Abstract
Purpose
To evaluate surgical treatments and outcomes in a multi-institutional cohort of neonates with Hirschsprung’s Disease (HD).
Methods
Using the Pediatric Health Information System (PHIS) from 1999–2009, neonates diagnosed with HD were identified and classified as having a single stage pull-through (SSPT) or multi-stage pull-through (MSPT). Diagnosis and classification algorithms and clinical variables and outcomes were validated by multi-institutional chart review. Groups were compared using logistic regression modeling and propensity-score matched analysis to account for baseline differences between groups.
Results
1,555 neonates with HD were identified; 77.2% underwent SSPT and 22.8% underwent MSPT. Misclassification of disease or surgical treatment was <2%. Rates of SSPT increased over time (p=0.03). Compared to SSPT, patients undergoing MSPT had significantly lower birth weights and higher rates of prematurity, non-HD gastrointestinal anomalies, enterocolitis, and preoperative mechanical ventilation. Patients undergoing MSPT had significantly higher rates of readmissions (58.5% vs. 37.9%) and additional operations (38.7% vs. 26%). Results were consistent in the propensity-score matched analysis.
Conclusion
Most neonates with HD undergo SSPT. In patients with similar observed baseline characteristics, MSPT was associated with worse outcomes suggesting that some infants currently selected to undergo MSPT may have better outcomes with SSPT. However, there remains a subgroup of MSPT patients who were too ill to be adequately compared to SSPT patients; for this subgroup of severely ill infants with HD, MSPT may be the best option.
Keywords: Hirschsprung’s disease, Single stage pull-through, Multi-stage pull-through, Primary pull-through, Pediatric Health Information System, PHIS, Outcomes
Surgical management of neonatal Hirschsprung’s disease (HD) is typically performed with either a single stage pull-through (SSPT) consisting of an early primary colo-anal reconstruction in the neonatal period, or a multi-stage pull-through (MSPT) characterized by a leveling colostomy followed by delayed colo-anal reconstruction later in infancy. Over time, SSTPs have been performed more frequently with SSPTs now the most commonly performed procedures (1). This transition to predominantly performing SSTP has occurred without evidence from prospective trials comparing SSPT and MSPT. Most reports have been retrospective reviews at one or several centers (2–10). At this point, the widespread adoption of SSPT in clinical practice precludes the development of a rigorously designed multi-center prospective trial to directly compare these two options (11). Furthermore, the rarity of Hirschsprung’s disease coupled with its treatment at a large number of centers further challenges the feasibility and utility of a prospective clinical trial.
Administrative datasets represent a source for developing large multi-institutional cohorts of patients with rare diseases (12,13). However, reliance on administrative data alone raises concerns about the accuracy of those data and whether treatment recommendations should be based on such studies. To address this, comparative effectiveness studies may be performed by combining the administrative data with multi-institutional chart validation of key variables and outcomes (13–16). Several groups have used this approach with data from the Pediatric Health Information System (PHIS) database (13, 17–19). The objective of this study was to use the PHIS and multi-institutional chart validation to compare outcomes between SSPT and MSPT in a multicenter cohort of infants with Hirschsprung’s disease. We hypothesized that (1) rates of SSPT are increasing; (2) patients selected to undergo MSPT are more severely ill; and (3) in patients with similar severity of illness, SSTP may lead to more long term morbidity.
Methods
Study Design
We performed a retrospective multi-institutional cohort study to evaluate surgical treatment patterns and compare outcomes of SSTP and MSTP in infants with Hirschsprung’s Disease (HD). Our primary outcomes were readmission rate and rate of additional operations within 2 years after pull-through. Secondary outcomes were rates of post-operative enterocolitis, surgical site infections (SSI), small bowel obstruction (SBO), anastomotic leak, and hospital charges and costs. Charges were calculated as the total billed charges for inpatient care from the index admission through 2 years after the pull-through procedure. These charges were converted to costs by using the hospital-specific ratios of cost to charge (RCC) estimates for the total cost of each inpatient stay. These ratios are reported to the Center for Medicare and Medicaid Services (CMS) and used to convert reported charges to estimates of their true economic costs. Cost data for each hospital were further adjusted for the regional wage index as reported by the CMS. Neither the charges nor costs described in this study include physician charges.
Cohort Identification and Validation
This study utilized the PHIS which includes comprehensive administrative data from 44 free-standing children’s hospitals, including demographics, diagnoses and procedures using International Classification of Diseases 9, Clinical Modification codes (ICD-9-CM) (20). Date-stamped billing data for imaging, procedures, laboratory tests, medications, and supplies are also included, and encrypted medical record numbers enable longitudinal tracking of individual patients across hospital encounters.
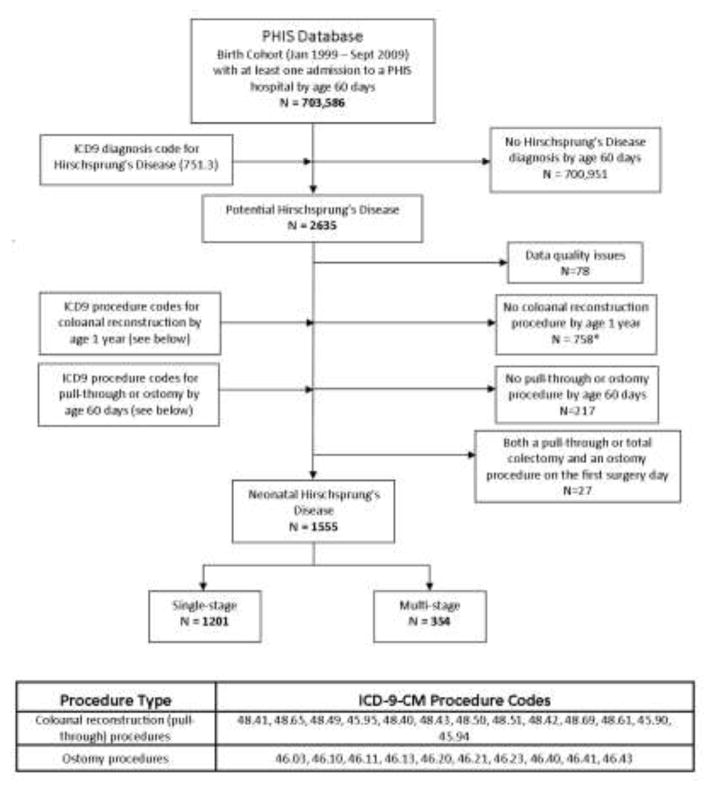
Figure 1 outlines the methodology used for cohort identification. The PHIS was queried for patients born between January 1999 and September 2009 who had at least one hospital admission by 60 days of life associated with the ICD-9-CM diagnosis code for Hirschsprung’s disease (ICD-9-CM 751.3). This age criteria ensured that only patients with HD diagnosed in the neonatal period would be included. Any patient without a subsequent ICD-9-CM procedure code for colo-anal reconstruction within 1 year of life was excluded under one of two assumptions: (1) without a definitive procedure for HD within 1 year, it was unlikely that the diagnosis code indicated actual presence of disease, or (2) delayed pull-through in these patients may be related to either longer segment Hirschsprung’s disease, total colonic aganglionosis, or severe comorbid illnesses. The cohort was then divided into the two treatment groups: SSPT patients were identified as having a colo-anal reconstruction procedure code as their first HD related procedure; MSPT patients were identified as having a stoma creation procedure code as their first procedure followed by a colo-anal reconstruction procedure code on a later date. In order to reflect the decision to perform either a SSPT or MSPT in infants with similar physiology and risks, this search strategy excluded patients managed with rectal irrigations who underwent a single stage pull-through after 60 days of life because of the potential for overlap between patients undergoing SSPT with MSPT patients coming back for their pull-through procedures.
Figure 1.
Identification of study cohort of neonatal Hirschsprung’s Disease patients. *Includes patients who had an ostomy procedure by age 60 days but who died at that admission (N=2) or died at a later admission (N=22) before age 1, without ever having a pull-through procedure.
To ensure the validity of the PHIS data, medical record chart validation of patient characteristics, diagnoses, treatments, and outcomes was performed at 3 Children’s Hospital Association member-hospitals. Medical records were reviewed to confirm the data available in PHIS on the diagnosis of HD, type of repair (SSPT or MSPT), and clinical variables and outcomes. The chart review validation study was approved by each participating hospital’s Institutional Review Board (Nationwide Children’s Hospital, Children’s Hospital of Philadelphia, and Monroe Carell Jr. Children’s Hospital at Vanderbilt).
Statistical Analysis
Pre-operative and operative variables were compared between treatment groups using two sample t-tests or Wilcoxon rank sum tests for continuous variables and Pearson chi square tests for categorical variables. Marginal logistic regression models accounting for patient clustering within hospitals were used to compare the effect of SSPT versus MSPT on our primary outcomes; marginal linear regression models were used to compare log transformed hospital charges and costs between groups. Additional operations included redo pull-through, ostomy procedures, colectomies, biopsies of the small or large intestine, colonoscopies, and anal procedures including dilation, myomectomy, or sphincterotomy. Because morbidity in the MSPT group includes complications that can occur between the time of stoma creation and definitive colo-anal reconstruction, we compared the groups from the time of initial surgery (either stoma or pull-through) until 2 years after pull-through procedure (excluding the planned pull-through procedure and its associated admission in the MSPT group for our primary outcomes). To assess the impact of having a longer follow-up period in the MSPT group, we performed a sensitivity analysis to compare outcomes looking only at the two year time period after pull-through. Since the results of both of these analyses were similar, we only report outcomes for the analysis from time of initial surgery through 2 years after pull-through.
A propensity score matched analysis was performed in order to control for potential differences in pre-operative exposures between the two treatment groups. In order to include variables with missing data, multiple imputation was performed by a Markov Chain Monte Carlo method (21). Propensity scores were estimated using a separate logistic regression model including all pre-operative variables with p<0.20 in bivariate analyses and first-order interactions with p<0.10 for each of 20 imputed datasets. Propensity scores were then averaged and SSPT and MSPT patients were matched using 1:1 nearest neighbor matching within calipers of width equal to 0.25 times the standard deviation of the logit of the propensity score (22,23). Patients without an eligible match were excluded. Standardized differences for the pre-operative variables in the matched groups were computed and were all ≤0.10 (22,24). Because of ambiguity in the diagnosis codes and timing of enterocolitis during the initial admission, propensity score matching was performed with and without this variable. Since results were similar, we report the matched cohort that included the enterocolitis variable. Also, in an attempt to minimize potential confounding caused by the presence of a greater number of long-segment HD patients in the MSPT group, we carried out a sensitivity analysis designed to exclude the majority of patients with long segment HD that undergo MSPT. Based on reported average ages of 10–14 months at the time of pull-through in patients with long segment HD undergoing MSPT, we performed a similar propensity score matched analysis including only patients who had a pull-through procedure by 6 months of age (25–28).
All analyses were performed using SAS v9.3 (SAS Institute, Cary, NC). The propensity score matching was performed using the “gmatch” SAS macro (29). All tests were 2-tailed and p<0.05 was considered statistically significant.
Results
Cohort Identification and Validation
The PHIS search strategy outlined in Figure 1 identified 1,555 infants with Hirschsprung’s disease. The diagnosis of HD was confirmed for all patients at all three validating institutions (n=133). As far as treatment group assignment into MSPT or SSPT, all 47 patients were assigned to the correct treatment group at one hospital, while 1 out of 30 and 1 out of 56 were incorrectly assigned at each of the other hospitals. This yielded an overall treatment group misclassification rate of 1.5% (Table 1). With a few exceptions, overall misclassification rates for specific demographic and clinical pre-operative characteristics were low across all 3 validating institutions (Table 1). For example, the proportion of patients across all 3 hospitals with discrepant values was: 0% for gender, 0% for prematurity, 0–4% for the various types of congenital anomalies, and 10% for gestational age; however, the misclassification rate was 20% for birth weight and 14% for date of first HD surgery. The overall misclassification rate for need for at least one additional operation was 5.3%; for redo pull-through, it was 0%.
Table 1.
Multi-institutional Validation Study (table)
| Hospital 1 | Hospital 2 | Hospital 3 | Total Mismatch |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Variable | Number of Mismatches |
Number of Patients |
% | Number of Mismatches |
Number of Patients |
% | Number of Mismatches |
Number of Patients |
% | Overall Misclassification Rate |
| Treatment group | 1 | 56 | 1.8% | 0 | 47 | 0.0% | 1 | 30 | 3.3% | 1.5% |
| DOB | 2 | 56 | 3.6% | 1 | 47 | 2.1% | 2 | 30 | 6.7% | 3.8% |
| Race | 6 | 56 | 10.7% | 8 | 45 | 17.8% | 6 | 30 | 20.0% | 15.3% |
| Gestational agea | 6 | 54 | 11.1% | 2 | 19 | 10.5% | 0 | 8 | 0.0% | 9.9% |
| Premature | 0 | 56 | 0.0% | 0 | 46 | 0.0% | 0 | 30 | 0.0% | 0.0% |
| Birth Weightb | 12 | 56 | 21.4% | 6 | 25 | 24.0% | 2 | 19 | 10.5% | 20.0% |
| Gender | 0 | 56 | 0.0% | 0 | 47 | 0.0% | 0 | 30 | 0.0% | 0.0% |
| Congenital anomalies | ||||||||||
| Neurologic | 0 | 56 | 0.0% | 2 | 47 | 4.3% | 1 | 30 | 3.3% | 2.3% |
| Cardiac | 3 | 56 | 5.4% | 1 | 47 | 2.1% | 1 | 30 | 3.3% | 3.8% |
| Pulmonary | 0 | 56 | 0.0% | 0 | 47 | 0.0% | 0 | 30 | 0.0% | 0.0% |
| Renal | 2 | 56 | 3.6% | 1 | 47 | 2.1% | 0 | 30 | 0.0% | 2.3% |
| Chromosomal | 0 | 56 | 0.0% | 1 | 47 | 2.1% | 1 | 30 | 3.3% | 1.5% |
| Date of first surgeryc | 14 | 56 | 25.0% | 3 | 47 | 6.4% | 2 | 30 | 6.7% | 14.3% |
| Post-Op TPN | 3 | 55 | 5.5% | 33 | 39 | 84.6% | 12 | 30 | 40.0% | 38.7% |
| Diarrhea | 28 | 55 | 50.9% | 23 | 47 | 48.9% | 10 | 30 | 33.3% | 46.2% |
| Constipation | 26 | 55 | 47.3% | 20 | 47 | 42.6% | 4 | 30 | 13.3% | 37.9% |
| Enterocolitis | 7 | 55 | 12.7% | 5 | 47 | 10.6% | 4 | 30 | 13.3% | 12.1% |
| Incontinence | 6 | 55 | 10.9% | 0 | 47 | 0.0% | 2 | 30 | 6.7% | 6.1% |
| Anastomotic Leak | 1 | 55 | 1.8% | 6 | 47 | 12.8% | 1 | 30 | 3.3% | 6.1% |
| Stricture | 9 | 55 | 16.4% | 3 | 47 | 6.4% | 3 | 30 | 10.0% | 11.4% |
| Dilation | 9 | 55 | 16.4% | 6 | 47 | 12.8% | 4 | 30 | 13.3% | 14.4% |
| SBO | 3 | 55 | 5.5% | 5 | 47 | 10.6% | 1 | 30 | 3.3% | 6.8% |
| Redo pull-through | 0 | 58 | 0.0% | 0 | 47 | 0.0% | 0 | 30 | 0.0% | 0.0% |
| Reoperation | 3 | 56 | 5.4% | 4 | 47 | 8.5% | 0 | 30 | 0.0% | 5.3% |
±1 week considered a match;
±100 g considered a match;
±1 days consider a match
Overall Cohort: Population Characteristics
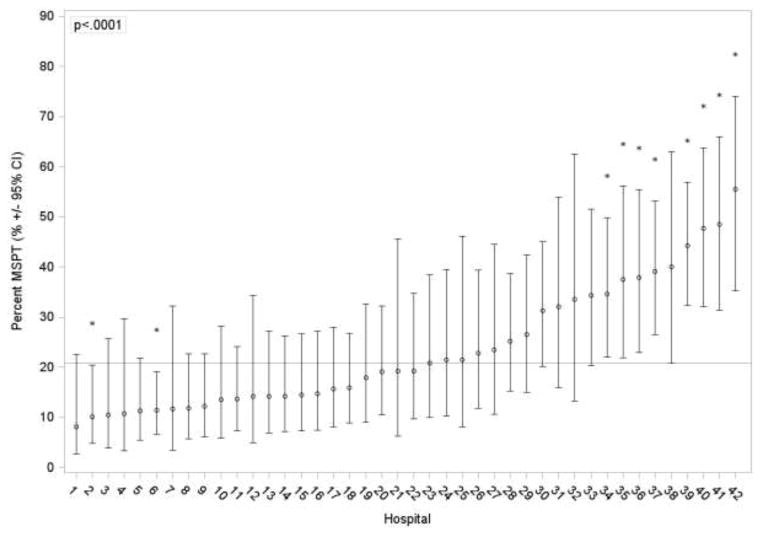
The proportion of patients undergoing SSPT significantly increased over time (p=0.03); during 1999–2001 69% of patients underwent SSPT, whereas during 2007–2009 78% underwent SSPT. In addition, there was significant variability (p<0.0001) in the proportion of patients undergoing SSPT at each PHIS hospital during the study period with a median of 81% and a range of 33–100% across hospitals (Figure 2).
Figure 2.
Variability across PHIS hospitals in the percent of patients who undergo multi-stage pull-through procedures. Unadjusted estimates and 95% CIs from a mixed effects logistic regression model with hospital specific intercepts are represented by the open circles and solid error bars. The asterisks denote hospitals that were significantly higher or lower than average (as shown by the reference line).
Clinical characteristics including demographics, pre-operative variables, and comorbid conditions for the overall cohort and each treatment group are shown in Table 2. As compared to patients undergoing SSPT, patients undergoing MSPT more often had government insurance, lower birth weight, were younger at their first surgery, and had a shorter length of stay prior to their first operation. Patients undergoing MSPT also had higher rates of prematurity, non-HD gastrointestinal anomalies, enterocolitis at their first admission, and preoperative mechanical ventilation.
Table 2.
Population Characteristics of Hirschsprung’s Disease Cohort Before and After Propensity Score Matching (table)
| Total Cohort | PS Matched Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Cohort N=1555 | Single-Stage N=1201 | Multi-Stage N=354 | P | Total Cohort N=558 | Single-Stage N=279 | Multi-Stage N=279 | P | |
| Male gender (N, %) | 1217 (78.3) | 941 (78.4) | 276 (78.0) | 0.88 | 434 (77.8) | 214 (76.7) | 220 (78.9) | 0.54 |
| Insurance Status (N, %) | ||||||||
| Government | 707 (45.5) | 521 (43.4) | 186 (52.5) | 0.006 | 273 (48.9) | 138 (49.5) | 135 (48.4) | 0.97 |
| Private Insurance | 558 (35.9) | 442 (36.8) | 116 (32.8) | 190 (34.1) | 94 (33.7) | 96 (34.4) | ||
| Other | 290 (18.7) | 238 (19.8) | 52 (14.7) | 95 (17.0) | 47 (16.9) | 48 (17.2) | ||
| Birth weight (mean, SD)a | 3292 (563) | 3323 (535) | 3190 (635) | 0.001 | 3246 (586) | 3257 (568) | 3235 (603) | 0.78 |
| Gestational age in weeks (median, IQR)b | 39 (38, 40) | 39 (38, 40) | 39 (37, 40) | 0.07 | 39 (38, 40) | 39 (37, 40) | 39 (37, 40) | 0.85 |
| Pre-op LOS in days (median, IQR) | 5 (2, 8) | 5 (2, 8) | 4 (1, 7) | 0.02 | 4 (2, 7) | 4 (1, 6) | 4 (2, 7) | 0.49 |
| Age in days at first HD surgery (median, IQR) | 12 (7, 24) | 12 (7, 24) | 11 (5, 22) | 0.0005 | 10 (6, 21) | 10 (7, 20) | 11 (6, 21) | 0.59 |
| Congenital heart disease (N, %) | 247 (15.9) | 181 (15.1) | 66 (18.6) | 0.11 | 96 (17.2) | 46 (16.5) | 50 (17.9) | 0.65 |
| Trisomy 21 (N, %) | 177 (11.4) | 129 (10.7) | 48 (13.6) | 0.14 | 59 (10.6) | 26 (9.3) | 33 (11.8) | 0.34 |
| Prematurity (N, %) | 94 (6.1) | 63 (5.3) | 31 (8.8) | 0.01 | 36 (6.5) | 18 (6.5) | 18 (6.5) | 1.0 |
| GI anomaly (other than HD) (N, %) | 88 (5.7) | 60 (5.0) | 28 (7.9) | 0.04 | 41 (7.4) | 22 (7.9) | 19 (6.8) | 0.63 |
| Meconium ileus (N, %) | 79 (5.1) | 57 (4.8) | 22 (6.2) | 0.27 | 29 (5.2) | 12 (4.3) | 17 (6.1) | 0.34 |
| Genital anomaly (N, %) | 41 (2.6) | 29 (2.4) | 12 (3.4) | 0.34 | 19 (3.4) | 8 (2.9) | 11 (3.9) | 0.48 |
| Renal anomaly (N, %) | 37 (2.4) | 27 (2.3) | 10 (2.8) | 0.53 | 18 (3.2) | 9 (3.2) | 9 (3.2) | 1.0 |
| Limb anomaly (N, %) | 33 (2.1) | 25 (2.1) | 8 (2.3) | 0.84 | 16 (2.9) | 10 (3.6) | 6 (2.2) | 0.31 |
| Neurologic anomaly (N, %) | 21 (1.4) | 14 (1.2) | 7 (2.0) | 0.29 | 9 (1.6) | 3 (1.1) | 6 (2.2) | 0.50 |
| Auditory anomaly (N, %) | 15 (1.0) | 12 (1.0) | 3 (0.9) | 1.0 | 5 (0.9) | 2 (0.7) | 3 (1.1) | 1.0 |
| Respiratory anomaly (N, %) | 14 (0.9) | 10 (0.8) | 4 (1.1) | 0.54 | 5 (0.9) | 2 (0.7) | 3 (1.1) | 1.0 |
| Other chromosomal anomalies (N, %) | 10 (0.6) | 9 (0.8) | 1 (0.3) | 0.47 | 2 (0.4) | 1 (0.4) | 1 (0.4) | 1.0 |
| Enterocolitis (N, %) | 143 (9.2) | 87 (7.2) | 56 (15.8) | <0.0001 | 63 (11.3) | 34 (12.2) | 29 (10.4) | 0.50 |
| Pre-op mechanical ventilation (N, %) | 142 (9.1) | 88 (7.3) | 54 (15.3) | <0.0001 | 69 (12.4) | 36 (12.9) | 33 (11.8) | 0.70 |
N=1252,
N=797.
PHIS: Pediatric Health Information System; IQR: interquartile range; GI: gastrointestinal; TPN: total parenteral nutrition.
P values for comparisons of the single-stage vs. multi-stage groups are from the chi-squared test or Fisher exact test for categorical variables and the t test or Mann Whitney U test for continuous variables.
Overall Cohort: Surgical Treatments and Outcomes
Surgical treatments and outcomes for the overall cohort and each treatment group are shown in Table 3. The most commonly performed procedure overall and within each treatment group was the Soave pull-through. Patients in the MSPT group more commonly underwent a Duhamel pull-through and patients in the SSPT more commonly underwent a laparoscopic assisted pull-through.
Table 3.
Surgical Procedures and Post-Operative Outcomes for Hirschsprung’s Disease Cohort Before and After Propensity Score Matching (table)
| Variable, N (%) | Total Cohort | PS Matched Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Cohort N=1555 | Single-Stage N=1201 | Multi-Stage N=354 | P | Total Cohort N=558 | Single-Stage N=279 | Multi-Stage N=279 | P | |
| Type of pull-through | ||||||||
| Soave | 851 (54.7) | 668 (55.6) | 183 (51.7) | <0.0001 | 313 (56.1) | 164 (58.9) | 149 (53.4) | <0.0001 |
| Duhamel | 91 (5.9) | 14 (1.2) | 77 (21.8) | 58 (10.4) | 2 (0.7) | 56 (20.1) | ||
| Other | 613 (39.4) | 519 (43.2) | 94 (26.6) | 187 (33.5) | 113 (40.5) | 74 (26.5) | ||
| Laparoscopic pull-through | 199 (12.8) | 184 (15.3) | 15 (4.2) | <0.0001 | 51 (9.1) | 40 (14.3) | 11 (3.9) | <0.0001 |
| Total LOS of initial surgical admission in days (median, IQR) | 12 (7, 18) | 11 (7, 17) | 12 (8, 20) | 0.001 | 12 (8, 18) | 11 (7, 17) | 12 (8, 20) | 0.02 |
| Transfusion received on day of pull-through | 30 (1.9) | 30 (2.5) | 15 (1) | 0.10 | 7 (1.3) | 7 (2.5) | 15 (5.0) | 0.13 |
| Additional operation through 2 years after pull-througha | 449 (28.9) | 312(26.0) | 137(38.7) | <0.0001 | 197 (35.3) | 84 (30.1) | 113(40.5) | 0.007 |
| Redo pull-througha | 170 (10.9) | 143 (11.9) | 27 (7.6) | 0.75 | 51 (9.1) | 30 (10.8) | 21 (7.5) | 0.61 |
| Anal dilationa | 47 (3.0) | 31 (2.6) | 16 (4.5) | 0.12 | 23 (4.1) | 9 (3.2) | 14 (5) | 0.27 |
| Any readmission through 2 years after pull-througha | 662 (42.6) | 455(37.9) | 207(58.5) | <0.0001 | 272 (48.7) | 107(38.4) | 165(59.1) | <0.0001 |
| Surgical Site Infectionb | 65 (4.2) | 32 (2.7) | 33 (9.3) | <0.0001 | 34 (6.1) | 9 (3.2) | 25 (9) | 0.007 |
| Anastomotic leakb | 65 (4.2) | 41 (3.4) | 24 (6.8) | 0.0005 | 38 (6.8) | 16 (5.7) | 22 (7.9) | 0.33 |
| Strictureb | 83 (5.3) | 67 (5.6) | 16 (4.5) | 0.34 | 32 (5.7) | 19 (6.8) | 13 (4.7) | 0.21 |
| Small Bowel Obstructionb | 82 (5.3) | 33 (2.8) | 49 (13.8) | <0.0001 | 45 (8.1) | 8 (2.9) | 37 (13.3) | <0.0001 |
| Enterocolitisc | 430 (27.7) | 309 (25.7) | 121 (34.2) | <0.0001 | 170 (30.5) | 72 (25.8) | 98 (35.1) | 0.003 |
Includes procedures performed or readmissions up to two years following the initial pull through procedure.
Diagnoses could occur at any admission, including the initial admission where HD was diagnosed.
Diagnoses only at admissions subsequent to the initial admission where HD was diagnosed.
PHIS: Pediatric Health Information System; LOS: length of stay; IQR: interquartile range; PS: propensity score
Excluding the planned pull-through procedure and its associated admission in the MSPT group, patients who underwent MSPT were more likely to be readmitted (58.5% vs. 37.9%, p<0.0001, OR 2.40, 95% CI 1.90, 3.03) and to require at least one additional operation besides their pull-through procedure (38.7% vs. 26.0%, p<0.0001, odds ratio (OR) 2.18, 95% CI 1.63, 2.90) (Table 3). In addition, patients in the MSPT group had higher rates of SSI, post-operative enterocolitis, and SBO (Table 3). Compared to the SSPT group, patients in the MSPT group had higher total adjusted hospital charges (median, IQR: $120,290 (72,599–189,084) vs. $70,313 (41,899–115,468), p<0.0001) and costs ($57,107 (34,476–90,119) vs. $33,774 (18,739–57,027), p<0.0001) for overall inpatient care from the index admission through 2 years after the pull-through procedure.
Propensity Matched Cohort Development
In the overall cohort, significant predictors of undergoing MSPT that were used for propensity score matching included: preoperative mechanical ventilation, lower gestational age, lower birth weight, enterocolitis, preoperative ICU stay in a non-neonatal ICU (e.g. Cardiac ICU), earlier year of treatment, and a lower hospital rate of SSPTs during the study period. Propensity score matching identified a cohort of 558 patients with no significant differences between the two groups in terms of demographics or comorbid anomalies and conditions (Table 2). Of note, we could not match 21% of patients in the MSPT group because there were insufficient numbers of SSPT patients with propensity scores high enough to match these patients.
Propensity Matched Cohort: Surgical Treatments and Outcomes
The Soave pull-through was the most common colo-anal reconstruction procedure performed (56.1%). Patients in the MSPT more commonly underwent a Duhamel pull-through procedure and patients in the SSPT more commonly underwent a laparoscopic assisted pull-through procedure (Table 3).
Patients who underwent MSPT were more likely to be readmitted (59.1% vs. 38.4%, p<0.0001, OR 2.32, 95% CI 1.72, 3.12) and to require at least one additional operation (40.5% vs. 30.1%, p=0.007, OR 1.55, 95% CI 1.13, 2.14) (Table 3). MSPT patients were also more likely than SSPT patients to have a SSI, SBO, and post-operative enterocolitis. The MSPT group had higher total adjusted hospital charges (median, IQR: $119, 991 (75,091–186,573) vs. $71,012 (39,360–122,575), p<0.0001) and costs ($58,072 (33,971–84,239) vs. $33,038 (17,189–60,505), p<0.0001) for overall inpatient care.
Propensity Matched Cohort: Sensitivity Analysis
To remove confounding due to the unmeasured association between long-segment HD and MSPT, a propensity score matched cohort analysis including only patients who had a pull-through by age 6 months was performed. In this matched analysis (n=173 in each group), the results remained similar favoring SSPT for all outcomes except that the difference between groups in the rate of post-operative enterocolitis was reduced (MSPT 30.1% vs. SSPT 24.9%, p=0.19).
Discussion
Using the Pediatric Health Information System (PHIS) in conjunction with multi-institutional chart validation of key variables and outcomes, we have developed a large multi-institutional cohort of infants with Hirschsprung’s disease and accurately characterized them as having undergone either SSPT or MSPT. The size and diversity of this unique cohort allowed us to characterize practice variation and perform longitudinal outcome studies in both the overall cohort and in propensity score matched groups. This study demonstrated that (1) the rates of SSPT are increasing; (2) patients selected to undergo MSPT are more severely ill; and (3) amongst patients with similar severity of illness, MSPT was associated with increased morbidity.
The rate of SSPT increased over time with almost 80% of infants with Hirschsprung’s treated at PHIS hospitals now undergoing SSPT. This increase in performing SSPT is consistent with other reports of practice trends both in the United States and abroad (30–33). In addition to identifying this change in practice, our analysis further demonstrates significant variability in the proportion of patients undergoing SSPT at each PHIS hospital. Therefore, it is likely that different indications are being used across PHIS hospitals to select patients for MSPT. Consequently, some patients selected for MSPT would likely have been treated with SSPT if they were treated at a different center. Identifying causes for this practice variation in the future may allow for more consistent care of patients with Hirschsprung’s disease across institutions. In the overall cohort, patients undergoing MSPT had worse outcomes across almost all variables. In particular, the MSPT group had more readmissions, surgical site infections, small bowel obstructions, episodes of enterocolitis, and additional operations by 2 years after the pull-through procedure. However, patients undergoing MSPT were also more severely ill, suggesting that the operating surgeons may be selecting the sicker, more complicated patients for MSPT. These findings are consistent with previous reports that demonstrate that patients selected to undergo MSPT were often those considered too ill to undergo a SSPT (30–33). Commonly reported factors influencing the decision for MSPT include the presence of enterocolitis and either long-segment Hirschsprung’s disease or total colonic aganglionosis. Since there is only one ICD-9 diagnosis code encompassing all cases of Hirschsprung’s disease, we could not directly account for the impact of the length of the Hirschsprung’s segment in our analyses. However, our study did confirm the presence of enterocolitis as an important factor in selecting patients for MSPT. Therefore, we included enterocolitis occurring prior to the first surgery performed to treat Hirschsprung’s disease (ostomy or pull-through) as a preoperative risk factor in order to assess its impact on the treatment choice and outcomes after pull-through. Using this study design, it is possible that the overall morbidity of SSPT to a patient may be underestimated by the reported post-operative outcomes in this study.
In order to account for differences in severity of illness and comorbid conditions between patients undergoing SSTP and MSPT in the overall cohort of patients, we performed a propensity score matched analysis. This type of analysis is particularly useful in large cohort studies of patients with diverse baseline characteristics because it identifies and removes patients from the analysis who are dissimilar across treatment groups. In contrast to a multi-variable risk adjustment model that estimates the effects of a treatment on an outcome in the entire cohort while controlling for differences in baseline characteristics, a propensity score matched analysis creates treatment groups with similar risk profiles that can then be compared to assess differences in outcomes. Propensity score matching uses multi-variable regression modeling to identify factors significantly associated with a higher likelihood of undergoing a specific treatment and generates a score that reflects the likelihood of receiving that treatment. The scores are then used to match patients with similar profiles thus simulating what randomization does in a clinical trial – i.e. to create groups with balanced baseline characteristics (22,23,34). In this study, propensity scores were generated to reflect the likelihood of patients undergoing MSPT, then patients with similar scores undergoing SSPT and MSPT were matched and compared. In these matched groups MSPT continued to have worse outcomes.
Since long-segment HD is considered an important determinant of whether or not a surgeon will perform a SSPT or MSPT, we attempted to account for this variable despite its absence from the dataset (31). Prior publications reporting on long-segment HD have demonstrated that these infants tend to undergo their pull-through procedures at average age of 10–14 months (25–28). In addition, in our multi-institutional chart review, patients with long segment disease in the MSPT group had their pull-through procedures after 6 months of age. Based on this, we performed an additional propensity score matched analysis including only patients who had a pull-through procedure by 6 months of age. This analysis yielded similar results to those of the original propensity-score matched analysis. Combined, these propensity score matched analyses suggest that some infants who undergo MSPT procedures may have better outcomes with SSPT despite the presence of other congenital anomalies or comorbid conditions. However, there remains a subgroup of MSPT patients who are too ill to be adequately compared to the SSPT patients in this cohort (i.e. the patients who had very high propensity scores). For this subgroup of severely ill infants with HD, MSPT may be the best option.
Using the PHIS to study rare congenital diseases, such as Hirschsprung’s disease, allows for rapid and inexpensive development of a large multi-institutional cohort of patients from a geographically diverse group of tertiary Children’s hospitals (12). The dataset includes longitudinal patient-level data on diagnoses, procedures, and resource utilization which can be used to assess practice variability and differences in resource utilization. In addition, when cohort development is combined with institutional chart validation, the PHIS may be used to perform descriptive and comparative effectiveness studies (13–16).
There are several limitations of our study that center around the use of administrative data. First, the types of analyses that can be performed are limited by the availability of ICD-9-CM codes that can capture clinically important variables and outcomes. For example, in the current study, we could neither assess the impact of the length of the Hirschsprung’s segment on the selection of an operative procedure nor its effect on outcome. We also were unable to stratify each treatment group based on the type of specific repair that was performed or the approach used (transanal vs. abdominal vs. combination); this may account for the higher rates of certain complications (e.g. small bowel obstruction) in the MSPT group as this group may include more open and fewer transanal approaches. In addition, the impact of the type of surgical procedure on relevant functional outcomes, such as fecal incontinence and constipation, and on need for inclinic treatments, such as Botox injections, cannot reliably be assessed using administrative data. The effects of these variables and assessment of these outcomes will require more resource intense longitudinal clinical registries or clinical trials. Second, although substantial quality control measures are in place to ensure the validity of data within the PHIS, there is still a possibility for misclassification bias. In addition, there can be variability in terms of the clarity or ambiguity of what each ICD-9-CM code represents and when it is used. To determine the rates and potential impact of misclassification bias and miscoding, we performed medical record reviews at three separate institutions. Third, the ICD-9-CM diagnosis codes used as exposures and outcomes are associated with an entire admission and not date-stamped; therefore, within a given admission, it is not possible to determine if a diagnosis occurred before or after a procedure. To minimize this issue, we attempted to use procedure and billing codes which are date stamped to define variables whenever possible. Fourth, within the PHIS, patients can only be followed longitudinally at one institution, so care received at a different institution (PHIS or non-PHIS) would not be included.
Conclusions
This study demonstrates that current practice across major freestanding children’s hospitals is to perform SSPT for most infants diagnosed with HD, with MSPT reserved for more severely ill patients. Comparisons of patients with similar baseline characteristics based on propensity score matching demonstrate that MSPT was associated with increased rates of readmissions and reoperations. These results suggest that some patients currently selected to undergo MSPT may have better outcomes if they underwent a SSPT. However, there was a subgroup of MSPT patients who were too ill and did not have comparable matches in the SSPT group; for this subgroup of severely ill infants with HD, MSPT likely remains the better option. Future studies should focus on characterizing factors that can be used to identify patients who would benefit from a MSPT. This may reduce the number of newborns receiving colostomies and undergoing multiple procedures, and may lead to improved outcomes. In addition, further multi-institutional studies to determine the impact of single versus multi-staged pull-through on functional outcomes are warranted.
Acknowledgments
This project is supported by NIH 5T32HL098039-03 (Dr. Sulkowski) and intramural funding from the Research Institute at Nationwide Children’s Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huang EY, Tolley EA, Blakely ML, et al. Changes in hospital utilization and management of Hirschsprung disease: analysis using the kids’ inpatient database. Annals of Surgery. 2013 Feb;257(2):371–375. doi: 10.1097/SLA.0b013e31827ee976. [DOI] [PubMed] [Google Scholar]
- 2.So HB, Schwartz DL, Becker JM, et al. Endorectal “pull-through” without preliminary colostomy in neonates with Hirschsprung’s disease. Journal of Pediatric Surgery. 1980 Aug;15(4):470–471. doi: 10.1016/s0022-3468(80)80755-x. [DOI] [PubMed] [Google Scholar]
- 3.Carcassonne M, Guys JM, Morrison-Lacombe G, et al. Management of Hirschsprung’s disease: curative surgery before 3 months of age. Journal of Pediatric Surgery. 1989 Oct;24(10):1032–1034. doi: 10.1016/s0022-3468(89)80209-x. [DOI] [PubMed] [Google Scholar]
- 4.Cilley RE, Statter MB, Hirschl RB, et al. Definitive treatment of Hirschsprung’s disease in the newborn with a one-stage procedure. Surgery. 1994 May;115(5):551–556. [PubMed] [Google Scholar]
- 5.Langer JC, Fitzgerald PG, Winthrop AL, et al. One-stage versus two-stage Soave pull-through for Hirschsprung’s disease in the first year of life. Journal of Pediatric Surgery. 1996 Jan;31(1):33–36. doi: 10.1016/s0022-3468(96)90315-2. discussion 36–37. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox DT, Bruce J, Bowen J, et al. One-stage neonatal pull-through to treat Hirschsprung’s disease. Journal of Pediatric Surgery. 1997 Feb;32(2):243–245. doi: 10.1016/s0022-3468(97)90187-1. discussion 245–247. [DOI] [PubMed] [Google Scholar]
- 7.Pierro A, Fasoli L, Kiely EM, et al. Staged pull-through for rectosigmoid Hirschsprung’s disease is not safer than primary pull-through. Journal of Pediatric Surgery. 1997 Mar;32(3):505–509. doi: 10.1016/s0022-3468(97)90617-5. [DOI] [PubMed] [Google Scholar]
- 8.Georgeson KE, Cohen RD, Hebra A, et al. Primary laparoscopic-assisted endorectal colon pull through for Hirschsprung’s disease: a new gold standard. Annals of Surgery. 1999 May;229(5):678–682. doi: 10.1097/00000658-199905000-00010. discussion 682–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramesh JC, Ramanujam TM, Yik YI, et al. Management of Hirschsprung’s disease with reference to one-stage pull-through without colostomy. Journal of Pediatric Surgery. 1999 Nov;34(11):1691–1694. doi: 10.1016/s0022-3468(99)90646-2. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum DH, Cilley RE, Sherman NJ, et al. A decade of experience with the primary pull through for Hirschsprung Disease in the newborn period: a multicenter analysis of outcomes. Annals of Surgery. 2000 Sep;232(3):372–380. doi: 10.1097/00000658-200009000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattei P. Hirschsprung’s Disease. In: Wyllie R, Hyams J, editors. Pediatric Gastrointestinal and Liver Disease. 4. Saunders; 2011. pp. 576–582. [Google Scholar]
- 12.Sulkowski JP, Deans KJ, Asti L, et al. Using the Pediatric Health Information System to study rare congenital pediatric surgical diseases: development of a cohort of esophageal atresia patients. Journal of Pediatric Surgery. 2013 Sep;48(9):1850–1855. doi: 10.1016/j.jpedsurg.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 13.Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009 Feb;123(2):636–642. doi: 10.1542/peds.2008-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo Re V, 3rd, Haynes K, Goldberg D, et al. Validity of diagnostic codes to identify cases of severe acute liver injury in the US Food and Drug Administration’s Mini-Sentinel Distributed Database. Pharmacoepidemiology and Drug Safety. 2013 Aug;22(8):861–872. doi: 10.1002/pds.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson KE, Beaton SJ, Andrade SE, et al. Methods of linking mothers and infants using health plan data for studies of pregnancy outcomes. Pharmacoepidemiology and Drug Safety. 2013 Jul;22(7):776–782. doi: 10.1002/pds.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan ST, Fuchs DC, Shelton RC, et al. Identifying suicidal behavior among adolescents using administrative claims data. Pharmacoepidemiology and Drug Safety. 2013 Jul;22(7):769–775. doi: 10.1002/pds.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mongelluzzo J, Mohamad Z, Ten Have TR, et al. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008 May 7;299(17):2048–2055. doi: 10.1001/jama.299.17.2048. [DOI] [PubMed] [Google Scholar]
- 18.Fox D, Morrato E, Campagna EJ, et al. Outcomes of laparoscopic versus open fundoplication in children’s hospitals: 2005–2008. Pediatrics. 2011 May;127(5):872–880. doi: 10.1542/peds.2010-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerardi KE, Auger KA, Shah SS, et al. Discordant antibiotic therapy and length of stay in children hospitalized for urinary tract infection. Journal of Hospital Medicine. 2012 Oct;7(8):622–627. doi: 10.1002/jhm.1960. [DOI] [PubMed] [Google Scholar]
- 20.Kittle K, Currier K, Dyk L, et al. Using a pediatric database to drive quality improvement. Seminars in Pediatric Surgery. 2002 Feb;11(1):60–63. doi: 10.1053/spsu.2002.29367. [DOI] [PubMed] [Google Scholar]
- 21.Schafer JL. Multiple imputation: a primer. Statistical Methods in Medical Research. 1999 Mar;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 22.Faries DE, Leon AC, Haro JM, et al. Analysis of Observational Health Care Using SAS. Cary, N.C: SAS Institute; 2010. [Google Scholar]
- 23.Mitra R, Reiter JP. A comparison of two methods of estimating propensity scores after multiple imputation. Statistical Methods in Medical Research. 2012 Jun 11; doi: 10.1177/0962280212445945. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum PR. Design of Observational Studies. New York: Springer; 2010. [Google Scholar]
- 25.Escobar MA, Grosfeld JL, West KW, et al. Long-term outcomes in total colonic aganglionosis: a 32-year experience. Journal of Pediatric Surgery. 2005 Jun;40(6):955–961. doi: 10.1016/j.jpedsurg.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 26.Ieiri S, Suita S, Nakatsuji T, et al. Total colonic aganglionosis with or without small bowel involvement: a 30-year retrospective nationwide survey in Japan. Journal of Pediatric Surgery. 2008 Dec;43(12):2226–2230. doi: 10.1016/j.jpedsurg.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 27.Choe EK, Moon SB, Kim HY, et al. Outcomes of surgical management of total colonic aganglionosis. World Journal of Surgery. 2008 Jan;32(1):62–68. doi: 10.1007/s00268-007-9270-5. [DOI] [PubMed] [Google Scholar]
- 28.Cobellis G, Noviello C, Cruccetti A, et al. Staged laparoscopic-assisted endorectal pull-through for long segment Hirschprung’s disease and total colonic aganglionosis. Minerva Pediatrica. 2011 Jun;63(3):163–167. [PubMed] [Google Scholar]
- 29.GMATCH [computer program] Mayo Clinic Division of Biomedical Statistics and Informatics; 2004. [Google Scholar]
- 30.Bradnock TJ, Walker GM. Evolution in the management of Hirschsprung’s disease in the UK and Ireland: a national survey of practice revisited. Annals of the Royal College of Surgeons of England. 2011 Jan;93(1):34–38. doi: 10.1308/003588410X12771863936846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keckler SJ, Yang JC, Fraser JD, et al. Contemporary practice patterns in the surgical management of Hirschsprung’s disease. Journal of Pediatric Surgery. 2009 Jun;44(6):1257–1260. doi: 10.1016/j.jpedsurg.2009.02.050. discussion 1260. [DOI] [PubMed] [Google Scholar]
- 32.Suita S, Taguchi T, Ieiri S, et al. Hirschsprung’s disease in Japan: analysis of 3852 patients based on a nationwide survey in 30 years. Journal of Pediatric Surgery. 2005 Jan;40(1):197–201. doi: 10.1016/j.jpedsurg.2004.09.052. discussion 201–192. [DOI] [PubMed] [Google Scholar]
- 33.Singh SJ, Croaker GD, Manglick P, et al. Hirschsprung’s disease: the Australian Paediatric Surveillance Unit’s experience. Pediatric Surgery International. 2003 Jun;19(4):247–250. doi: 10.1007/s00383-002-0842-z. [DOI] [PubMed] [Google Scholar]
- 34.Kuss O, Legler T, Borgermann J. Treatments effects from randomized trials and propensity score analyses were similar in similar populations in an example from cardiac surgery. Journal of Clinical Epidemiology. 2011 Oct;64(10):1076–1084. doi: 10.1016/j.jclinepi.2011.01.005. [DOI] [PubMed] [Google Scholar]