Abstract
Background:
Physicians have prescribed anticholinergic agents such as benztropine, procyclidine, biperiden and trihexyphenidyl for treatment and prophylaxis of antipsychotic-induced extrapyramidal symptoms (EPS) for decades. Anticholinergic agents can however worsen tardive dyskinesia and cause many adverse effects, including cognitive impairment. Previous studies of anticholinergic discontinuation in patients with schizophrenia receiving antipsychotics have yielded a wide range of EPS relapse rates. Improvement in cognition after anticholinergic withdrawal was observed in some studies.
Objective:
This study evaluated the effect of anticholinergic discontinuation on movement disorders, cognition and general psychopathology after a 4-week taper in 20 outpatients with schizophrenia or schizoaffective disorder treated with antipsychotics.
Results:
Eighteen of twenty patients successfully discontinued their anticholinergic medication; two did not because of akathisia. Repeated measures analysis of variance did not show a significant effect of anticholinergic discontinuation on total Extrapyramidal Symptoms Rating Scale score or on the Parkinsonism, Akathisia, Dystonia or Tardive Dyskinesia subscales. However, significant improvement was found on the Brief Assessment of Cognition in Schizophrenia composite z score at weeks 6, 8 and 12 compared with baseline. Significant improvements were seen on the motor and the symbol-coding tasks. No significant effects were observed on the Positive and Negative Syndrome Scale, Clinical Global Impression – Severity and Clinical Global Impression – Improvement scales.
Conclusion:
In this 12-week study of anticholinergic discontinuation in 20 outpatients with schizophrenia or schizoaffective disorder, gradual decrease and discontinuation of anticholinergics led to a positive effect on cognition. There were no adverse consequences on general psychopathology and no significant differences for 18 of 20 subjects on movement disorders.
Keywords: anticholinergics, antipsychotics, cognition, extrapyramidal symptoms, schizophrenia
Introduction
Movement disorders, including extrapyramidal symptoms (EPS; parkinsonism, dystonia, akathisia and tardive dyskinesia), are very common adverse effects in patients taking antipsychotics [Margolese et al. 2005]. Physicians have long prescribed anticholinergic agents such as benztropine, procyclidine, biperiden and trihexyphenidyl for the treatment and prophylaxis of antipsychotic-induced EPS [Rashkis and Smarr, 1957]. There is evidence of their efficacy in treating antipsychotic-induced parkinsonism (tremors, rigidity and bradykinesia) and acute dystonias [Haddad and Dursun, 2008]. Evidence of their efficacy in treating akathisia is more limited [Miller et al. 2000]. Certain studies [Klawans and Rubovits, 1974; Gerlach and Thorsen, 1976, Chouinard et al. 1979; Straker, 1980] suggest that anticholinergic agents may worsen tardive dyskinesia, at least acutely, and that anticholinergic dose reduction or discontinuation can lead to an acute improvement in the dyskinesia. Anticholinergic agents have a long list of side effects which includes xerostomia, blurred vision, xeropthalmia, constipation, flushed skin, delayed micturition, urinary retention, sexual dysfunction and cognitive impairment [Bezchlibnyk-Butler et al. 2009; Cancelli et al. 2009]. In addition, abuse of anticholinergic agents can cause euphoria and psychosis [Smith, 1980; Barsoum et al. 2000; Caplan et al. 2007]. The consensus among the medical community is that prophylaxis of EPS with anticholinergics is generally not indicated in patients receiving antipsychotics [World Health Organization, 1990].
In 1991, Lavin and Rifkin reviewed 15 double-blind placebo-controlled studies of anticholinergic agent discontinuation in patients receiving antipsychotics and described relapse rates of EPS ranging between 7% and 71%. All of these studies were conducted with first-generation antipsychotics and anticholinergic discontinuation was often performed abruptly. EPS were measured with various scales. Lavin and Rifkin commented that most of these studies had serious methodological and statistical flaws. The authors of the placebo-controlled studies had divergent opinions with some authors suggesting a trial of anticholinergic discontinuation once EPS have been stable for 3 months [Klett and Caffey, 1972] and others believing anticholinergics were necessary for clinical stability [Jellinek et al. 1981] .
After 1991, four studies [Horiguchi and Nishimatsu, 1992; Double et al. 1993; Ben Hadj Ali et al. 1995; Ungvari et al. 1999] of anticholinergic discontinuation in patients receiving first-generation antipsychotics reported relapse rates of EPS between 10% and 77%, with the highest rate of relapse found in a group of patients on depot fluphenazine or pipothiazine who had their anticholinergic abruptly withdrawn [Ben Hadj Ali et al. 1995]. More recently, two studies [Mori et al. 2002; Ogino et al. 2011] included patients on second-generation antipsychotics and reported relapse rates of EPS of 33% and 4%, respectively, after discontinuation of anticholinergics.
Four studies [Baker et al. 1983; Mori et al. 2002; Drimer et al. 2004; Ogino et al. 2011] have examined the impact of anticholinergic discontinuation on cognition of patients receiving antipsychotics. The scales used were the Wechsler Memory Scale, Digit Span Distractibility Test, Alzheimer’s Disease Assessment Scale – Cognitive, and the Japanese version of Brief Assessment of Cognition in Schizophrenia. All of these studies showed an improvement in cognition after anticholinergic discontinuation and two studies included a control group in which no significant improvement occurred [Baker et al. 1983; Ogino et al. 2011].
Based on the results of the above studies, in a recent review we suggested a trial of gradual anticholinergic discontinuation in patients with stable movement disorders [Desmarais et al. 2012]. Most studies of anticholinergic withdrawal in schizophrenia have not used very descriptive scales for the measurement of EPS and few have conjointly evaluated cognition. This study, a prospective open study of anticholinergic agent discontinuation, does both. The primary goal was to evaluate the need for these medications. The primary outcome measure was changes in EPS as measured by the Extrapyramidal Symptoms Rating Scale (ESRS) [Chouinard and Margolese, 2005]. The secondary objective was to evaluate potential cognitive benefits of withdrawing anticholinergics in this population using the Brief Assessment of Cognition in Schizophrenia (BACS) [Keefe et al. 2004] and to evaluate the effect on the positive and negative symptoms of schizophrenia, measured by the Positive and Negative Syndrome Scale (PANSS) [Kay et al. 1987], and general condition, measured by the Clinical Global Impression (CGI) [Guy, 1976]. The present study is the first anticholinergic discontinuation study to combine the ESRS and BACS, and the first one to examine EPS and cognition in a North American population.
Following recent studies such as the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) [Lieberman et al. 2005] and Cost Utility of The Latest Antipsychotics in Severe Schizophrenia (CUtLASS) [Jones et al. 2006], which have shown that some first-generation antipsychotics may be as effective as certain second-generation antipsychotics in some patients, and given the propensity of second-generation antipsychotics to cause metabolic disturbances, many psychiatrists are now prescribing first-generation antipsychotics more often. This brings movement disorders and their management back to the forefront. Furthermore, cognition is one of the main domains of schizophrenia and it is imperative that we know which factors can influence it positively or negatively.
Methods and measures
Methods
This prospective study was conducted at the McGill University Health Centre in Montreal, Quebec, Canada from July 2010 to July 2011 after acceptance of the protocol by the Research Ethics Board of the McGill University Health Centre. The study was conducted in compliance with the Declaration of Helsinki, ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use) guidelines for Good Clinical Practice, and the Canadian Tri-Council Policy Statement on Ethical Conduct for Research Involving Humans. Written informed consent was obtained from each patient prior to entry into the study.
Study participants
Twenty patients were recruited from the Schizophrenia Tertiary Services outpatient clinic of the McGill University Health Centre. Male and female patients were eligible for the study if they were aged between 18 and 64, had a diagnosis of schizophrenia or schizoaffective disorder according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) [American Psychiatric Association, 2000]; were on long-term maintenance treatment with antipsychotics and anticholinergics for at least 1 year, dosage constant for at least 2 months; had not had their psychotherapeutic medication changed for 2 months prior to study entry; and were able to provide written informed consent. Patients with comorbid DSM-IV-TR diagnoses other than substance abuse or dependence, mental retardation, delirium, dementia or amnestic disorder were eligible provided that their condition had been stable for at least 3 months. Pregnant or lactating women were excluded as well as patients with significant medical disease incompatible with the study and patients who participated in a trial with an investigational drug in the 2 months preceding the trial. Patients had to have sufficient knowledge of English or French to be able to participate in cognitive testing.
Study design
Patients were assessed at baseline with the ESRS, BACS or its French version, the Brève Evaluation de la Cognition en Schizophrénie (BECS) [Bralet et al. 2007], depending which language they were more fluent in, PANSS and CGI – Severity subscale (CGI-S). Their anticholinergic medication was then gradually withdrawn over the first 4 weeks of the study as follows: reduction to approximately 75% of starting dose for the first week, 50% the second week, 25% the third week and 12.5% the fourth week. Patients were allowed to take extra doses (‘prn’) of their anticholinergic agent for intolerable EPS. Patients were asked about taking extra doses at each visit. Patients who still required four or more extra doses per week at week 8 were withdrawn from the study. The participants’ treating psychiatrists were encouraged to keep doses of other psychotropic medications constant during the study. The ESRS was administered again at weeks 1, 2, 4, 6, 8 and 12. The BACS was repeated at weeks 6, 8 and 12. CGI-S and CGI – Improvement subscale (CGI-I) were rated at weeks 2, 4, 6, 8 and 12. Two additional visits, at weeks 3 and 10, were available at the psychiatrist’s discretion. The tests were administered by three psychiatrists (JED, LB, HCM).
Measures
The ESRS is recognized as a valid scale for the measurement of drug-induced EPS and is widely used in clinical research [Chouinard and Margolese, 2005]. Its sensitivity and validity were substantiated through clinical trials with antipsychotics and antiparkinsonian agents, amongst other medications [Chouinard and Margolese, 2005]. Its specificity was examined through path analyses [Moller et al. 1995] and analysis of covariance PANSS factor changes [Marder et al. 1997]. The BACS has been used in over 30 clinical trials of patients with schizophrenia [Keefe et al. 2008]. It includes tests assessing processing speed (through verbal fluency, a token motor task and symbol coding), reasoning and problem solving (through the Tower of London test), verbal memory (through list learning) and working memory (through digit sequencing). Its administration takes about 30 min. The BACS was shown to be a reliable, valid and comparable measure with high test–retest reliability in patients with schizophrenia and controls, and it is as sensitive as a standard 2.5 h battery in patients with schizophrenia [Keefe et al. 2008]. The French version of the BACS, the BECS, was validated in French-speaking patients with schizophrenia, with scores on the different tasks strongly correlating with subscores obtained on a standard battery [Bralet et al. 2007]. BACS and BECS raw scores were converted to z scores using data from 404 healthy controls from the BACS Norms and Standardization study [Keefe et al. 2008]. Studies have shown that the PANSS is a reliable scale [Kay et al. 1988], has criterion-related as well as construct validity [Kay et al. 1988; Peralta and Cuesta, 1994]. The CGI-S and CGI-I are widely used in schizophrenia research.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences version 20.0. Normality was assessed by examining the normal quantile plots of each measure. Normally distributed data were analyzed by one-way repeated-measures analysis of variance (ANOVA). The Greenhouse–Geisser correction was employed when the assumption of sphericity was violated. A p value of less than 0.05 indicated statistical significance. Significant effects were decomposed using Bonferroni’s method. Nonnormally distributed data were analyzed with Friedman’s test. Ninety-five percent confidence intervals (CIs) were calculated as well as effect sizes. Power analyses were performed using G*Power 3 [Faul et al. 2007]. We first conducted an intent-to-treat analysis with last observation carried forward to accommodate for missing data and then supplemented our results with a completer analysis. Subanalyses were conducted on the patients who experienced clinically significant EPS at baseline and those who did not. Another subanalysis compared BACS/BECS z scores of patients tested in French with those tested in English.
Results
Baseline characteristics
The characteristics of study participants at baseline are listed in Table 1. While patient records did not always contain a clear reason why the anticholinergic was initially prescribed, it appears that for the majority of patients, it was intended for treatment of actual EPS as opposed to prophylaxis of EPS.
Table 1.
Baseline characteristics of study participants (N = 20).
| N = 20 (SD) | |
|---|---|
| Sex | 14 M / 6 F |
| Age in years | 52.7 (7.8) |
| Race | |
| Caucasian | 18 |
| Latin American | 1 |
| Black Caribbean | 1 |
| Diagnosis | |
| Schizophrenia | 17 |
| Schizoaffective disorder | 3 |
| Highest level of education attained | |
| University degree | 2 |
| College degree | 4 |
| High-school degree | 14 |
| Age at onset of illness in years | 27.5 (11.1) |
| Length of illness in years | 25.2 (12.2) |
| Number of hospitalizations | 4.9 (5.3) |
| Anticholinergic medication | |
| Procyclidine | 18 |
| Benztropine | 2 |
| Dose of anticholinergic in mg* | 7.3 (3.3) |
| Length of uninterrupted treatment with anticholinergic in months | 71.8 (58.0) |
| Antipsychotic | 20 |
| Haloperidol | 1 |
| Loxapine | 1 |
| Zuclopenthixol long-acting injectable | 1 |
| Clozapine | 4 |
| Olanzapine (including orally disintegrating) | 6 |
| Quetiapine (including XR) | 4 |
| Risperidone | 6 |
| Risperidone long-acting injectable | 5 |
| Ziprasidone | 3 |
| Combination 2 antipsychotics | 7 |
| Combination 3 antipsychotics | 2 |
| Mood stabilizer | 8 |
| Divalproex sodium | 2 |
| Gabapentin | 4 |
| Lithium | 2 |
| Antidepressants | 3 |
| Fluoxetine | 1 |
| Paroxetine | 1 |
| Venlafaxine XR | 1 |
| Sedatives | 6 |
| Clonazepam | 3 |
| Diazepam | 1 |
| Lorazepam | 1 |
| Trazodone | 1 |
In procyclidine equivalents where 1 mg of benztropine = 2 mg of procyclidine [Stanilla and Simpson, 2009].
SD, standard deviation; XR, extended release.
Anticholinergic discontinuation
Eighteen of twenty patients successfully discontinued their anticholinergic. The two patients who were still using more than four anticholinergic doses per week at week 8 were withdrawn from the study as per protocol. Both patients had significant akathisia when they tried to further lower their anticholinergic medication. These two patients required an additional visit at week 3 for akathisia and for difficulty tapering their anticholinergic. Another patient was seen at week 3 for a transient increase in psychosis. One patient was seen at week 10 for transient suicidal ideation.
As needed doses of anticholinergic medications (‘prn’)
Eleven patients took ‘prn’ doses of anticholinergic medication during the study, including the two patients who had to be removed from the study. Among the nine patients who took prn doses but completed the study, three took only 1 prn, two patients required 2, one patient took 3, one took 7 and one took 18. The patient who took 18 prn doses reduced his use to 1 per week by the end of the study at week 12. The most common reason cited for taking a prn was akathisia or anxiety. Prn doses had variable effects on symptoms depending on the patient.
Effects of discontinuation on movement disorders
The results of the ESRS and subscales are shown in Table 2. Repeated-measures ANOVA did not show a significant change over time in the total ESRS score [F (6, 114) = 0.492, p = 0.813] or on the Parkinsonism [F (6, 114) = 1.247, p = 0.288] and Akathisia [F (6, 114) = 0.827, p = 0.551] subscales. Post hoc power analysis revealed that the study had 80% power to detect a medium effect size (d = 0.66) for within-subject change in ESRS scores (α = 0.05, two tailed). Data for the Dystonia and Tardive Dyskinesia subscales were analyzed with a Friedman’s test, as the quantile plots deviated from normality. Friedman’s test did not show a significant change over time in these measures [χ2 (6) = 7.114, p = 0.310 and χ2 (6) = 3.764, p = 0.709, respectively].
Table 2.
Mean ESRS total and subscale scores (SD) across time.
| ESRS | Week 0 (N = 20) | Week 1 (N = 20) | Week 2 (N = 20) | Week 4 (N = 19 + 1 LOCF) | Week 6 (N = 20) | Week 8 (N = 20) | Week 12 (N = 18 + 2 LOCF) | F or χ2 statistic | df | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Total score | 24 (12.5) | 23.4 (12.9) | 24.3 (13.0) | 23.9 (13.3) | 23.6 (13.5) | 25.5 (15.7) | 24.4 (12.1) | F = 0.492 | 6, 114 | 0.813 |
| Parkinsonism | 9.1 (5.7) | 9.5 (6.0) | 9.25 (5.8) | 9.8 (6.3) | 9.2 (6.0) | 10.7 (6.5) | 9.7 (6.3) | F = 1.247 | 6, 114 | 0.288 |
| Akathisia | 1.7 (1.8) | 1.6 (1.4) | 2.0 (1.8) | 1.7 (1.8) | 2.1 (1.7) | 1.8 (1.9) | 2.2 (1.7) | F = 0.827 | 6, 114 | 0.551 |
| Dystonia | 0.8 (1.6) | 0.8 (1.6) | 0.8 (1.5) | 0.9 (1.6) | 0.7 (1.5) | 1.1 (1.7) | 0.8 (1.6) | χ2 = 7.114 | 6 | 0.310 |
| Tardive dyskinesia | 3.6 (3.4) | 3.3 (3.6) | 3.6 (4.0) | 3.2 (3.5) | 3.2 (3.7) | 3.4 (3.9) | 3.4 (4.1) | χ2 = 3.764 | 6 | 0.709 |
ESRS, Extrapyramidal Symptoms Rating Scale; LOCF, last observation carried forward; SD, standard deviation.
A completer analysis performed with the 18 participants who succeeded in discontinuing their anticholinergic yielded similar results.
Subanalyses were conducted with patients who met criteria for movement disorders at baseline. Presence of parkinsonism, dystonia or tardive dyskinesia was determined by a score of 3 or greater on one ESRS item or a score of 2 on two items. Akathisia was considered present with a score of 3 or more on the combined score of subjective and objective akathisia. [Chouinard and Margolese, 2005]. At baseline, 17 (85%), 6 (30%), 2 (10%) and 10 (50%) patients exhibited parkinsonism, akathisia, dystonia and tardive dyskinesia, respectively.
Repeated-measures ANOVA performed on the data of the 17 patients exhibiting parkinsonism at baseline did not show a significant change over time, F (6, 96) = 1.5, p = 0.186. Among the three patients who did not have parkinsonism at baseline, one transiently met criteria for parkinsonism at week 6.
Repeated-measures ANOVA with Greenhouse–Geisser correction conducted on the data of the six patients with akathisia at baseline did not show a significant change over time, F (3.010, 15.051) = 1.956, p = 0.164. Among the 14 patients who did not have akathisia at baseline, 6 developed akathisia, which was transient in 4 of these patients, including the 2 patients who terminated the study early. Data of the 14 patients who did not have akathisia at baseline were analyzed with Friedman’s test which showed no change over time, χ2 (6) = 8.069, p = 0.233.
Throughout the study, there were no changes in the dystonia subscale score of the two patients with dystonia at baseline. Two other patients transiently met the criteria for dystonia during the study.
The data of the 10 patients who had tardive dyskinesia at baseline were analyzed with repeated-measures ANOVA, which did not show a significant change over time, F (6, 54) = 0.383, p = 0.887. Two of these 10 patients no longer met criteria for tardive dyskinesia by week 12. One patient who did not exhibit tardive dyskinesia at week 0 met criteria for the disorder at week 12. Data of the 10 patients who did not have tardive dyskinesia at baseline were analyzed with Friedman’s test which showed no change over time, χ2 (6) = 2.978, p = 0.812.
Effects of discontinuation on cognition
Intent-to-treat analysis with last observation carried forward
The results of the BACS/BECS composite z scores are illustrated in Figure 1. Repeated measures ANOVA showed a significant change over time [F (3, 57) = 9,878, p < 0.001] with an effect size of 0.342. Subsequent pairwise comparison tests conducted using Bonferroni’s method indicated that the mean BACS/BECS composite z scores at weeks 6, 8 and 12 were significantly higher than the mean BACS/BECS composite z score at baseline (p = 0.029, p = 0.001 and p = 0.002, respectively) with mean differences of –0.287 (95% CI –0.023 to –0.552), –0.416 (95% CI –0.146 to –0.686) and –0.517 (95% CI –0.163 to –0.871), respectively. Table 3 shows the results on the different tasks. Repeated-measures ANOVA with Greenhouse–Geisser correction showed a significant change over time on the motor task [F (2.010, 38.196) = 5.141, p = 0.010] with an effect size of 0.213. Subsequent pairwise comparison tests conducted using Bonferroni’s method showed that the mean motor task z score at week 12 was significantly higher than the mean motor task z score at week 0 (p = 0.023), with a mean difference of –0.476 and a 95% CI from –0.049 to –0.902. Repeated-measures ANOVA yielded a significant change over time on the symbol coding task with F (3, 57) = 4.219, p = 0.009 and an effect size of 0.182. Subsequent pairwise comparison tests with Bonferroni’s method showed that the mean symbol-coding task z score at week 12 was significantly higher than the mean symbol-coding task z score at week 0 (p = 0.043) with a mean difference of –0.279 and a 95% CI from –0.006 to –0.552. Repeated-measures ANOVA showed a significant change over time for verbal memory and digit sequencing with F (3, 57) = 4.494, p = 0.007, effect size of 0.191, and F (3, 57) = 2.836, p = 0.046, effect size of 0.130. However, none of the pairwise comparisons were significant (p > 0.05). Repeated-measures ANOVA failed to show a significant change over time for verbal fluency, F (3, 57) = 2.246 (p = 0.093). The Tower of London was analyzed with a Friedman’s test, as the quantile plots deviated from normality. Friedman’s test did not show a significant change over time on the Tower of London, χ2 (3) =0.708, p = 0.871.
Figure 1.
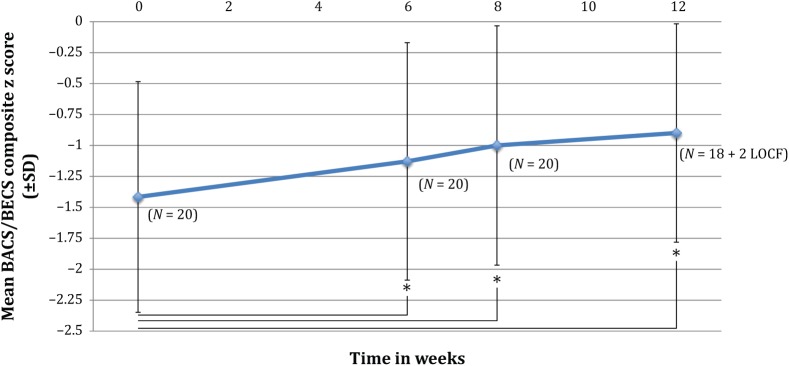
Mean BACS/BECS composite z score as a function of time.
BACS, Brief Assessment of Cognition in Schizophrenia; BECS, Brève Evaluation de la Cognition en Schizophrénie; LOCF, last observation carried forward; SD, standard deviation.
* Indicates significant difference from baseline (p < 0.05 with Bonferroni correction).
Table 3.
Mean z scores of BACS/BECS tasks (SD) across time.
| Week 0 (N = 20) | Week 6 (N = 20) | Week 8 (N = 20) | Week 12 (N = 18 + 2 LOCF) | F or χ2 statistic | df | p | |
|---|---|---|---|---|---|---|---|
| Verbal memory | −1.43 (0.95) | −1.24 (0.81) | −0.84 (1.01) | −0.94 (0.72) | F = 4.494 | 3, 57 | 0.007* |
| Digit sequencing | −1.10 (0.81) | −0.79 (1.00) | −0.73 (1.03) | −0.71 (0.96) | F = 2.836 | 3, 57 | 0.046* |
| Motor task | −0.73 (0.97) | −0.57 (0.97) | −0.50 (0.96) | −0.26 (1.09) | F = 5.141$ | 2.010, 38.196 | 0.010* |
| Verbal fluency | −1.01 (0.75) | −0.75 (0.90) | −0.78 (0.82) | −0.74 (0.89) | F = 2.246 | 3, 57 | 0.093 |
| Symbol coding | −1.02 (0.87) | −0.90 (1.00) | −0.82 (0.97) | −0.74 (0.90) | F = 4.219 | 3, 57 | 0.009* |
| Tower of London | −0.40 (1.26) | −0.29 (1.03) | −0.36 (1.40) | −0.23 (1.17) | χ2 = 0.708 | 3 | 0.871 |
Indicates statistical significance.
With Greenhouse–Geisser correction.
BACS, Brief Assessment of Cognition in Schizophrenia; BECS, Brève Evaluation de la Cognition en Schizophrénie; SD, standard deviation.
Completer analysis
Similar results were obtained through a completer analysis performed with the 18 patients who were able to discontinue their anticholinergic agent.
Language
A two-way, mixed-design ANOVA, with language (French or English) as a between-subject factor, and time as a within-subject factor, did not yield a significant language by time interaction, F (3, 54) = 0.374, p = 0.772 when using data from all 20 participants.
Effects of discontinuation on psychopathology
Two patients had a transient decrease of at least 20% in their PANSS total score. One patient had a transient increase of at least 20% in his PANSS total score after the dose of one of his antipsychotics was halved. Mean total PANSS and subscale scores are shown in Table 4. Repeated-measures ANOVA with Greenhouse–Geisser correction did not show a significant change over time in the PANSS total score, F (1.778, 33.777) = 2.100, p = 0.143 or any of the PANSS subscales: positive, F (3, 57) = 1.997, p = 0.125, negative (with Greenhouse–Geisser correction), F (1.885, 35.824) = 0.935, p = 0.397, general psychopathology F (3, 57) = 1.536, p = 0.215. Similar results were obtained in the completer analysis.
Table 4.
Mean PANSS total and subscale scores (SD) across time.
| Week 0 (N = 20) | Week 6 (N = 20) | Week 8 (N = 20) | Week 12 (N = 18 + 2 LOCF) | F statistic | df | p | |
|---|---|---|---|---|---|---|---|
| PANSS total | 66.5 (11.7) | 63.3 (11.2) | 64.3 (11.7) | 63.3 (10.5) | 2.100$ | 1.778, 33.777 | 0.143 |
| Positive subscale | 15.4 (3.7) | 14.5 (3.3) | 14.8 (4.0) | 14.3 (3.4) | 1.997 | 3, 57 | 0.125 |
| Negative subscale | 19.3 (6.4) | 18.4 (5.5) | 18.5 (6.1) | 19.2 (5.9) | 0.935$ | 1.885, 35.824 | 0.397 |
| General psychopathology subscale | 31.9 (5.1) | 30.4 (6.3) | 31.1 (6.2) | 29.9 (5.5) | 1.536 | 3, 57 | 0.215 |
With Greenhouse–Geisser correction.
LOCF, last observation carried forward; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation.
No patients had more than a one-point variation on the CGI-S throughout the study. At study termination, all patients obtained a score of 4 (no change) on the CGI-I except for one patient who scored 3 (minimally improved) and one who obtained 5 (minimally worse). The latter had become hypomanic after his general practitioner prescribed prednisone for an exacerbation of his pulmonary disease. Median CGI-S and CGI-I scores were 4 (moderately ill and no change, respectively) at all measurements. The CGI-S and CGI-I were analyzed with a Friedman’s test, as the quantile plots deviated from normality. Friedman’s test showed no statistically significant change over time on both the CGI-S, χ2 (5) = 1.875, p = 0.866 and the CGI-I, χ2 (4) = 2.667,p = 0.615. The completer analysis yielded comparable results.
Discussion
In this study of anticholinergic medication withdrawal, most patients (90%) were able to discontinue their anticholinergic without a significant effect on movement disorders and psychopathology. As a group, a significant improvement in cognition was observed post discontinuation. In a similar study, Ogino and colleagues obtained a significant improvement in BACS scores for the discontinuation group and no significant changes in the control group [Ogino et al. 2011]. The effect size of improvement in cognition in their study was 0.42 for the discontinuation group (p = 0.002). Our results are consistent with their findings; we obtained a similar effect size: 0.34 (p < 0.001). Our results on the BACS/BECS suggest that even removal of modest doses of anticholinergics may benefit patients. Ogino and colleagues did not find significant differences in EPS in the discontinuation group between baseline and endpoint [Ogino et al. 2011]. Likewise, we did not observe a significant worsening of EPS on the ESRS and its subscales. This suggests that long-term anticholinergics may be unnecessary in most patients receiving antipsychotics.
Our study has several limitations, lack of a control group being an obvious one. It then becomes difficult to rule out a practice effect on the cognitive measure. However, as stated above, our results are similar to those of Ogino and colleagues, whose study did include a control group [Ogino et al. 2011]. Also, in a study exploring the reliability and sensitivity of the BACS in patients and controls, Keefe and colleagues did not observe significant practice effects on the verbal fluency and Tower of London tests when alternate versions were administered. Likewise, no significant practice effects were detected on other measures without alternate versions, except for the symbol-coding task, which showed a significant practice effect (standard deviation of 0.25 in patients with schizophrenia).
Our sample of patients likely had fewer cognitive deficits than generally found in schizophrenia; their mean BACS/BECS composite z score was –1.416 at baseline, which is higher than the –1.94 obtained by Ogino and colleagues [Ogino et al. 2011]. It may be interesting to repeat the current study with more cognitively impaired patients to see if a greater effect is obtained.
The French and English versions of the BACS differ slightly, which could have influenced the results. However, we conducted a two-way ANOVA comparing the results of patients tested in French with those tested in English. Given that no significant difference was obtained, we are confident that performing cognitive testing in two languages did not influence the overall results in a significant way.
Another study limitation is the small sample size. However, our study had adequate power to detect a medium effect size on the ESRS total score. This suggests that many patients can successfully withdraw anticholinergic agents. The patients in our study had relatively low ESRS scores at baseline so our results may not extrapolate to patients with more significant movement disorders. Our study did not detect a significant difference on the PANSS total and subscale scores, or on the CGI-S and CGI-I. However, the results on the PANSS positive score approached significance (p = 0.125). Ogino and colleagues found a statistically significant improvement on the general psychopathology subscale, indicating a possible benefit of removing anticholinergics on psychopathology. Given that anticholinergics can cause psychosis, an improvement in positive symptoms following their withdrawal appears plausible. However, without a control group, improvement in psychopathology may be the result of more intensive monitoring by clinicians and not attributable to the change in medications.
One may note that our patients were on relatively small doses of anticholinergics at baseline. It would be interesting to conduct a similar study with patients who have a high anticholinergic burden.
Ratings on the measures, especially the ESRS, may have been influenced by the use of prn doses of anticholinergics. We permitted use of prn doses in case patients experienced severe dystonic reactions, such as torticollis, oculo-gyric crisis and opisthotonus. However, most patients who took prn doses did so in an attempt to decrease akathisia or anxiety. Given that anticholinergics do not have good efficacy in treating akathisia and are not a treatment for anxiety, it is not surprising that most patients who took prn doses obtained little benefit from them. This coupled with the lack of serious adverse reactions with a gradual decrease of anticholinergics under close monitoring suggests that the use of prn doses does not need to be a component of future studies. The medication taper rate was variable between patients, which may also have affected the results. From a clinical perspective, the use of punctual prn doses of anticholinergics may be preferable to regular use of this class of medication. Allowing patients to take prn doses may also alleviate anxiety created by the withdrawal of a medication. Compliance was not formally assessed in this study and is another variable that could affect these findings. Further studies of anticholinergic discontinuation should ideally have a control group, monitor compliance and include measures of quality of life and daily functioning.
Investigators are currently trying to find novel compounds to improve cognition in schizophrenia. This study suggests that the first step in improving cognition should be a careful review of medications and gradual removal of agents with anticholinergic properties that have questionable benefits. As greater inclusion of patients with schizophrenia in society is advocated, clinicians must be attentive to their patients’ needs and minimize both the unnecessary use of medications and medication adverse effects that could possibly hinder a return to school or employment.
Acknowledgments
Thank you to Dr Richard Keefe (Duke University Medical Center) and Dr Marie-Cécile Bralet (CHI Clermont de l’Oise) for providing the English and French versions of the BACS. All authors were involved in writing the protocol of the study. Dr Desmarais performed the literature search and wrote the first draft of the manuscript. Dr Desmarais and Professor Annable undertook the statistical analysis. All authors have contributed to and have approved the final manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Julie Eve Desmarais: none. Dr Linda Beauclair: Hoffman-LaRoche, Janssen, Lunbeck, Otsuka, EnVivo, Amgen. Prof. Lawrence Annable: none. Marie-Claire Bélanger: none. Dr Theodore T. Kolivakis: AstraZeneca, Eli Lilly, BMS, Pfizer, Lundbeck, GlaxoSmithKline. Dr Howard C. Margolese: BMS, Hoffman-LaRoche, Janssen, Pfizer, AstraZeneca, Novartis, Sunovion, Lundbeck.
Contributor Information
Julie Eve Desmarais, Clinical Psychopharmacology and Therapeutics Unit, Allan Memorial Institute, McGill University Health Centre, 1025 Pine Avenue West, Montreal, Quebec, Canada H3A 1A1.
Linda Beauclair, Clinical Psychopharmacology and Therapeutics Unit, Allan Memorial Institute, McGill University Health Centre, Montreal, Quebec, Canada Department of Psychiatry, McGill University, Montreal, Quebec, Canada.
Lawrence Annable, Clinical Psychopharmacology and Therapeutics Unit, Allan Memorial Institute, McGill University Health Centre, Montreal, Quebec, Canada Department of Psychiatry, McGill University, Montreal, Quebec, Canada.
Marie-Claire Bélanger, Clinical Psychopharmacology and Therapeutics Unit, Allan Memorial Institute, McGill University Health Centre, Montreal, Quebec, Canada Department of Psychiatry, McGill University, Montreal, Quebec, Canada.
Theodore T. Kolivakis, Clinical Psychopharmacology and Therapeutics Unit, Allan Memorial Institute, McGill University Health Centre, Montreal, Quebec, Canada Department of Psychiatry, McGill University, Montreal, Quebec, Canada
Howard C. Margolese, Clinical Psychopharmacology and Therapeutics Unit, Allan Memorial Institute, McGill University Health Centre, Montreal, Quebec, Canada Department of Psychiatry, McGill University, Montreal, Quebec, Canada
References
- American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision. Washington, DC: American Psychiatric Publishing Inc. [Google Scholar]
- Baker L., Cheng L., Amara I. (1983) The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry 143: 584–590. [DOI] [PubMed] [Google Scholar]
- Barsoum A., Kolivakis T., Margolese H., Chouinard G. (2000) Diphenhydramine (Unisom), a central anticholinergic and antihistaminic: abuse with massive ingestion in a patient with schizophrenia. Can J Psychiatry 45: 846–847. [PubMed] [Google Scholar]
- Ben Hadj Ali B., Dogui M., Ben Ammou S., Loo H. (1995) [Antiparkinson drugs in neuroleptic treatment: comparative study of progressive and abrupt withdrawal]. Encephale 21: 209–215. [PubMed] [Google Scholar]
- Bezchlibnyk-Butler K., Jeffries J., Virani A. (2009) Clinical Handbook of Psychotropic Drugs, 18th revised edition Oxford: Hogrefe and Huber Publishers. [Google Scholar]
- Bralet M., Falissard B., Neveu X., Lucas-Ross M., Eskenazi A., Keefe R. (2007) Validation of the French version of the BACS (the Brief Assessment of Cognition in Schizophrenia) among 50 French schizophrenic patients. Eur Psychiatry 22: 365–370. [DOI] [PubMed] [Google Scholar]
- Cancelli I., Beltrame M., Gigli G., Valente M. (2009) Drugs with anticholinergic properties: cognitive and neuropsychiatric side-effects in elderly patients. Neurol Sci 30: 87–92. [DOI] [PubMed] [Google Scholar]
- Caplan J., Epstein L., Quinn D., Stevens J., Stern T. (2007) Neuropsychiatric effects of prescription drug abuse. Neuropsychol Rev 17: 363–380. [DOI] [PubMed] [Google Scholar]
- Chouinard G., De Montigny C., Annable L. (1979) Tardive dyskinesia and antiparkinsonian medication. Am J Psychiatry 136: 228–229. [DOI] [PubMed] [Google Scholar]
- Chouinard G., Margolese H. (2005) Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res 76: 247–265. [DOI] [PubMed] [Google Scholar]
- Desmarais J., Beauclair L., Margolese H. (2012) Anticholinergics in the era of atypical antipsychotics: short-term or long-term treatment? J Psychopharmacol 26: 1167–1174. [DOI] [PubMed] [Google Scholar]
- Double D., Warren G., Evans M., Rowlands R. (1993) Efficacy of maintenance use of anticholinergic agents. Acta Psychiatr Scand 88: 381–384. [DOI] [PubMed] [Google Scholar]
- Drimer T., Shahal B., Barak Y. (2004) Effects of discontinuation of long-term anticholinergic treatment in elderly schizophrenia patients. Int Clin Psychopharmacol 19: 27–29. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A., Buchner A. (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral and biomedical sciences. Behav Res Methods 39: 175–191. [DOI] [PubMed] [Google Scholar]
- Gerlach J., Thorsen K. (1976) The movement pattern of oral tardive dyskinesia in relation to anticholinergic and antidopaminergic treatment. Int Pharmacopsychiatry 11: 1–7. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976) ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education and Welfare. [Google Scholar]
- Haddad P., Dursun S. (2008) Neurological complications of psychiatric drugs: clinical features and management. Hum Psychopharmacol 23(Suppl. 1): 15–26. [DOI] [PubMed] [Google Scholar]
- Horiguchi J., Nishimatsu O. (1992) Usefulness of antiparkinsonian drugs during neuroleptic treatment and the effect of clonazepam on akathisia and parkinsonism occurred after antiparkinsonian drug withdrawal: a double-blind study. Jpn J Psychiatry Neurol 46: 733–739. [DOI] [PubMed] [Google Scholar]
- Jellinek T., Gardos G., Cole J. (1981) Adverse effects of antiparkinson drug withdrawal. Am J Psychiatry 138: 1567–1571. [DOI] [PubMed] [Google Scholar]
- Jones P., Barnes T., Davies L., Dunn G., Lloyd H., Hayhurst K., et al. (2006) Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUTLASS 1). Arch Gen Psychiatry 63: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Kay S., Fiszbein A., Opler L. (1987) The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276. [DOI] [PubMed] [Google Scholar]
- Kay S., Opler L., Lindenmayer J. (1988) Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenics. Psychiatry Res 23: 99–110. [DOI] [PubMed] [Google Scholar]
- Keefe R., Goldberg T., Harvey P., Gold J., Poe M., Coughenour L. (2004) The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity and comparison with a standard neurocognitive battery. Schizophr Res 68: 283–297. [DOI] [PubMed] [Google Scholar]
- Keefe R., Harvey P., Goldberg T., Gold J., Walker T., Kennel C., et al. (2008) Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res 102: 108–115. [DOI] [PubMed] [Google Scholar]
- Klawans H., Rubovits R. (1974) Effect of cholinergic and anticholinergic agents on tardive dyskinesia. J Neurol Neurosurg Psychiatry 37: 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klett C., Caffey E., Jr (1972) Evaluating the long-term need for antiparkinson drugs by chronic schizophrenics. Arch Gen Psychiatry 26: 374–379. [DOI] [PubMed] [Google Scholar]
- Lieberman J., Stroup T., McEvoy J., Swartz M., Rosenheck R., Perkins D., et al. (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209–1223. [DOI] [PubMed] [Google Scholar]
- Marder S., Davis J., Chouinard G. (1997) The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry 58: 538–546. [DOI] [PubMed] [Google Scholar]
- Margolese H., Chouinard G., Kolivakis T., Beauclair L., Miller R. (2005) Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 1: pathophysiology and mechanisms of induction. Can J Psychiatry 50: 541–547. [DOI] [PubMed] [Google Scholar]
- Miller C., Fleischhacker W. (2000) Managing antipsychotic-induced acute and chronic akathisia. Drug Saf 22: 73–81. [DOI] [PubMed] [Google Scholar]
- Moller H., Muller H., Borison R., Schooler N., Chouinard G. (1995) A path-analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients. A re-evaluation of the North American Risperidone Study. Eur Arch Psychiatry Clin Neurosci 245: 45–49. [DOI] [PubMed] [Google Scholar]
- Mori K., Yamashita H., Nagao M., Horiguchi J., Yamawaki S. (2002) Effects of anticholinergic drug withdrawal on memory, regional cerebral blood flow and extrapyramidal side effects in schizophrenic patients. Pharmacopsychiatry 35: 6–11. [DOI] [PubMed] [Google Scholar]
- Ogino S., Miyamoto S., Tenjin T., Kitajima R., Ojima K., Miyake N., et al. (2011) Effects of discontinuation of long-term biperiden use on cognitive function and quality of life in schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 35: 78–83. [DOI] [PubMed] [Google Scholar]
- Peralta V., Cuesta M. (1994) Psychometric properties of the Positive and Negative Syndrome Scale (PANSS) in schizophrenia. Psychiatry Res 53: 31–40. [DOI] [PubMed] [Google Scholar]
- Rashkis H., Smarr E. (1957) Protection against reserpine-induced parkinsonism. Am J Psychiatry 113: 1116. [DOI] [PubMed] [Google Scholar]
- Smith J. (1980) Abuse of the antiparkinson drugs: a review of the literature. J Clin Psychiatry 41: 351–354. [PubMed] [Google Scholar]
- Stanilla J., Simpson G. (2009) Chapter 34: Drugs to treat extrapyramidal side effects. In: Schatzberg A.F., Nemeroff C.B. (eds), The American Psychiatric Publishing Textbook of Psychopharmacology, fourth edition Arlington, VA: American Psychiatric Publishing Inc, pp. 669–694. [Google Scholar]
- Straker M. (1980) Clinical factors in tardive dyskinesia. Psychiatr J Univ Ottawa 5: 28–33. [Google Scholar]
- Ungvari G., Chiu H., Lam L., Pang A., Chung D., Li S., et al. (1999) Gradual withdrawal of long-term anticholinergic antiparkinson medication in Chinese patients with chronic schizophrenia. J Clin Psychopharmacol 19: 141–148. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1990) Prophylactic use of anticholinergics in patients on long-term neuroleptic treatment. A consensus statement. Br J Psychiatry 156: 412. [PubMed] [Google Scholar]


