Abstract
The use of 18F-sodium fluoride (18F-NaF) with positron emission tomography-computed tomography (PET/CT) is increasing. This resurgence of an old tracer has been fueled by several factors including superior diagnostic performance over standard 99mTc-based bone scintigraphy, growth in the availability of PET/CT imaging systems, increase in the number of regional commercial distribution centers for PET radiotracers, the recent concerns about potential chronic shortages with 99mTc based radiotracers, and the recent decision by the Centers for Medicare and Medicaid Services to reimburse for 18F-NaF PET/CT for evaluation of patients with known or suspected bone metastases through the National Oncologic PET Registry. The major goal of this article is to review the current evidence on the diagnostic utility of 18F-NaF in the imaging assessment of bone and joint in a variety of clinical conditions.
Keywords: 18F-NaF, fluoride, PET, CT, bone, joint
Standard bone scintigraphy with 99mTc-based radiotracers (e.g. 99mTc methylene diphosphonate or MDP) remains one of the most common imaging procedures worldwide for a variety of disorders. These conditions include imaging evaluation for infection (e.g. osteomyelitis), non-infectious inflammation (e.g. arthritis), trauma, metabolic bone disease, benign and malignant neoplasms, and specific states in children. Both planar technique and in selected cases, single photon emission tomography (SPECT) are performed. The scan may also include dynamic image data acquisition (3-phase) and involve only a specific region of the body or the whole skeleton. The procedure guidelines and the common imaging findings with various conditions using standard bone scintigraphy have been reviewed extensively in many excellent reviews and medical imaging textbooks (1). Bone scintigraphy is most effective when correlated to other relevant imaging modalities such as radiography, computed tomography (CT), and magnetic resonance imaging (MRI) that often assists with improving specificity.
Despite the historical legacy and widespread current clinical practice that employs 99mTc-based bone scintigraphy, the use of positron emission tomography (PET) with 18F-sodium fluoride (18F-NaF) is increasing. 18F-NaF was introduced in 1962 and approved for clinical use by the U.S. Food and Drug Administration in 1972 (2, 3). However, despite the early recognition of the superior diagnostic performance of 18F-NaF, the tracer was not widely used in view of limited supply and relatively high cost, the technical limitations of gamma cameras with high-energy photon imaging and, at the time, the paucity of PET scanners. Since 2001, when combined PET/computed tomography (PET/CT) scanners became commercially available, the use of this imaging system with the most common PET radiotracer, 18F-fluorodeoxyglucose (18F-FDG) has rapidly increased, particularly in the evaluation of patients with cancer. The growing availability of PET/CT scanners, establishment of commercial regional distribution centers for PET radiotracers, and the recent problems with shortages of 99mTc-labeled tracers, prompted a resurgence of use of 18F-NaF in bone scintigraphy (4–6). A major reinforcement in use was also the decision by the Centers for Medicare and Medicaid Services on February 7, 2011, to reimburse for 18F-NaF PET/CT through the coverage with evidence development program managed by the National Oncologic PET Registry (NOPR) to assess the effect of 18F-NaF PET/CT on referring physicians’ intended management of patients with known or suspected bone metastases (7).
In this article, we review the mechanism of uptake, biodistribution, and dosimetry of 18F-NaF followed by a concise summary of the published evidence on its utility in a variety of bone and joint conditions.
Biodistribution and Radiation Dosimetry
Czernin and colleagues summarized the mechanism of 118F-NaF uptake in the bone (8). 18F-NaF is rapidly cleared from plasma in a biexponential manner with most of the tracer retained by bone after a single pass. The tracer uptake by the bone is due to chemisorption with exchange of 18F− ion for OH− ion on the surface of the hydroxyapatite matrix of bone forming fluoroapatite and migration of 18F− ion into the crystalline matrix of bone. There is minimal binding to serum protein and rapid renal clearance that contributes to the high quality of images with high bone-to-background ratio in a shorter time than for standard 99mTc-based tracers (9, 10). 18F-NaF may be used with dynamic PET data acquisition to obtain quantitative measurements of the tracer pharmacokinetics that then can be useful in specific research and clinical applications (11–16).
Procedure guideline for use of 18F-NaF PET/CT has been published (17). The radiotracer is injected intravenously with an adult activity dose of 185–370 MBq (5–10 mCi). Imaging may begin after 30–45 min of uptake phase in patients with normal renal function. The effective dose for 18F-NaF is 0.024 mSv/MBq (0.089 mrem/mCi). The effective dose is 8.9 mSv (0.89 rem) for a typical activity dose of 370 MBq (10 mCi). This effective dose is approximately 70% higher than the effective dose of 0.0057 mSv/MBq (0.021 rem/mCi) for 99mTc-MDP, which translates to an effective dose of 5.3 mSv (0.53 rem) for a typical activity of 925 MBq (25 mCi) (17). However, given the high bone-to-background ratio (i.e. high signal-to-noise ratio) of 18F-NaF, the effective dose may be lowered without major untoward effect on image quality by reducing the injected activity (e.g. by about half) such that the effective dose would then be comparable to that for 99mTc-MDP (18). Bladder receives the largest radiation dose of 0.22 mGy/MBq (0.81 rad/mCi) with voiding interval of 3.5 h (17).
Pediatrics
The guidelines for clinical indications, image acquisition, image processing, and image interpretation of bone scans in children with either 99mTc-labeled radiotracers and 18F-NaF PET have been published (19). Pediatric activity is typically weight-based at 2.22 MBq/kg (0.06 mCi/kg), with a range of 18.5–185 MBq (0.5–5 mCi). The effective dose is 0.086 mSv/MBq (0.32 rem/mCi) for a 5-year old child. The urinary bladder receives the largest radiation dose of 0.61 mGy/MBq (2.3 rad/mCi) at a voiding interval 3.5 h (17).
In view of the higher sensitivity and excellent quality images of 18F-NaF PET in a shorter amount of time in comparison to standard bone scintigraphy, this imaging modality may be an excellent alternative to 99mTc MDP bone scans for assessment of bone pain and diseases in children (20). A major use of bone scintigraphy in children is for assessment of skeletal trauma in child abuse (21). Drubach et al performed a retrospective study of the diagnostic utility of 18F-NaF PET in 22 patients younger than 2 years. Skeletal survey was obtained in all patients at baseline and in 14 patients during follow-up (22). The reference standard was based on independent interpretation of baseline and follow-up skeletal survey studies. 18F-NaF PET demonstrated sensitivities of 85% for all fractures, 92% for thoracic fractures, 93% for posterior rib fractures, and 67% for classic metaphyseal lesions (CMLs). In contrast, the baseline skeletal survey showed corresponding sensitivities of 72%, 68%, 73%, and 80%, respectively. Therefore, 18F-NaF PET/CT was overall advantageous over skeletal survey for detection of almost all suspicious fractures except for CMLs that may require an initial radiographic skeletal survey.
Localization and appropriate treatment of back pain in young children and adolescents are also important clinical endeavors (23). In an investigation of 94 young patients between the ages of 4 and 26 years, 18F-NaF PET was able to localize the possible cause of back pain in 55% of patients (24). In descending order of occurrence, these conditions included pars interarticularis/pedicle stress, spinous process injury, vertebral body ring apophyseal injury, stress at a transitional vertebra-sacral articulation, and sacroiliac joint inflammation/stress.
Benign Bone, Joint and Other Diseases
18F-NaF PET may be useful in the metabolic evaluation of benign bone, joint, and other clinical conditions. Furthermore, the CT portion of the 18F-NaF PET/CT provides not only precise information on anatomic localization but also may reveal previously unknown clinically significant extraosseous findings in about 11% of patients (25).
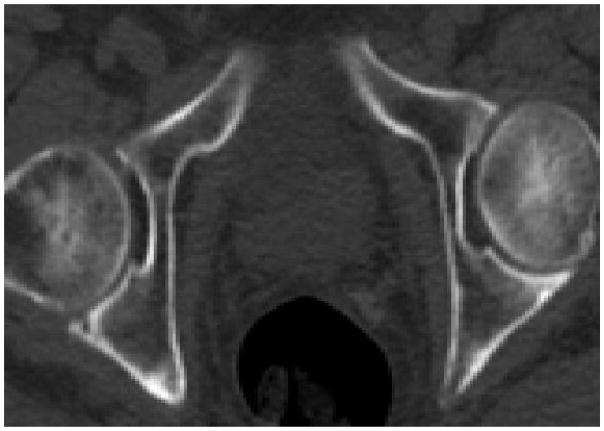
During evaluation of patients for osseous metastatic disease, it is often observed that there is high 18F-NaF uptake in joint degenerative and arthritic changes (Fig. 1). This commonly observed feature has been used to investigate the potential utility of 18F-NaF PET/CT in this clinical setting. For example, 18F-NaF PET may be useful in the detection of bone remodeling in early stage osteoarthritis of the temporomandibular and hip joints, ankylosing spondylitis, patellofemoral compartment pain, spontaneous osteonecrosis of the knee, femoral head osteonecrosis, characterization of bone mineralization around joint arthroplasties, early detection of aspetic loosening of total knee arthroplasty, differentiation of septic from aseptic loosening of total hip arthroplasty, and determination of treatment efficacy of bone active agents in patients with diminished bone mineral density (26–36). The osteointegration of cancellous bone allografts in bone defect fillers around the acetabular component of the loosened total hip replacement may also be monitored quantitatively with 18F-NaF PET (38, 39). Similar findings have been reported for assessment of perfusion and viability of osseous (e.g. revascularized fibula graft) and osseocutaneus flaps for mandibular reconstruction and for allogenic bone grafts of the limbs (40–42). In patients with recurrent back pain after spinal fusion surgery (e.g. due to failed posterior lumbar interbody fusion), 18F-NaF PET/CT may have a role in identifying abnormalities that require surgical reintervention (43, 44).
Fig. 1.
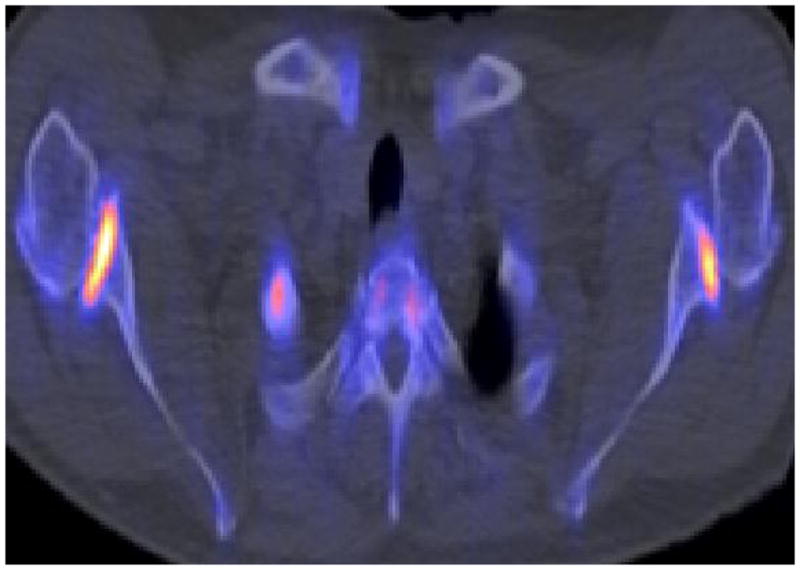
Benign causes of increased 18F-NaF uptake; (A) enthesopathy, (B) degenerative joint disease, and (C) osteophytosis.
In a proof-of-principle investigation, 18F-NaF PET has been shown to be superior to other imaging modalities in characterization of pulmonary alveolar microlithiasis, a rare disease in which multiple microscopic calcium phosphate microliths are deposited in the alveoli (45). Other pilot studies of patients with either otosclerosis or hyperostosis cranialis interna have shown that the metabolic activities of the affected bones, as measured by 18F-NaF PET, may be useful for assessment of these clinical conditions (46, 47). Moreover, 18F-NaF PET/CT appears to be the most accurate imaging modality and advantageous over contrast-enhanced MRI and cone beam CT in the assessment of the bisphosphonate-induced osteonecrosis of the jaw (48).
Since 18F-NaF is taken up at sites of calcification, another interesting potential application may be in the assessment of atherosclerosis (49). In fact, use of PET with 18F-FDG and 18F-NaF may provide important insight into the evolutionary phases of the atherosclerotic plaque from a vulnerable inflammatory state to development of plaque microcalcification, which then helps to understand the complex biology of plaque rupture (50). Dweck and colleagues prospectively evaluated 119 volunteers with and without aortic valve disease and scanned them for determination of coronary calcium score as well as 18F-NaF and 18F-FDG uptake levels (51). Patients with increased coronary 18F-NaF uptake level had higher rates of prior cardiovascular events, angina, and Framingham risk scores, suggesting that coronary 18F-NaF uptake may represent a new imaging biomarker for plaque assessment. In another study that employed both 18F-NaF and 18F-FDG PET, the coincident uptake of both radiotracers was noted in only 6.5% of arterial atherosclerotic lesion suggesting that these radiotracers may provide distinct information on the spectrum of the pathophysiologic processes involved in atherosclerosis (52).
Malignant Diseases
There is growing convincing evidence that 18F-NaF PET/CT is diagnostically superior to standard 99mTc-based bone scintigraphy for more accurate detection and determination of the extent osseous metastatic disease in a variety of cancers (53–60). A recent meta-analysis of 10 studies comparing standard bone scintigraphy with 18F-NaF PET/CT reported a patient-based pooled sensitivity of 96% and pooled specificity of 98%, respectively, and a lesion-based pooled sensitivity of 97% and pooled specificity of 98%, which were substantially better compared to 99mTc-based bone scintigraphy (61). Despite the superior diagnostic performance of 18F-NaF PET in comparison to standard bone scan, it should be noted that the well-recognized entities of “superscan” and treatment-related “flare” are similar between these imaging modalities (62, 63).
Combined 18F-NaF fluoride and 18F-FDG PET/CT has also been proposed for assessment of both osseous and soft tissue lesions in the setting of a single imaging session for streamlining of care and potential reduction in the overall imaging cost (64–67). However, additional experience will be needed before such single-injection maneuver is adopted in view of issues such as dual tracer interference in semi-quantitative uptake measurements and potential challenge in comparing baseline dual-tracer studies to post-treatment single or dual-tracer scans.
In this section, we briefly review the major reports on the utility of 18F-NaF PET or PET/CT in various cancers.
Head and Neck Cancer
In an investigation of 80 patients with head and neck cancer who were at increased risk for metastases, 18F-NaF PET/CT and 18F-FDG PET/CT were performed within 2 weeks of each other (68). Both scans showed similar lesion-based sensitivity (69.4% vs. 57.1%, p=0.126). Combined information detected more lesions than either scan alone, however, there was no statistically significant difference with patient-based analysis.
Thyroid Cancer
Ota et al evaluated 11 patients with differentiated thyroid carcinoma with suspected bone metastases after total thyroidectomy who underwent 131I radioiodine therapy (69). Standard bone scintigraphy and 18F-NaF PET/CT were performed and the findings were compared to those on radioiodine scan and CT (or MRI if available) as standard of reference. 18F-NaF PET/CT was significantly more sensitive in detecting bone metastases from thyroid cancer in comparison to planar bone scan. However, another investigation showed only limited osteosclertic bone reaction from thyroid cancer metastases on 18F-NaF PET (70)
Lung Cancer
Standard 99mTc-based bone scan with and without SPECT were compared to 18F-NaF PET in 53 patients with small cell lung cancer or locally advanced non-small cell lung cancer for detection of vertebral bone metastases (71). Of the twelve patients with vertebral metastases, bone scan produced 6 false negatives, SPECT produced 1 false-negative and 18F-NaF PET resulted in no false-negatives. Moreover, 18F-NaF PET impacted clinical management in 11% of patients.
Breast Cancer
18F-NaF PET/CT has been shown to be superior to CT alone for detection of bone metastases from breast cancer (72). The investigation by Piccardo and colleagues compared 18F-NaF PET/CT and CT alone in 39 breast cancer patients with bone metastases (73). A repeat CT at 12 month served as standard of reference. The sensitivity and specificity for 18F-NaF PET/CT were 91% and 91%, respectively. The sensitivity and specificity for CT alone were 77% and 93%, respectively.
Interestingly, it appears that there is an age-related change in the amount of 18F-NaF uptake in the bone of pre- and post-menopausal women (e.g. positive association between humeral shaft uptake and advancing age and negative association between lumbar spine uptake and advancing age)(74). As such, the background normal bone activity and tumor-to-background activity ratio may be different in pre- and post-menopausal women with bone metastases from breast cancer.
Hepatocellular Carcinoma
An investigation from Taiwan compared 18F-NaF PET/CT and standard 99mTC-based bone scintigraphy in 34 patients with hepatocellular carcinoma (74). On a lesion-based basis, 18F-NaF PET/CT had higher accuracy of detecting lesions in comparison to bone scan (96% versus 75%, p=0.0001). Moreover, 18F-NaF PET/CT had also prognostic value in terms of overall survival while bone scan did not.
Multiple Myeloma
An early study of 7 patients with either whole-body MRI or whole-body radiographic survey as standard of reference identified 89% of the lesions on 18F-NaF PET/CT (76). Sachpekidis et al performed a similar study with 18F-FDG PET/CT serving as reference standard (77). The correlation of lesion detection was only 39% between 18F-NaF PET/CT and 18F-FDG PET/CT, suggesting that 18F-FDG PET/CT may be more useful in the skeletal assessment of patients with multiple myeloma.
Bladder Cancer
A report of 48 patients with urinary bladder carcinoma compared standard planar and SPECT bone scintigraphy with 18F-NaF PET/CT (78). The sensitivity and specificity for detection of bone metastases were 82.4% and 64.5% for standard planar bone scan, 88.2% and 74.2% for SPECT/CT and 100% and 87.1% for 18F-NaF PET/CT, respectively. 18F-NaF PET/CT also changed the clinical management in 35% of patients. The study concluded that 18F-NaF PET/CT may serve as a cost-effective screening procedure for detection of skeletal metastases in high-risk patients with urinary bladder carcinoma.
Prostate Cancer
Bone is the most common site for metastases from prostate cancer. Most of these bone metastases appears osteoblastic (sclerotic) on radiography. Roudier et al demonstrated through bone histomorphometry that the osteolytic-osteopenic component of the osteoblastic lesions, which are woven bone formed directly from the tumor stroma and not from the adjacent bone surface, might be responsible for the frequent fractures observed phenotypically as skeletal related events with their associated significant morbidity and cost (79).
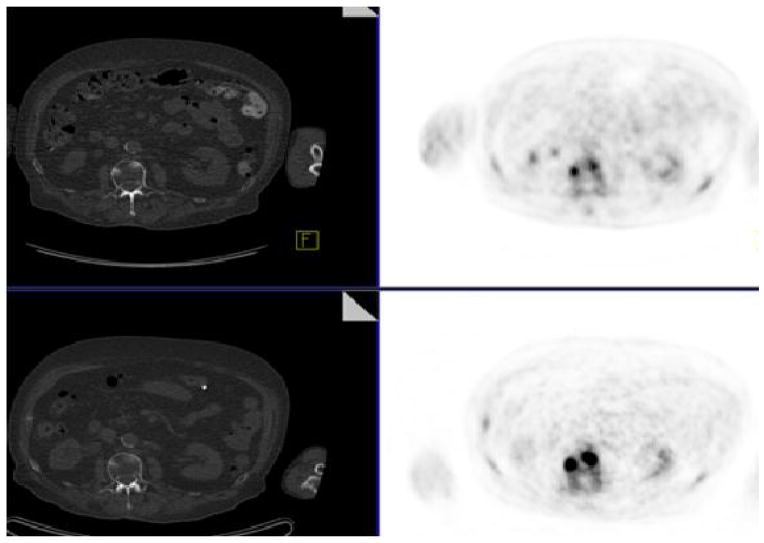
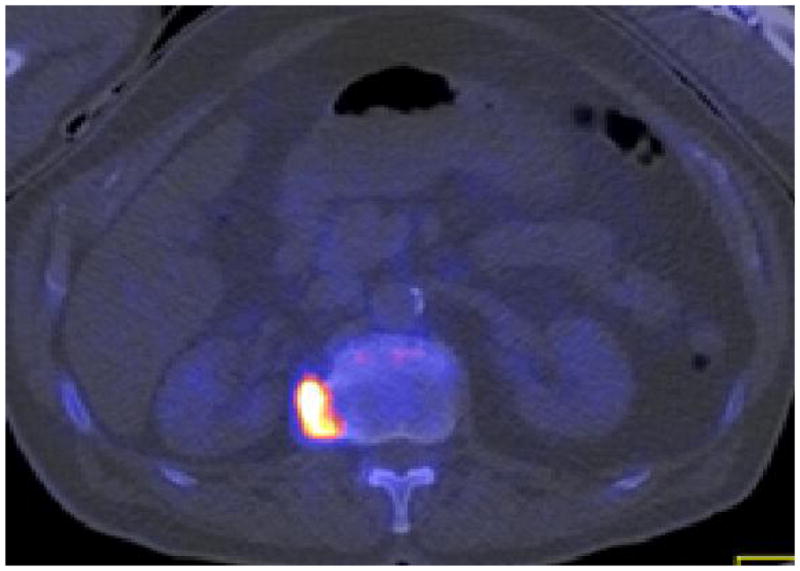
Even-Sapir and colleagues compared 99mTc-MDP planar bone scintigraphy, SPECT, 18F-NaF PET, and 18F-NaF PET/CT in 25 men with newly diagnosed prostate cancer (Gleason scores of 8 or higher or serum prostate specific antigen (PSA) levels of 20 ng/mL or higher or nonspecific sclerotic lesions on CT) and 19 patients who were referred for evaluation of suspected recurrence or progression of disease (80). A clinical follow-up of 6–15 months was used as reference standard. In a patient based analysis, the sensitivity and specificity were 70% and 57%, respectively, for planar bone scintigraphy, 92% and 82% for SPECT, 100% and 62% for 18F-NaF PET, and 100% and 100% for 18F-NaF PET/CT. The high sensitivity and specificity of 18F-NaF PET/CT may also allow for detection of occult bone metastases that are missed on standard bone scintigraphy, at lower PSA ranges than conventionally recognized. In a recent investigation for our PET/CT Center at the University of Southern California, we reported a true-positive detection rate of 16.2% for occult osseous metastases in 37 men with biochemical recurrence of prostate cancer who had negative conventional scans (Fig. 2) (81).
Fig. 2.

Biochemical recurrence of prostate cancer with negative conventional 99mTc-MDP bone scintigraphy. 18F-NaF PET/CT demonstrated focal hyperactivity (arrow) in the left acetabulum corresponding to subtle sclerosis on CT; (A) CT, (B) 18F-NaF PET, (C) fused PET/CT.
A recent investigation compared 99mTc-MDP bone scintigraphy, 18F-fluorocholine PET/CT and 18F-NaF PET/CT with MRI as reference standard in detecting spine metastases from prostate cancer (82). The sensitivity and specificity were 51% and 82% for bone scintigraphy, 85% and 91% for 18F-fluorocholine, and 93% and 54% for 18F-NaF PET/CT. The authors concluded that combined 18F-NaF PET/CT and 18F-fluorocholine PET/CT is accurate in this clinical setting and superior to standard bone scintigraphy. Similar results have been reported by others suggesting that practice guidelines should include 18F-NaF PET/CT and (11C or 18F)-choline PET/CT in preference over 99mTc-based bone scan for detection and monitoring of bone metastases in patients with prostate cancer (83–85).
Beheshti and colleagues compared 18F-fluorocholine PET/CT and 18F-NaF PET/CT in 38 men with prostate cancer, 17 preoperatively at the time of initial diagnosis and 21 patients postoperatively for suspected recurrence (86). There was moderate agreement between the two radiotracers on both lesion-based (kappa=0.57) and patient-based (kappa=0.76) analyses. The authors also noted a significant negative correlation between tracer intensity as measured by standardized uptake value and the CT density of the lesions as measured in Hounsfield units for both tracers. The sensitivity and specificity were 81% and 93% for 18F-NaF PET/CT and 77% and 91% for 18F-fluorocholine, respectively. The authors concluded that while 18F-fluorocholine might be superior for detection of early marrow metastases, but 18F-NaF PET/CT might provide additional useful information when 18F-fluorocholine is negative at sites of suspicious sclerotic lesions.
The early results of the prospective data that were collected under Medicare’s Coverage with Evidence Development policy using the NOPR process in men with known prostate cancer showed high overall impact in the management of these patients (87). The patients were examined at the time of initial staging (Group I, n=1024), suspected first bone metastasis (Group II, n=1997), and suspected progression of osseous metastases (Group III, n=510). The post 18F-NaF PET management plans changed in 77% of Group I, 52% of Group II, and 71% of Group III, respectively. The overall change in intended management ranged from 44% to 52%. Even after adjusting for prior plans for additional imaging evaluation (imaging-adjusted impact), the overall impact ranged from 12% to 16%. Bone metastases were recorded in 14%, 29%, and 76%, of patients in Groups, I, II, and III, respectively.
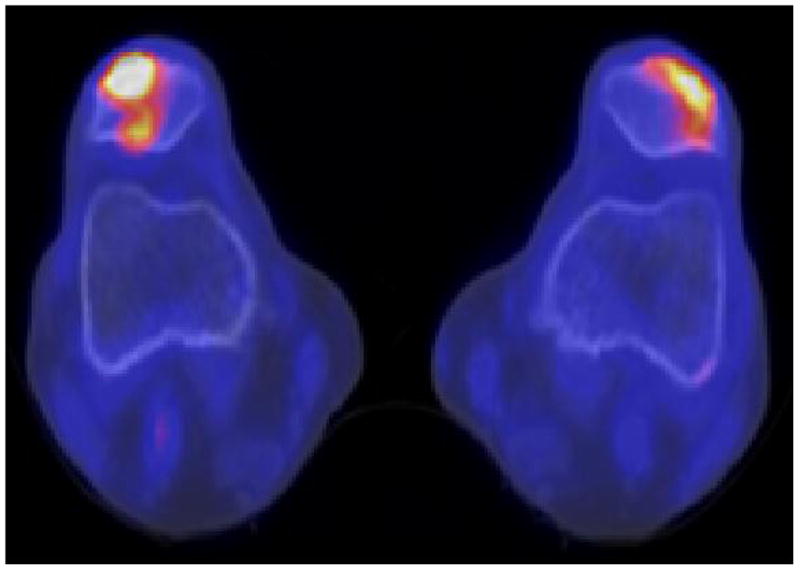
With respect to treatment response evaluation in metastatic prostate cancer, one small cases series demonstrated that 18F-NaF PET/CT may be useful for semi-quantitative assessment of response to alpha particle treatment with the recently FDA approved 223Ra-dichloride in castrate resistant prostate cancer (88). Additional studies will be needed to define the role of 18F-NaF PET/CT in assessment therapy response in patients with metastatic prostate cancer (Fig. 3).
Fig. 3.
Metastatic prostate cancer, (A, Bottom) before (PSA 13.3 ng/mL) and (B, Top) after (PSA 9.6 ng/mL) androgen deprivation therapy. Treatment induced increase in sclerosis and decrease in 18F-NaF uptake of the vertebral lesions. Left Panel: CT at bone window level, Right Panel: 18F-NaF PET.
Conclusion
In comparison to 99mTc-based bone scintigraphy, 18F-NaF PET/CT provides higher diagnostic performance with higher quality images within a shorter period from injection time and with relatively similar radiation dosimetry, albeit at currently higher scan cost. However, this higher cost may be at least partly be mitigated by increased patient throughput, avoidance of performing additional separate CT scans, and increased availability, access, and use of PET/CT, particularly in an environment at risk for potential chronic 99mTc shortages.
Acknowledgments
This work was partly supported by grant R01-CA111613 (PI: H. Jadvar) from the National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fischer DR. Musculoskeletal imaging using fluoride PET. Semin Nucl Med. 2013;43:427–33. doi: 10.1053/j.semnuclmed.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Blau M, Nagler W, Bender M. Fluorine-18: a new isotope for bone scanning. J Nucl Med. 1962;3:332–334. [PubMed] [Google Scholar]
- 3.Grant FD, Fahey FH, Packard AB, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 4.Ho CK, Hawkins RA, Dahlbom M, et al. Whole body skeletal imaging with [18F]fluoride ion and PET. J Comput Assist Tomogr. 1993;17:34–41. doi: 10.1097/00004728-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Sheth S, Colletti PM. Atlas of sodium fluoride PET bone scans. Clin Nucl Med. 2012;37:e110–116. doi: 10.1097/RLU.0b013e31824c81ee. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Schiepers C, Lake R, et al. Clinical utility of 18F-fluoride PET/CT in benign and malignant bone diseases. Bone. 2012;50:128–139. doi: 10.1016/j.bone.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 7. [accessed April 11, 2014]; http://www.cancerpetregistry.org/status.htm.
- 8.Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med. 2010;51:1826–1829. doi: 10.2967/jnumed.110.077933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Even-Sapir E, Mishani E, Flusser G, et al. 18Ffluoride positron emission tomography and positron emission tomography/computed tomography. Semin Nucl Med. 2007;37:462–469. doi: 10.1053/j.semnuclmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Toegel S, Hoffmann O, Wadsak W, et al. Uptake of bone-seekers is solely associated with mineralization. A study with 99mTc-MDP, 153Sm-EDTMP and 18F-fluoride on osteoblasts. Eur J Nucl Med Mol Imaging. 2006;33:491–494. doi: 10.1007/s00259-005-0026-x. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins RA, Choi Y, Huang SC, et al. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–642. [PubMed] [Google Scholar]
- 12.Schiepers C, Nuyts J, Bormans G, et al. Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18 fluoride PET. J Nucl Med. 1997;38:1970–1976. [PubMed] [Google Scholar]
- 13.Schiepers C, Broos P, Miserez M, et al. Measurement of skeletal flow with positron emission tomography and 18F-fluoride in femoral head osteonecrosis. Arch Orthop Trauma Surg. 1998;118:131–135. doi: 10.1007/s004020050332. [DOI] [PubMed] [Google Scholar]
- 14.Blake GM, Park-Holohan SJ, Cook GJ, et al. Quantitative studies of bone with the use of 18F-fluoride and 99Tc-methylene diphosphonate. Semin Nucl Med. 2001;31:28–49. doi: 10.1053/snuc.2001.18742. [DOI] [PubMed] [Google Scholar]
- 15.Berding G, Burchert W, van den Hoff J, et al. Evaluation of the incorporation of bone grafts used in maxillofacial surgery with [18F]fluoride ion and dynamic positron emission tomography. Eur J Nucl Med. 1995;22:1133–1140. doi: 10.1007/BF00800595. [DOI] [PubMed] [Google Scholar]
- 16.Kurdziel KA, Shih JH, Apolo AB, et al. The kinetics and reproducibility of 18F-sodium fluoride for oncology using current PET camera technology. 2012;53:1175–1184. doi: 10.2967/jnumed.111.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segall G, Delbeke D, Stabin M, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scan 1. 0. J Nucl Med. 2010;51:1813–20. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 18.Ohnona J, Mchaud L, Balogova S, et al. Can we achieve a radionuclide radiation dose equal to or less than that of 99mTc-hydroxymethane diphosphonate bone scintigraphy with a low dose 18F-sodium fluoride time-of-flight PET of diagnostic quality. Nucl Med Commun. 2013;34:417–425. doi: 10.1097/MNM.0b013e32835fcd9d. [DOI] [PubMed] [Google Scholar]
- 19.Stauss J, Hahn K, Mann M, et al. Guidelines for pediatric bone scanning with 99mTc-labelled radiopharmaceuticals and 18F-fluoride. Eur J Nucl Med Mol Imaging. 2010;37:1621–1628. doi: 10.1007/s00259-010-1492-3. [DOI] [PubMed] [Google Scholar]
- 20.Drubach LA, Connolly SA, Palmer EL., 3rd skeletal scintigraphy with 18F-NaF PET for the evaluation of bone pain in children. AJR Am J Rontgenol. 2011;197:713–719. doi: 10.2214/AJR.11.6670. [DOI] [PubMed] [Google Scholar]
- 21.Drubach LA, Sapp MV, Laffin S, et al. Fluorine-18 NaF PET imaging of child abuse. Pediatr Radiol. 2008;38:776–779. doi: 10.1007/s00247-008-0885-y. [DOI] [PubMed] [Google Scholar]
- 22.Drubach LA, Johnston PR, Newton AW, et al. Skeletal trauma in child abuse: detection with 18F-NaF PET. Radiology. 2010;255:173–181. doi: 10.1148/radiol.09091368. [DOI] [PubMed] [Google Scholar]
- 23.Ovadia D, Metser U, Loevshitz G, et al. back pain in adolescents: assessment with integrated 18F-fluoride positron-emission tomography-computed tomography. J Peditar Orthop. 2007;27:90–93. doi: 10.1097/01.bpo.0000242438.11682.10. [DOI] [PubMed] [Google Scholar]
- 24.Lim R, Fahey FH, Drubach LA, et al. early experience with fluorine-18 sodium fluoride bone PET in young patients with back pain. J Pediatr Orthop. 2007;27:277–282. doi: 10.1097/BPO.0b013e31803409ba. [DOI] [PubMed] [Google Scholar]
- 25.Chen CJ, Ma SY. Prevalence of clinically significant extraoosseous findings on unenhanced CT portions of 18F-fluoride PET/CT bone scans. Scientific World Journal. 2012;2012:979867. doi: 10.1100/2012/979867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi N, Inaba Y, Choe H, et al. Use of F-18 fluoride PET to differentiate septic from aseptic loosening in total hip arthroplasty patients. Clin Nucl Med. 2011;36:e156–161. doi: 10.1097/RLU.0b013e3182291ae7. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi N, Inaba Y, Tateishi U, et al. New application of 18F-fluoride PET for the detection of bone remodeling in early stage osteoarthritis of the hip. Clin Nucl Med. 2013;38:e379–383. doi: 10.1097/RLU.0b013e31828d30c0. [DOI] [PubMed] [Google Scholar]
- 28.Ullmark G, Nilsson O, Maripuu E, et al. Analysis of bone mineralization on uncemented femoral stems by [18F]-fluoride-PET: a randomized clinical study of 16 hips in 8 patients. Acta Orthop. 2013;84:138–44. doi: 10.3109/17453674.2013.786632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JW, Lee SM, Kim SJ, et al. Clinical utility of fluoride-18positron emission tomography/CT in temporomandibular disorder with osteoarthritis: comparisons with 99mTc-MDP bone scan. Dentomaxillofac Radiol. 2013;42:29292350. doi: 10.1259/dmfr/29292350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frost ML, Moore AE, Siddique M, et al. 18F-fluoride PET as a noninvasive imaging biomarker for determining treatment efficacy of bone active agents at the hip: a prospective, randomized, controlled clinical study. J Bone Miner Res. 2013;28:1337–1347. doi: 10.1002/jbmr.1862. [DOI] [PubMed] [Google Scholar]
- 31.Fischer DR, Pfirrmann CW, Zubler V, et al. High bone turnover assessed by 18F-fluoride PET/CT in the spine and sacroiliac joints of patients with ankylosing spondylitis: comparison with inflammatory lesions detected by whole body MRI. EJNMMI Res. 2012;2:38. doi: 10.1186/2191-219X-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strobel K, Fischer DR, Tamborrini G, et al. 18F-fluoride PET/CT for detection of sacroilitis of ankylosing spondylitis. Eur J Nucl Med Mol Imaging. 2010;37:1760–1765. doi: 10.1007/s00259-010-1464-7. [DOI] [PubMed] [Google Scholar]
- 33.Draper CE, Quon A, Fredericson M, et al. Comparison of MRI and 18F-NaF PET/CT in patients with patellofemoral pain. J Magn Reson Imaging. 2012;36:928–932. doi: 10.1002/jmri.23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchida K, Nakajima H, Miyazaki T, et al. Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by 18F-fluoride PET. J Nucl Med. 2009;50:1808–1814. doi: 10.2967/jnumed.109.062570. [DOI] [PubMed] [Google Scholar]
- 35.Aratake M, Yoshifumi T, Takahashi A, et al. Evaluation of lesion in a spontaneous osteonecrosis of the knee using 18F-fluoride positron emission tomography. Knee Surg Sports Tramautol Arthrosc. 2009;17:53–59. doi: 10.1007/s00167-008-0641-8. [DOI] [PubMed] [Google Scholar]
- 36.Sterner T, Pink R, Freudenberg L, et al. The role of [18F]fluoride positron emission tomography in the early detection of aspetic loosening of total knee arthroplasty. Int J Surg. 2007;5:99–104. doi: 10.1016/j.ijsu.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Choe H, Inaba Y, Kobayashi N, et al. use of 18F-flluoride PET to determine the appropriate tissue sampling region for improved sensitivity of tissue examinations in cases of suspected periprosthetic infection after total hip arthroplasty. Acta Orthop. 2011;82:427–432. doi: 10.3109/17453674.2011.594232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piert M, Winter E, Becker GA, et al. Allogenic bone graft viability after hip revision arthroplasty assessed by dynamic [18F]fluoride ion positron emission tomography. Eur J Nucl Med. 1999;26:615–624. doi: 10.1007/s002590050429. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein P, Beuthien-Baumann B, Kotzerke J, et al. Periacetabular bone metabolism following hip revision surgery. PET-based evaluation of allograft osteointegration. Nuklearmedizin. 2014 doi: 10.3413/Nukmed-0607-13-06. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Muller S, Gosau M, Strobel D, et al. Assessment of bone microcirculation by contrast-enhanced ultrasound (CEUS) and 18F-positron emission tomography/computed tomography in free osseous and osseocutaneus flaps for mandibular reconstruction: preliminary results. Clin Hemorheol Microcirc. 2011;49:115–128. doi: 10.3233/CH-2011-1462. [DOI] [PubMed] [Google Scholar]
- 41.Brenner W, Vernon C, Conrad EU, et al. Assessment of the metabolic activity of bone grafts with (18F)F-fluoride PET. Eur J Nucl Med Mol Imaging. 2004;31:1291–1298. doi: 10.1007/s00259-004-1568-z. [DOI] [PubMed] [Google Scholar]
- 42.Berding G, Schliephake H, van den Hoff J, et al. Assessment of the incorporation of revascularized fibula grafts used for mandibular reconstruction with F-18-PET. Nuklearmedizin. 2001;40:51–58. [PubMed] [Google Scholar]
- 43.Quon A, Dodd R, Iagaru A, et al. Initial investigation of 18F-NaF PET/CT for identification of vertebral sites amenable to surgical revision after spinal fusion surgery. Eur J Nucl Med Mol Imaging. 2012;39:1737–1744. doi: 10.1007/s00259-012-2196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brans B, Weijers R, Halders S, et al. Assessment of bone graft incorporation by 18F-fluoride positron emission tomography/computed tomography in patients with persisting symptoms after posterior lumbar interbody fusion. EJNMMI Res. 2012;30:42. doi: 10.1186/2191-219X-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahoo MK, Karunanithi S, Bal CS. Pulmonary alveolar microlithiasis: imaging characteristics of planar and SPECT/CT bone scan versus 18F-FDG and 18F-sodium fluoride PET/CT scanning. Jpn J Radiol. 2013;31:766–769. doi: 10.1007/s11604-013-0250-4. [DOI] [PubMed] [Google Scholar]
- 46.Waterval JJ, Van Dongen TM, Stokroos RJ, et al. Bone metabolic activity in hyperostosis cranialis interna measured with 18F-fluoride PET. Eur J Nucl Med Mol Imaging. 2011;38:884–893. doi: 10.1007/s00259-010-1655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waterval JJ, Van Dongen TM, Stokroos RJ, et al. Bone metabolic activity in hyperostosis cranialis interna measured with 18F-fluoride PET. Eur J Nucl Med Mol Imaging. 2011;38:884–893. doi: 10.1007/s00259-010-1655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guggenberger R, Fischer DR, Metzler P, et al. Biphosphonate-induced osteonecrosis of the jaw: comparison of disease extent on contrast-enhanced MR imaging, [18F]fluoride PET/CT, and cone beam CT imaging. AJNR Am J Neuroradiol. 2013;34:1242–1247. doi: 10.3174/ajnr.A3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurata S, Tateishi U, Shizukuishi K, et al. Assessment of atherosclerosis in oncologic patients using 18F-fluoride PET/CT. Ann Nucl Med. 2013;27:481–486. doi: 10.1007/s12149-013-0706-8. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Dilsizian V. Integrated PET/CT imaging of vulnerable atherosclerotic plaques: microcalcification with sodium fluoride and inflammation with fluorodeoxyglucose. Curr Cardiol Rep. 2013;15:364. doi: 10.1007/s11886-013-0364-4. [DOI] [PubMed] [Google Scholar]
- 51.Dweck MR, Chow MW, Joshi NV, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 52.Derlin T, Toth Z, Papp L, et al. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, vascular calcification in atherosclerotic plaque: a dual-tracer PET/CT study. J Nucl Med. 2011;52:1020–1027. doi: 10.2967/jnumed.111.087452. [DOI] [PubMed] [Google Scholar]
- 53.Even-Sapir E, Metser U, Flusser G, et al. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride Pet and 18F-fluoride PET/CT. J Nucl Med. 2004;45:272–278. [PubMed] [Google Scholar]
- 54.Iagaru A, Mittra E, Mosci C, et al. Combined 18F-fluoride and 18F-FDG PET/CT scanning for evaluation of malignancy: results of an international multicenter trial. J Nucl Med. 2013;54:176–183. doi: 10.2967/jnumed.112.108803. [DOI] [PubMed] [Google Scholar]
- 55.Damle NA, Bal C, Bandopadhyaya GP, et al. The role of 18F-fluoride PET/CT in the detection of bone metastases in patients with breast, lung, prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol. 2013;31:262–269. doi: 10.1007/s11604-013-0179-7. [DOI] [PubMed] [Google Scholar]
- 56.Bortot DC, Amorim BJ, Oki GC, et al. 18F-fluoride PET/CT is highly effective for excluding bone metastases even in patients with equivocal bone scintigraphy. Eur J Nucl Med Mol Imaging. 2012;39:1730–1736. doi: 10.1007/s00259-012-2195-8. [DOI] [PubMed] [Google Scholar]
- 57.Withofs N, Grayet B, Tancredi T, et al. 18F-fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl Med Commun. 2011;32:168–176. doi: 10.1097/MNM.0b013e3283412ef5. [DOI] [PubMed] [Google Scholar]
- 58.Segall GM. PET/CT with sodium 18F-fluoride for management of patients with prostate cancer. J Nucl Med. 2014;55:531–533. doi: 10.2967/jnumed.113.133546. [DOI] [PubMed] [Google Scholar]
- 59.Cook GJ, Fogelman I. detection of bone metastases in cancer patients by 18F-fluoride and 18F-fluorodeoxyglucose positron emission tomography. Q J Nucl Med. 2001;45:47–52. [PubMed] [Google Scholar]
- 60.Shirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40:1623–1629. [PubMed] [Google Scholar]
- 61.Tateishi U, Morita S, Taguri M, et al. A meta-analysis of (18F)F-fluoride positron emission tomography for assessment of metastatic bone tumor. Ann Nucl Med. 2010;24:523–531. doi: 10.1007/s12149-010-0393-7. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Tafti BA, Shaba W, et al. Superscan pattern of F-18 sodium fluoride PET/CT study in a case of prostate cancer. Clin Nucl Med. 2011;36(11):1046–8. doi: 10.1097/RLU.0b013e3182291c3d. [DOI] [PubMed] [Google Scholar]
- 63.Wade AA, Scott JA, Kuter I, et al. Flare response in 18F-fluoride ion PET bone scanning. AJR Am J Rontgenol. 2006;186:1783–1786. doi: 10.2214/AJR.05.0225. [DOI] [PubMed] [Google Scholar]
- 64.Hoegerle S, Juengling F, Otte A, et al. Combined FDG and [F-18]fluoride whole-body PET: a feasible two-in-one approach to cancer imaging? Radiology. 1999;209:253–258. doi: 10.1148/radiology.209.1.9769840. [DOI] [PubMed] [Google Scholar]
- 65.Iagaru A, Mittra E, Yaghoubi SS, et al. Novel strategy for a cocktail 18F-fluoride and 18F-FDG PET/CT scan for evaluation of malignancy: results of the pilot-phase study. J Nucl Med. 2009;50:501–505. doi: 10.2967/jnumed.108.058339. [DOI] [PubMed] [Google Scholar]
- 66.Iagaru A, Mittra E, Dick DW, et al. Prospective evaluation f (99m)Tc MDP scintigraphy, (18F)F NaF PET/CT, and (18F)F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol. 2012;14:252–259. doi: 10.1007/s11307-011-0486-2. [DOI] [PubMed] [Google Scholar]
- 67.Iagaru A, Young P, Mittra E, et al. Pilot prospective evaluation of 99mTc-MDP scintigraphy, 18F NaF PET/CT, 18F-FDG PET/CT and whole-body MRI for detection of skeletal metastases. Clin Nucl Med. 2013;38:e290–296. doi: 10.1097/RLU.0b013e3182815f64. [DOI] [PubMed] [Google Scholar]
- 68.Chan SC, Wang HM, Ng SH, et al. utility of 18F-fluoride PET/CT and 18F-FDG PET/CT in the detection of boney metastases in heightened-risk head and neck cancer patients. J Nucl Med. 2012;53:1730–1735. doi: 10.2967/jnumed.112.104893. [DOI] [PubMed] [Google Scholar]
- 69.Ota N, Kato K, Iwano S, et al. Comparison of 18F-fluoride PET/CT, 18F-FDG PET/CT and bone scintigraphy (planar and SPECT) in detection of bone metastases of differentiated thyroid cancer: a pilot study. Br J Radiol. 2014;87:20130444. doi: 10.1259/bjr.20130444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schirrmeister H, Buck A, Guhlmann A, et al. Anatomical distribution and sclerotic activity of bone metastases from thyroid cancer assessed with F-18 sodium fluoride positron emission tomography. Thyroid. 2011;11:677–683. doi: 10.1089/105072501750362754. [DOI] [PubMed] [Google Scholar]
- 71.Schirrmeister H, Glatting G, Hetzel J, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and (18F)-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2011;42:1800–1804. [PubMed] [Google Scholar]
- 72.Petren-Mallmin M, Andreasson I, Ljunggren O, et al. Skeletal metastases from breast cancer: uptake of 18F-fluoride measured with positron emission tomography in correlation with CT. Skeletal Radiol. 1999;27:72–76. doi: 10.1007/s002560050340. [DOI] [PubMed] [Google Scholar]
- 73.Piccardo A, Altrinetti V, Bacigalupo L, et al. Detection of metastatic bone lesions in breast cancer patients: fused (18)F-Fluoride-PET/MDCT has higher accuracy than MDCT. Preliminary experience. Eur J Radiol. 2012;81:2632–8. doi: 10.1016/j.ejrad.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 74.Kurata S, Shizukuishi K, Tateishi U, et al. Age-related changes in pre- and postmenopausal women investigated with 18F-fluoride PET—a preliminary study. Skeletal Radiol. 2012;41:947–953. doi: 10.1007/s00256-011-1318-9. [DOI] [PubMed] [Google Scholar]
- 75.Yen RF, Chen CY, Cheng MF, et al. The diagnostic and prognostic effectiveness of F-18 sodium fluoride PET-CT in detecting bone metastases for hepatocellular carcinoma patients. Nucl Med Commun. 2010;31:637–645. doi: 10.1097/MNM.0b013e3283399120. [DOI] [PubMed] [Google Scholar]
- 76.Nishiyama Y, Tateishi U, Shizukuishi K, et al. Role of 18F-fluoride PET/CT in the assessment of multiple myeloma initial experience. Ann Nucl Med. 2013;27:78–83. doi: 10.1007/s12149-012-0647-7. [DOI] [PubMed] [Google Scholar]
- 77.Sachpekidid C, Goldschmidt H, Hose D, et al. PET/CT studies of multiple myeloma using 18F-FDG and 18F-NaF: comparisons of distribution patterns and tracers’ pharmacokinetics. Eur J Nucl Med Mol Imaging. 2014 doi: 10.1007/s00259-014-2721-y. Epub ahead of publication. [DOI] [PubMed] [Google Scholar]
- 78.Chakraborty D, Bhattacharya A, Mete UK, et al. Comparison of 18F-fluoride PET/CT and 99mTc-MDP bone scan in the detection of skeletal metastases in urinary bladder carcinoma. Clin Nucl Med. 2013;38:616–621. doi: 10.1097/RLU.0b013e31828da5cc. [DOI] [PubMed] [Google Scholar]
- 79.Roudier MP, Morrissey C, True LD, et al. Histopathological assessment of prostate cancer bone osteoblastic metastases. J Urol. 2008;180:1154–60. doi: 10.1016/j.juro.2008.04.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 81.Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastases in biochemical recurrence of prostate cancer. Clin Nucl Med. 2012;37:637–643. doi: 10.1097/RLU.0b013e318252d829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poulsen MH, Petersen H, Hoilund-Carlsen PF, et al. Spine metastases in prostate cancer: comparison of [99m Tc]MDP whole body bone scintigraphy, [18F]choline PET/CT, and [18F]NaF PET/CT. BJU Int. 2013 doi: 10.1111/bju.12599. Epub ahead of publication. [DOI] [PubMed] [Google Scholar]
- 83.Wondergem M, van der Zant FM, van der Ploeg T, et al. A literature review of 18F-fluoride PET/CT and 18F-choline or 11C-choline PET/CT for detection of bone metastases in patients with prostate cancer. Nucl Med Commun. 2013;34:935–945. doi: 10.1097/MNM.0b013e328364918a. [DOI] [PubMed] [Google Scholar]
- 84.Kjolhede H, Ahlgren G, Almquist H, et al. Combined 18F-fluorocholine and 18F-fluoride positron emission tomography/computed tomography imaging for staging of high-risk prostate cancer. BJU Int. 2012;110:1501–1516. doi: 10.1111/j.1464-410X.2012.11123.x. [DOI] [PubMed] [Google Scholar]
- 85.Langsteger W, Balogova S, Hachette V, et al. Fluor choline (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. Q J Nucl Med Mol Imaging. 2011;55:448–457. [PubMed] [Google Scholar]
- 86.Beheshti M, Vail R, Walden Berger P, et al. Detection of bone metastases in patients with prostate cancer by 18F-fluorocholine and 18F-fluoride PET/CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766–1774. doi: 10.1007/s00259-008-0788-z. [DOI] [PubMed] [Google Scholar]
- 87.Hillier BE, Siegel BA, Hanna L, et al. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the National Oncologic PET Registry. J Nucl Med. 2014 doi: 10.2967/jnumed.113.130005. Epub ahead of publication. [DOI] [PubMed] [Google Scholar]
- 88.Cook G, Jr, parker C, Chua S, et al. 18F-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with 223Rad-chloride (Alpharadin) EJNMMI Res. 2011;1:4. doi: 10.1186/2191-219X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]