C spectroscopic acquisition (TR = 2000 ms, 15° flip angle, 2048 readout points, 4.96-kHz bandwidth, 100 repetitions, and 20-mm slice thickness) persisted throughout the agent lifetime. Two veterinary staff performed 200-μL injections of the HP agent over a ~10-s duration. The agent volumes were independently drawn and immediately injected into the mice to reduce signal loss from T1 relaxation.
Results
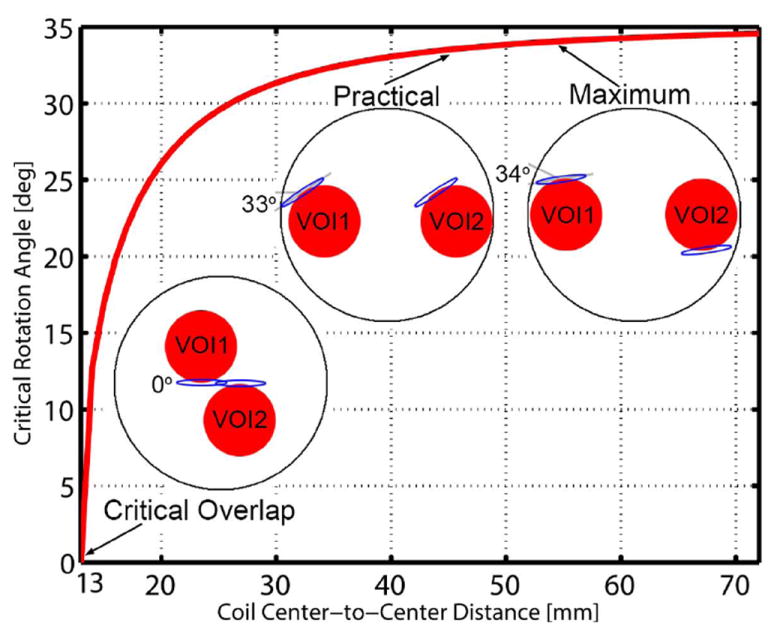
The rotation angle required for optimal decoupling varied from 0° (i.e. coplanar) for coils critically overlapped (22) with 13-mm center-to-center separations, up to 34° for the maximum (55-mm) separation permitted by the 72-mm volume coil inner diameter (Figure 4). The close proximity of coils with 55-mm separation to the inner bore of the transmit coil requires that one animal be inverted so that identical coil sensitivities to the anatomy of interest are achieved. By reducing coil separation to 45 mm, the geometric relationship between coils and animals can be consistent, permitting a more practical configuration for animal handling and support (Figures 2 and 4). Based on simulations, intervolume sensitivity was 49% for a 13-mm separation and 1.5% for a 55-mm separation. A 45-mm coil separation has asymmetric intervolume sensitivities which were 1.7% and 3.2% for the two coils respectively. These low intervolume sensitivities, the ability to optimally decouple coils by rotation, and the compatibility with conventional animal handling procedures led us to proceed with a 45-mm separation.
Figure 4.
Simulation to evaluate optimal geometric decoupling between surface coils. The common angle of rotation required to minimize coupling ranged from 0° at critical overlap (13-mm center-to-center separation) to 34° at maximum (55-mm) separation. A practical solution with 45-mm separation, that avoided the requirement for animals to be distinctly rotated, required 33° coil rotations to achieve optimal isolation.
As predicted by simulation, rotating the fabricated coils by approximately 33° resulted in more than 46 dB of isolation. Low input impedance preamplifiers provided an additional ~19 dB of decoupling, and active detuning circuitry provided more than 50 dB of decoupling from the transmit coil. S21 measurements between the carefully-rotated and tuned transmit volume coil and each receive coil (with preamplifiers removed) were -22 ± 6 dB for the 13C configuration and -40 ± 7 dB for the 1H imaging configuration. Scanner-based measurements indicated approximately 2.5% and 1.4% intervolume sensitivity for the geometry with 45-mm coil separation. These low intervolume sensitivities and the excellent isolation permitted the encode-by-channel approach. Unloaded-to-loaded quality factors (QU/QL) were approximately 49/43, indicating that 88% of losses were due to the coil and only 12% of the losses were due to the sample. Linewidths resulting from the mean shim and the pre-calibrated shim were both below 0.2 ppm, implying a negligible impact on metabolite differentiation.
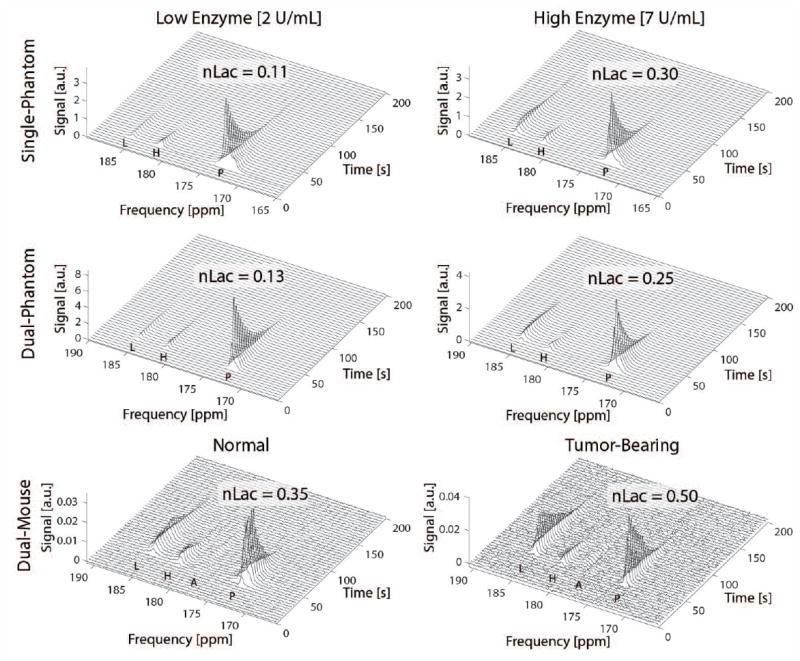
Spectral time courses from the single- and dual-volume acquisitions are shown in Figure 5. Resulting pyruvate linewidths from the enzyme phantoms were 0.25 ppm and 0.52 ppm for the dual-volume measurements compared with 0.45 ppm and 0.46 ppm for the single-volume measurements, and all configurations used the shim pre-calibrated on a 70-mm spherical phantom. Normalized lactate (nLac) as defined by the total cumulative HP lactate signal divided by the total HP carbon signal for the single- and dual-volume measurements were: nLac2U, single = 0.11, nLac 2U,dual = 0.13; nLac7U,single = 0.30, and nLac7U,dual = 0.25. The 11.8% and 12.9% variation among single and dual-volume measurements were consistent with those performed on single-volume experiments alone (26). Kinetic data agreed with results from phantom and bench-based measurements indicating minimal inductive coupling and low intervolume sensitivity for the encode-by-channel approach. Analyses of synthetic dual-volume datasets indicated minor worst-case errors due to coupling and intervolume sensitivity on nLac calculations (%error7U,primary = 1.5% and %error2U,primary = 3.9%) for the dynamic phantoms with high and low LDH concentrations. HP 13C MRS was successfully performed on two animals simultaneously. As shown in Figure 5, conversion of infused HP [1-13C] pyruvate into lactate was observed in both animals, with higher conversion measured in the tumor-bearing animal (nLactumor = 0.50) compared to the normal animal (nLacnormal = 0.35).
Figure 5.
Dynamic spectra from [1-13C] pyruvate infusion into enzyme phantoms acquired with a conventional single-coil approach (top row) and with the dual-coil method (middle row) developed in this work. Higher lactate production results from a higher enzyme concentration (right) compared to a lower concentration (left), and resulting data quality is similar between acquisition methods. In vivo results from a dual-mouse acquisition are shown in the bottom row. Higher pyruvate to lactate conversion is evident in the tumor-bearing mouse (right) compared to the normal mouse (left), although lactate signal is still relatively strong in the nape of the normal mouse. There is no evidence of detrimental coupling or intervolume sensitivity that would compromise data collection using this dual-volume configuration. Pyruvate, pyruvate hydrate, alanine, and lactate are indicated by P, H, A, and L labels respectively.
Discussion
We have established the initial feasibility of applying a multianimal methodology to improve the throughput of preclinical HP MR. The combination of an inductively decoupled pair of 13C coil array elements and a broadband multi-channel receiver permitted multivolume MRS with high sensitivity using an encode-by-channel approach. Although the inner diameter of the transmit volume coil restricted the number of mice that could be accommodated, a modest yet respectable two-fold throughput improvement was achieved compared to a conventional approach, with minimal compromise in data quality.
Prior to in vivo demonstration, enzyme phantoms of varying activity were used to mimic metabolism in a controlled environment. Synthetic datasets were generated from single-volume acquisitions, providing a means to quantitatively evaluate the impact of intervolume sensitivity and inductive coupling on dual-volume kinetic analysis. Simulations indicated less than a 4% worst-case error in nLac calculations. Although this error is minor and well below the expected variability associated with HP agent injections (26), methods to further reduce data contamination due to multi-volume acquisitions could also be applied. For instance, a SENSE-like approach (27) in which coil sensitivities and coupling matrices are estimated to identify and eliminate signal originating from secondary VOIs (11) could be incorporated.
Other methods could exploit selective excitation to further differentiate the spatial origin of multivolume MRS data. A so called encode-by-slice method would not only be complementary to the encode-by-channel approach used in this work, but could potentially offer an alternative approach for multianimal MRS on systems with a single 13C channel. Animals could be staggered such that their anatomies of interested would be contained in distinct slices. Surface coils with mutually exclusive coil sensitivities to each VOI could be combined into an effective coil, reducing SNR but requiring only one receive channel. A potential drawback of this approach is the requirement for additional RF excitations that may unnecessarily deplete the HP signal in all animals.
Based on a limited number trials, we noticed both a higher and a lower linewidth for the dual-volume measurements compared to those achieved by single-volume measurement, which was counter intuitive to the expectation that single-volume performance would be superior. This anomaly can potentially be explained by the use of only one global shim for all measurements. The phantom enclosures remained empty prior to experimentation to leave room for agent mixing, offering no practical means for shimming optimization on single-volume runs.
With two animals placed in close proximity, the global shim readily permitted the differentiation of metabolites produced by [1-13C] pyruvate. This was not surprising due to the large chemical shifts between metabolites of interest, however narrower linewidths may be required for other 13C-labeled substrates or label positions, such as [2-13C] pyruvate (28). An optimal multi-volume shimming approach would likely require an array of individual gradient coil inserts placed at each VOI (11,29). However, this would further decrease available packing density, placing additional constraints on throughput. Dedicating receive arrays (22,30) to each animal (16,20) could improve spatial coverage, enhance sensitivity, or permit accelerated acquisitions to more efficiently acquire data that is continually decaying (31). The use of arrays could also aid in the optimization of spectroscopic imaging strategies suited for multivolume HP experiments.
The high-throughput platform developed in this work was designed for use on a 7-T preclinical scanner. Benefits of the high field strength include wider spectral separation and enhanced 1H signal-to-noise ratio (SNR). Drawbacks include the need for high-bandwidth sequences and shorter T1 relaxation of HP pyruvate due to chemical shielding anisotropy. Clinical scanners should be excellent candidates for high-throughput HP acquisitions (18,32) due to their large bore volume and the overall insensitivity of HP signal to field strength.
A limitation that must be further studied involves fast and repeatable agent distribution to multiple volumes simultaneously. Experiments in this study required two trained veterinary staff to perform agent injections, and human errors involved with extracting precisely 200 μL and inconsistent injection delays caused variations in the enzyme phantom measurements (26). In general, HP 13C agent injections should be made quickly to reduce T1signal decay. Agents with substantially longer T1 relaxation times, such as HP 15N choline (33) or HP silicon (34) could relax timing constraints of agent administration, but also require lengthy polarization times and could therefore substantially benefit from high-throughput multianimal approaches.
Motivations for this work were that the throughput of HP 13C experiments is significantly limited by the polarization build-up time and that a large fraction of the fixed agent is wasted for small-animal investigations. To address the agent generation aspect of this problem, a multi-sample polarizer that improves throughput and streamlines workflow has recently become commercially available. Nonetheless, large output volumes for preclinical applications are wasteful and multivolume methods more efficiently utilize the agent volume. Substantial cost reductions using these approaches would help promote the integration of HP MR into routine biological investigations and more readily permit longitudinal evaluation of metabolic changes to confidently evaluate disease progression and response to therapy.
Acknowledgments
This work was supported in part by the National Institutes of Health (P30-CA016672) and the Cancer Prevention and Research Institute of Texas (RP-101243-P5). Funding for MSR as an Odyssey Fellow was supported by the Odyssey Program and The Estate of C.G. Johnson, Jr. at The University of Texas M.D. Anderson Cancer Center.
References
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, Deberardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 2011;13(2):81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci Transl Med. 2013;5(198):198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92(3):329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 6.Albers MJ, Bok R, Chen AP, Cunningham CH, Zierhut ML, Zhang VY, Kohler SJ, Tropp J, Hurd RE, Yen YF, Nelson SJ, Vigneron DB, Kurhanewicz J. Hyperpolarized 13C lactate, pyruvate, and alanine: noninvasive biomarkers for prostate cancer detection and grading. Cancer Res. 2008;68(20):8607–8615. doi: 10.1158/0008-5472.CAN-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson ET, Gordon JW, Erickson MG, Fain SB, Rowland IJ. Dynamic nuclear polarization system output volume reduction using inert fluids. J Magn Reson Im. 2011;33(4):1003–1008. doi: 10.1002/jmri.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowers CR, Weitekamp D. Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J Am Chem Soc. 1987;109(18):5541–5542. [Google Scholar]
- 9.Hovener JB, Chekmenev EY, Harris KC, Perman WH, Robertson LW, Ross BD, Bhattacharya P. PASADENA hyperpolarization of 13C biomolecules: equipment design and installation. MAGMA. 2009;22(2):111–121. doi: 10.1007/s10334-008-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S, Larson PE, Vancriekinge M, Leach AM, Park I, Leon C, Zhou J, Shin PJ, Reed G, Keselman P, von Morze C, Yoshihara H, Bok RA, Nelson SJ, Kurhanewicz J, Vigneron DB. Rapid sequential injections of hyperpolarized [1-(1)(3)C]pyruvate in vivo using a sub-kelvin, multi-sample DNP polarizer. Magn Reson Imaging. 2013;31(4):490–496. doi: 10.1016/j.mri.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bock NA, Konyer NB, Henkelman RM. Multiple-mouse MRI. Magn Reson Med. 2003;49(1):158–167. doi: 10.1002/mrm.10326. [DOI] [PubMed] [Google Scholar]
- 12.Bock NA, Nieman BJ, Bishop JB, Henkelman RM. In vivo multiple-mouse MRI at 7 Tesla. Magn Reson Med. 2005;54(5):1311–1316. doi: 10.1002/mrm.20683. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez MS, Bankson JA. A practical method for 2D multiple-animal MRI. J Magn Reson Im. 2007;26(4):1162–1166. doi: 10.1002/jmri.21112. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez MS, Ragan DK, Kundra V, Bankson JA. Feasibility of multiple-mouse dynamic contrast-enhanced MRI. Magn Reson Med. 2007;58(3):610–615. doi: 10.1002/mrm.21348. [DOI] [PubMed] [Google Scholar]
- 15.Bock NA, Zadeh G, Davidson LM, Qian B, Sled JG, Guha A, Henkelman RM. High-resolution longitudinal screening with magnetic resonance imaging in a murine brain cancer model. Neoplasia. 2003;5(6):546–554. doi: 10.1016/s1476-5586(03)80038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez MS, Esparza-Coss E, Bankson JA. Multiple-mouse MRI with multiple arrays of receive coils. Magn Reson Med. 2010;63(3):803–810. doi: 10.1002/mrm.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beuf O, Jaillon F, Saint-Jalmes H. Small-animal MRI: signal-to-noise ratio comparison at 7 and 1.5 T with multiple-animal acquisition strategies. Magma. 2006;19(4):202–208. doi: 10.1007/s10334-006-0048-9. [DOI] [PubMed] [Google Scholar]
- 18.Bernardo M, Kobayashi H, Metzger G, Koyama Y, Shaw C, Thomasson D, Choyke P. In-vivo multiple mouse MRI using parallel receive-only coils on a 3.0 T clinical scanner for molecular imaging research. Proceedings of the 14th Annual Meeting of ISMRM; Seattle, WA, USA. 2003. p. 2604. [Google Scholar]
- 19.Bernardo M, Kramer-Marek G, Shenoy N, Seidel J, Green MV, Capala J, Choyke P. Dual Mouse 8-Element Coil Array and Bed for Sequential Multimodality PET, SPECT, CT, and MRI of Multiple Mice. Proceedings of the 19th Annual Meeting of ISMRM; Montreal, QC, CA. 2011. p. 6539. [Google Scholar]
- 20.Ramirez MS, Lai SY, Bankson JA. A throughput-optimized array system for multiple-mouse MRI. NMR Biomed. 2013;26(3):237–247. doi: 10.1002/nbm.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blaimer M, Breuer F, Mueller M, Heidemann RM, Griswold MA, Jakob PM. SMASH, SENSE, PILS, GRAPPA: how to choose the optimal method. Top Magn Reson Imaging. 2004;15(4):223–236. doi: 10.1097/01.rmr.0000136558.09801.dd. [DOI] [PubMed] [Google Scholar]
- 22.Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990;16(2):192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 23.Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci U S A. 2007;104(50):19773–19777. doi: 10.1073/pnas.0706235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golman K, in ’t Zandt R, Thaning M. Real-time metabolic imaging. Proc Natl Acad Sci U S A. 2006;103(30):11270–11275. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris T, Eliyahu G, Frydman L, Degani H. Kinetics of hyperpolarized 13C1-pyruvate transport and metabolism in living human breast cancer cells. Proc Natl Acad Sci U S A. 2009;106(43):18131–18136. doi: 10.1073/pnas.0909049106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker CM, Lee J, Ramirez MS, Schellingerhout D, Millward S, Bankson JA. A catalyzing phantom for reproducible dynamic conversion of hyperpolarized [1-(1)(3)C]-pyruvate. PLoS One. 2013;8(8):e71274. doi: 10.1371/journal.pone.0071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 28.Hu S, Yoshihara HA, Bok R, Zhou J, Zhu M, Kurhanewicz J, Vigneron DB. Use of hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate to probe the effects of the anticancer agent dichloroacetate on mitochondrial metabolism in vivo in the normal rat. Magn Reson Imaging. 2012;30(10):1367–1372. doi: 10.1016/j.mri.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda Y, Utsuzawa S, Kurimoto T, Haishi T, Yamazaki Y, Kose K, Anno I, Marutani M. Super-parallel MR microscope. Magn Reson Med. 2003;50(1):183–189. doi: 10.1002/mrm.10515. [DOI] [PubMed] [Google Scholar]
- 30.Keil B, Wiggins GC, Triantafyllou C, Wald LL, Meise FM, Schreiber LM, Klose KJ, Heverhagen JT. A 20-channel receive-only mouse array coil for a 3 T clinical MRI system. Magn Reson Med. 2011;66(2):584–595. doi: 10.1002/mrm.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohliger MA, Larson PE, Bok RA, Shin P, Hu S, Tropp J, Robb F, Carvajal L, Nelson SJ, Kurhanewicz J, Vigneron DB. Combined parallel and partial fourier MR reconstruction for accelerated 8-channel hyperpolarized carbon-13 in vivo magnetic resonance Spectroscopic imaging (MRSI) J Magn Reson Im. 2013;38(3):701–713. doi: 10.1002/jmri.23989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitsuda M, Yamaguchi M, Furuta T, Nabetani A, Hirayama A, Nozaki A, Niitsu M, Fujii H. Multiple-animal MR imaging using a 3T clinical scanner and multi-channel coil for volumetric analysis in a mouse tumor model. Magn Reson Med Sci. 10(4):229–237. doi: 10.2463/mrms.10.229. [DOI] [PubMed] [Google Scholar]
- 33.Cudalbu C, Comment A, Kurdzesau F, van Heeswijk RB, Uffmann K, Jannin S, Denisov V, Kirik D, Gruetter R. Feasibility of in vivo 15N MRS detection of hyperpolarized 15N labeled choline in rats. Phys Chem Chem Phys. 2010;12(22):5818–5823. doi: 10.1039/c002309b. [DOI] [PubMed] [Google Scholar]
- 34.Cassidy MC, Chan HR, Ross BD, Bhattacharya PK, Marcus CM. In vivo magnetic resonance imaging of hyperpolarized silicon particles. Nat Nanotechnol. 2013;8(5):363–368. doi: 10.1038/nnano.2013.65. [DOI] [PubMed] [Google Scholar]


