Abstract
The transcription factor brachyury is a major driver of epithelial to mesenchymal transition in human carcinoma cells. It is overexpressed in several human tumor types versus normal adult tissues, except for testes and thyroid. Overexpression is associated with drug resistance and poor prognosis. Previous studies identified a brachyury HLA-A2 cytotoxic T-lymphocyte epitope. The studies reported here describe an enhancer epitope of brachyury. Compared to the native epitope, the agonist epitope: (a) has enhanced binding to MHC class I, (b) increased the IFN-γ production from brachyury-specific T cells, (c) generated brachyury-specific T cells with greater levels of perforin and increased proliferation, (d) generated T cells more proficient at lysing human carcinoma cells endogenously expressing the native epitope, and (e) achieved greater brachyury-specific T-cell responses in vivo in HLA-A2 transgenic mice. These studies also report the generation of a heat-killed recombinant Saccharomyces cerevisiae (yeast) vector expressing the full-length brachyury gene encoding the agonist epitope. Compared to yeast-brachyury (native) devoid of the agonist epitope, the yeast-brachyury (agonist) enhanced the activation of brachyury-specific T cells, which efficiently lysed human carcinoma cells. In addition to providing the rationale for the recombinant yeast-brachyury (agonist) as a potential vaccine in cancer therapy, these studies also provide the rationale for the use of the agonist in (a) dendritic cell (DC) vaccines, (b) adjuvant or liposomal vaccines, (c) recombinant viral and/or bacterial vaccines, (d) protein/polypeptide vaccines, (e) activation of T cells ex vivo in adoptive therapy protocols, and (f) generation of genetically engineered targeted T cells.
Keywords: Agonist epitope, Brachyury, Cytotoxic T lymphocytes (CTL), Epithelial to mesenchymal transition (EMT), Immunotherapy, Saccharomyces cerevisiae vector
Introduction
The epithelial to mesenchymal transition (EMT) has been identified in preclinical studies to be important in the process of carcinoma invasion and metastasis as well as in drug resistance. Several transcription factors that have been shown to mediate EMT, such as twist, slug and snail, are also associated with poor prognosis of multiple human tumor types [1–3]. Some of these transcription factors are expressed in normal adult tissues at a level similar to their expression in tumors [4]; thus, their potential as therapeutic targets is unknown at this time.
The transcription factor brachyury was initially identified as a molecule relevant to the formation of the mesoderm during murine embryonic development, which involves conversion of epithelial cells into mesenchymal cells [5]. It is thus a mediator of a normal physiologic EMT. Subsequent studies revealed brachyury to be expressed in a range of human tumors, with limited levels in human adult testes and thyroid, and little or no expression in other normal adult tissues [4, 6]. In vitro studies showed that high levels of brachyury expression in a range of human carcinoma cells correlated with a more mesenchymal/fibroblastoid morphology, ability to migrate and invade in in vitro assays, and expression of the mesenchymal markers [7]; silencing of brachyury led to a reversion of these phenomena [7–10]. These and other studies have defined brachyury as a master driver of EMT in human carcinomas. Analyses of cloned populations of human carcinoma cells have shown brachyury to be associated with drug resistance [10].
Brachyury expression has been demonstrated in a number of carcinomas, but the expression levels vary by tumor type. Studies of biopsy specimens of human lung carcinomas have shown brachyury to be expressed at higher levels in high-grade lesions [9]. Immunohistochemistry (IHC) studies of human breast carcinoma lesions using a brachyury-specific monoclonal antibody have shown low to moderate brachyury expression in primary tumors with a high level of expression in regional lymph node and distal metastases, and brachyury expression was associated with poor prognosis [11]. High levels of brachyury expression have also been associated with poor prognosis of lung [12], prostate [13] and colon [14] carcinomas, and with tamoxifen resistance in breast carcinomas [11]. High levels of brachyury have also been found in human chordomas [15].
Transcription factors such as brachyury, however, are generally believed to be difficult to target with small molecule targeted therapies due to their nuclear location and lack of a specific groove for the tight binding of a small molecule inhibitor. An alternative approach to target transcription factors is vaccine-mediated T-cell therapy. Previous studies have identified an HLA-A2 class I brachyury peptide that is capable of inducing human CD8+ cytotoxic T lymphocytes (CTL) in vitro [6]; these T cells were shown to be capable of selectively lysing a range of brachyury expressing human carcinoma cell lines [6]. These studies demonstrated that brachyury polypeptides are transported through the cytoplasm to the cell surface in the context of 9–10 mer peptide—major histocompatibility complex (MHC) class I complexes for T-cell recognition. While class I HLA-A2 peptides restrict their use to HLA-A2 positive patients, this allele is present in approximately 50 % of the Caucasian population [16].
A heat-killed recombinant Saccharomyces cerevisiae (yeast) vaccine expressing brachyury has also been developed that expresses the entire brachyury protein and can activate both human brachyury-specific CD8+ and CD4+ T cells in vitro [17]. This vaccine is being evaluated in a phase I clinical trial in patients with advanced cancers (NCT01519817) [18–20].
Subsequent to these studies, an effort was undertaken to enhance the endogenous immunogenicity of brachyury. One way to accomplish this is to alter the amino acid anchor residue of a 9- to 10-mer peptide to strengthen its binding to the MHC-class I molecule [21, 22]. Such enhancer agonist epitopes must be capable of augmenting T-cell functions such as cytokine production over the native epitope; more importantly, T cells activated with the agonist must also recognize the native epitope endogenously expressed on tumor cells and be able to lyse these cells. The studies reported here describe the identification of a brachyury agonist epitope that is capable of enhancing the generation and activation of brachyury-specific CD8+ T cells. Also reported is the characterization of a heat-killed recombinant yeast vector expressing the whole brachyury gene and containing the brachyury enhancer epitope. These studies provide the rationale for the potential use of the agonist epitope in peptide-based vaccines, a recombinant yeast vaccine, other vaccine platforms, or engineered T cells targeting brachyury.
Materials and methods
Patients
For the establishment of brachyury peptide-specific T-cell lines, we utilized peripheral blood mononuclear cells (PBMCs) from two patients (patients #1 and #2) with prostate cancer enrolled in a previously described clinical trial of PSA-TRICOM vaccine in combination with ipilimumab (NCT00113984) [23, 24]. An institutional review board of the National Institutes of Health (NIH) Clinical Center had approved the procedures, and informed consent was obtained in accordance with the Declaration of Helsinki.
Cell culture
The human pancreatic carcinoma cell lines CFPAC-1 and ASPC-1, the human lung cancer cell line H441, the human colon carcinoma cell line SW620, and the human breast carcinoma cell line MDA-MB-231 were purchased from American Type Culture Collection (ATCC) (Manassas, VA). The cultures were free of Mycoplasma and were maintained as recommended by ATCC. K562/A2.1, a human myelogenous leukemia cell line that expresses a transfected genomic clone of HLA-A2.1, was obtained from C. Britten (Johannes Gutenberg-University of Mainz, Mainz, Germany) [25]. The transport deletion mutant cell line T2 transfected with the HLA-A2 gene was provided by Dr. Peter Cresswell (Yale University School of Medicine, New Haven, CT) [26]. K562/A2.1 and T2 cells were Mycoplasma free and were maintained in RPMI 1640 complete medium and in Iscove’s modified Dulbecco’s complete medium [supplemented with 10 % fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Technologies, Inc, Carlsbad, CA)], respectively.
Peptides
The 9-mer brachyury HLA-A2 binding peptide (WLLPGTSTL), herein called “native,” had been previously identified and evaluated for binding and stability [6]. An agonist peptide, designated “agonist,” was produced by changing the anchor residue at position 254 from leucine to valine, increasing the predicted one-half-time dissociation of the peptide/MHC complex according to the BIMAS algorithm [27] (Fig. 1a). Peptides were synthesized at >95 % purity by CPC Scientific (Sunnyvale, CA).
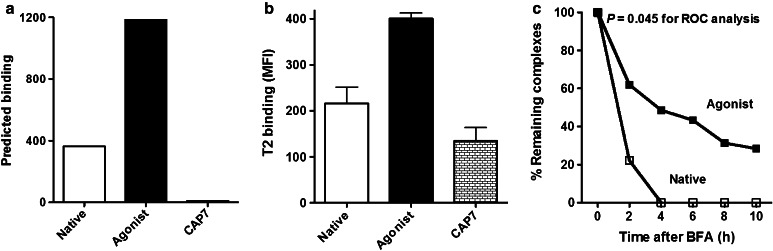
Fig. 1.
Predicted and actual binding of the brachyury peptides, and stability of the peptide binding to the HLA-A2 molecule. a Predicted binding of the native (WLLPGTSTL) and agonist (WLLPGTSTV) brachyury peptides. The results are expressed as the estimated half time of dissociation of a peptide containing this sequence from an HLA-A2 molecule using BIMAS software. CAP7 is an HLA-A3 binding peptide, which served as a negative control for the assay. b Actual binding of peptides to MHC (HLA-A2) on T2 cells. Results are expressed as mean ± SD of fluorescence intensity (MFI) of anti-HLA-A2 antibody. All peptides were used at a concentration of 12.5 µg/ml. c Stability of brachyury native and agonist peptide binding to the HLA-A2 molecule. T2 cells were incubated with the native and agonist peptides at 25 µg/ml for 18 h, then treated with brefeldin A (BFA) and analyzed by flow cytometry at the indicated time points for the presence of surface peptide–HLA-A2 complexes. The percentages of remaining complexes are shown, compared to the MFI at time point 0 (100 %). ROC curve analysis showed area under the curve (AUC) 0.8472 ± 0.1416 (SE) with significance level P = 0.045
Peptide binding and stability
Peptide binding and stability were evaluated by the accumulation of HLA-A2 on the surface of T2 cells as determined by an increase in the mean fluorescence intensity (MFI) [28]. HLA-A2 binding peptide NGEP [29] and an HLA-A3 binding peptide served as positive and negative controls, respectively. Samples were run on a BD FACScan and analyzed with CellQuest software (BD). For the stability assay, the percentage of remaining peptide–MHC complexes was calculated by dividing the MFI at each time point by the initial MFI (time point 0 = 100 %).
Saccharomyces cerevisiae (yeast) vector
Saccharomyces cerevisiae (yeast) were transfected with a plasmid expressing full-length brachyury with or without the substitution of leucine to valine at position 254 (GlobeImmune, Louisville, CO).
Culture of dendritic cells from PBMCs
HLA-A2 healthy donor PBMCs were obtained from heparinized blood. PBMCs were separated using lymphocyte separation medium gradient (Organon Teknika, Durham, NC), according to the manufacturer’s instructions. Dendritic cells (DCs) were prepared from PBMCs as previously described [30].
Generation of T-cell lines
Peptide-generated T-cell lines
A modification of the protocol described by Tsang et al. [31, 32] was used to generate brachyury-specific CTL. IFN-γ production by T-cell cultures was measured using a human IFN-γ ELISA kit (Invitrogen, Grand Island, NY), 24 h after restimulation.
Yeast-generated T-cell lines
A modification of the protocol described by Remondo et al. [33] was used to generate brachyury-specific CTL using yeast. Autologous DCs were incubated with yeast at a 1:1 ratio for 48 h. The culture was gently rinsed to remove excess yeast, and PBMCs were added at an effector-to-APC ratio of 10:1. Irradiated autologous EBV-transformed B cells pulsed with the corresponding peptide were used as APCs after the third IVS cycle.
T-cell phenotyping
The brachyury agonist-specific T-cell lines were examined for antigen-specificity and perforin production. T cells (2 × 105) were stained with FITC-conjugated anti-CD8 (BD) and either a PE-conjugated agonist-specific tetramer (NIH Tetramer Core Facility, Atlanta, GA) or a negative control tetramer (Beckman Coulter, Fullerton, CA). Cells stained for perforin production were surface stained with FITC-conjugated anti-CD8 (BD) and then permeabilized using a Fixation/Permeabilization kit (eBioscience, San Diego, CA) according to the manufacturer’s instructions. Cells then underwent intracellular staining with PE-conjugated anti-perforin antibody or isotype control (BD). In addition, T-cell lines were stained with CD8-V421 (BD), CD45RA-PerCP-Cy5.5 (eBioscience), CCR7-AF700 (R&D Systems, Minneapolis, MN), CD27-PE (BD), Perforin-APC (BioLegend, San Diego, CA) and Ki67-FITC (BD), and 1x105 cells were captured on an LSRII (BD) and analyzed using FlowJo 9.0.1 software (Tree Star Inc, Ashland, OR).
Cytotoxicity assay
Cytotoxicity assays were performed as previously described [34]. For cold target inhibition, K562/A2.1 cells were treated with or without 20 µg/ml of agonist peptide for 2 h and then added at a 10:1 ratio with the targets. Antibody-blocking experiments were performed by pre-treating target cells with 10 µg/ml of anti-HLA-A2 antibody or isotype control UPC-10 for 1 h.
Immunizations, in vitro cytotoxicity assay, and cytokine release assay
Adult female A2.1/Kb transgenic mice [35] were vaccinated s.c. 3× weekly with 100 µl Montanide ISA VG 51 emulsion (Seppic, Fairfield, NJ) containing 0, 10, 50, or 100 µg brachyury agonist or native peptide. Two weeks after the final vaccination, two spleens were harvested and pooled from each vaccination group. Single-cell suspensions were prepared and grown for 6 days in complete RPMI containing 10 µg/ml of the immunizing peptide. These cells were then co-incubated in round-bottomed 96-well plates with 111In-labeled, A2.1/Kb-expressing Jurkat cells [36] pulsed with 10 µg/ml of brachyury agonist, brachyury native, or control peptide (CMV pp65495–503 or influenza M158–66). After overnight incubation, a Wizard2 gamma counter (PerkinElmer) was used to measure 111In release. Cytotoxicity was calculated as % specific lysis = [(experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm)] × 100. Results from triplicate wells were combined and reported as mean ± SEM. Unused effector cells were placed in complete RPMI overnight without peptide and then restimulated with 10 µg/ml of the same brachyury or control peptides. Each restimulation culture consisted of 2 × 105 effector cells, 1 × 106 naïve A2.1/Kb transgenic mouse splenocytes as APCs (irradiated at 20 Gy), and 10 µg/ml peptide in 1 ml complete RPMI. Supernatant samples were collected after 2 days, centrifuged to remove contaminating cells, and then tested for mouse IFN-γ levels using a standard ELISA kit (Thermo Scientific, Waltham, MA).
Results
The predicted and actual binding to T2 cells of the previously described [6] native (Tp2) brachyury 9-mer HLA class I binding peptide (WLLPGTSTL) is shown in Fig. 1a, b. Modification of a single amino acid at the position 9 anchor residue from leucine (L) to valine (V) (WLLPGTSTV) predicted a greater binding to HLA-A2 class I (Fig. 1a) [27]. The actual binding assay to T2 cells revealed an increased T2 binding of the agonist peptide (Fig. 1b). A comparison of the sequences of the native and agonist epitopes in a protein database [Basic local alignment search tool (BLAST)] showed similar results for both peptides, with 100 % homology to the brachyury protein, and not to other proteins in the database. Titration of the native and agonist peptides for binding to T2 cells from 25 to 1.65 µg/ml (twofold dilutions) showed greater binding of the potential agonist at each concentration. To determine the HLA-A2 “off rate,” i.e., avidity of each peptide, T2 cells were incubated with each peptide for 18 h, then treated with brefeldin A (BFA) and analyzed by flow cytometry at various time points to detect the presence of surface peptide–HLA-A2 complexes. As seen in Fig. 1c, the agonist peptide was more stable at each time point than the native peptide (P = 0.045 for ROC curve analysis).
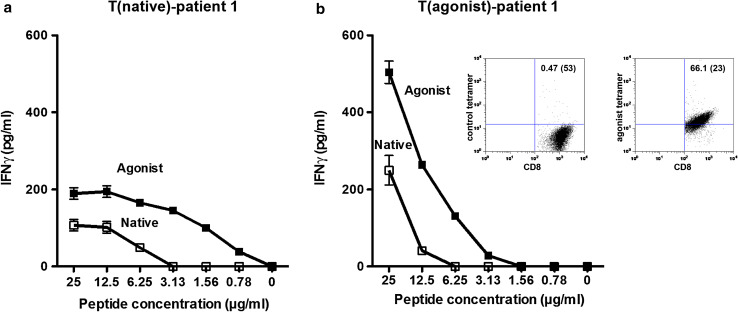
Functional studies of the peptides were initiated by determining the ability of each to activate T cells to produce IFN-γ. A CD8+ T-cell line was generated from PBMCs from a prostate cancer patient previously vaccinated with PSA-TRICOM vaccine and ipilimumab [23] by pulsing autologous DCs with the native brachyury peptide. As seen in Fig. 2a, DCs pulsed with agonist peptide were more efficient than DCs pulsed with the native peptide at activating T cells to produce IFN-γ at each peptide concentration. Approximately tenfold less agonist peptide than the native peptide enabled T-cell IFN-γ production of 100 pg/ml. A T-cell line from the same prostate cancer patient was then generated with the agonist peptide, using the same methods. As seen in Fig. 2b, DCs pulsed with the agonist peptide again elicited higher levels of IFN-γ from the T-cell line than DCs pulsed with the native peptide. A tetramer generated against the agonist peptide showed 66 % binding of CD8+ T cells generated with the agonist peptide vs. 0.47 % binding to control tetramer (Fig. 2b, insert).
Fig. 2.
Functional analysis of T-cell lines generated with the brachyury native and agonist peptides. a The agonist peptide induced greater IFNγ production by a T-cell line generated with native peptide from a prostate carcinoma patient (T(native)-patient #1), 24 h after stimulation with native or agonist peptide. Results are expressed as mean ± SD. b The agonist peptide induced greater IFNγ production by an agonist T-cell line generated from the same patient (T(agonist)-patient #1), 24 h after stimulation with native or agonist peptide. T-cell lines were cultured at a concentration of 1 × 105/ml. The ratio of APC/T cells was 3:1. Supernatants were collected after 24 h. Results are expressed as mean ± SD. Insert: T(agonist)-patient #1 was analyzed for CD8 expression and tetramer binding by flow cytometry 10 days after stimulation. Tetramer binding was 66.1 %, and binding to a negative control tetramer was 0.47 %
Previous studies [6, 17] have shown that human T-cell lines generated using the native peptide have the ability to lyse a variety of brachyury positive, HLA-A2 positive human carcinoma lines. The two T-cell lines generated in the present study using the native and agonist peptides were then analyzed for the ability to lyse human carcinoma cells endogenously expressing the native brachyury epitope. While some lysis was seen against the CFPAC-1 human pancreatic carcinoma cell line (brachyury+, HLA-A2+) using the T-cell line generated with the native peptide, a higher degree of lysis was seen using the T-cell line generated using the agonist peptide (Fig. 3a). As an indication of the MHC-restricted character of their cytotoxic function, both T-cell lines were unable to lyse the ASPC1 pancreatic carcinoma line (brachyury+, HLA-A2neg) (Fig. 3b). The T-cell line generated using the agonist peptide was also able to efficiently lyse the human lung carcinoma line H441 (Fig. 3c) and the human colon carcinoma line SW620 (Fig. 3d), both of which are brachyury+, HLA-A2+. Brachyury expression in these tumor cell lines had previously been evaluated by RT-PCR [6].
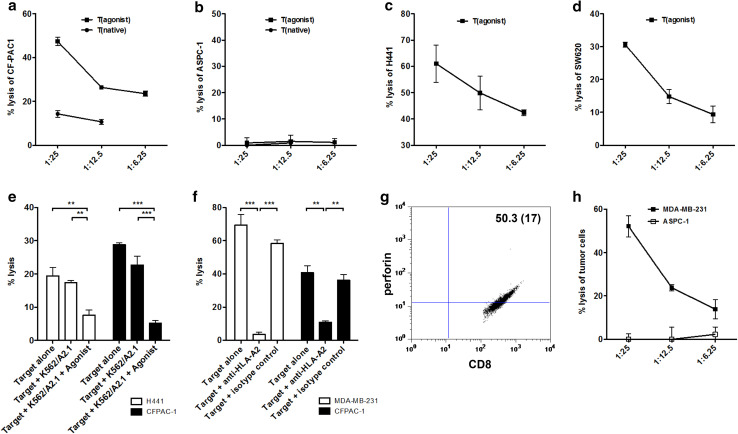
Fig. 3.
The brachyury agonist peptide-specific T-cell line lysed tumor cell targets more efficiently than the brachyury native peptide-specific T-cell line, and the lysis could be specifically blocked by cold target inhibition or the addition of anti-HLA-A2 antibody. The peptide-specific T-cell lines lysed several different human carcinoma cell lines; CFPAC-1 (pancreatic carcinoma, HLA-A2+, brachyury+), H441 (lung carcinoma, HLA-A2+, brachyury+), SW620 (colon carcinoma, HLA-A2+, brachyury+), and MDA-MB-231 (breast carcinoma, HLA-A2+, brachyury+). The native and agonist peptide T-cell lines were generated from the same patient #1 with prostate cancer and used at IVS4. The native peptide-derived T-cell line grew poorly and could therefore not be evaluated in all assays. a Native and agonist brachyury T-cell line lysis of CFPAC-1. b There was no native or agonist brachyury T-cell line lysis of ASPC-1 (pancreatic carcinoma, HLA-A2neg, brachyury+). c Agonist brachyury T-cell line lysis of H441. d Agonist brachyury T-cell line lysis of SW620. The results are from individual experiments with triplicate determinations and are expressed as % specific lysis ± SD at indicated effector-to-target (E/T) ratios. e The brachyury+, HLA-A2+ tumor cell lines H441 (lung carcinoma) and CFPAC-1 (pancreatic carcinoma) were used as targets, alone or with K562/A2.1 cells (cold targets) ± peptide at a concentration of 20 µg/ml. K562/A2.1 cells were added at a 10:1 ratio with tumor cells. Target cell lysis was inhibited by the addition of K562/A2.1 cells exposed to peptide, but not without peptide. The results are from individual experiments with triplicate determinations and are expressed as mean % specific lysis ± SD at an effector-to-target ratio of 25:1. f The brachyury+, HLA-A2+ tumor cell lines MDA-MB-231 (breast carcinoma) and CFPAC-1 (pancreatic carcinoma) were incubated for 1 h alone, or in the presence of anti-HLA-A2 antibody (10 μg/ml) or a negative control antibody (UPC-10, 10 μg/ml) before the 111In release assay was performed. The results are from an individual experiment with triplicate determinations and are expressed as mean % specific lysis ± SD at an effector-to-target ratio of 25:1. g, h Perforin expression and tumor cell lysis by a T-cell line generated with the yeast brachyury agonist, T-yeast (agonist), from patient #2 with prostate cancer, assayed at IVS6. g T-yeast (agonist) was examined for perforin expression in CD8+ cells on day 5 post-stimulation. Results are expressed as % positive cells of the live gate (MFI). h This T-cell line was used to target two different human carcinoma cell lines: MDA-MB-231 (breast carcinoma, HLA-A2+, brachyury+) and ASPC-1 (pancreatic carcinoma, HLA-A2neg, brachyury+). The results are from individual experiments with triplicate determinations and are expressed as % specific lysis ± SD at the indicated effector-to-target (E/T) ratios
Cold target inhibition studies were conducted to validate the nature of the HLA-A2 restriction of the lysis observed with the agonist peptide-derived T-cell line. The lysis of both the H441 lung carcinoma line and the CFPAC-1 pancreatic carcinoma line was inhibited by the addition of K562/A2.1 cells exposed to peptide, with no appreciable reduction in lysis when the K562/A2.1 cells were not exposed to peptide (Fig. 3e). The lysis of CFPAC-1 and the human breast carcinoma line MDA-MB-231 (brachyury+, HLA-A2+) could be specifically inhibited by the addition of anti-HLA-A2 monoclonal antibody (Fig. 3f), thus providing additional evidence for the MHC-class I A2 restriction of the lysis, and that T cells generated using the agonist peptide could lyse tumor cells endogenously expressing the native epitope.
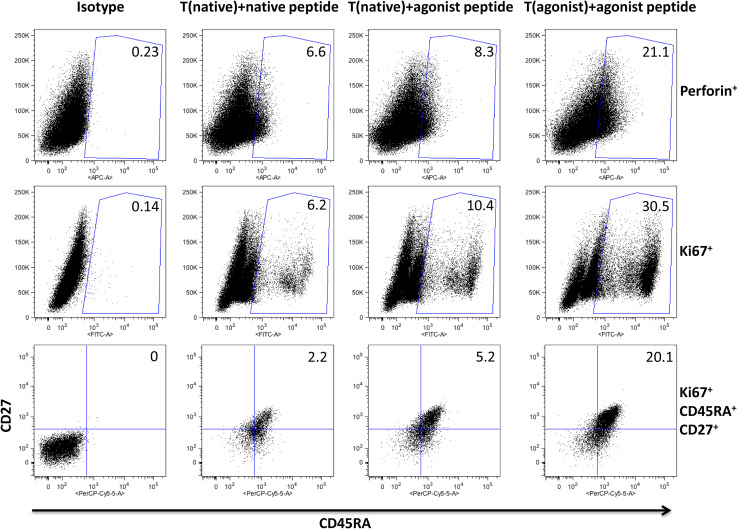
Studies were then conducted to better examine the differences that may exist in the generation of a human CD8+ T-cell line from the same individual and at the same time using APCs pulsed with either the native or agonist brachyury peptide. As seen in the middle row of Fig. 4, the T-cell line generated with the native peptide and stimulated with the native peptide showed 6.2 % Ki67 positive CD8+ T cells, while the same cell line stimulated with the agonist peptide showed 10.4 % Ki67 positive CD8+ T cells, indicating a higher degree of proliferation when stimulated with the agonist vs. native peptide. The T-cell line generated with the agonist peptide and stimulated with that same peptide had a fivefold increase in Ki67 positive cells compared to the T-cell line generated and stimulated with the native peptide (Fig. 4, middle row). Similar findings were observed with regard to the levels of perforin (Fig. 4, top row), revealing more cells with higher potential lytic activity when T cells are either established with or activated with the agonist peptide vs. the native peptide. The same analysis was performed on T-cell lines from an additional patient and showed 93.6 % perforin expressing CD8+ T cells on day 5 after stimulation (data not shown).
Fig. 4.
Phenotypic analysis of brachyury native and agonist peptide-generated T-cell lines. T-cell lines were generated with brachyury native and agonist peptides from the same patient #2 with prostate cancer and were designated T(native) and T(agonist), respectively. At IVS 5 T(native) was stimulated with either native or agonist peptide, and T(agonist) was stimulated with agonist peptide. On day 5 after stimulation, phenotypic analysis by flow cytometry was performed. The experiment was repeated three times, and representative FACS plots are shown. For the first two rows, cells were first gated on CD8+ and the percent positive out of the CD8 gate is shown in each plot. For the third row, cells were first gated on CD8+/Ki67+/CCR7neg and the percent positive shown is of total CD8
The studies shown on the bottom row of Fig. 4 reveal that T cells derived with the native peptide and stimulated with the agonist peptide have a greater number of CCR7neg/CD45RA+/CD27+ cells. Moreover, cells generated with the agonist peptide and stimulated with the agonist peptide have a sevenfold greater level of CCR7neg/CD45RA+/CD27+ cells than CD8+ T cells generated and stimulated with the native peptide. There is evidence that the loss of CD27 from CD45RA+ TEMRA cells is a terminal differentiation event that correlates with loss of telomerase activity and telomere shortening [37, 38]. The presence of a significant population of Ki67+/CD45RA+/CD27+ T cells thus suggests that the highly proliferative population induced by stimulation with the agonist peptide is not a terminally differentiated effector population, but rather a population that can maintain proliferative potential to expand the pool of antigen-specific effectors.
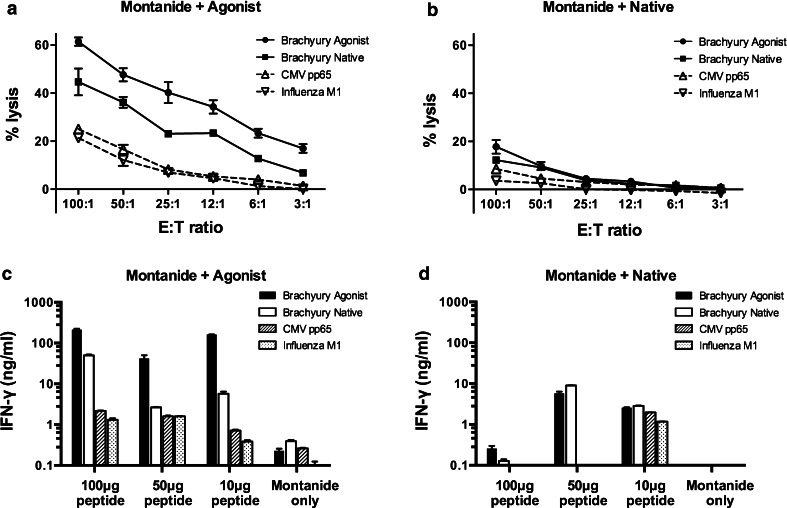
In order to investigate the immunogenicity of the brachyury agonist in vivo, A2.1/Kb transgenic mice were vaccinated three times weekly with the agonist or native peptide mixed with the vaccine adjuvant Montanide. Splenocytes from mice vaccinated with the brachyury agonist peptide were highly cytotoxic against Jurkat target cells (A2.1/Kb+) pulsed with the agonist peptide (Fig. 5a). Those same T cells also recognized to a lesser extent target cells pulsed with the native brachyury peptide; two viral-derived peptides were used as controls (Fig. 5a). Splenocytes from mice vaccinated with Montanide mixed with native brachyury peptide (Fig. 5b) failed to lyse targets in a peptide-specific manner. Splenic T cells from mice vaccinated with the agonist peptide (Fig. 5c) produced much higher levels of IFN-γ than T cells from mice vaccinated with the native peptide (Fig. 5d). For the native peptide, stimulation with 100 µg of either peptide resulted in lower levels of IFN-γ than stimulation with 50 µg of peptide, and this is most likely due to overactivation of the T cells. Furthermore, the agonist peptide consistently reactivated T cells from agonist peptide-vaccinated mice more strongly than the native peptide. Taken together, these results indicate that the brachyury agonist peptide activates T cells in vivo and in vitro more effectively than the native peptide.
Fig. 5.
Immunogenicity of brachyury-derived epitopes in HLA-A2.1 transgenic mice. Mice were vaccinated with Montanide emulsion mixed with 100 µg brachyury agonist peptide (a), brachyury native peptide (b), or Montanide alone. Two weeks after the final immunization, splenocytes were harvested from each vaccination group and stimulated in vitro with either brachyury agonist peptide or brachyury native peptide at a concentration of 10 µg/ml. Six days later, these cells were used as effectors in an 111In-release cytotoxicity assay. Radiolabeled Jurkat target cells (A2.1/Kb+) were pulsed with 10 µg/ml brachyury agonist (filled circle), brachyury native (filled square), CMV pp65 (open triangle), or Influenza M1 peptide (inverted open triangle). Results shown are the mean ± SEM of triplicate wells. Additional effector cells from the brachyury agonist peptide-stimulated cultures (c) and brachyury native peptide-stimulated cultures (d) were starved overnight in complete RPMI without peptide and then restimulated the following day with 10 µg/ml brachyury agonist, brachyury native, CMV pp65, or Influenza M1 peptide. Supernatants were collected 2 days later, and IFN-γ levels were determined by ELISA. Results are shown as mean ± SD of duplicates
We have recently characterized in preclinical studies a heat-killed recombinant S. cerevisiae (yeast) brachyury vaccine [17]. A recombinant yeast brachyury vector has now been constructed that transcribes the entire brachyury gene with the residue change at position 254 to create the agonist epitope. As shown in Table 1, DCs derived from the same donor were exposed to either recombinant yeast (native) containing the entire brachyury gene with the native epitope, or recombinant yeast (agonist), which means it contains the brachyury gene encoding the agonist epitope. Yeast (control) is wild-type yeast containing no brachyury protein. As seen in Table 1a, at each yeast/DC ratio the yeast (agonist) was more efficient in activating brachyury-specific T cells originally generated with the native peptide to produce IFN-γ. The same phenomenon was observed employing the activation of brachyury-specific T cells from the same individual, but derived using the agonist epitope (Table 1b). There was approximately a five- to eightfold increase in the IFN-γ production of T cells if they were derived from DCs pulsed with agonist epitope and stimulated with the yeast (agonist) compared to T cells derived from DCs pulsed with the native epitope and stimulated with the yeast (native). Control yeast did not stimulate the brachyury-specific T-cell lines to produce IFN-γ.
Table 1.
Allogeneic DCs treated with recombinant yeast brachyury (agonist) can stimulate brachyury-specific T cells to greater levels than DCs treated with yeast brachyury (native)
| DC | Treatment | Yeast/DC ratio | T-cell line | IFNγ (pg/ml) |
|---|---|---|---|---|
| (a) T-cell line (native) stimulated with yeast (native) and yeast (agonist) | ||||
| + | Yeast (native) | 10:1 | + | 131 |
| + | Yeast (native) | 5:1 | + | 162 |
| + | Yeast (native) | 1:1 | + | 95 |
| + | Yeast (agonist) | 10:1 | + | 321 |
| + | Yeast (agonist) | 5:1 | + | 330 |
| + | Yeast (agonist) | 1:1 | + | 176 |
| + | Yeast (native) | 10:1 | − | <15.6 |
| + | Yeast (control) | 10:1 | + | <15.6 |
| (b) T-cell line (agonist) stimulated with yeast (native) and yeast (agonist) | ||||
| + | Yeast (native) | 10:1 | + | 775 |
| + | Yeast (native) | 5:1 | + | 728 |
| + | Yeast (native) | 1:1 | + | 278 |
| + | Yeast (agonist) | 10:1 | + | 1196 |
| + | Yeast (agonist) | 5:1 | + | 1095 |
| + | Yeast (agonist) | 1:1 | + | 547 |
| + | Yeast (agonist) | 10:1 | − | <15.6 |
| + | Yeast (control) | 10:1 | + | <15.6 |
Allogeneic HLA-A2-positive DCs from a healthy donor were treated with yeast brachyury (native), yeast brachyury (agonist), or empty yeast vector for 48 h at the indicated yeast to DC ratios, and then used to stimulate brachyury-specific T-cell lines from patient #2 derived by stimulation with the native (a) and agonist (b) peptides, designated T-cell lines T (native) and T (agonist). The DC to T-cell ratio was 1:10. Results are expressed in pg/ml/2 × 105 T cells
A T-cell line generated by treating DCs from PBMCs of a prostate cancer patient to the recombinant yeast (agonist) displayed 50.3 % perforin+ CD8+ cells (Fig. 3g). The same T-cell line also showed the ability to lyse human breast carcinoma cells (MDA-MB-231, brachyury+, HLA-A2+) but not the brachyury+, HLA-A2neg pancreatic carcinoma cell line ASPC-1 (Fig. 3h), indicating the MHC HLA-A2 class I restriction of the lysis.
Discussion
It should be noted that the development of an enhancer agonist epitope is not as simple as producing a peptide with altered binding affinity based on computer algorithms. Recent studies in our laboratory have shown [32] that some potential “agonist” peptides with the highest predicted binding to MHC-class I HLA-A2 (a) can have relatively poor HLA-A2 binding in in vitro assays, (b) cannot activate T cells to induce IFN-γ, and/or (c) cannot generate T cells that lyse tumor cells endogenously expressing the native antigen. Some predicted “agonist” epitopes have also demonstrated antagonist activity. There is always the possibility that a novel agonist epitope will not cross-react with the native epitope endogenously expressed on tumor cells. Here, we have shown that four different human tumor cell lines that endogenously express the native epitope are lysed by the agonist-specific T cells, so indeed these T cells recognize and bind the native epitope. In addition, the lysis of tumor cells expressing the native epitope could be blocked by the addition of K562/A2.1 cells pulsed with the agonist epitope (see Fig. 3e). In making the agonist epitope, we also did not alter the T-cell receptor, only the affinity for HLA-A2.
We have previously shown that tumor cells that express no brachyury cannot be lysed by brachyury-specific T cells [6]. However, there is not always a direct correlation between the amount of brachyury expression and the degree of CTL lysis when using different tumor cell lines. Other factors have been shown previously to be involved in T-cell killing of tumor cells, such as (a) HLA class I level on tumor, (b) level and/or presence of accessory molecules such as Fas and ICAM, and (c) processing of peptide via transporter molecules to peptide–MHC complexes. Also, we have recently shown [39] that extremely high levels of brachyury in some tumor cell lines result in resistance to killing by chemotherapeutic agents and immune attack.
The studies reported here provide the rationale to employ the agonist brachyury epitope in a range of vaccine modalities. The agonist peptide described here can be used with (a) dendritic cell vaccines, (b) as transgenes in viral, bacterial or yeast vector vaccines, (c) conventional or experimental adjuvants, (d) for the in vitro activation of T cells for adoptive T-cell transfer protocols, (e) for T-cell receptor genetically engineered T cells for adoptive transfer protocols, or (f) in protein- or polypeptide-based vaccines.
The studies reported here also demonstrate that the brachyury agonist epitope can be appropriately processed and expressed in the context of a peptide–MHC complex by human DCs exposed to the recombinant yeast brachyury vector to activate brachyury-specific T cells. As shown, compared to the native epitope, both the brachyury agonist peptide and the brachyury agonist yeast vector can generate T cells capable of enhanced IFN-γ production and lysis of tumor cells endogenously expressing the native epitope.
Previous studies have shown that recombinant yeast is relatively easy to engineer, produce and purify. They contain the recombinant protein, are heat-killed, and are thus relatively safe compared to other forms of cancer therapy [40]. Previous studies have shown that recombinant yeast are efficiently taken up by human or murine DCs, which are consequently matured to express high levels of costimulatory molecules and produce high levels of type I cytokines [41, 42]. Preclinical studies have shown that yeast-CEA can activate human CD8+ and CD4+ T cells in vitro, and show anti-tumor activity versus CEA-expressing murine tumors [33, 42]. A Phase I trial with recombinant yeast-CEA has been completed [40], and a Phase II trial is ongoing in patients with medullary thyroid cancer (NCT01856920) [43]. Using either the native or agonist peptides, or yeast constructs containing these epitopes, we have generated CD8+ T-cell lines specific for the brachyury peptides. This was accomplished using PBMCs from both healthy donors and cancer patients. Specific T-cell lines were generated from PBMCs from 4/9 healthy donors and 5/5 cancer patients using the brachyury yeast constructs, and 3/6 healthy donors and 8/8 cancer patients using the brachyury peptides. In total, brachyury-specific CD8+ T-cell lines were generated from 9/14 individuals with brachyury yeast, and 11/14 individuals with brachyury peptides. Thus, no difference was seen. However, since the yeast-brachyury constructs express the entire brachyury protein, we were also able to generate brachyury-specific CD4+ T cells from 3/9 healthy donors using the yeast-brachyury constructs.
Previous clinical trials with a CEA-based vaccine and a PSA-based vaccine have revealed that patients mounted a post-vaccination brachyury-specific CD8+ response not seen pre-vaccination [23, 40]. This may be the result of cross-priming of brachyury from lysed tumor cells to antigen-presenting cells. These studies, however, provide additional evidence of the potential immunogenicity of brachyury in humans. The studies described here provide the rationale for the use of the brachyury agonist epitope to enhance the immunogenicity of brachyury in the use of a range of anticancer vaccine strategies.
Acknowledgments
Grant support was provided by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, and a Cooperative Research and Development Agreement between the National Cancer Institute and GlobeImmune, Inc. The authors thank Diane J. Poole, Garland Davis and Curtis Randolph for technical assistance and Debra Weingarten for editorial assistance in the preparation of this manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
Abbreviations
- ATCC
American Type Culture Collection
- BFA
Brefeldin A
- BLAST
Basic local alignment search tool
- CTL
Cytotoxic T lymphocyte
- DCs
Dendritic cells
- EMT
Epithelial to mesenchymal transition
- IHC
Immunohistochemistry
- MFI
Mean fluorescence intensity
- MHC
Major histocompatibility complex
- PBMCs
Peripheral blood mononuclear cells
Footnotes
Jo A. Tucker and Caroline Jochems have contributed equally to this paper as primary authors. Jeffrey Schlom and Kwong-Yok Tsang have contributed equally to this paper as senior authors.
References
- 1.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 2.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton DH, Litzinger MT, Fernando RI, Huang B, Palena C. Cancer vaccines targeting the epithelial-mesenchymal transition: tissue distribution of brachyury and other drivers of the mesenchymal-like phenotype of carcinomas. Semin Oncol. 2012;39(3):358–366. doi: 10.1053/j.seminoncol.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kispert A, Koschorz B, Herrmann BG. The T protein encoded by brachyury is a tissue-specific transcription factor. EMBO J. 1995;14(19):4763–4772. doi: 10.1002/j.1460-2075.1995.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, Baranova AV, Kozlov AP, Schlom J. The human T-box mesodermal transcription factor brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13(8):2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 7.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120(2):533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palena CSJ (2013) Target: brachyury, a master driver of epithelial-to-mesenchymal transition (EMT). In: Marshall J (ed) Cancer therapeutic targets: SpringerReference (www.springerreference.com). Springer, Berlin. doi:10.1007/SpringerReference_3676052014-03-1406:40:12UTC
- 9.Roselli M, Fernando RI, Guadagni F, Spila A, Alessandroni J, Palmirotta R, Costarelli L, Litzinger M, Hamilton D, Huang B, Tucker J, Tsang KY, Schlom J, Palena C. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res. 2012;18(14):3868–3879. doi: 10.1158/1078-0432.CCR-11-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang B, Cohen JR, Fernando RI, Hamilton DH, Litzinger MT, Hodge JW, Palena C. The embryonic transcription factor brachyury blocks cell cycle progression and mediates tumor resistance to conventional antitumor therapies. Cell Death Dis. 2013;4:e682. doi: 10.1038/cddis.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palena C, Roselli M, Litzinger MT, Ferroni P, Costarelli L, Spila A, Cavaliere F, Huang B, Fernando RI, Hamilton DH, Jochems C, Tsang KY, Cheng Q, Kim Lyerly H, Schlom J, Guadagni F (2014) Overexpression of the EMT driver brachyury in breast carcinomas: association with poor prognosis. J Natl Cancer Inst 106(5). doi:10.1093/jnci/dju054 [DOI] [PMC free article] [PubMed]
- 12.Haro A, Yano T, Kohno M, Yoshida T, Koga T, Okamoto T, Takenoyama M, Maehara Y. Expression of Brachyury gene is a significant prognostic factor for primary lung carcinoma. Ann Surg Oncol. 2013;20(Suppl 3):S509–S516. doi: 10.1245/s10434-013-2914-9. [DOI] [PubMed] [Google Scholar]
- 13.Pinto F, Pertega-Gomes N, Pereira MS, Vizcaino JR, Monteiro P, Henrique RM, Baltazar F, Andrade RP, Reis RM (2014) T-box transcription factor brachyury is associated with prostate cancer progression and aggressiveness. Clin Cancer Res. doi:10.1158/1078-0432.CCR-14-0421 [DOI] [PubMed]
- 14.Kilic N, Feldhaus S, Kilic E, Tennstedt P, Wicklein D, Wasielewski R, Viebahn C, Kreipe H, Schumacher U. Brachyury expression predicts poor prognosis at early stages of colorectal cancer. Eur J Cancer. 2011;47(7):1080–1085. doi: 10.1016/j.ejca.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, Boshoff C, Flanagan AM. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209(2):157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 16.Ellis JM, Henson V, Slack R, Ng J, Hartzman RJ, Katovich Hurley C (2000) Frequencies of HLA-A2 alleles in five U.S. population groups. Predominance of A*02011 and identification of HLA-A*0231. Hum Immunol 61(3):334–340 [DOI] [PubMed]
- 17.Hamilton DH, Litzinger MT, Jales A, Huang B, Fernando RI, Hodge JW, Ardiani A, Apelian D, Schlom J, Palena C. Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury–yeast vaccine. Oncotarget. 2013;4(10):1777–1790. doi: 10.18632/oncotarget.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heery CR, Singh H, Marte JL, Madan RA, O’Sullivan Coyne GH, Farsaci B, Rodell TC, Palena C, Schlom J, JL G (2014) NCI experience using yeast–brachyury vaccine (GI-6301) in patients (pts) with advanced chordoma. 2014 ASCO Annual Meeting J Clin Oncol 32:5 s (suppl; abstr 3081)
- 19.Open label study to evaluate the safety and tolerability of GI-6301 (whole heat-killed recombinant yeast modified to express brachyury protein) in adults with solid tumors (NCT01519817) http://clinicaltrials.gov/show/NCT01519817
- 20.Singh H, Heery CR, Marte JL, Farsaci B, Madan RA, O’Sullivan Coyne GH, Palena C, Rodell TC, Schlom J, Gulley JL (2014) A phase I study of a yeast-based therapeutic cancer vaccine, GI-6301, targeting brachyury in patients with metastatic carcinoma. J Clin Oncol 32 (suppl; abstr e14026)
- 21.Grey HM, Ruppert J, Vitiello A, Sidney J, Kast WM, Kubo RT, Sette A. Class I MHC-peptide interactions: structural requirements and functional implications. Cancer Surv. 1995;22:37–49. [PubMed] [Google Scholar]
- 22.Terasawa H, Tsang KY, Gulley J, Arlen P, Schlom J. Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clin Cancer Res. 2002;8(1):41–53. [PubMed] [Google Scholar]
- 23.Madan RA, Mohebtash M, Arlen PM, Vergati M, Rauckhorst M, Steinberg SM, Tsang KY, Poole DJ, Parnes HL, Wright JJ, Dahut WL, Schlom J, Gulley JL. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaccine and antibody treatment of prostate cancer (NCT00113984). http://www.clinicaltrials.gov/ct2/show/NCT00113984?term=NCT00113984&rank=1
- 25.Britten CM, Meyer RG, Kreer T, Drexler I, Wolfel T, Herr W. The use of HLA-A*0201-transfected K562 as standard antigen-presenting cells for CD8(+) T lymphocytes in IFN-gamma ELISPOT assays. J Immunol Methods. 2002;259(1–2):95–110. doi: 10.1016/S0022-1759(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 26.DeMars R, Rudersdorf R, Chang C, Petersen J, Strandtmann J, Korn N, Sidwell B, Orr HT. Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci USA. 1985;82(23):8183–8187. doi: 10.1073/pnas.82.23.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152(1):163–175. [PubMed] [Google Scholar]
- 28.Nijman HW, Houbiers JG, Vierboom MP, van der Burg SH, Drijfhout JW, D’Amaro J, Kenemans P, Melief CJ, Kast WM. Identification of peptide sequences that potentially trigger HLA-A2.1-restricted cytotoxic T lymphocytes. Eur J Immunol. 1993;23(6):1215–1219. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 29.Cereda V, Poole D, Palena C, Das S, Bera T, Remondo C, Gulley J, Arlen P, Yokokawa J, Pastan I, Schlom J, Tsang K. New gene expressed in prostate: a potential target for T cell-mediated prostate cancer immunotherapy. Cancer Immunol Immunother. 2010;59(1):63–71. doi: 10.1007/s00262-009-0723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokokawa J, Bera TK, Palena C, Cereda V, Remondo C, Gulley JL, Arlen PM, Pastan I, Schlom J, Tsang KY. Identification of cytotoxic T-lymphocyte epitope(s) and its agonist epitope(s) of a novel target for vaccine therapy (PAGE4) Int J Cancer. 2007;121(3):595–605. doi: 10.1002/ijc.22698. [DOI] [PubMed] [Google Scholar]
- 31.Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, Schlom J. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87(13):982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 32.Jochems C, Tucker JA, Vergati M, Boyerinas B, Gulley JL, Schlom J, Tsang KY. Identification and characterization of agonist epitopes of the MUC1-C oncoprotein. Cancer Immunol Immunother. 2014;63(2):161–174. doi: 10.1007/s00262-013-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remondo C, Cereda V, Mostbock S, Sabzevari H, Franzusoff A, Schlom J, Tsang KY. Human dendritic cell maturation and activation by a heat-killed recombinant yeast (Saccharomyces cerevisiae) vector encoding carcinoembryonic antigen. Vaccine. 2009;27(7):987–994. doi: 10.1016/j.vaccine.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokokawa J, Palena C, Arlen P, Hassan R, Ho M, Pastan I, Schlom J, Tsang KY. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clin Cancer Res. 2005;11(17):6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitiello A, Marchesini D, Furze J, Sherman LA, Chesnut RW. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991;173(4):1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irwin MJ, Heath WR, Sherman LA. Species-restricted interactions between CD8 and the alpha 3 domain of class I influence the magnitude of the xenogeneic response. J Exp Med. 1989;170(4):1091–1101. doi: 10.1084/jem.170.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, van Lier RA. Evidence that human CD8 + CD45RA + CD27- cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11(7):1027–1033. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 38.Plunkett FJ, Franzese O, Finney HM, Fletcher JM, Belaramani LL, Salmon M, Dokal I, Webster D, Lawson AD, Akbar AN. The loss of telomerase activity in highly differentiated CD8 + CD28-CD27-T cells is associated with decreased Akt (Ser473) phosphorylation. J Immunol. 2007;178(12):7710–7719. doi: 10.4049/jimmunol.178.12.7710. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton DH, Huang B, Fernando RI, Tsang KY, Palena C. WEE1 inhibition alleviates resistance to immune attack of tumor cells undergoing epithelial-mesenchymal transition. Cancer Res. 2014;74(9):2510–2519. doi: 10.1158/0008-5472.CAN-13-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilusic M, Heery CR, Arlen PM, Rauckhorst M, Apelian D, Tsang KY, Tucker JA, Jochems C, Schlom J, Gulley JL, Madan RA. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother. 2014;63(3):225–234. doi: 10.1007/s00262-013-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernstein MB, Chakraborty M, Wansley EK, Guo Z, Franzusoff A, Mostbock S, Sabzevari H, Schlom J, Hodge JW. Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine. 2008;26(4):509–521. doi: 10.1016/j.vaccine.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 42.Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, Guo Z, Quick D, Franzusoff A, Greiner JW, Schlom J, Hodge JW. Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res. 2008;14(13):4316–4325. doi: 10.1158/1078-0432.CCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A phase 2 study of GI-6207 in patients with recurrent medullary thyroid cancer (NCT01856920). http://clinicaltrials.gov/ct2/show/NCT01856920?term=NCT01856920&rank=1