Abstract
Objective
This was the first Indian multicenter study at six specialty hospitals, to assess the real-life usage of the vaginal ring in daily clinical practice.
Methods
This open-label, prospective, single-arm, nonrandomized, interventional study enrolled 252 women aged >18 years, seeking contraception with no contraindications to the use of combined hormonal contraceptive. Women were provided the ring with a monthly follow-up schedule for three cycles. Cycle control, acceptability, tolerability, and safety assessments were recorded at each visit.
Results
Regular menstrual bleeding was reported by 76.2 % (192/252) at baseline. In study completers, regular bleeding was seen in 94.1 % (192/204), 97.5 % (199/204), and 98 % (200/204) in the 1st, the 2nd, and the 3rd cycles, respectively. Most (94.2 % [195/207]) women were very satisfied or satisfied with the ring, and 93.2 % (193/207) would recommend it to others. No pregnancies or serious adverse events were reported.
Conclusion
The study demonstrated that NuvaRing® is a highly effective contraceptive method with an excellent cycle control. It is well tolerated and accepted by Indian women.
Keywords: NuvaRing®, Contraceptive, Acceptability, Cycle control, Efficacy
Introduction
The prevalence of modern contraceptive methods in India is reported to be 48.5 %, of which, condoms form 5.2 %, combined oral contraceptives (COCs) 3.1 %, intra-uterine devices (IUD) 1.7 %, injectables 0.1 %, and the majority constituted by sterilization (male and female) 38.3 % [1]. Among the above mentioned methods, the effectiveness of COCs is well established for contraception. However, usage of COCs is low due to its multiple shortcomings, the most significant one being an obligation for daily intake, which may lead to poor compliance. The other disadvantage is that due to oral intake, COCs undergo first-pass metabolism, and any interference with gastrointestinal absorption may also lead to fluctuations in the hormone levels [2]. It has been observed that 47 % of users missed one or more pills per cycle, and 22 % users sought physician’s advice for incidence of side effects [3].
To overcome these limitations, various nonoral hormonal contraceptive methods were investigated. Most of these are progestogen-only methods requiring a medical procedure and trained personnel’s intervention for use, and may lead to inherent unpredictable bleeding pattern, which make them less acceptable to women [4]. On the contrary, NuvaRing® is a combined contraceptive vaginal ring meant for single-month use and contains etonogestrel (11.7 mg) and ethinylestradiol (2.7 mg) in an Evatane® core. After placement in the vagina, each ring releases on average 0.120 mg/day etonogestrel and 0.015 mg/day ethinylestradiol over a 3-week period of use. Its main mode of contraception is inhibition of ovulation [5]. Over the last decade, the excellent contraceptive efficacy, tolerability, and acceptability of NuvaRing® have been established in large-scale studies conducted in Europe and North America [6–8]. Data in support of NuvaRing® use in daily clinical practice have been generated from observational studies carried out in European populations [9, 10].
NuvaRing® use is associated with several advantages which include use of lower doses of hormones in a controlled drug delivery, avoidance of hepatic first-pass metabolism and gastrointestinal interferences, and once-a-month use. Although NuvaRing® was introduced in India (2009), there was little information available for use of NuvaRing® in daily clinical practice. This was the first multicenter study which was undertaken to understand the real-life usage of the ring in daily clinical practice.
Materials and Methods
This was a multicenter, open-label, prospective, single-arm, nonrandomized, observational study. The study was carried out at six centers across India, involving 252 women in multispecialty hospitals.
The study was initiated after getting approval from the Institutional Review Board (IRB)/Independent Ethics Committee (IEC), in compliance with local laws. Ethical principles that have their origin in the World Medical Association Declaration of Helsinki, and all applicable local laws, rules, and regulations relating to the conduct of the study were followed. The study was conducted from December 2011 to December 2012 in compliance with the protocol (#P07733). Prior to including any woman in the clinical study or performing any study-related procedure, free and expressed informed consent was obtained in writing.
Population
Women (≥18 years) who were at risk of pregnancy and were seeking contraception without having any contraindication to the use of NuvaRing® (as specified in NuvaRing® prescribing information [India]) were enrolled in the study. At the baseline visit (Visit 1), demographic data, gynecological history, medical history, and reasons for NuvaRing® use were recorded prior to commencement of NuvaRing®use. A “new user” was defined as a woman who had not used any hormonal contraceptive method(s) within the last month. A “switcher” was defined as a woman who used any hormonal contraceptive method within the last month. The recruitment of the women was done by participating gynecologists. After providing a voluntary informed consent, eligible women were enrolled in the study and were provided a prescription for NuvaRing®. The physician dispensed the product to the woman which was reimbursed by the sponsor.
The follow-up visits were conducted at the end of each menstrual cycle, i.e., at Week 4 (Visit 2), Week 8 (Visit 3), and Week 12 (Visit 4), or at premature discontinuation.
Ring Use
Women were provided NuvaRing® for maximum of three menstrual cycles. One NuvaRing® was provided for each menstrual cycle (3 weeks of ring use, and 1 week of ring-free period). All women received a user leaflet and verbal instruction by the consulting gynecologists on appropriate ring use. Gynecologists demonstrated the method of insertion and the removal of the ring.
Study Assessments
Assessment of menstrual cycle control was based on variables of menstrual characteristics, and intermenstrual bleeding was recorded at each of the follow-up visits (after every cycle). Menstrual characteristics measured were bleeding pattern (regular/irregular), duration (average number of days), and intensity (average number of pads used/day). Intermenstrual bleeding was measured by incidence (yes or no), duration (number of bleeding or spotting days), and intensity (average number of pads used per day). Vaginal bleeding was classified as spotting or bleeding based on the number of pads required per day. Spotting was defined as when the requirement was ≤1 pad, and if bleeding required ≥2 pads.
Compliance
Women were considered to be compliant if NuvaRing® had been used for 21 days × 24 (± 48) hours and withdrawn after 7 days × 24 (±24) hours. The number of hours of temporary ring removal was recorded.
Acceptability
Acceptability assessment included responses to questions having variables on three domains: ease of use, sexual comfort, and satisfaction. Ease of use (insertion and removal) was measured by patient’s description of insertion and removal. Sexual comfort was measured by feeling of NuvaRing® by the user at any time, during intercourse, by the partner during intercourse and partner objections to NuvaRing®; overall satisfaction was measured by patient’s description as very satisfied, satisfied, neutral, unsatisfied, very unsatisfied, further usage, and recommendation to others.
Efficacy
Contraceptive efficacy was assessed by measuring the incidence of pregnancy due to contraception failure during the study at follow-up visits (Visits: 2–4). Pregnancy was confirmed by hCG qualitative analysis using strip and/or other test(s), e.g., ultrasound, at the discretion of the treating gynecologists.
Tolerability
Assessment was based on measurement of woman’s vital signs (body weight and blood pressure) at Visit 1 and Visit 4. Safety variables assessed included all adverse events (AEs), such as any abnormal laboratory values that led to hospitalization, resulted in change in dosing, withdrawal from the study, or events that were medically significant. Information concerning AEs was solicited from women by the investigator or study personnel at all visits. Ring expulsion was captured as an AE.
Physician Perspective
Six parameters namely, ease of education, how to use, ease of use (insertion and removal), overall user’s acceptance, the maximum benefit of NuvaRing® for women, and overall satisfaction as a physician was recorded.
Statistical Analysis
Safety population consisted of all women who inserted NuvaRing® at least once during the study period. Women who completed the study as per protocol were considered as per protocol population (PP) referred as study completers. Descriptive statistics for continuous data included mean ± standard deviation (SD), and the range (minimum, maximum). For categorical data, number of women (n) and percentages were calculated. The statistical analysis was done using the software SAS version 9.1.3 (SAS Institute, Cary, NC, USA). All AEs were coded using MedDRA Version 14.1.
Results
Subject Disposition
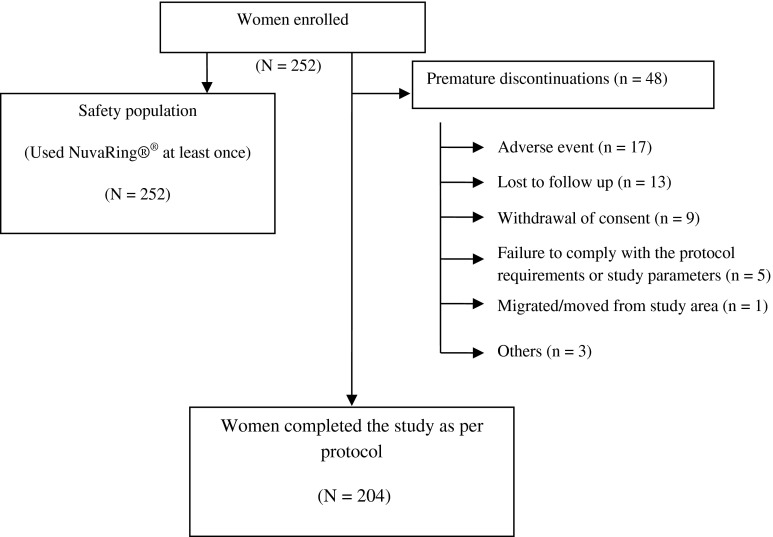
Two-hundred and fifty-two women constituted the safety population who had used NuvaRing® at least once. For a per protocol analysis, data of 204 women were considered who completed the study. Reason for discontinuation in 48 women is described in Fig. 1.
Fig. 1.
User acceptability for the ring contraceptive–per protocol population
The baseline demographic characters and previous contraception methods used by study population are presented in Tables 1 and 2, respectively. There were 118 (46.8 %) women who had history of abortion.
Table 1.
Baseline characteristics of study population
| Characteristics | Study population (N = 252) |
|---|---|
| Mean age(range), Years | 29.5 (19–46) |
| Weight (kg) | 58.91 |
| Systolic blood pressure (mmHg) + SD | 112.3 + 8.75 |
| Diastolic blood pressure (mmHg) + SD | 73.1 + 6.71 |
| Graviditya (%) | 85.7 |
| Abortiona (%) | 46.8 |
| Spontaneous (%) | 24 |
| Artificial (%) | 35.2 |
| Ectopic pregnancy (%) | |
| Yes | 2.0 % |
Denominator is the number of subjects in safety population
a N = 218
Table 2.
Previous contraceptive methods used by study population
| Characteristics | Study population (N = 252) |
|---|---|
| Contraception method (%) used previously | |
| Yes | 63.9 |
| Previous method (%) | |
| Barrier | 55.9 |
| Combined oral contraceptive | 19.9 |
| Fertility awareness method | 1.9 |
| copper-intrauterine device | 22.4 |
| Satisfaction with previous method (%) | |
| Satisfied | 24.2 |
| Neutral (ok) | 53.4 |
| Unsatisfied | 19.9 |
| Very unsatisfied | 1.9 |
| Ever used combined oral contraceptive (%) | |
| Yes | 23.6 |
| New user (%) | 83.3 |
| Switcher (%) | 16.7 |
Denominator is the number of subjects in safety population
Contraceptive Efficacy
There were no pregnancies reported during the study.
Compliance
Mean duration of use of NuvaRing® ranged from 21.1 to 21.2 days across all cycles in 204 women. Incidence of ring expulsion was low during this study. During the first cycle, one subject removed the ring after three days due to a muscular spasm. Another subject used the ring for one day during the third cycle due to expulsion. Incidences of temporary ring removal were reported by four women during the study. The durations of these temporary ring removal periods were 20 min, 2 h, 24 h, and 96 h.
Cycle Control
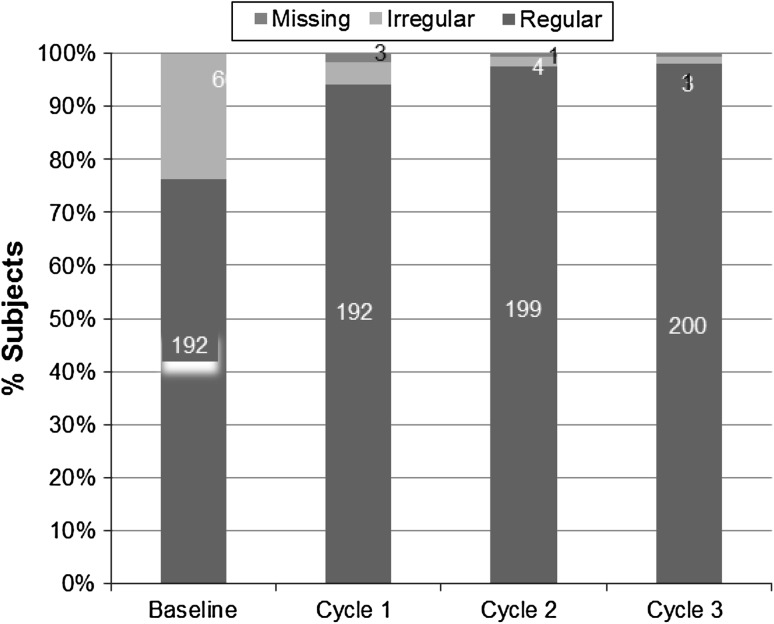
At baseline, 76.2 % of the women reported having a regular menstrual cycle. In study completers, while using NuvaRing®, this figure rose to 94.1 % (192/204) after first cycle. The increase in proportion of women having regular cycle was greater in the second and the third cycles, 97.5 % (199/204), and 98 % (200/204), respectively. Occurrence of irregular bleeding was very low, i.e., in 4.4 % (9/204), 2.0 % (4/204), and 1.5 % (3/204) women in first, the second, and the third cycles, respectively (Fig. 2). In safety population, regular bleeding was observed in 81.7 % (206/252), 95.8 % (204/213), and 97.1 % (201/207) in the first, the second, and the third cycles, respectively.
Fig. 2.
Bleeding patterns during the study
At baseline, the average cycle length was 32.1 days, and the average duration of menstruation was 4.2 days. The mean cycle length and mean duration of menstruation decreased to 26.8 ± 25 days and 3.6 ± 0.9 days, respectively, through three cycles in women using NuvaRing® (Table 3).
Table 3.
Menstrual characteristics after NuvaRing® use-per protocol population
| Statistics | Baseline | First cycle (N = 204) | Second cycle (N = 204) | Third cycle (N = 204) | |
|---|---|---|---|---|---|
| Average cycle length (days) | Mean (SD) | 32.1 (9.92) | 26.7 (4.06) | 26.9 (3.43) | 26.8 (2.54) |
| Average duration of menstruation (days) | Mean (SD) | 4.2 (2.04) | 3.8 (1.24) | 3.7 (1.13) | 3.6 (0.98) |
| Intensity of menstruation (pad[s]/day) | Mean (SD) | 2.0 | 2.0 | 2.0 | 2.0 |
Denominator is the number of women in each cycle
Intensity of menstruation was the same throughout the three cycles, i.e., 2 pads/day (Table 3).
Throughout the study period, intermenstrual bleeding was rare in study completers (Table 4) and safety population, with incidences of 4.4 % (11/252), 0.5 % (1/252), and 1 % (2/252) subjects in the first, the second, and the third cycles, respectively.
Table 4.
Intermenstrual bleeding-per protocol population
| Parameter | Statistics | First cycle (N = 204) | the second cycle (N = 204) | Third cycle (N = 204) |
|---|---|---|---|---|
| Incidence | ||||
| Yes | N (%) | 5 (2.5) | 0 | 1 (0.5) |
| No | N (%) | 199 (97.5) | 203 (99.5) | 203 (99.5) |
| Duration of bleeding (days) | Mean (SD) | 4.0 | ||
| Duration of spotting (days) | Mean (SD) | 5.5 (4.2) | 3.0 | |
Denominator for incidence is the number of women in each cycle
Acceptability
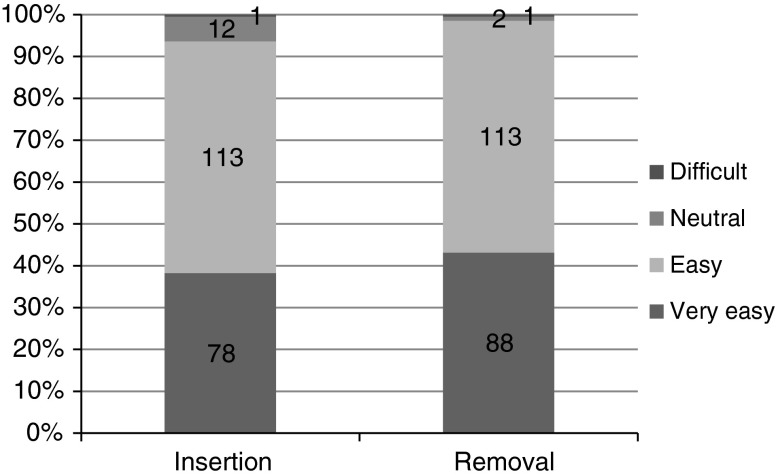
The proportion of women who considered that ring insertion was very easy or easy was increased from the first cycle to the third cycle from 69.1 % (141/204) to 93.6 % (191/204). Majority of the women considered ring removal as very easy or easy: 97 % (198/204) in the first cycle, 98 % (200/204) in the second cycle, and 98.5 % (201/204) in the third cycle (Fig. 3).
Fig. 3.
Convenience of insertion and removal of ring after the third cycle
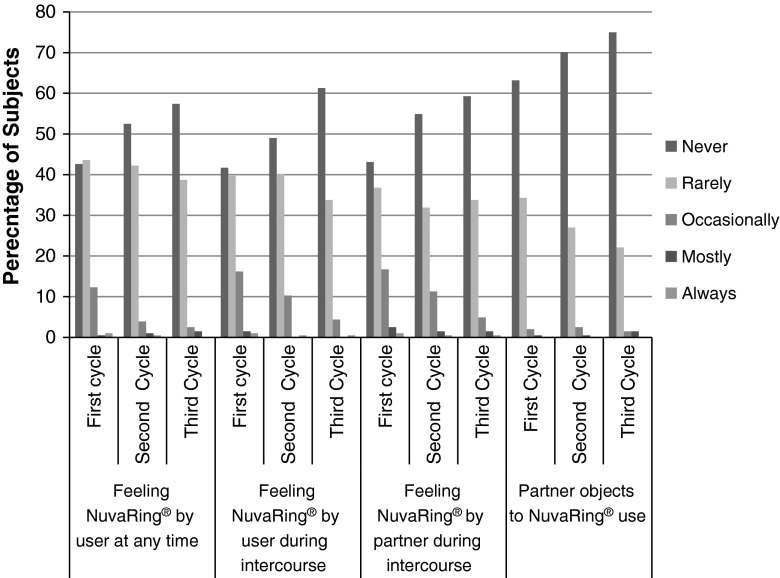
The proportions of women who reported that they never or rarely felt the ring at any time were 86.3 % (176), 94.6 % (193), and 96.1 % (196) in the first, the second, and the third cycles, respectively. The proportions of women who reported that they never or rarely felt the ring during intercourse were 81.4 % (166), 89.2 % (182) and 95.1 % (194) in the first, the second, and the third cycles, respectively. Proportion of women who occasionally felt the ring during intercourse decreased from the first (16.2 % [33]) to the third cycle (4.4 % [9]). The proportions of women whose partner never or rarely felt the ring during intercourse were 79.9 % (163), 86.8 % (177), and 93.1 % (190) in the first, the second and the third cycles, respectively. Most of women’s partner (75 %) never objected to NuvaRing®. Partners of very few women (1.5 %) mostly felt the ring during an intercourse (Fig. 4).
Fig. 4.
User acceptability for the ring contraceptive–per protocol population
Overall satisfaction with the method during the use was high with NuvaRing®. After the the third cycle, 94.2 % (195/207) of women were very satisfied or satisfied, and 93.2 % (193/207) would recommend the ring to others. The proportion of women who wished to continue the ring use after the third cycle was 76.8 % (159/207).
After the completion of the third cycle, 22.7 % (47/207) women said no for further usage with the commonest reason being plan for conceiving in 42.5 % (20/47) of women. Other reasons stated were NuvaRing® was expensive (21.3 % [10/47)]; planning for permanent/long duration method of contraception (14.9 % [7/47]).
Safety and Tolerability
Increase in mean body weight was approximately 0.42 (SD: 1.4) kg over three cycles of ring use. Mean increases observed in systolic and diastolic blood pressures over three cycles were 3.9 (SD: 9.0) and 2.1(SD: 7.3) mmHg, respectively.
There was no report of any serious AE throughout the study. At least one AE was reported by 18.6 % [47/252] women. At least one AE was reported by 11.9 % (30) subjects in the “reproductive system and breast disorders” system organ class (SOC). AEs most commonly reported under this included, vaginal bleeding/spotting in 5.2 % (13), vulvovaginal pruritus in 2.8 % (7), and vaginal discharge in 2.4 % (6) subjects. Premature discontinuation due to AEs was observed in 6.7 % (17/252). The commonest AEs are summarized in Table 5. A majority of the AEs were classified as mild (41), 16 were moderate, and only 3 were severe. Classification of AEs based on relationship suggested that 24 AEs were judged as unlikely related, 29 as possibly related, and 5 were judged as probably related to NuvaRing® use.
Table 5.
Summaries of all the AEs reported during the study of safety population (N = 252)
| Adverse Events | N | % |
|---|---|---|
| Nausea | 4 | 1.6 |
| Device expulsion | 3 | 1.2 |
| Pyrexia | 3 | 1.2 |
| Urinary tract infection | 2 | 0.8 |
| Weight increased | 3 | 1.2 |
| Dysuria | 2 | 0.8 |
| Breast discomfort | 2 | 0.8 |
| Menorrhagia | 3 | 1.2 |
| Metrorrhagia | 3 | 1.2 |
| Vaginal discharge | 6 | 2.4 |
| Vaginal bleeding/spotting | 13 | 5.2 |
| Vulvovaginal pain | 2 | 0.8 |
| Vulvovaginal pruritus | 7 | 2.8 |
Denominator is the number of subjects in the safety population
Physician’s Perspective
Physician’s perspective regarding educating ring use was very easy or easy for majority of women (89.7 %[226/252]); about ring insertion and removal were either very easy or easy for 85.7 % (216/252) and 93.25 % (235/252) women, respectively. Physician’s perspective about overall user’s acceptance was very good or good for 82.1 % (207/252) women. As reported by physicians, the most beneficial aspect of ring use was once a month contraceptive for 71.0 % (179/252) women, low side effects for 21.8 % (55/252), cycle control for 17.5 % (44/252) women, vaginal administration for 11.5 % (29/252) women, and low dosage for 6.3 % (16/252) women. Overall, the physicians were very satisfied or satisfied for 88.1 % women.
Discussion
The present observational, multicenter study was aimed to generate data on the usage of NuvaRing® by Indian women in a real-life setting. A majority of the women (85.7 %) had a history of gravidity and parity which suggests that a majority of users who adopted NuvaRing® had at least one child. History of induced abortion was reported in 46.8 % of women despite contraceptive usage in the majority (63.9 %).
There were no reports of pregnancies in this study suggesting high efficacy of NuvaRing®. Control of menstrual bleeding is a major factor for choosing any contraceptive method. Assessments of menstrual characteristics for three cycles indicated favorable cycle control. Regular incidences of bleeding were observed in 94.1 % (192/204), 97.5 % (199/204), and 98 % (200/204) women in the first, the second, and the third cycles, respectively. The present study showed a decrease in the incidence of irregular bleeding from 4.4 % to 1.5 % by the end of the third cycle which is supported by data achieved in clinical practice as well [9, 10]. There were few incidences of intermenstrual bleeding (≤2.5 %) during the three cycles of use; these were mainly restricted to spotting, which is slightly higher than the previously published single-center Indian study [4]. Most probably, the excellent cycle control in spite of its low level of hormone is due to constant serum concentration of hormone in the period of NuvaRing® use [9]. User acceptability is a very significant aspect to the success of a contraceptive method. Women found the ring use, i.e., both insertion and removal convenience. Most of the women, considering all three cycles, found ring insertion as well as removal as very easy or easy, which is consistent with the results of the multinational studies [7–10].
The contraceptive ring was never or rarely felt by majority of the women (>85 %) at any time. A majority (≥80 %) of the women or their partners never or rarely felt the ring during intercourse. Furthermore, partners of most of the women (>97 %) never or rarely objected to NuvaRing® use. After the third cycle, 94.2 % [195/207] of women were very satisfied or satisfied; the proportion of women who would recommend the ring to others was 93.2 % (193/207); and the proportion of women who said yes for further usage of the ring after the third cycle was 76.8 % (159/207). These data are in line with the multinational study on NuvaRing® where 85 % of women were satisfied with the ring, and 90 % would recommend its use to others after 13 cycles [7].
Based on physician’s perspective, the most beneficial aspect of ring use was once-a-month contraceptive method for a high proportion [71.0 % (179/252)] of women, and overall user’s acceptance for user was favorable for a majority of women. Compliance with ring use (ranging from 21.1 to 21.2 days) was close to the recommended ring insertion for a duration of three weeks. There were small increases from baseline in both body weight and blood pressure at the end of the study, consistent with what is observed with NuvaRing and other combined hormonal contraceptives. A majority of the AEs were mild in nature. Only 6.7 % (17) women discontinued the study due to AEs, and this percentage is lower (15.1 %) than that was observed in the large scale multicenter study data [6]. The limitations of a small sample size with a follow-up period of only 3 months make assessments for efficacy limited; however, this is the only multicenter study to date from India which clearly demonstrated the benefits of the vaginal ring.
In conclusion, NuvaRing® is an effective contraceptive with excellent cycle control. It has been well tolerated and accepted by Indian women with good compliance.
Acknowledgments
The authors thank the participating study investigators, Dr. Suchita Meherishi and Dr. Vijayalakshmi. This study was supported by Organon (India) Private Ltd, a subsidiary of Merck & Co. Inc., Whitehouse Station, NJ, USA.
Subrat Ray M.D.
is presently the Associate Director Medical Affairs (Women’s Health) at MSD in India. Dr Ray is a medical graduate from Utkal University and completed his post-graduation (M.D) in Pharmacology from Seth G.S. Medical College, Mumbai University (2001).
Dr Ray has over 12 years of experience in Pharmaceutical Industry in Medical Affairs, Clinical research and Pharmacovigilance in India, Bangladesh and Sri Lanka. In the field of women’s Health, he has research publications in newer contraceptives, counselling approach to improve acceptance of modern methods. He was instrumental for collaboration program with Family Welfare Committee of FOGSI for development of structured counselling programs in contraception. Dr Ray has been facilitator for national train the trainer workshops (Single rod Implant) for Ministry of Health & Family Welfare, of Bangladesh and Sri Lanka. His other therapy experience includes newer fertility drugs as well as impact of IVF treatment burden, anabolic hormones in wasting related diseases.

Footnotes
Clinical Trials Registry of India (CTRI) number: NCT01490190.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division (2011). World Contraceptive Use 2010 (POP/DB/CP/Rev2010). http://www.un.org/esa/population/publications/wcu2010/Main.html Accessed 20 Nov 2013.
- 2.Ahrendt HJ, Nisand I, Bastianelli C, et al. Efficacy, acceptability and tolerability of the combined contraceptive ring, NuvaRing®, compared with an oral contraceptive containing 30 µg of ethinyl estradiol and 3 mg of drospirenone. Contraception. 2006;74:451–457. doi: 10.1016/j.contraception.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg MJ, Waugh MS, Burnhill MS. Compliance, counseling and satisfaction with oral contraceptives: a prospective evaluation. Fam Plann Perspect. 1998;30:89–92. doi: 10.2307/2991665. [DOI] [PubMed] [Google Scholar]
- 4.Soni A, Garg S, Bangar R. Efficacy, user acceptability, tolerability, and cycle control of a combined contraceptive vaginal ring: the Indian perspective. J Obstet Gynecol India. 2013 doi: 10.1007/s13224-013-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NuvaRing®Full Prescribing Information New Jersey (USA): Merck Sharp &Dohme Corp., a subsidiary of Merck &Co., Inc., Oct 2013. http://www.merck.com/product/usa/pi_circulars/n/nuvaring/nuvaring_pi.pdf Accessed 20 Nov 2013.
- 6.Roumen FMJE, Apter D, Mulders TMT, et al. Efficacy, tolerability, and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyloestradiol. Hum Reprod. 2001;16:469–475. doi: 10.1093/humrep/16.3.469. [DOI] [PubMed] [Google Scholar]
- 7.Dieben TOM, Roumen FMJE, Apter D. Efficacy, cycle control, and user acceptability of a novel contraceptive vaginal ring. Obstet Gynecol. 2002;100:585–592. doi: 10.1016/S0029-7844(02)02124-5. [DOI] [PubMed] [Google Scholar]
- 8.Novak A, De la Loge C, Abetz L, et al. The combined contraceptive vaginal ring, NuvaRing®: an international study for user acceptability. Contraception. 2003;67:187–194. doi: 10.1016/S0010-7824(02)00514-0. [DOI] [PubMed] [Google Scholar]
- 9.Roumen FMJE, op ten Berg MMT, Hoomans EHM. The combined contraceptive vaginal ring NuvaRing®: first experience in daily clinical practice in the Netherlands. Eur JContracept Reprod Health Care. 2006; 11:14–22. [DOI] [PubMed]
- 10.Merki-Feld GS, Hund M. Clinical experience with NuvaRing® in daily practice in Switzerland: cycle control and acceptability among women of all reproductive ages. Eur J Contracept Reprod Health Care. 2007;12:240–247. doi: 10.1080/13625180701440180. [DOI] [PubMed] [Google Scholar]