Abstract
The soluble adenylyl cyclase (sAC) catalyzes the conversion of ATP into cyclic AMP (cAMP). Recent studies have shed new light on the role of sAC localized in mitochondria and its product cAMP, which drives mitochondrial protein phosphorylation and regulation of the oxidative phosphorylation system and other metabolic enzymes, presumably through the activation of intra-mitochondrial PKA. In this review article, we summarize recent findings on mitochondrial sAC activation by bicarbonate (HCO3−) and calcium (Ca2+) and the effects on mitochondrial metabolism. We also discuss putative mechanisms whereby sAC-mediated mitochondrial protein phosphorylation regulates mitochondrial metabolism.
Keywords: sAC, mitochondria, cAMP, PKA, protein phosphorylation
Introduction
In 1975, Braun and Dods described for the first time the presence in the cytosol of a “soluble” adenylyl cyclase (sAC), distinct from the transmembrane adenylyl cyclase (tmAC), which could be stimulated by Mn2+ and potentiated by calcium (Ca2+). These initial findings were made in rat seminiferous tubules and epididymal sperm [1, 2]. Indeed, the gene encoding for sAC is highly expressed in testis, where the protein mostly consists of a shorter fragment (48 kDa) of the full-length sAC (187 kDa) [3]. Functionally sAC is a cyclase that converts ATP in cyclic AMP (cAMP), the ubiquitous second messenger responsible for many signaling cascades in the cells. The presence of a soluble form of adenylyl cyclase allows cAMP to be produced in defined subcellular compartments, where it serves local signaling functions [3]. The catalytic active portions of sAC (C1 and C2) are conserved in cyanobacteria and myxobacteria, suggesting an evolutionary continuity, between the bacterial and mammalian sAC-cAMP signaling systems.
The enzymatic activity of sAC can be directly stimulated by bicarbonate (HCO3−) in vivo and in vitro [4] and by Ca2+, in a concentration dependent manner [5, 6]. Furthermore, it has been shown that sAC reflects alterations in intracellular ATP fluctuation in HEK293 cells [7]. Taken together, these findings suggest that sAC can be considered as a putative metabolic sensor. Recently the crystal structure of human sAC apo state was described [8, 9], including the mechanism of catalysis and the binding site of the HCO3− activation and regulators. The structure shows how HCO3− binds and activates sAC and how sAC can be inhibited by a drug [8, 9].
sAC was shown to localize to various subcellular compartments, including mitochondria, centrioles, mitotic spindles, mid-bodies and nuclei [10], suggesting that each one of these cell compartments contains a cAMP target. In particular, the role of sAC in mitochondria started to emerge in the recent years. Several laboratories have demonstrated that cAMP is generated in the mitochondrial matrix, where it regulates mitochondrial metabolism, coupling the CO2 generation in the Krebs cycle with the activity of the oxidative phosphorylation machinery [11–13]. It needs to be noted, however, that sAC and PKA, as well as the phosphodiesterase PDE2, are not reported in the MitoCarta compendium of the mitochondrial proteome [14]. The lack of predicted mitochondrial localization on the basis of canonical import signals in the primary protein sequences suggests that sAC and PKA enter mitochondria through alternative, still poorly understood, mechanisms. Alternatively, there could be unidentified splice isoforms with mitochondrial targeting. In the following sections, some of the issues raised around the presence of these proteins in mitochondria will be discussed in more detail.
Once generated by sAC, cAMP inside the cell stimulates its cellular effector protein kinase A (PKA) responsible for protein phosphorylation. In mitochondria, the activity of many enzymes and carriers is modulated by phosphorylation [15, 16]. The mechanisms underlying the regulation of these post-translational modifications are emerging, but are yet to be described in complete detail. Our current understanding of this process is based on the evidence that cAMP is locally produced inside mitochondria by sAC, where it activates a kinase, presumably PKA, which then phosphorylates mitochondrial proteins, thereby regulating their function [12, 17, 18]. Here, we review and discuss current evidence on the localization of sAC in mitochondria and on the role of sAC-generated cAMP in mitochondria in the regulation of energy metabolism.
sAC localization in mitochondria
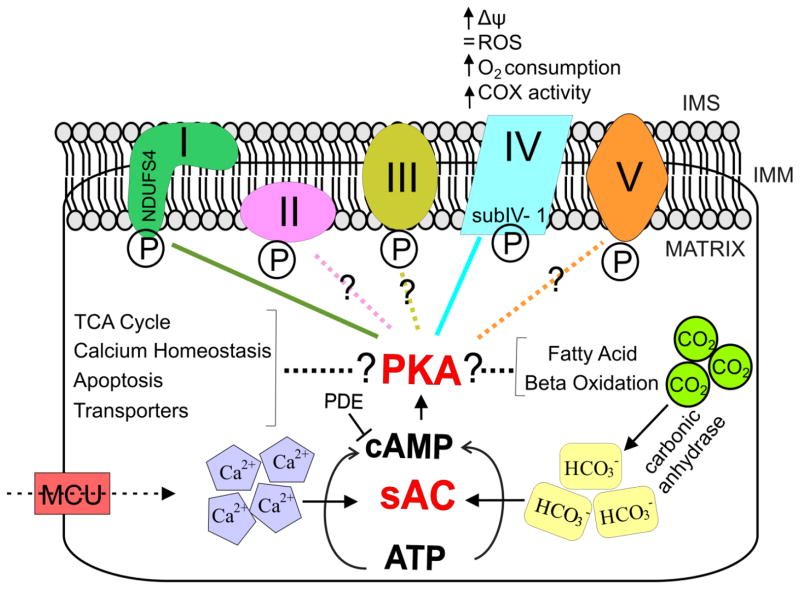
The first evidence that sAC is localized in mitochondria was shown in COS7 cells stained with both αN-term and αC-term sAC antisera and the mitochondrial marker MitoTracker green [10]. In this work, sAC was also detected in mitochondria of cardiac and skeletal muscle. Functionally, our group proposed for the first time that cAMP contained in the mitochondrial matrix is not generated in the cytosol and transported across the inner membrane, as previously suggested [19], because the latter is impermeant to cAMP. Instead, we showed that matrix cAMP originated inside the mitochondria by the action of sAC, in response to metabolically generated carbon dioxide [11]. Other groups have confirmed the presence of this signaling pathway in the mitochondrial matrix, using different approaches, such as cAMP specific FRET sensors [12, 18]. It was also shown that sAC activation increases ATP production in cells and isolated mitochondria [12, 17]. The oxidative phosphorylation (OXPHOS) activities, such as ATP production, O2 consumption, and cytochrome oxidase (COX) activity, are decreased by sAC inhibition, whereas sAC activation by HCO3− and Ca2+ stimulates OXPHOS [12, 17]. These authors concluded that the sAC-cAMP signaling pathway represents a metabolic sensor that modulates OXPHOS and ROS generation. Figure 1 shows a schematic representation of the putative sAC-cAMP signaling pathway in mitochondria (Fig. 1).
Figure 1. sAC-cAMP-PKA pathway in mitochondria.
sAC converts ATP in cAMP, the ubiquitous second messenger, which is degraded by phosphodiesterase (PDE). sAC is activated by Ca2+ (e.g. overexpression of the mitochondrial calcium uniporter, MCU) and HCO3− originated from the CO2 derived from the krebs cycle spontaneously or by the action of carbonic anhydrase. It was proposed that cAMP activates PKA, leading to phosphorylation of the NDUFS4 subunit of complex I (CI, green line), thereby regulating CI activity. sAC also induces phosphorylation of subunit COXIV-1 of complex IV (blue line), leading to an increase in COX activity, membrane potential (Δψ), O2 consumption and ATP production, without altering ROS level. It needs to be noted that the evidence that PKA is the sAC effector in mitochondria is contradictory and other kinases may be involved. Other OXPHOS complexes are phosphorylated, but whether this involves the mitochondrial sAC-signaling pathway is still unknown. Furthermore, proteins related to TCA cycle, calcium homeostasis, apoptosis, transporters, fatty acid oxidation and β-oxidation are also phosphorylated.
sAC regulation by HCO3− in mitochondria
The role of sAC in energy metabolism started to emerge when the sensitivity to HCO3− was discovered [4]. HEK293 cell line expressing the full-length sAC cDNA accumulated cAMP upon sAC stimulation with NaHCO3. On the contrary, tmsAC was insensitive to HCO3−. The latest is generated inside the mitochondria via the action of the enzyme carbonic anhydrase that coverts CO2 derived from the Krebs cycle. Our group has investigated cAMP production in mouse liver isolated mitochondria in the presence of 30 mM HCO3−, which is in the range of the physiological intra-mitochondrial HCO3− concentration. Under these conditions, COX activity and ATP synthesis were significantly increased [11], indicating a stimulation of the OXPHOS system by cAMP generated within mitochondria. We noted that, since OXPHOS activity is increased by both HCO3− and by exogenously generated CO2, in a carbonic anhydrase dependent manner, these effects could not be due to pH changes (or ionic strength), because HCO3− addition increases, while CO2 addition decreases the pH of the experimental medium, which (because the ΔpH is constant) is followed by the pH of the matrix [11].
Di Benedetto and colleagues recently showed that CHO cells transfected with the cAMP sensor 4mtH30 targeted to mitochondria and treated with 50mM NaHCO3 displayed a biphasic kinetic of cAMP generation, with an initial rise followed by a plateau [12]. Cells pretreated with the sAC inhibitor hydroxyl-estradiol and stimulated with HCO3− showed a significant decrease in cAMP generation. Taken together, these data demonstrated a direct effect of HCO3− in activating sAC and cAMP production in mitochondria.
sAC regulation by Ca2+ in mitochondria
Jaiswal and colleagues showed that sAC in the cytosol is regulated by Ca2+ in mature human spermatozoa in a concentration dependent manner (between 10−8 and 10−4 M). They also showed sAC regulation by Ca2+ in HEK293 cells treated with a Ca2+ ionophore (which induces Ca2+ influx from the extracellular media) or carbachol (which recruits Ca2+ from intracellular stores) [5]. The recent development of targeted cAMP sensors [12, 18] allowed for a better understanding of the cAMP responses inside mitochondria and for the investigation of Ca2+ and cAMP crosstalk in the regulation of energy metabolism.
Recently, Di Benedetto and coworkers demonstrated that cAMP is produced inside mitochondria using a mitochondrially targeted cAMP sensor (4mtH30) properly targeted to the mitochondria [12]. In order to assess if Ca2+ could stimulate cAMP inside mitochondria, CHO cells were transfected with 4mtH30, derived from CFP-Epac(δDEP-CD)-YFP probe with a mitochondrial targeting signal added to its N-terminus [20]. Cytosolic Ca2+ increase from intracellular stores release, induced by the IP3-generating agonist ATP in combination with the SERCA pump inhibitor TBHQ, resulted in a marked increase in mitochondrial cAMP signal, supporting the notion that Ca2+ stimulates sAC inside mitochondria [12].
Overexpression of the mitochondrial Ca2+ uniporter (MCU) leads to an increase in mitochondrial Ca2+ accumulation [21, 22]. Accordingly, Di Benedetto and coworkers showed that overexpression of MCU resulted in an increase in mitochondrial cAMP, while MCU silencing decreased mitochondrial cAMP [12]. Moreover, increased frequency and amplitude of mitochondrial Ca2+ oscillations triggered an increase of matrix cAMP in primary cultured neonatal rat cardiomyocytes transfected with mtH30 sensor [12].
ATP production increases when sAC is overexpressed in cells. Di Benedetto and coworkers demonstrated that ATP concentration increases in mitochondria with targeted sAC expression (mtsAC cells) [12], using a luciferase sensor, mtLUC, targeted to mitochondria [23]. Mitochondrial ATP dynamics was measured upon Ca2+ stimulation in control and mtsAC HeLa cells. Under this condition both the rate and the extent of ATP concentration were upregulated, demonstrating that Ca2+ stimulated-ATP depends, at least in part, on sAC, as Ca2+ regulates directly some of the Krebs cycle dehydrogenases, having per se a stimulatory effect [24]. To further investigate if this mechanism is PKA-dependent, a PKA inhibiting peptide was targeted to the mitochondria (mt-PKI). The overexpression of mt-PKI induced a decrease in ATP concentration in both control and mtsAC overexpressing cells. These data confirm that the target of mitochondrial cAMP is PKA or a PKA-like kinase.
Taken together the work described above showed that Ca2+ modulates components of the cAMP pathway within mitochondria, including sAC and PKA. It is certainly an interesting finding that the physiology of the two major cell regulators Ca2+ and cAMP takes place inside the mitochondria with interconnected pathway [25].
sAC-cAMP effectors in mitochondria
A number of protein families have been identified as cAMP effectors in mammalian cells. Upon cAMP binding, exchange proteins directly activated by cAMP (Epac) 1 and 2 serve as guanine nucleotide exchange factors for the small GTP-ases Rap1 and Rap2 [26, 27]. A large family of cyclic-nucleotide-activated ion channels have been described, which can be divided into three major subgroups; hyperpolarization-activated cyclic-nucleotide-modulated (HCN) channels, voltage independent cyclic-nucleotide-gated (CNG) channels and K+ channels called EAG and EAG-like channels, however modulation of the latter by cAMP is less well established [26].
The best understood regulatory effects of cAMP are mediated by activation of the cAMP-dependent protein kinases (PKAs). PKA exists primarily as an inactive tetrameric holoenzyme, consisting of two of each catalytic (C) and regulatory (R) subunits. cAMP binding on the regulatory subunits triggers the release of the active C subunits, which then phosphorylate specific serine and threonine residues of target proteins [28]. There is no single consensus sequence that predicts phosphorylation sites, however the Arg-Arg-X-Ser is common and highly similar domains are contained within the vast majority of known sites [29]. The cellular distribution of PKA largely depends on the R subunit isoforms. Type IIα and IIβ are mostly bound to anchor proteins, while type Iα and Iβ are considered being more diffusedly cytosolic, but unspecific anchoring of both types (see below) and type I specific anchor sites have also been described to play important roles in signaling [30–33]. Depending on cell type and subcellular localization of the enzyme, the cAMP dependent protein phosphorylation by PKA is responsible for a wide variety of cellular responses [34–36]. Investigations of subcellular fractions revealed the existence of mitochondrial protein phosphorylation activity [37, 38] in different sub-mitochondrial fractions [39]. Nevertheless, especially for the matrix compartment the nature of the cAMP effector is controversial. The evidence both supporting and undermining the idea of PKA being the effector of cAMP in different mitochondrial compartments are reviewed below.
Evidence of A-kinase anchoring proteins (AKAPs) targeted to mitochondria may be a good indication that PKA is the effector of cAMP in these organelles. AKAPs are often part of heterogeneous complexes together with the R subunit of PKA, including mRNAs, tyrosine phosphatase(s) and tyrosine kinase(s) [40]. Their role is the control of spatial distribution and specificity of PKA activity throughout the cell, allowing for efficient transduction of the cAMP signal. The AKAP1 gene translates into several splice variants, of which three were found to be associated to mitochondria. Both S-AKAP 84 [41] and AKAP 121 [42] bind RII subunits, while D-AKAP1 [43] binds both RI and RII, furthermore all three variants share a targeting sequence on their N terminals, directing them to the outer mitochondrial membrane (OMM). AKAP 121 was also demonstrated to be present in the matrix by immunoelectron microscopy [44] and to regulate oxidative phosphorylation through COX [45]. Other AKAPs, such as D-AKAP2, co-localize with cytochrome c upon immunostaining [46]. The mechanism by which D-AKAP2 is targeted to mitochondria is still unclear. Recently, it was also shown that AKAP79, known to be associated to the plasma membrane and lacking a mitochondrial targeting sequence [47], is present in mitochondria isolated from human placenta [48]. Finally, two proteins were discovered to act as AKAPs, as a secondary function. The Rab subfamily of Ras proteins regulates membrane dynamics upon activation by GTP. Rab 32, uniquely in the subfamily, is an important player in mitochondrial fission and also binds PKA [49]. The sphingosine kinase interacting protein (SKIP), which interestingly specifically binds the RI (mostly cytosolic) subunit, was shown to be present in the intermembrane space (IMS) and the mitochondrial matrix [50, 51].
Although a few of the AKAPs mentioned above were reported also in the mitochondrial matrix, evidence best supports they are associated to the cytosolic surface and IMS of mitochondria, where small molecules of the cytosol can diffuse without constraint; therefore, theoretically both tmAC and sAC can produce cAMP affecting PKA in these domains. In the mitochondrial matrix however, sAC is the only source of cAMP described to date. There are numerous reports of matrix PKA activity revealed by protein phosphorylation upon radiolabelling [39, 52–60], by immunoblotting and immunocytochemistry [44, 61] and by immuno-electron microscopy [62]. Sardanelli et al. estimated that ~90% of total mitochondrial PKA activity was present in the matrix [44]. More recently, a novel method to assess PKA activity specifically was developed by applying coumarin-derived fluorescent peptides as PKA substrates [63]. In this work, the relative PKA activities of the outer surface, IMS and matrix of isolated bovine heart mitochondria were measured to be 9:6:85 percent, respectively. Our group was first to examine the impact of pharmacological effectors of PKA on mitochondrial parameters, and found that oxidative phosphorylation was activated by the agonist 8Br-cAMP; and inactivated by inhibitors (H89, myristoylated PKI) of PKA, while 8CPT methyl-cAMP, a specific activator of Epac had no effect [11]. The inhibitory effects of H89, PKI and additionally Rp-8-CPT-cAMP on matrix ATP levels were confirmed by Di Benedetto and colleagues, however they did not achieve activation of matrix ATP by 8Br-cAMP or 8-CPT-6-Phe-cAMP [12]. It is worth to note, that matrix ATP concentration is only one of the numerous factors that contribute to the regulation of the kinetics of ATP production in mitochondria [64], and may very well not be the only one affected by protein phosphorylation, furthermore elevated matrix ATP may not always correlate with elevated ATP output through the adenine nucleotide translocase [65].
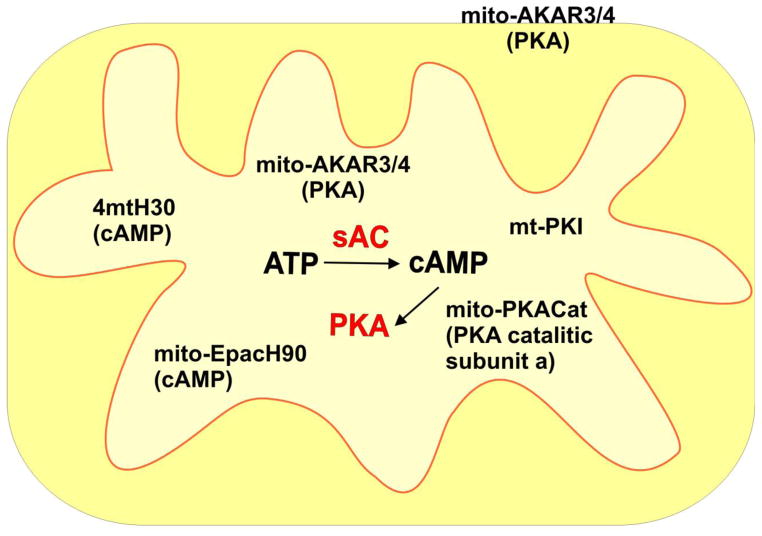
The fact that PKA subunits are devoid of discernible mitochondrial targeting sequences undermines the idea of their presence in the matrix. Di Benedetto et al. reported failure to show mitochondrial localization of GFP tagged PKA subunits (unpublished results in [25]). Furthermore, PKA activity could not be measured by matrix targeted A-kinase activity reporter (AKAR) 3 and 4 [18]. AKARs are FRET based sensors containing a PKA specific substrate, which upon phosphorylation, due to conformational changes, increase their energy transfer [66, 67]. To confirm that the negative result was not due to the sensor, AKAR 4 was shown to respond to genetically engineered PKA targeted to the matrix, which could be inhibited by co-expression of mito-PKI. These results suggest that a yet undiscovered cAMP effector exhibiting PKA-like responses to pharmacological manipulation could be responsible for phosphorylation events in the mitochondrial matrix, as proposed by Di Benedetto et al. [25]. Figure 2 summarizes the genetically encoded probes to study the function of sAC-cAMP-PKA inside mitochondria (Fig. 2), as reported in recent publications by Di Benedetto and Lefkimmiatis [12, 18].
Figure 2. Genetically-encoded tools to study the mitochondrial sAC-cAMP pathway.
Recently, new tools were developed to investigate the mitochondrial sAC-cAMP pathway in living cells. The diagram schematically depicts the genetically encoded probes. To detect cAMP in the matrix H30 and H90 were targeted to the mitochondria matrix (4mtH30 and mito-EpacH90) by adding 4 copies of the mitochondrial targeting sequence of subunit VIII of the human cytochrome oxidase (COX8) at the N-terminus of the Epac1-cAMP sensor. The same strategy was used to target AKAR3 and AKAR4, the FRET-based sensor to measure PKA activity, and PKI-mCherry (mt-PKI) and PKA-Cat-mCherry to the matrix [12, 18]. To target PKA, AKAR3 and AKAR4 to the OMM the targeting peptide used was yTom70 [18].
Degradation of cAMP in mitochondria
In order for an autonomous cAMP signaling cascade to operate properly in mitochondria, mechanisms for degrading the cAMP generated by sAC must also exist. Phosphodiesterase (PDE) activity, indicated by cAMP hydrolyzing activity in the matrix, which was sensitive to a non-selective PDE inhibitor, IBMX, was first reported in mitochondria along with the presence of sAC [11]. More recently, isolated mouse brain and liver mitochondria were shown to lose cAMP and cGMP hydrolysis, when treated with BAY 60, an inhibitor of the PDE2A isoform. The mitochondrial localization of the second splice variant of PDE2A to the mitochondrial matrix was also demonstrated [68, 69]. The inhibition of PDE2A increases oxygen consumption and ATP production in isolated mitochondria [68]. Hydrogen sulfide at low levels was proposed as a physiological effector of PDE2A in mitochondria [69]. These findings may offer new intriguing approaches in the treatment of a wide variety of afflictions, in which bioenergetic incompetence contributes to pathology, by developing specific and safe inhibitors of mitochondrial PDE2A2.
Mitochondrial phosphoproteins and metabolism
Phosphorylation represents an important regulatory mechanism for mitochondrial protein function. In particular, as discussed above, reversible phosphorylation of mitochondrial proteins is a player in OXPHOS modulation. Many mitochondrial proteins are known to be phosphorylated [16, 70–74], but the relation between phosphorylation and function is still not completely understood.
Contrasting results suggest that phosphorylation by PKA can both inhibit and activate OXPHOS. Papa and coworkers demonstrated that the NDUFS4 accessory subunit of complex I (CI) is phosphorylated by PKA (Fig. 1). Patients harboring mutation in this subunit displayed decrease CI activity and decreased cAMP-dependent phosphorylation [75, 76]. Bender and Kandenbach showed that the allosteric ATP inhibition of CIV in bovine heart is switched on by cAMP dependent phosphorylation of sub II and Vb by PKA, and switched off by protein phosphatase 1 [77]. Moreover, the cytochrome c oxidase results in a serine and threonine phosphorylation via PKA of subunit I, which was correlated with inhibition of the enzyme in the presence of ATP [78]. Our group proposed that PKA phosphorylates COXIV-I at serine 58 and enhances COX activity [79]. This COX regulation by phosphorylation of COXIV-1 subunit was due to prevention of COX allosteric inhibition by ATP (Fig. 1). Furthermore, overexpression of sAC targeted to the mitochondrial matrix of COXIV-I deficient cells dramatically improved mitochondrial function and stabilized a long-term metabolic adaptation. This was interpreted as the result of constitutive stimulation of cAMP production in mitochondria triggering adaptation mechanisms in COX deficient cells, which upregulated OXPHOS function and reduced the need for enhanced ROS-dependent OXPHOS biogenesis [79]. In a therapeutic perspective, mitochondrial sAC activation or cAMP degradation inhibition in COX deficient cells could be a viable strategy to improve COX deficiency in vivo [17].
Recent work investigated the correlation between phosphorylation and function in mitochondria from mouse liver and human skeletal muscle [73, 80]. Not only OXPHOS proteins are included in these studies, but also metabolism related proteins indirectly connected with mitochondria. However, their relation with sAC-PKA remains unknown so far. Grimsrud and coworkers reveled that phosphorylation is a key mechanism in regulating ketogenesis (phosphorylation of Serine 456 on Hmgcs2) in obese and type 2 diabetes mice [80]. The acute mitochondria phosphorylation changes during fasting and re-feeding of mice. Together, these data show that Hmgcs2 S456, the enzyme that catalyzes the rate-limiting step in ketogenesis, is an important phosphorylation target, which enhances enzymatic activity during increased ketogenic demand.
Phosphorylation was shown in human skeletal muscle mitochondrial proteins involved in oxidative phosphorylation, TCA cycle, lipid metabolism, amino acid degradation, importers and transporters, calcium homeostasis, and apoptosis (Fig. 1) [73]. Using different phosphopeptide enrichment techniques and mass spectrometry, Zhao and coworkers identified 176 different phosphorylation sites in 86 mitochondrial proteins. The major proportion of the identified phospho-sites involves potential substrates of PKA, but also CKII, PKC or DNAPK. PKA activity has been shown in human mitochondria [15, 81] regulating protein expression, biogenesis and apoptosis. As discussed above, the presence of PKA in mitochondria is still controversial. Detection of mitochondrial protein kinases is difficult due to their concentration relative to proteins. Moreover, the lack of a highly specific antibody and possible contamination from associated cell compartments limit the possibility of clearly dissecting the nature of the kinases involved [82].
Conclusions and future perspectives
The discovery of sAC within the mitochondria opens new avenues to study metabolic regulation dependent on HCO3− and Ca2+ stimulation via the sAC-cAMP pathway. It needs to be noted, however, that the studies of sAC function in mitochondria described above were conducted only in cell culture models or isolated mitochondria. Currently, there is no hard evidence on the relation between sAC function, mitochondrial protein phosphorylation, and metabolic regulation in vivo. Two different mouse models of sAC deletion are available [83, 84]. In the first one, the catalytic domain C1 was knocked out (sAC C1KO), whereas in the second the C2 catalytic domain was abolished. Investigating mitochondrial function and protein phosphorylation in different tissues of these mice will shed new lights on the functions of sAC in mitochondria in vivo. Therefore, different approaches will need to be devised to specifically investigate the intra-mitochondrial effects of sAC modulation. Since total sAC KO will likely affects extra-mitochondrial function that may indirectly affects mitochondrial metabolism, models where sAC is exclusively depleted or increased in mitochondria would be very useful for a better understanding of mitochondrial and metabolic regulation. In addition, more work is necessary to investigate the effect of sAC on OXPHOS complexes and TCA cycle, fatty acid transporters, beta oxidation, amino acid degradation, calcium homeostasis, etc., [73] in vitro and in vivo under different relevant conditions that affect metabolism, such as high and low calories intake or exposure to cold or in conditions where the energy metabolism is compromised by aging or disease. Such studies could provide insights on the potential significance of modulating sAC and cAMP in mitochondria as a therapeutic tool for metabolic and degenerative diseases that affect metabolism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braun T. The effect of divalent cations on bovine spermatozoal adenylate cyclase activity. J Cyclic Nucleotide Res. 1975;1:271–281. [PubMed] [Google Scholar]
- 2.Braun T, Dods RF. Development of a Mn-2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc Natl Acad Sci U S A. 1975;72:1097–1101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 5.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci U S A. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. The Journal of biological chemistry. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 7.Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, Tso P, Holz GG, Sharp GW, Levin LR, Buck J. CO2/HCO3(−)- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J Biol Chem. 2013;288:33283–33291. doi: 10.1074/jbc.M113.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinboelting S, Diaz A, Moniot S, van den Heuvel J, Weyand M, Levin LR, Buck J, Steegborn C. Crystal structures of human soluble adenylyl cyclase reveal mechanisms of catalysis and of its activation through bicarbonate. Proc Natl Acad Sci U S A. 2014;111:3727–3732. doi: 10.1073/pnas.1322778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saalau-Bethell SM, Berdini V, Cleasby A, Congreve M, Coyle JE, Lock V, Murray CW, O’Brien MA, Rich SJ, Sambrook T, Vinkovic M, Yon JR, Jhoti H. Crystal Structure of Human Soluble Adenylate Cyclase Reveals a Distinct, Highly Flexible Allosteric Bicarbonate Binding Pocket. ChemMedChem. 2014 doi: 10.1002/cmdc.201300480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 11.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell metabolism. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. Mitochondrial Ca(2)(+) uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell metabolism. 2013;17:965–975. doi: 10.1016/j.cmet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. cAMP and mitochondria. Physiology. 2013;28:199–209. doi: 10.1152/physiol.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagliarini DJ, Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci. 2006;31:26–34. doi: 10.1016/j.tibs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Covian R, Balaban RS. Cardiac mitochondrial matrix and respiratory complex protein phosphorylation. Am J Physiol Heart Circ Physiol. 2012;303:H940–966. doi: 10.1152/ajpheart.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acin-Perez R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO molecular medicine. 2009;1:392–406. doi: 10.1002/emmm.200900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefkimmiatis K, Leronni D, Hofer AM. The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J Cell Biol. 2013;202:453–462. doi: 10.1083/jcb.201303159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 25.Di Benedetto G, Pendin D, Greotti E, Pizzo P, Pozzan T. Ca2+ and cAMP cross-talk in mitochondria. J Physiol. 2014;592:305–312. doi: 10.1113/jphysiol.2013.259135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gancedo JM. Biological roles of cAMP: variations on a theme in the different kingdoms of life. Biological reviews of the Cambridge Philosophical Society. 2013;88:645–668. doi: 10.1111/brv.12020. [DOI] [PubMed] [Google Scholar]
- 27.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SS, Zhang P, Steichen JM, Keshwani MM, Kornev AP. PKA: lessons learned after twenty years. Biochim Biophys Acta. 2013;1834:1271–1278. doi: 10.1016/j.bbapap.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shabb JB. Physiological substrates of cAMP-dependent protein kinase. Chemical reviews. 2001;101:2381–2411. doi: 10.1021/cr000236l. [DOI] [PubMed] [Google Scholar]
- 30.Angelo R, Rubin CS. Molecular characterization of an anchor protein (AKAPCE) that binds the RI subunit (RCE) of type I protein kinase A from Caenorhabditis elegans. The Journal of biological chemistry. 1998;273:14633–14643. doi: 10.1074/jbc.273.23.14633. [DOI] [PubMed] [Google Scholar]
- 31.Burgers PP, Ma Y, Margarucci L, Mackey M, van der Heyden MA, Ellisman M, Scholten A, Taylor SS, Heck AJ. A small novel A-kinase anchoring protein (AKAP) that localizes specifically protein kinase A-regulatory subunit I (PKA-RI) to the plasma membrane. The Journal of biological chemistry. 2012;287:43789–43797. doi: 10.1074/jbc.M112.395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circulation research. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 33.Skalhegg BS, Tasken K, Hansson V, Huitfeldt HS, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994;263:84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- 34.McKnight GS. Cyclic AMP second messenger systems. Current opinion in cell biology. 1991;3:213–217. doi: 10.1016/0955-0674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 35.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 36.Edelman AM, Blumenthal DK, Krebs EG. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- 37.Lee PC, Jungmann RA. Ontogeny of cyclic AMP-dependent protein phosphokinase during hepatic development of the rat. Biochim Biophys Acta. 1975;399:265–276. doi: 10.1016/0304-4165(75)90257-3. [DOI] [PubMed] [Google Scholar]
- 38.Kleitke B, Sydow H, Wollenberger A. Evidence for cyclic AMP-dependent protein kinase activity in isolated guinea pig and rat liver mitochondria. Acta biologica et medica Germanica. 1976;35:K9–K17. [PubMed] [Google Scholar]
- 39.Henriksson T, Jergil B. Protein kinase activity and endogenous phosphorylation in subfractions of rat liver mitochondria. Biochim Biophys Acta. 1979;588:380–391. doi: 10.1016/0304-4165(79)90346-5. [DOI] [PubMed] [Google Scholar]
- 40.Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17:279–287. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q, Lin RY, Rubin CS. Organelle-specific targeting of protein kinase AII (PKAII). Molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J Biol Chem. 1997;272:15247–15257. doi: 10.1074/jbc.272.24.15247. [DOI] [PubMed] [Google Scholar]
- 42.Feliciello A, Rubin CS, Avvedimento EV, Gottesman ME. Expression of a kinase anchor protein 121 is regulated by hormones in thyroid and testicular germ cells. J Biol Chem. 1998;273:23361–23366. doi: 10.1074/jbc.273.36.23361. [DOI] [PubMed] [Google Scholar]
- 43.Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem. 1997;272:8057–8064. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- 44.Sardanelli AM, Signorile A, Nuzzi R, Rasmo DD, Technikova-Dobrova Z, Drahota Z, Occhiello A, Pica A, Papa S. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006;580:5690–5696. doi: 10.1016/j.febslet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, Garbi C, Castaldo I, Annunziato L, Avvedimento EV, Feliciello A. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol Biol Cell. 2006;17:263–271. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Sunahara RK, Krumins A, Perkins G, Crochiere ML, Mackey M, Bell S, Ellisman MH, Taylor SS. Cloning and mitochondrial localization of full-length D-AKAP2, a protein kinase A anchoring protein. Proc Natl Acad Sci U S A. 2001;98:3220–3225. doi: 10.1073/pnas.051633398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HY, Tao J, Shumay E, Malbon CC. G-Protein-coupled receptor-associated A-kinase anchoring proteins: AKAP79 and AKAP250 (gravin) European journal of cell biology. 2006;85:643–650. doi: 10.1016/j.ejcb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Ma MP, Thomson M. Protein Kinase A Subunit alpha Catalytic and A Kinase Anchoring Protein 79 in Human Placental Mitochondria. Open Biochem J. 2012;6:23–30. doi: 10.2174/1874091X01206010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovanich D, van der Heyden MA, Aye TT, van Veen TA, Heck AJ, Scholten A. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. Chem biochem : a European journal of chemical biology. 2010;11:963–971. doi: 10.1002/cbic.201000058. [DOI] [PubMed] [Google Scholar]
- 51.Means CK, Lygren B, Langeberg LK, Jain A, Dixon RE, Vega AL, Gold MG, Petrosyan S, Taylor SS, Murphy AN, Ha T, Santana LF, Tasken K, Scott JD. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc Natl Acad Sci U S A. 2011;108:E1227–1235. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verdanis A. Protein kinase activity at the inner membrane of mammalian mitochondria. J Biol Chem. 1977;252:807–813. [PubMed] [Google Scholar]
- 53.Dimino MJ, Bieszczad RR, Rowe MJ. Cyclic AMP-dependent protein kinase in mitochondria and cytosol from different-sized follicles and corpora lutea of porcine ovaries. J Biol Chem. 1981;256:10876–10882. [PubMed] [Google Scholar]
- 54.Danko SJ, Markwell JP. Protein phosphorylation in plant mitochondria. Plant physiology. 1985;79:311–314. doi: 10.1104/pp.79.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgess JW, Yamada EW. cAMP-dependent protein kinase isozymes with preference for histone H2B as substrate in mitochondria of bovine heart. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1987;65:137–143. doi: 10.1139/o87-019. [DOI] [PubMed] [Google Scholar]
- 56.Muller G, Bandlow W. cAMP-dependent protein kinase activity in yeast mitochondria, Zeitschrift fur Naturforschung. C. Journal of biosciences. 1987;42:1291–1302. doi: 10.1515/znc-1987-11-1224. [DOI] [PubMed] [Google Scholar]
- 57.Muller G, Bandlow W. Protein phosphorylation in yeast mitochondria: cAMP-dependence, submitochondrial localization and substrates of mitochondrial protein kinases. Yeast. 1987;3:161–174. doi: 10.1002/yea.320030304. [DOI] [PubMed] [Google Scholar]
- 58.Sardanelli AM, Technikova-Dobrova Z, Speranza F, Mazzocca A, Scacco S, Papa S. Topology of the mitochondrial cAMP-dependent protein kinase and its substrates. FEBS Lett. 1996;396:276–278. doi: 10.1016/0014-5793(96)01112-x. [DOI] [PubMed] [Google Scholar]
- 59.Technikova-Dobrova Z, Sardanelli AM, Papa S. Phosphorylation of mitochondrial proteins in bovine heart. Characterization of kinases and substrates. FEBS Lett. 1993;322:51–55. doi: 10.1016/0014-5793(93)81109-d. [DOI] [PubMed] [Google Scholar]
- 60.Technikova-Dobrova Z, Sardanelli AM, Stanca MR, Papa S. cAMP-dependent protein phosphorylation in mitochondria of bovine heart. FEBS Lett. 1994;350:187–191. doi: 10.1016/0014-5793(94)00760-8. [DOI] [PubMed] [Google Scholar]
- 61.Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc Natl Acad Sci U S A. 2005;102:13915–13920. doi: 10.1073/pnas.0502878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwoch G, Trinczek B, Bode C. Localization of catalytic and regulatory subunits of cyclic AMP-dependent protein kinases in mitochondria from various rat tissues. Biochem J. 1990;270:181–188. doi: 10.1042/bj2700181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agnes RS, Jernigan F, Shell JR, Sharma V, Lawrence DS. Suborganelle sensing of mitochondrial cAMP-dependent protein kinase activity. Journal of the American Chemical Society. 2010;132:6075–6080. doi: 10.1021/ja909652q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Metelkin E, Demin O, Kovacs Z, Chinopoulos C. Modeling of ATP-ADP steady-state exchange rate mediated by the adenine nucleotide translocase in isolated mitochondria. FEBS J. 2009;276:6942–6955. doi: 10.1111/j.1742-4658.2009.07394.x. [DOI] [PubMed] [Google Scholar]
- 65.Chinopoulos C, Konrad C, Kiss G, Metelkin E, Torocsik B, Zhang SF, Starkov AA. Modulation of F0F1-ATP synthase activity by cyclophilin D regulates matrix adenine nucleotide levels. FEBS J. 2011;278:1112–1125. doi: 10.1111/j.1742-4658.2011.08026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun. 2006;348:716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- 67.Depry C, Allen MD, Zhang J. Visualization of PKA activity in plasma membrane microdomains. Molecular bioSystems. 2011;7:52–58. doi: 10.1039/c0mb00079e. [DOI] [PubMed] [Google Scholar]
- 68.Acin-Perez R, Russwurm M, Gunnewig K, Gertz M, Zoidl G, Ramos L, Buck J, Levin LR, Rassow J, Manfredi G, Steegborn C. A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. The Journal of biological chemistry. 2011;286:30423–30432. doi: 10.1074/jbc.M111.266379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modis K, Panopoulos P, Coletta C, Papapetropoulos A, Szabo C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem Pharmacol. 2013;86:1311–1319. doi: 10.1016/j.bcp.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 70.Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boja ES, Phillips D, French SA, Harris RA, Balaban RS. Quantitative mitochondrial phosphoproteomics using iTRAQ on an LTQ-Orbitrap with high energy collision dissociation. J Proteome Res. 2009;8:4665–4675. doi: 10.1021/pr900387b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balaban RS. The mitochondrial proteome: a dynamic functional program in tissues and disease states. Environ Mol Mutagen. 2010;51:352–359. doi: 10.1002/em.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao X, Leon IR, Bak S, Mogensen M, Wrzesinski K, Hojlund K, Jensen ON. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol Cell Proteomics. 2011;10:M110 000299. doi: 10.1074/mcp.M110.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen R, Fearnley IM, Peak-Chew SY, Walker JE. The phosphorylation of subunits of complex I from bovine heart mitochondria. The Journal of biological chemistry. 2004;279:26036–26045. doi: 10.1074/jbc.M402710200. [DOI] [PubMed] [Google Scholar]
- 75.De Rasmo D, Signorile A, Larizza M, Pacelli C, Cocco T, Papa S. Activation of the cAMP cascade in human fibroblast cultures rescues the activity of oxidatively damaged complex I. Free Radic Biol Med. 2012;52:757–764. doi: 10.1016/j.freeradbiomed.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 76.Papa S, Sardanelli AM, Cocco T, Speranza F, Scacco SC, Technikova-Dobrova Z. The nuclear-encoded 18 kDa (IP) AQDQ subunit of bovine heart complex I is phosphorylated by the mitochondrial cAMP-dependent protein kinase. FEBS Lett. 1996;379:299–301. doi: 10.1016/0014-5793(95)01532-9. [DOI] [PubMed] [Google Scholar]
- 77.Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 78.Helling S, Vogt S, Rhiel A, Ramzan R, Wen L, Marcus K, Kadenbach B. Phosphorylation and kinetics of mammalian cytochrome c oxidase. Mol Cell Proteomics. 2008;7:1714–1724. doi: 10.1074/mcp.M800137-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acin-Perez R, Gatti DL, Bai Y, Manfredi G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 2011;13:712–719. doi: 10.1016/j.cmet.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grimsrud PA, Carson JJ, Hebert AS, Hubler SL, Niemi NM, Bailey DJ, Jochem A, Stapleton DS, Keller MP, Westphall MS, Yandell BS, Attie AD, Coon JJ, Pagliarini DJ. A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Cell Metab. 2012;16:672–683. doi: 10.1016/j.cmet.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huttemann M, Lee I, Samavati L, Yu H, Doan JW. Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochimica et biophysica acta. 2007;1773:1701–1720. doi: 10.1016/j.bbamcr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Phillips D, Aponte AM, Covian R, Balaban RS. Intrinsic protein kinase activity in mitochondrial oxidative phosphorylation complexes. Biochemistry. 2011;50:2515–2529. doi: 10.1021/bi101434x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, Strik AM, Kuil C, Philipsen RL, van Duin M, Conti M, Gossen JA. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J, Martinez J, Milner TA, Buck J, Levin LR. Neuronal expression of soluble adenylyl cyclase in the mammalian brain. Brain research. 2013;1518:1–8. doi: 10.1016/j.brainres.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]