Abstract
Background
This study was conducted to evaluate the efficacy and safety of once-monthly continuous erythropoietin receptor activator (CERA) for maintenance of stable haemoglobin (Hb) levels in adult chronic renal anaemia patients on dialysis according to local clinical judgment in Turkey.
Methods
This was a prospective, open-label, single-arm, multi-centre study conducted in 20 centres in Turkey. After a 4-week screening period, eligible patients receiving conventional erythropoiesis-stimulating agents were converted to monthly intravenous CERA and entered a 16-week CERA dose-titration period (DTP) followed by an 8-week efficacy evaluation period (EEP) and a 4-week safety follow-up. The primary endpoint was the proportion of patients whose Hb concentration remained stable within ±1.0 g/dL of their reference Hb and within the range of 10.0–12.0 g/dL during the EEP.
Results
A total of 173 patients were screened, 132 entered the DTP and 84 completed the study. Thirty-nine patients [46.4% (95% confidence interval: 35.5–57.7%)] maintained stable target Hb concentrations. The mean change in time-adjusted average Hb concentration was 0.29 ± 1.08 g/dL between baseline and the EEP. The mean CERA monthly dose was 112.4 ± 76.78 µg during the EEP, and the CERA dose was adjusted in 39 patients (36.4%). Eleven patients (8.4%) reported 13 treatment-related adverse events, the most frequent adverse events being infections and infestations, gastrointestinal and vascular disorders.
Conclusions
Once-monthly CERA maintains stable Hb concentrations in chronic renal anaemia patients on dialysis in Turkey. The study results confirm the known efficacy and safety profile of CERA.
Keywords: anaemia, dialysis, erythropoietin receptor, kidney disease
Introduction
Erythropoiesis-stimulating agents (ESAs) are a well-accepted treatment option for anaemia of chronic kidney disease (CKD) [1–5]. Two types of recombinant human erythropoietin have been available for clinical use since ESAs first became available on the market: epoetin alpha and epoetin beta [6, 7]. Both epoetin alpha and epoetin beta are effective but short-acting. Subsequently, ESAs with an extended duration of action were developed—darbepoetin alfa and methoxy polyethylene glycol-epoetin beta.
In contrast to erythropoietin, continuous erythropoietin receptor activator (CERA) shows a different activity at the receptor level characterized by a slower association to and faster dissociation from the receptor, which results in a considerably increased half-life allowing longer (one-month) dosing intervals [8–11]. CERA has a prolonged half-life after intravenous (i.v.) and subcutaneous (s.c.) administration [12].
In Phase III trials, CERA has demonstrated efficacy in anaemia correction when administered every 2 weeks [13, 14] and every 4 weeks [15] and maintaining haemoglobin (Hb) concentrations within the target range when administered once-monthly for Hb maintenance in patients with renal anaemia [16–19].
In this study, we aimed to assess the maintenance of stable Hb concentrations with once-monthly i.v. CERA in haemodialysis patients with chronic renal anaemia previously treated with ESAs. We also aimed to evaluate the safety and tolerability of once-monthly i.v. CERA in these patients.
Materials and methods
Study design and population
This single-arm, open-label study was performed in 20 centres in Turkey. The inclusion criteria were as follows: age 18 years or older, diagnosis of chronic renal anaemia Hb concentration between 10.0 and 12.0 g/dL, adequate iron status [serum ferritin >100 ng/mL and transferrin saturation (TSAT) >20% or hypochromic red cells <10%], continuous i.v. or s.c. maintenance of epoetin alpha, epoetin beta or darbepoetin alpha therapy with the same dosing interval during the previous month and no change in total (calculated) weekly dose, regular long-term haemodialysis therapy with the same mode of dialysis for at least the previous 3 months, Kt/V ≥1.0 or urea reduction ratios of ≥55% at screening for haemodialysis patients. Exclusion criteria were as follows: transfusion of red blood cells during the previous 2 months, poorly controlled hypertension, significant acute or chronic bleeding, active malignant disease, haemolysis, haemoglobinopathies, folic acid deficiency, vitamin B12 deficiency, platelet count >500 × 109/L or <100 × 109/L, pure red cell aplasia, epileptic seizure during previous 6 months, congestive heart failure (New York Heart Association Classification Class IV), myocardial infarction or stroke, severe or unstable coronary artery disease, severe liver disease at investigator's discretion, during the previous 3 months, uncontrolled hyperparathyroidism or symptomatic secondary hyperparathyroidism, pregnancy or lactation period, women of childbearing potential without effective contraception, participation in a clinical trial during the previous 3 months, known hypersensitivity to recombinant human erythropoietin, polyethylene glycol or to any constituent of the study medication, planned elective surgery during the study period, temporary (untunneled) dialysis access catheter, active serious infection and/or use of injectable antibiotics during the previous 8 weeks and known clinically significant hypercoagulability states.
Written informed consent was provided from all patients included in the study. The study was conducted in compliance with the Declaration of Helsinki and Good Clinical Practice. The study was approved by the Ankara University Faculty of Medicine Ethics Committee and the Ministry of Health.
After a 4-week screening period, during which mean Hb levels were maintained between 10.0 and 12.0 g/dL on their current ESA treatment, eligible patients were converted to once-monthly i.v. CERA. Patients entered a 16-week CERA dose-titration period (DTP) followed by a 8-week efficacy evaluation period (EEP) and 4-week safety follow-up (Figure 1).
Fig. 1.
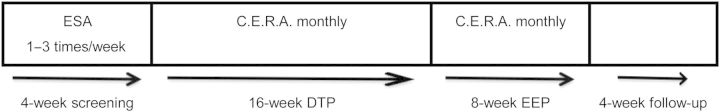
Study design. ESA, erythropoiesis-stimulating agent; DTP, dose-titration period; EEP, efficacy evaluation period.
Efficacy and safety parameters
The primary efficacy parameter was the proportion of patients in the per protocol (PP) population who maintained their mean Hb concentration within ±1.0 g/dL of their reference Hb and between 10.0 and 12.0 g/dL during the EEP. Secondary efficacy parameters were the percentage of patients whose Hb concentrations remained within the range of 10.0–12.0 g/dL throughout the EEP, the median time spent in the target Hb range of 10.0–12.0 g/dL during the EEP, the mean change in time-adjusted average Hb concentration between baseline and the EEP or DTP and the mean monthly and cumulative CERA dose to maintain target Hb level throughout the EEP.
The safety parameters evaluated in the study included incidence of adverse events, changes and abnormalities in laboratory safety parameters and incidence of red blood cell transfusions during the CERA treatment phase.
Study medication
CERA (MIRCERA®, Roche, Istanbul, Turkey) was administered i.v. on Day 0 and Weeks 4, 8, 12, 16 and 20. Starting dose of CERA was 120 µg when previous weekly epoetin <8000 IU or darbepoetin alpha <40 µg, 200 µg when previous weekly epoetin 8–16 000 IU or darbepoetin alpha 40–80 µg and 360 µg when previous weekly epoetin >16 000 IU or darbepoetin alpha >80 µg. The dose of CERA was adjusted to maintain the individual patient's Hb within a range of ±1.0 g/dL of the reference Hb concentration and between 10.0 and 12.0 g/dL throughout the DTP and the EEP (Weeks l–24).
Iron supplementation
Patients were required to be iron replete to enter into this study and were required to maintain an adequate iron status throughout the study period. Patient iron status was monitored by assessing serum ferritin and TSAT. Initiation or intensification of iron supplementation was permitted in the case of iron deficiency during the study. Iron deficiency was defined as serum ferritin <100 ng/mL and TSAT <20% (or percentage of hypochromic red blood cells >10%).
Iron supplementation was performed according to individual centre practice. To avoid iron toxicity, iron supplementation was temporarily discontinued in patients with serum ferritin >800 ng/mL or TSAT >50% until serum ferritin decreased to <800 ng/mL and TSAT to <50%.
Statistical analysis
The sample size calculation was based on an initial estimate of 72% for patients maintaining Hb concentration within ±1.0 g/dL and a two-sided 95% confidence interval (CI). The sample size of 200 patients was calculated for the study protocol before the first patient was screened. However, there were only 173 patients screened and 132 patients treated with CERA. With 132 patients, the power of the study is reduced to 65%.
Patients who received at least one dose of CERA (Week 0) and for whom data for at least one follow-up variable were available were included in the intention-to-treat (ITT) population. The safety population was defined as all patients who received at least one dose of the trial medication and underwent a safety follow-up, whether withdrawn prematurely or not. The PP population was all patients in the safety population with the exception of patients with <3 recorded Hb values during the EEP, patients missing an administration of CERA during Weeks 16–24, patients withdrawn before the end of the EEP, patients with inadequate iron status defined as mean serum ferritin <100 ng/mL or mean TSAT ≤20% or mean hypochromic red blood cells ≥10% during EEP.
For the primary endpoint, the reference Hb value was defined on the basis of all assessments at Weeks 4, 3, 2, 1 and 0. For Hb measurements H0, … , Hn taken at time point's t0, … , tn, the time-adjusted average Hb value was calculated. Data missing at the end of the EEP was handled using the last observation carried forward method, including any data missing due to withdrawal of patients following red blood cell transfusion.
Results
Study population
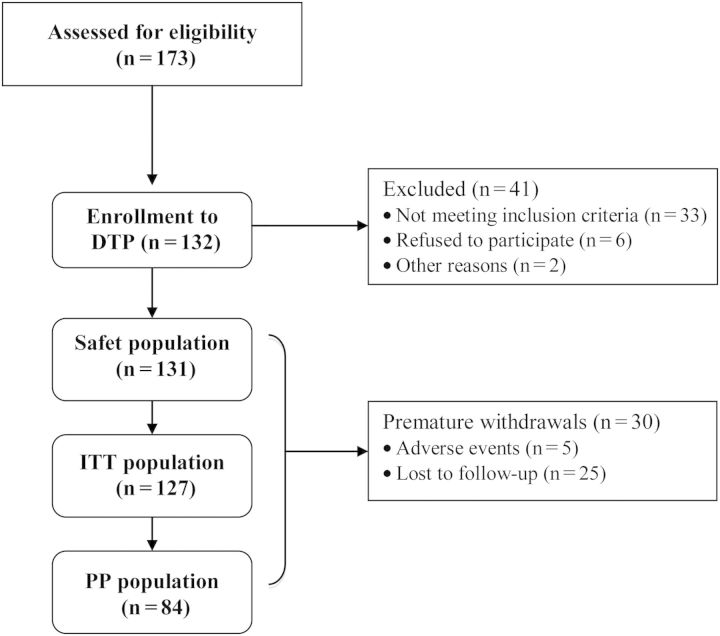
A total of 173 patients were screened for eligibility to participate in the trial. Of these, 33 failed screening, 6 refused treatment, did not cooperate or withdrew consent, and 2 experienced an adverse event. Thus, 132 were enrolled in the trial and entered the DTP (Figure 2). Thirty patients discontinued the study treatment prematurely. Five patients withdrew from the study due to adverse events [ischaemic stroke, anaemia, renal transplantation, gastrointestinal haemorrhage (two patients)].
Fig. 2.
Study population. ITT, intent to treat; PP, per protocol.
The mean age of 127 patients in the ITT population was 50.5 ± 13.9 years, and the most common aetiology for CKD was hypertension (34.4%). The mean baseline Hb level was 11.1 ± 0.6 g/dL. The mean Kt/V and urea reduction ratio were 1.6 ± 0.4 and 68.1 ± 20.6, respectively, showing that dialysis was generally adequate (Table 1). There was no patient with Kt/V < 1.0. Among the safety population, the most commonly prescribed ESA prior to commencement of CERA was epoetin alpha followed by epoetin beta and darbepoetin alpha.
Table 1.
Demographics and baseline data of study patients
| Result | |
|---|---|
| Age (years), mean ± SD | 50.5 ± 13.9 |
| Gender, n (%) | |
| Female | 63 (49.6%) |
| Male | 64 (50.4%) |
| Aetiology of CKD, n (%) | |
| Hypertension | 45 (34.4%) |
| Unknown | 34 (26.0%) |
| Diabetes | 29 (22.1%) |
| Glomerulonephritis | 14 (10.7%) |
| Interstitial nephritis/pyelonephritis | 12 (9.2%) |
| Others | 20 (15.3%) |
| Dosage of ESA before switch to CERA, mean ± SD (n) | |
| Darbepoetin alpha | 40.7 ± 54.4 µg (n = 23) |
| Epoetin alpha | 6540 ± 5358 IU (n = 65) |
| Epoetin beta | 7698 ± 2932 IU (n = 43) |
| Laboratory findings, n (%) | |
| Hb (g/dL) | 11.1 ± 0.6 |
| Haematocrit (fraction) | 0.33 ± 0.02 |
| Erythrocyte MCV (fL) | 90.5 ± 6.14 |
| White blood cell number (109/L) | 6.7 ± 1.7 |
| Platelet number (109/L) | 214.2 ± 67.1 |
| Serum ferritin (µg/L) | 675.7 ± 413.8 |
| TSAT (g/L) | 34.8 ± 15.7 |
| Iron concentration (µmol/L) | 12.4 ± 4.7 |
| Total iron-binding capacity (µmol/L) | 34.8 ± 11.4 |
| TSAT (%) | 34.8 ± 15.7 |
| Albumin (g/L) | 39.9 ± 4.8 |
| Creatinine (µmol/L) | 712.7 ± 232.31 |
| C-reactive protein (mg/L) | 11.4 ± 27.1 |
| Phosphate (mmol/L) | 1.5 ± 0.5 |
| Potassium (mmol/L) | 5.1 ± 0.9 |
| Kt/V | 1.6 ± 0.4 |
| Urea reduction ratio | 68.1 ± 20.6 |
Efficacy results
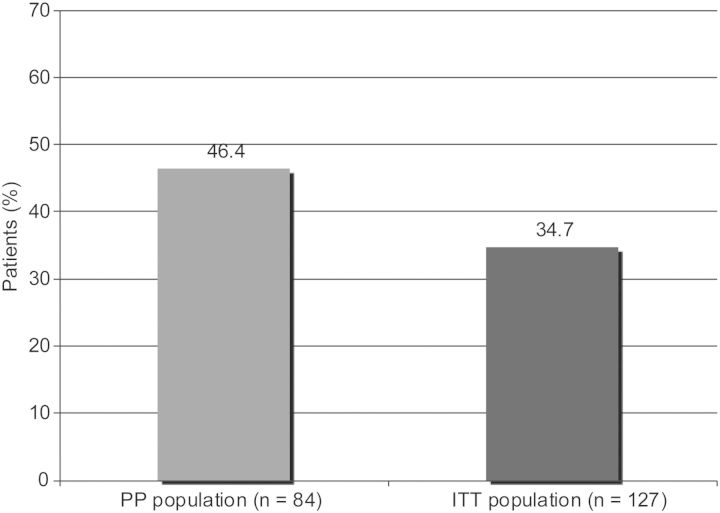
Thirty-nine patients [46.4% (95% CI: 35.5–57.7%)] in the PP population maintained a mean Hb concentration within ±1.0 g/dL of the reference Hb concentration and between 10.0 and 12.0 g/dL during the EEP. This proportion of patients in the ITT population during the EEP was 34.7% (44 patients; 95% CI: 26.4–43.6%) (Figure 3). The percentage of patients whose Hb concentrations remained within the range of 10.0–12.0 g/dL throughout the EEP was 51.2% (65 patients), with a 95% CI of 42.2–60.2%. Thirty-three patients maintained all individual Hb values between 10.0 and 12.0 g/dL throughout the EEP. The median time spent in the target range of 10.0–12.0 g/dL was 38 days (mean: 33.1 ± 16.89 days) during the EEP and 62 days (mean: 60.4 ± 32.69 days) during the DTP.
Fig. 3.
Percentage of patients with a mean Hb concentration within ±1.0 g/dL of the reference Hb concentration and between 10.0 and 12.0 g/dL.
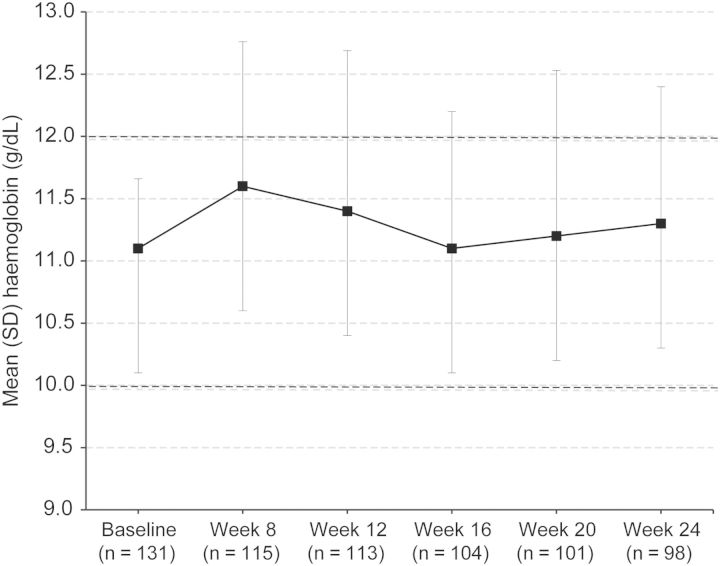
Figure 4 shows the mean Hb concentration during the DTP and EEP. The mean change in time-adjusted average Hb concentration was 0.29 ± 1.08 g/dL between baseline and the EEP, and 0.52 ± 0.90 g/dL between baseline and DTP (Figure 4). The successive change in Hb concentration was 0.46 ± 0.41 g/dL during the DTP and 0.45 ± 0.63 g/dL during the EEP.
Fig. 4.
Mean Hb concentration of patients over time.
The mean CERA monthly dose was 121.6 ± 47.13 µg during the DTP and 112.4 ± 76.78 µg during the EEP (Table 2). The median monthly dose decreased from 120 µg during the DTP to 100 µg during the EEP. The mean cumulative total dose up to Week 20 was 703.2 ± 313.24 µg. The mean CERA dose of patients who maintained all individual Hb values between 10.0 and 12.0 g/dL throughout the EEP was 103.5 ± 46.95 µg.
Table 2.
Dosage of CERA
| CERA dose (μg/month), mean ± SD (median) | Result |
|---|---|
| DTP | 121.6 ± 47.13 (120.0) |
| EEP | 112.4 ± 76.78 (100.0) |
| Cumulative CERA dose (μg), mean ± SD (median) | |
| Baseline | 144.0 ± 38.7 (120.0) |
| Week 24 | 703.2 ± 313.24 (695.0) |
| Dose adjustment requirement, n (%) | |
| DTP | |
| Total | 96 (75.6%) |
| Dose decreased | 38 (29.9%) |
| Dose increased | 18 (14.2%) |
| Dose decreased + increased | 40 (31.5%) |
| EEP | |
| Total | 39 (36.4%) |
| Dose decreased | 23 (21.5%) |
| Dose increased | 16 (15.0%) |
The mean serum ferritin level was 675.7 ± 413.79 and 682.9 ± 447.08 µg/L, and TSAT was 34.8 ± 15.7 and 36.5 ± 17.6% at baseline and final evaluation, respectively.
Safety results
Forty-four patients (33.6%) reported a total of 73 adverse events during treatment with CERA, and 11 patients (8.4%) had 13 treatment-related adverse events (Table 3). The most affected body system classes were infections and infestations (11% of all patients), gastrointestinal disorders (6%) and vascular disorders (5%). The most frequently occurring adverse events were hypertension (4%) and bronchitis (2%). Thirteen patients (9.9%) reported 15 serious adverse events. Two serious adverse events (ischaemic stroke and vomiting) were considered related to treatment with CERA, 12 serious adverse events occurred during the DTP (one occurred twice in one individual during the DTP) and 3 serious adverse events occurred during the EEP.
Table 3.
Summary of adverse events in the safety population (n = 131)
| Patients (%) | |
|---|---|
| Any adverse event | 44 (33.6%) |
| Serious adverse events | 13 (9.9%) |
| Adverse events leading to withdrawal | 5 (3.8%) |
| Treatment-related adverse events | 11a (8.4%) |
| Treatment-related serious adverse events | 2b (1.5%) |
| Withdrawals | 29 (22.1%) |
| Deaths | 0 (0.0%) |
aSteal syndrome, hypertension (four patients), arteriovenous fistula occlusion (two patients), leucopoenia, rhinitis, thrombocytopenia and white blood cell count decreased.
bIschaemic stroke, vomiting.
Discussion
This single-arm, open-label study demonstrates that stable Hb concentrations can be maintained in CKD patients on haemodialysis after switching from shorter-acting ESAs to once-monthly CERA.
CERA was created by integrating a large methoxy polyethylene glycol polymer chain into the erythropoietin molecule [8]. CERA has distinct binding properties at the erythropoietin receptor and a long half-life of ∼130 h, which allow administration of CERA at extended intervals (once-monthly) [12]. Extended dosing intervals may result in a more convenient and manageable schedule of administration than the more frequent dosing options available for currently available epoetin treatments [12]. Less frequent dosing regimens may also be associated with smoother control of Hb concentrations [14].
In the present study, the starting dose of CERA was 120–360 µg based on the dose of ESA, which is the starting dose preferred in the previous studies. In a study by Dellanna et al. [20], CERA starting dose was 125–200 µg in haemodialysis patients converting from short-acting ESAs to once-monthly CERA. Minutolo et al. [21, 22], however, reported that lower doses of CERA (75–100 µg/month) maintains Hb levels in non-dialysis CKD disease. Since we preferred standard initial dose of CERA, we cannot comment on the efficacy of lower conversion dose CERA.
The dose of CERA was adjusted to maintain Hb levels within a range of ±1.0 g/dL of the reference Hb concentration and between 10.0 and 12.0 g/dL. Similar to our study, in the STRIATA study, CERA doses were adjusted to maintain Hb levels within ±1.0 g/dL of baseline and between 10.0 and 13.5 g/dL [23].
Primary and secondary endpoints of the present study were also in agreement with previous studies such as Hb levels within 1 g/dL of the baseline Hb value [16, 17, 24], Hb levels within a target range [17] and mean change in Hb level between baseline and evaluation period [16, 17].
The results of this study show similarities with previously published studies demonstrating that CERA once-monthly provides maintenance of stable Hb levels within target range in CKD patients with chronic renal anaemia [16–18, 25]. Sulowicz et al. [17] and Levin et al. [16] reported that 66.1 and 68% of renal anaemia patients on dialysis treated with CERA once-monthly maintained Hb values within ±1 g/dL from baseline during the evaluation period. In a similar patient population, Carrera et al. [18] found that 64.1% of patients maintained average Hb levels ≥10.5 g/dL which did not decrease by >1 g/dL from baseline values, whereas this proportion was 40.1% in patients treated with once-monthly darbepoetin alfa. In a study with very similar study design and patient population to ours, Fliser et al. [25] reported 30.8 and 74.9% of patients achieved Hb levels within 11–12.5 and 10–13 g/dL during an 8-week evaluation period, with 82.9% of patients maintaining Hb concentration within ±1.0 g/dL of their mean Hb concentration. Results from these studies cannot be directly compared with results from our study, as the definition of Hb stability in our study was different; we found that 46.4% of patients in the PP population maintained their mean Hb concentration within ±1.0 g/dL of their reference Hb value and between 10.0 and 12.0 g/dL during the EEP and that 51.2% of patients in the ITT population maintained a mean Hb concentration between 10.0 and 12.0 g/dL throughout the EEP. In spite of some differences in study design and the definition of Hb stability of our study from those of previous studies, our findings supported the reports in literature that CERA once-monthly is effective in maintaining stable Hb levels within target range in CKD patients with chronic renal anaemia.
The mean change in time-adjusted average Hb concentration between baseline and the EEP in the ITT population was 0.29±1.08 g/dL. Sulowicz et al. [17] found that the mean change in Hb from baseline to the evaluation period was lower (0.131 g/dL) for once-monthly CERA.
Hb values remained stable throughout the evaluation phase in our study population. Similarly, in the previous clinical studies, CERA was shown to have comparable efficacy and safety profile to existing conventional ESAs or darbepoetin alfa in both dialysis and non-dialysis patients [13, 15, 17, 21, 25]. For example, In STRIATA study, i.v. CERA once every 2 weeks was found clinically non-inferior to darbepoetin alfa in maintaining Hb levels in patients on dialysis [23]. The PROTOS study, a randomized Phase III study in 572 patients, showed that s.c. CERA once or twice monthly successfully maintained stable Hb levels in patients who were on dialysis and randomly converted directly from epoetin [17]. The MIRACEL study showed that conversion from epoetin or darbepoetin to monthly CERA administration was practical, convenient and offer good control of Hb levels, regardless of the previous type of therapy or dosing frequency in over 400 haemodialysis patients [25]. The ARCTOS study showed that CERA once every 2 weeks was as effective as darbepoetin alfa once weekly for correcting anaemia in 324 ESA-naïve patients with CKD not on dialysis [13]. Minutolo et al. [21] also reported that conversion of darbepoetin to CERA maintains Hb levels in non-dialysis CKD patients. In a randomized controlled study, Roger et al. [15] compared monthly CERA and darbepoetin alfa once weekly or every 2 weeks in 307 non-dialysis CKD patients and reported that CERA successfully corrects anaemia and maintains stable Hb levels in non-dialysis CKD patients. CERA administered s.c. was also reported to be a good alternative for treating chronic anaemia in patients with CKD and intolerance to epoetin beta and darbepoetin alpha (single case report) [26].
The results from clinical studies showed comparable safety profile of CERA with conventional ESAs. In a pooled analysis in patients with CKD (n = 1789), CERA showed a safety profile comparable with that of other ESAs [27]. The present study also confirmed the good safety and tolerability profile of CERA. Less than 10% of patients reported treatment-related adverse events.
One of the limitations of this study was the low number of patients who completed the study according to protocol (84 patients). Another limitation was that iron supplementation was left to the discretion of the individual study centres. Inflammation and oxidative stress can limit or prevent the uptake of oral iron in the intestine [28]. Since CKD patients on dialysis often present with high levels of inflammation and oxidative stress [29], they are often limited in their ability to oral iron uptake. Thus, in centres where oral rather than i.v. iron was given to correct for iron deficiency, the patients’ responses to CERA may have been limited by iron levels too low for efficient erythropoiesis. In spite of these limitations, the present study is the first study evaluating CERA treatment in patients with chronic renal anaemia undergoing haemodialysis in Turkey.
As a conclusion, the results of this study confirm that once-monthly CERA treatment maintains stable Hb concentrations with a good safety and tolerability profile in patients with chronic renal anaemia undergoing haemodialysis.
Funding
This study was supported by Roche, Turkey.
Conflict of interest statement
Co-authors, Ertuǧrul Akbaş and Fatih Özdener are currently staff members of Roche, Istanbul; manufacturer of study drug (MIRCERA®). Other co-authors had no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper. The results presented in this paper have not been published previously in whole or part.
References
- 1.NKF-K/DOQI Clinical practice guidelines for anemia of chronic kidney disease. Am J Kidney Dis. 2001;37:182–238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 2.Fink J, Blahut S, Reddy M, et al. Use of erythropoietin before the initiation of dialysis and its impact on mortality. Am J Kidney Dis. 2001;37:348–355. doi: 10.1053/ajkd.2001.21305. [DOI] [PubMed] [Google Scholar]
- 3.European best practice guidelines for the management of anemia in patients with chronic renal failure. Nephrol Dial Transplant. 1999;14:1–50. [PubMed] [Google Scholar]
- 4.Eckardt KU. Pathophysiology of renal anemia. Clin Nephrol. 2000;53:2–8. [PubMed] [Google Scholar]
- 5.Tong EM, Nissenson AR. Erythropoietin and anemia. Semin Nephrol. 2001;21:190–203. doi: 10.1053/snep.2001.20939. [DOI] [PubMed] [Google Scholar]
- 6.Halstenson CE, Macres M, Katz SA, et al. Comparative pharmacokinetics and pharmacodynamics of epoetin alpha and epoetin beta. Clin Pharmacol Ther. 1991;50:702–712. doi: 10.1038/clpt.1991.210. [DOI] [PubMed] [Google Scholar]
- 7.Nissenson AR. Novel erythropoiesis stimulating protein for managing the anemia of chronic kidney disease. Am J Kid Dis. 2001;38:1390–1397. doi: 10.1053/ajkd.2001.29264. [DOI] [PubMed] [Google Scholar]
- 8.Macdougall IC. CERA (continuous erythropoietin receptor activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440. [PubMed] [Google Scholar]
- 9.Macdougall IC. Novel erythropoiesis-stimulating agents: a new era in anemia management. Clin J Am Soc Nephrol. 2008;3:200–207. doi: 10.2215/CJN.03840907. [DOI] [PubMed] [Google Scholar]
- 10.Panchapakesan U, Sumual S, Pollock C. Nanomedicines in the treatment of anemia in renal disease: focus on CERA (continuous erythropoietin receptor activator) Int J Nanomed. 2007;2:33–38. doi: 10.2147/nano.2007.2.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veng-Pedersen P, Freise KJ, Schmidt RL, et al. Pharmacokinetic differentiation of drug candidates using system analysis and physiological-based modelling. Comparison of C.E.R.A. and erythropoietin. J Pharm Pharmacol. 2008;60:1321–1334. doi: 10.1211/jpp/60.10.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdougall IC, Robson R, Opatrna S, et al. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:1211–1215. doi: 10.2215/CJN.00730306. [DOI] [PubMed] [Google Scholar]
- 13.Macdougall IC, Walker R, Provenzano R, et al. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clin J Am Soc Nephrol. 2008;3:337–347. doi: 10.2215/CJN.00480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinger M, Arias M, Vargemezis V, et al. Efficacy of intravenous methoxy polyethylene glycol-epoetin beta administered every 2 weeks compared with epoetin administered 3 times weekly in patients treated by hemodialysis or peritoneal dialysis: a randomized trial. Am J Kidney Dis. 2007;50:989–1000. doi: 10.1053/j.ajkd.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Roger SD, Locatelli F, Woitas RP, et al. C.E.R.A. once every 4 weeks corrects anemia and maintains hemoglobin in patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant. 2011;26:3980–3986. doi: 10.1093/ndt/gfr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin NW, Fishbane S, Cañedo FV, et al. Intravenous methoxy polyethylene glycol-epoetin beta for hemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA) Lancet. 2007;370:1415–1421. doi: 10.1016/S0140-6736(07)61599-2. [DOI] [PubMed] [Google Scholar]
- 17.Sulowicz W, Locatelli F, Ryckelynck JP, et al. Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol. 2007;2:637–646. doi: 10.2215/CJN.03631006. [DOI] [PubMed] [Google Scholar]
- 18.Carrera F, Lok CE, de Francisco A, et al. Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trial. Nephrol Dial Transplant. 2010;25:4009–4017. doi: 10.1093/ndt/gfq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler M, Martínez-Castelao A, Siamopoulos KC, et al. C.E.R.A. once every 4 weeks in patients with chronic kidney disease not on dialysis: the ARCTOS extension study. Hemodial Int. 2010;14:233–239. doi: 10.1111/j.1542-4758.2009.00421.x. [DOI] [PubMed] [Google Scholar]
- 20.Dellanna F, Winkler RE, Bozkurt F, et al. Dosing strategies for conversion of haemodialysis patients from short-acting erythropoiesis stimulating agents to once-monthly C.E.R.A.: experience from the MIRACEL study. Int J Clin Pract. 2011;65:64–72. doi: 10.1111/j.1742-1241.2010.02551.x. [DOI] [PubMed] [Google Scholar]
- 21.Minutolo R, Zamboli P, Chiodini P, et al. Conversion of darbepoetin to low doses of CERA maintains hemoglobin levels in non-dialysis chronic kidney disease patients. Blood Purif. 2010;30:186–194. doi: 10.1159/000321486. [DOI] [PubMed] [Google Scholar]
- 22.Minutolo R, Conte G, Cozzolino M, et al. Conversion from epoetin and darbepoetin to C.E.R.A. in non-dialysis CKD patients: a multicenter Italian prospective study in nephrology practice. Blood Purif. 2013;36:69–77. doi: 10.1159/000353607. [DOI] [PubMed] [Google Scholar]
- 23.Canaud B, Mingardi G, Braun J, et al. Intravenous C.E.R.A. maintains stable hemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol Dial Transplant. 2008;23:3654–3661. doi: 10.1093/ndt/gfn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinowitz B, Coyne DW, Lok CE, et al. C.E.R.A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am J Nephrol. 2008;28:280–289. doi: 10.1159/000111115. [DOI] [PubMed] [Google Scholar]
- 25.Fliser D, Kleophas W, Dellanna F, et al. Evaluation of maintenance of stable hemoglobin levels in hemodialysis patients converting from epoetin or darbepoetin to monthly intravenous C.E.R.A.: the MIRACEL study. Curr Med Res Opin. 2010;26:1083–1089. doi: 10.1185/03007991003666652. [DOI] [PubMed] [Google Scholar]
- 26.Davila Fajardo CL, Pena Ortega M, Cabeza Barrera J, et al. Methoxy polyethylene glycol-epoetin beta (Mircera) in the treatment of a patient with chronic kidney disease presenting late-onset hypersensitivity to other epoetins. Nefrologia. 2010;30:372–373. doi: 10.3265/Nefrologia.pre2010.Mar.10310. [DOI] [PubMed] [Google Scholar]
- 27.Locatelli F, Mann JF, Aldigier JC, et al. C.E.R.A.: safety profile: a pooled analysis in patients with chronic kidney disease. Clin Nephrol. 2010;73:94–103. doi: 10.5414/cnp73094. [DOI] [PubMed] [Google Scholar]
- 28.Munoz M, Villar I, Garcia-Erce JA. An update on iron physiology. World J Gastroenterol. 2009;15:4617–4626. doi: 10.3748/wjg.15.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaziri ND, Zhou XT. Potential mechanisms of adverse outcomes in trials of anemia correction with erythropoietin in chronic kidney disease. Nephrol Dial Transplant. 2009;24:1082–1088. doi: 10.1093/ndt/gfn601. [DOI] [PubMed] [Google Scholar]