Abstract
We report a novel drug delivery system composed of layer-by-layer (LBL) milk protein casein (CN) coated iron oxide nanoparticles. Doxorubicin (DOX) and indocyanine green (ICG) were selected as model drug molecules, which were incorporated into the inner polymeric layer, and subsequently coated with casein. The resulting casein coated iron oxide nanoparticles (CN-DOX/ICG-IO) were stable in the acidic gastric condition with the presence of gastric protease. On the other hand, the loaded drugs were released when the casein outer layer was gradually degraded by the intestinal protease in the simulated intestine condition. Such unique properties enable maintenance of the bioactivity of the drugs and thus enhance the drug delivery efficiency. Ex vivo experiments showed that the LBL CN-DOX-IO improved the translocation of DOX across microvilli and its absorption in the small intestine sacs. In vivo imaging of mice that were orally administered with these LBL CN-ICG-IO nanostructures further confirmed that the reported drug delivery vehicles could pass the stomach without significant degradation, and then accumulated in the small intestine. In addition, the magnetic iron oxide nanoparticle core offered an MRI contrast enhancing capability for in vivo imaging guided drug delivery. Therefore, the reported LBL CN-DOX/ICG-IO is a promising oral drug delivery nanoplatform, especially for drugs that are poorly soluble in water or degradable in the gastric environment.
1. Introduction
Oral delivery is considered an ideal drug administration route, because it not only avoids the discomfort and additional procedures associated with intravenous delivery injections, but also allows for delivery of non-water-soluble drugs.[1–3] However, oral delivery through organs in the gastrointestinal (GI) tract needs to overcome several obstacles, including: 1) the strong acidic gastric environment that reduces the drug stability and solubility; 2) the digestive enzymes that degrade drugs and decrease drug bioavailability; and 3) a mucus barrier that blocks drug penetration and subsequent tissue absorption.[4, 5] Even if a drug can be formulated for oral administration, it remains a challenge to deliver the drugs to a specific segment of the GI tract, such as the intestine, for maximal drug action. In clinical practice, certain drugs, for example those for treating Crohn’s disease, ulcerative colitis and chemotherapy medications, may need controlled release of the drug in the targeted areas or organs of the GI tract to increase the bioavailability and efficacy of the drug while reducing the toxicity to the normal organs and tissue.[6] The recent development of nanomaterial-based drug delivery systems has demonstrated improved oral delivery of various drug formulations by protecting drugs from premature degradation while releasing them in an organ specific and temporally controlled manner.[2, 4] For example, polymer micelle based nanocarrier enhanced the intestinal absorption of teniposide 4–6 fold.[7] Core shell corona lipoparticles improved insulin delivery in diabetic rats.[8]
Casein (CN) is a major protein ingredient in milk and can form micelle-like porous structures with the capacity of absorbing a large amount of small molecules, such as vitamins and minerals, for nutrient delivery.[9–16] It is an inexpensive and bio-safe natural product that has high amphiphilicity, good dispersibility and capability of rapid reconstitution in physiological media. Recently, we have developed a method to reconstruct casein proteins to cap magnetic iron oxide nanoparticles for cell targeted molecular imaging probes.[15] As a result, the casein coated iron oxide (CNIO) nanoparticles were found to be highly water soluble and stable in a wide range of pH (2.0–8.0) with substantial high relaxation properties and MRI contrast enhancing effect, due to the highly water permeable layer structures.[15]
Here we report the development of a layer-by-layer (LBL) construct composed of CN protected iron oxide nanoparticles loaded with an experimental hydrophobic drug, doxorubicin (DOX), for effective oral delivery of drug through the GI tract. This LBL CN-capped DOX-loaded iron oxide nanoparticle construct (CN-DOX-IO) has high stability in enzymatic and acidic conditions similar to the gastric environment. Meanwhile, it underwent enzyme-responsive release of payload drugs after the casein coating was enzymatically cleaved in conditions similar to the intestine. The uptake and cytotoxicity effect of LBL CN-DOX-IO were investigated through Caco-2 cells cultured as a confluent monolayer in vitro and mouse intestine tissues ex vivo. In vivo imaging of the mice fed with LBL nanoconstructs containing near infrared (NIR) indocyanine green (ICG) in place of DOX revealed that the developed LBL delivery vehicle is stable in the acidic conditions of the stomach, allowing improved delivery of drugs to the intestine.
2. Materials and Methods
2.1 Reagents and chemicals
All materials were used as received. Magnetic iron oxide nanoparticles (SHP-10), with an average core diameter of 10 nm and coated with amphiphilic triblock copolymer poly(maleic acid) and octadecene, were prepared as previously described.[17, 18] Doxorubicin (DOX) was purchased from Polymed Therapeutic INC, (Houston, TX). Pepsin powder, sodium dodecyl sulfate (SDS), N,N-dimethylformamide (DMF), and hydrochloric acid (HCl), dimethyl sulfoxide (DMSO) was obtained from Fisher Scientific (Rockford, IL). Dulbecco’s phosphate-buffered saline (PBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypsin (0.25%), fetal bovine serum (FBS) and L-glutamine were obtained from Mediatech INC (Herndon, VA). Casein (CN), indocyanine green (ICG), Dulbecco’s Modified Eagle’s Medium (DMEM), Penicillin-streptomycin solution, Trypsin-EDTA solution, Tritons X-100 and Hank’s balanced salt solution (HBSS) were purchased from Sigma (St. Louis, MO, USA).
2.2 Preparation of LBL casein coated IO nanoparticles loaded with doxorubicin
The process for preparing the layer-by-layer (LBL) assembled casein coated iron oxide nanoparticles loaded with drugs (DOX/ICG) (CN-DOX/ICG-IO) is illustrated in Scheme 1. The inner layer of amphiphilic polymer offered a coating layer for the IO nanoparticle core along with the space for embedding the hydrophobic small molecules DOX/ICG. CN fragments was then deposited and assembled into an outer layer that was stable in acidic conditions but can be broken down enzymatically.
Scheme 1.

Illustration of layer-by-layer assembled casein coated iron oxide nanoparticles loaded with drug (Doxorubicin/Indocyanine green).
Doxorubicin-loaded IO nanoparticles (DOX-IO)
SHP-IO nanoparticle suspension (1 mg/mL) was mixed with freshly prepared doxorubicin solution in methanol (1 mg/mL). The mixture was shaken and incubated for 2 h so that hydrophobic DOX could be incorporated into the hydrophobic layer of the amphiphilic coating polymer. Doxorubicin-loaded iron oxide nanoparticles (DOX-IO) were collected by centrifuging using the Ultra-4 centrifuge tube with a cut-off size of 100 kDa. Collected DOX-IO was rinsed several times with deionized water until no free DOX was detected in the rinsing solution.
Casein coated doxorubicin-loaded IO nanoparticles (CN-DOX-IO)
The outer layer of casein (CN) protein encapsulating DOX-IO was applied via the method modified from that reported previously.[15] Briefly, DOX-IO solution was mixed with CN solution at the weight ratio of Fe:CN=1:2. The mixture was kept at room temperature for 24 h to allow casein molecules to assemble on the surface of DOX-IO. Then a freshly prepared 0.4% glutaraldehyde solution was added to crosslink the casein molecules to form an outer layer on the surface of DOX-IO. After 2 h, the product of casein coated doxorubicin-loaded iron oxide nanoparticles (CN-DOX-IO) was collected by centrifuging using the Ultra-4 centrifuge tube with a cut-off size of 100 kDa, and washed with deionized water three times.
2.3 Characterizations of DOX-IO and CN-DOX-IO nanoparticles
The core diameters, hydrodynamic sizes and zeta potentials of the prepared DOX-IO and CN-DOX-IO were determined by transmission electron microscope (TEM, HitachiH-7500, accelerating voltage 75 kV) and dynamic light scattering (DLS, Malvern Zeta Sizer Nano S-90) instrument, respectively. Gel electrophoresis was performed to confirm the presence of casein coating in the CN-DOX-IO using 2% agarose gel. The percentage of DOX loaded on IONPs was determined by the weight ratio of loaded DOX to Fe. Samples were dissolved in 1 M HCl, and sonicated for 30 min, and then measured for the fluorescence intensity from DOX at 590 nm (λex=485 nm) with a microplate reader (Synergy 2 Multi-Mode Microplate Reader, BioTek, USA) to determine the amount of loaded DOX. The Fe concentration was determined by the phenanthroline-colorimetric method.[15]
2.4 Stability of CN-DOX-IO at different pH and enzymatic conditions mimicking gastric and small intestine
The stabilities of CN-DOX-IO at different pH and enzymatic conditions that mimic gastric and small intestinal environments were investigated with DOX-IO as the control. To test pH stability, the pH values of nanoparticle solutions were adjusted from 1.0 to 8.0 by titrating 1 M NaOH or HCl under rapid stirring. The pH adjusted nanoparticle solutions were then incubated for 30 min, followed by measuring the change of hydrodynamic sizes by DLS to determine if aggregation took place. Furthermore, the stability of CN-DOX-IO under gastric enzymatic digestion condition was performed by incubating nanoparticles with the gastric protease pepsin (0.1 and 1.5 mg/mL) at pH 2.0, 37 °C. The ratios of CN-DOX-IO:pepsin used in the experiments were 1:150 and 1:10 (w:w), respectively. After being incubated for 2 h, the proteolytic reaction was terminated by adjusting the pH back to 7.0 with 1 M NaHCO3. The hydrodynamic sizes of CN-DOX-IO were then measured by DLS and compared before and after being treated in gastric mimicking conditions. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to determine the presence and relative amount of the proteolyzed CN fragments in CN-DOX-IO solutions at different conditions. The stability studies of CN-DOX-IO in digestion conditions mimicking small intestine was performed similarly, except that the pH value was adjusted to pH 7.0, and pepsin was substituted by the intestine protease trypsin (0.5 and 2.5 mg/mL).
2.5 Enzymatic responsive drug release under pH conditions mimicking gastric and small intestine
We expect that DOX loaded in the inner layer of LBL CN-DOX-IO is protected by the casein outer layer from release unless the casein layer is disassembled. To study the release profile of DOX in the different pH conditions, drug loaded nanoparticle suspensions, i.e., DOX-IO or CN-DOX-IO, at a concentration equivalent to 5 μg/mL of free DOX (or 8.6 μM) were diluted to pH 2.0, 5.5 and 7.4 with deionized water. At different time points, the mixtures were centrifuged with ultra-4 centrifuge tubes (cut-off size 30 kDa), leaving DOX released from nanocarriers in the supernatant. The DOX content in the supernatant was then determined by measuring the fluorescence intensity (λem=590 nm) with a microplate reader and calibrated against a standard curve. For the DOX release profile under digestive conditions mimicking the gastric or small intestinal environment, drug loaded nanoparticle suspensions were incubated with the gastric protease pepsin (1.0 mg/mL, pH 2.0) solution for 2 h, followed by incubation with the intestine protease trypsin (2.5 mg/mL, pH 7.0) solution for another 6 h. The concentrations of released DOX in solutions were determined using the aforementioned measurement.
2.6 In vitro investigation of uptake of CN-DOX-IO by Caco-2 cell monolayer
The Caco-2 monolayer is widely used as an in vitro model of the human small intestinal mucosa to predict the absorption of orally administered drugs.[5, 19, 20] Caco-2 cells, purchased from the American Type Culture Collection (ATCC), were cultured following the vendor’s protocol. Cells were seeded in 8-well chamber slides and allowed to form a confluent monolayer. DOX-IO, CN-DOX-IO, and free DOX solutions were added into each well at a concentration of 17.2 μM DOX in Hank’s Solution. At each time point of incubation (i.e. 10, 30, or 60 min), the supernatant was removed, and cells were washed with ice-cold PBS and fixed with 4% paraformalin. 4′,6-diamidino-2-phenylindole (DAPI) staining for cell nucleus was then applied. The uptake of free DOX and DOX loaded nanoparticles by Caco-2 cells and their intracellular localization were investigated under a fluorescent microscope.
2.7 Ex vivo investigation of uptake of CN-DOX-IO by small intestine tissue
Nude mice (8 weeks old and weighed 16–17 g, N=3) were sacrificed, and the entire small intestine was immediately excised and flushed three times with PBS at room temperature. After the muscularis was removed, the intestine tissue sample was placed in warm (37°C) Tyrode’s solution.[21] A section of 2–3 cm of the jejunum was isolated and cultured with fresh oxygenated Tyrode’s solution containing CN-DOX-IO at 37 °C. DOX-IO and free DOX were also tested as controls. After 1 h, the sacs were washed with saline (0.9% NaCl solution), and placed into OCT compound (Sakura Finetek Europe BV, The Netherlands) to prepare frozen sections. The obtained small intestine mucosa tissue sections were processed with Prussian blue staining to determine the presence of iron containing nanocarriers in the tissue.[15]
2.8 In vivo imaging of oral administration of LBL CN-ICG-IO in mice
We used optical imaging to investigate the stability and distribution of the developed LBL nanoconstructs in the GI tract of live mice. In this case, DOX was substituted with near infrared (NIR) fluorescent indocyanine green (ICG) for in vivo NIR optical imaging.[22] ICG is a hydrophobic molecule similar to DOX, thus it can be incorporated into the amphiphilic polymer layer of IONP just as DOX The mice (N = 3/group) were fasted for 12 h and orally administered with CN-ICG-IO or ICG-IO at the dosage of 10 mg Fe/kg. NIR images were collected for the mice before and 3, 5, 7 h after administration of CN-ICG-IO by a Kodak FX in vivo imaging system (Carestream Health, Inc, New Haven, CT), using a 720 nm excitation and 790 nm emission filter set with a 3-minute exposure time. Mice were anesthetized by i.p. injection of a ketamine:xylazine mixture (95:5 mg/kg) during the imaging experiments. For each NIR image, a corresponding X-ray image was taken to provide a general anatomic overlay.
For in vivo MRI, images were obtained before and 3 h after oral administration on a 3 T MRI scanner (Tim Trio, Siemens, Erlangen, Germany) using a T2-weighted fast spin echo sequence. The parameter was TR = 3600 ms, TE = 86 ms, flip angle = 150, image matrix = 154 × 320, FOV = 70 × 120 mm2, slice thickness = 1 mm and number of averages = 3. In addition to confirming the MRI contrast enhancing effect of administered CN-DOX-IO construct, MRI offers clear soft tissue contrast and three dimensional anatomic details, thus allowing for identification of exact locations of organs in the mouse GI tracts as anatomic references for NIR imaging.
3. Results
3.1 Formation of LBL CN-DOX-IO nanoparticles
Figure 1A–C show TEM images of SHP-IO, DOX-IO and LBL CN-DOX-IO nanoparticles. The average diameters of the IO nanoparticle cores, measured from 100 individual nanoparticles in the TEM images, are calculated to be: 10.1±0.6, 10.4±0.5, and 10.4±0.6 for SHP-IO, DOX-IO and CN-DOX-IO, respectively. This indicates the core size of IO nanoparticles remained unchanged after DOX loading in the inner polymeric layer and subsequent assembly of the casein outer layer. The formation of LBL CN-DOX-IO was confirmed by the increased hydrodynamic sizes with increasing coating layers. After coating with amphiphilic polymer, hydrophobic IO nanoparticles were transferred into aqueous solution, and consequently the hydrodynamic size increased from 10.1 nm (IO, data not shown) to 18.8 nm (SHP-IO). However, the hydrodynamic size of DOX-IO (17.7±3.8 nm) was slightly smaller compared with that of SHP-IO, which was attributed to the contraction of the polymer coating when DOX was absorbed in the methanol/water mixture. The hydrodynamic sizes increased to 24.4±4.67 nm (CN-DOX-IO) after applying the outer layer of CN (Figure 1E). Furthermore, gel electrophoresis demonstrated a higher molecular weight and lower mobility of LBL CN-DOX-IO comparing to that of DOX-IO and SHP-IO (Figure 1F). It is notable that DOX-IO showed the highest mobility in electrophoresis because of its smallest size. Subsequently staining the gel with GelCode Blue evidenced a marked blue band in CN-DOX-IO, indicating the presence of protein coating on CN-DOX-IO. In contrast, no such band was observed for SHP-IO and DOX-IO (Figure 1F). The UV-Vis absorption spectra of both DOX-IO and CN-DOX-IO revealed the characteristic peak of DOX at 495 nm, as shown in Figure 1G, representing that DOX was successfully loaded on DOX-IO and remained in the LBL CN-DOX-IO structure when the casein outer layer formed. All three types of nanoparticles exhibited excellent water solubility and were stable in water over months of storage without aggregation/precipitation (Figure 1D).
Figure 1.

Representative TEM images of SHP-IO (A), DOX-IO (B), CN-DOX-IO (C); Pictures of aqueous solutions of SHP-IO, DOX-IO, CN-DOX-IO (D); DLS profiles of size distribution of SHP-IO, DOX-IO, CN-DOX-IO (E). Gel electrophoresis of SHP-IO, DOX-IO, CN-DOX-IO (upper), and corresponding GelCode Blue staining for the presence of proteins (F); (G) UV-Vis absorption spectra of SHP-IO, DOX-IO and CN-DOX-IO with distinctive peak of DOX indicated.
3.2 Stabilities of CN-DOX-IO at low pH and against gastric and small intestine enzymes
Selectively delivering drugs to the intestine requires conquering the gastric acidic and enzymatic conditions that may prematurely release, degrade and deactivate the drugs. We found that the prepared LBL CN-DOX-IO was stable over the pH range of 2.0–8.0. DLS measured hydrodynamic sizes of CN-DOX-IO (~30 nm) remained unchanged over this pH range (Figures 2A) except at the isoelectric point of pH 4.0, in which the hydrodynamic sizes increased to 100 nm, similar to the previously reported iron oxide nanoparticles coated with casein (CNIO).[15] At the isoelectric point of pH 4.0, monodispersed CN-DOX-IO formed reversible clusters, but not precipitation, the same as we observed in CNIO.[15] This reversible aggregation returned to the single dispersed form (with hydrodynamic size of 30 nm) by adjusting pH to lower than the isoelectric point, due to the presence of abundant positive/negative charged functional groups in CN. At pH 2.0, which is close to the pH condition of the stomach fluid, CN-DOX-IO remained single dispersed with a hydrodynamic size of 30 nm for more than 24 h. On the contrary, DOX-IO without the protective casein outer layer precipitated when pH changed to 2.0 and below, evidenced by the drastic increase of the hydrodynamic size (Figure 2B).
Figure 2.
Changes of the hydrodynamic size at different pH of CN-DOX-IO (A) and DOX-IO (B) nanoparticles measured by DLS. SDS-PAGE analysis of digested protein fragments from the coating layer of CN-DOX-IO after treated with gastric enzyme pepsin at pH 2.0 (C) and small intestine enzyme trypsin at pH 7.0 (D). Changes of hydrodynamic sizes of CN-DOX-IO after treated with pepsin at pH 2.0 (E) and trypsin at pH 7.0 (F) at different enzyme concentrations.
To examine the stability of the casein outer layer in CN-DOX-IO against digestive enzymes in stomach and its enzymatic-responsive degradation by the digestive enzymes in the small intestine, CN-DOX-IO was treated with pepsin, which is the protease in the gastric juice that breaks down protein to peptides, and trypsin, which is a duodenum secreted protease that degrades protein or peptides, at different enzyme concentrations at pH 2.0 (for pepsin) and 7.0 (for trypsin). SDS-PAGE gel electrophoresis was used to examine the protein or peptides digested by the enzymes. We found that the band of CN in SDS-PAGE gel was still observed (Figure 2C) after treatment at pH 2.0 with pepsin (0.1 and 1.5 mg/mL), which represented the physiological enzyme range of human gastric fluid (0.3–1.3 mg pepsin/mL). DLS measurements of CN-DOX-IO treated with pepsin at pH 2.0 also validated the stability of the reported LBL nanostructure in the mimicked gastric digestive system. CN-DOX-IO showed persistent dispersibility with unvaried hydrodynamic size after treated with pepsin (Figure 2E). On the other hand, SDS-PAGE showed different bands when treating CN-DOX-IO with trypsin at pH 7.0 (Figure 2D). The amount of intact CN in DOX-CN-IO decreased while short peptides and fragments of CN emerged in the SDS-PAGE gel. The size of newly appearing peptide fragments became smaller (lower molecular weight in SDS-PAGE gel) as the trypsin concentration further increased. DLS measurement showed a decrease in hydrodynamic size of CN-DOX-IO after treated with trypsin (Figure 2F), which further confirmed the breakdown of the casein outer layer. These results suggest that LBL CN-DOX-IO nanoparticles can remain intact under the acidic gastric condition with a protective casein outer layer resistant to the low pH and pepsin. In addition, the casein outer layer can be disassembled by the intestine protease trypsin at pH 7.0, thus exposing the inner layer of amphiphilic polymer, which is loaded with the hydrophobic drug (DOX). Therefore, this LBL delivery vehicle is suitable for oral drug delivery through the GI tract to the intestine.
3.3 Enzymatic responsive release of doxorubicin from CN-DOX-IO
Figure 3A shows the profiles of DOX released from the CN-DOX-IO nanoparticles and those from DOX-IO at pH 2.0, 5.5 and 7.4 without the presence of either pepsin or trypsin. Both of the DOX loaded nanoparticles exhibited very low release of DOX at pH 5.5 and 7.4, compared to that at pH 2.0. The increased release of DOX at lower pH (2.0) is caused by the protonated DOX, which has a higher solubility in water.[23, 24] Notably, CN-DOX-IO exhibited a slower and sustained release of DOX compared with DOX-IO at pH 2.0, referring to the effective protection of DOX by the casein outer layer. When cultured in the medium simulating gastric juice (pH 2.0, [Pep]=1.0 mg/mL), the initial rapid release of the encapsulated DOX in DOX-IO (62%) was significantly reduced to about 40% when casein outer layer was applied (Figure 3B). The results implied that the embedded DOX in the inner amphiphilic polymer layer in DOX-IO was shielded by the pH- and pepsin-stable casein outer layer. Alternatively, both DOX-IO and CN-DOX-IO showed sustained release of DOX in the simulated intestinal juice (pH 7.0, [Try]=2.5 mg/mL). As observed, DOX was released from CN-DOX-IO more effectively (~30% in 6 h) in the medium simulating intestinal juice than from DOX-IO (~15% in 6 h). The LBL CN-DOX-IO demonstrated a preferential and enzymatic-responsive drug release property by preventing the loss of drug in the acidic stomach, thus improving the efficacy of drug delivery to the small intestine.
Figure 3.

Comparison of release profiles of DOX from CN-DOX-IO and DOX-IO at different pH (A), in the simulated gastric juice (pH 2.0, [Pep]=1.0 mg/mL) and simulated intestinal juice (pH 7.0, [Try]=2.5 mg/mL) (B).
3.4 Uptake of CN-DOX-IO by Caco-2 cell monolayer
To investigate the cellular uptake of the released DOX from CN-DOX-IO by the small intestine in vitro, the monolayer of Caco-2 cells was incubated with CN-DOX-IO at a dosage of 17.2 μM for different lengths of time (10, 30 and 60 min). For comparison, cells incubated with DOX-IO and DOX were examined as controls. The intracellular accumulation of DOX was examined by the fluorescent microscope utilizing the fluorescence of DOX (Figure 4). After 10 min incubation, fluorescence signal from DOX was observed in the nucleus only in the cells treated with free DOX, owing to the high permeability of the small molecules (i.e. DOX), which facilitated rapid influx into the nucleus. For the cells treated with DOX-IO and CN-DOX-IO, the fluorescence signal of DOX was localized mostly in the cytoplasm instead of the nucleus after 10 min incubation, implying the nanocarriers were uptake through the endocytic process.[25–27] However, at 30 minutes after incubation, a small amount of DOX was observed to accumulate in the nucleus, which was ascribed to the slow release of DOX from DOX-IO and CN-DOX-IO,. After 60 min incubation, DOX was evidenced in both cytoplasm and nucleus of the cells treated with DOX-IO and CN-DOX-IO, demonstrating that the sustained release of DOX from the nanoparticles enabled the continuous accumulation of DOX in the nucleus.
Figure 4.
Representative fluorescence images collected from the Caco-2 cells incubated with free DOX, DOX-IO, CN-DOX-IO for 10, 30, and 60 min, showing the time dependent uptake of DOX-IO and CN-DOX-IO in the cytoplasm (pointed out by the white arrow heads), and the released DOX accumulated in the nucleus. Nuclei were stained for blue using DAPI. Right panel is the representative corresponding intensity profile analysis for each sample.
3.5 Uptake of CN-DOX-IO by small intestine tissue samples
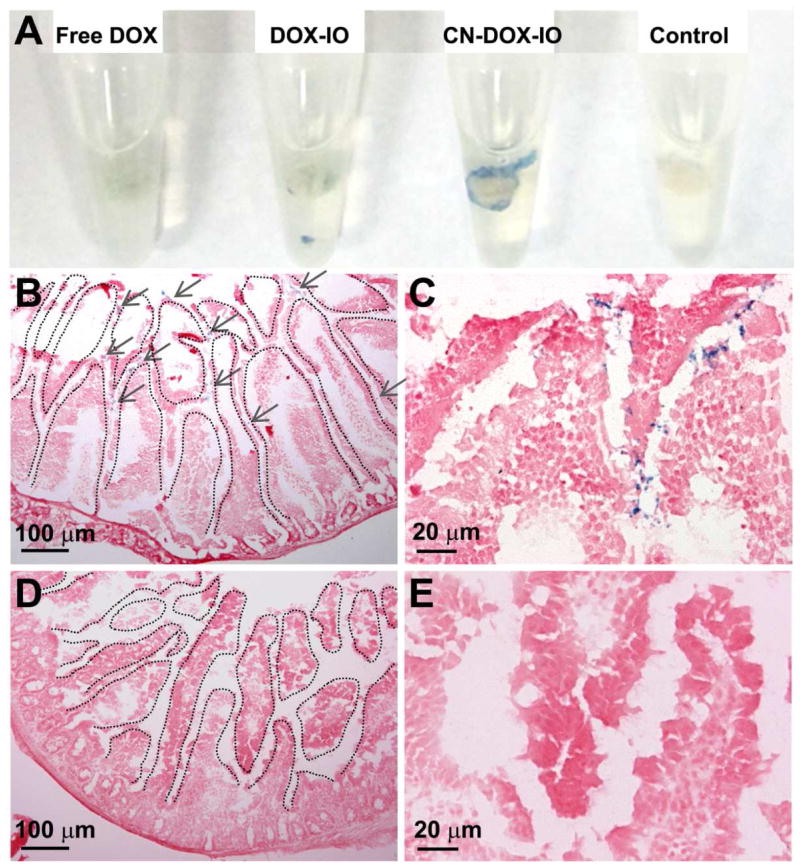
To determine the uptake and permeability of CN-DOX-IO in the small intestine, the jejunum villi of the mouse small intestine was sectioned and incubated with CN-DOX-IO and DOX-IO, respectively, for 1 h. Prussian blue staining for iron revealed the distribution of CN-DOX-IO in the villi of the small intestine as shown in Figure 5A–C. Intense blue staining was observed in the sacs treated with CN-DOX-IO, in comparison with that of the DOX-IO treated samples (Figure 5D–F). The result suggested that the casein outer layer may facilitate the interaction of nanoparticles with villi to increase the tissue uptake, subsequently enhancing the effective plasma concentration.
Figure 5.
Prussian blue staining for iron showed the uptake of CN-DOX-IO in intestine (A) and sections of small intestine tissue (B, C) comparing to DOX-IO treated ones (D, E).
3.6 Distribution and stability of CN-ICG-IO in mice observed with optical imaging
To study the stability and organ distribution profiles of the developed LBL drug delivery carriers in the GI tract in vivo, we used noninvasive NIR optical imaging to investigate mice fed with nanoparticles. CN-ICG-IO, in which DOX was substituted with indocyanine green (ICG), was orally administered to mice (N=3). ICG-IO was used as the control. Mouse stomach and intestine could be identified from the MRI images (Figure 6). The accumulation of magnetic nanoparticles was confirmed by the change of MRI contrast, i.e., the almost complete loss of signal or darkening in the T2-weighted MR images, after oral administration of nanoparticles. At 3 h after oral administration, the NIR signal of ICG was found mostly in the intestine of the mice administered with CN-ICG-IO. However, the signal was mainly observed in the area of the stomach for the ICG-IO treated group (Figure 6), revealing the release of ICG from ICG-IO in the stomach. At 5 h after administration, NIR imaging showed that CN-ICG-IO reached into the ileum and spread further in the intestine. However, the NIR signal in the stomach was still highest in the animals treated with ICG-IO, while only a slight increase of NIR signal was observed in the small intestine. For the ICG-IO treated group, the signal in the stomach remained even up to 7 hours, for the reason that the precipitation of ICG-IO formed and deposited in the crypts of gastric pits (Figure S1). The results from noninvasive NIR imaging thus further support that CN-ICG-IO could sustain in acidic gastric conditions, allowing for preferential delivery of the payload drugs to the small intestine. In addition, MRI contrast generated by iron oxide nanoparticles potentially enables the monitoring of drug delivery by MRI (Figure 7).
Figure 6.

Representative NIR images of mice feed with CN-ICG-IO, ICG-IO, showing the failure of ICG-IO delivering to small intestine, due to the lack of duration in stomach, thus trapped and release ICG in the stomach. The stomach was pointed out by yellow arrow heads. On the left, an average intensity Z-stack of MR image was supplied to show the location of stomach (outlined with dash-dot line and pointed out by yellow arrow head).
Figure 7.

Representative MR images of mice before and after oral administration of CN-ICG-IO. Severe signal distort (pointed out by red arrow head) was observed in small intestine due to the high concentration of magnetic nanoparticles delivered.
4. Discussions
Polymer and liposome-based nanoplatforms have been explored for the effective oral delivery of therapeutic agents, including proteins,[8, 28] nucleic acids,[29] and insoluble drugs.[30, 31] The LBL structured nanocarriers presented in this work provide a potential approach for preferential drug delivery to the intestine, with good stability in the low pH stomach fluid and enhanced mucosal/membrane penetration, mainly attributed to the casein coating of the LBL structure. Early studies have revealed that the layered coating structure could protect the drug more efficiently to realize the sustained release.[32] Meanwhile, most layered structures were microsized or had positive surface charge, which were unstable in the stomach, thus not suitable for the intestinal-specific drug delivery. In this study, the LBL-CN-DOX-IO nanoparticles showed a significant reduction of the initial rapid release of DOX from the amphiphilic inner polymer layer in the low pH conditions of the stomach fluid. Therefore, a higher amount of payload drug was retained for delivery and release in the intestine.
In addition to the protective function of the outer casein layer, casein has been reported to enhance the cellular uptake by penetrating the plasma membrane in an energy-independent fashion.[33] Indeed, significant enhanced permeability of LBL CN-DOX-IO was observed in the ex vivo experiment using small intestine sacs treated with different nanoconstructs, in which we observed more LBL CN-DOX-IO delivered deeply into the villi pits compared to DOX-IO without the casein outer layer. Although the mechanism by which casein improved the cell uptake and tissue penetration remained unclear, the LBL CN-DOX-IO likely has the ability to penetrate the mucus, which is one of the major obstacles for intestinal drug delivery. This enhanced absorption in intestinal villi was further confirmed by the histological analysis with Prussian blue staining. Obvious blue dots stained by iron could be observed in the intestinal villi 3 h after oral administration of the LBL construct, but not in those treated with DOX-IO (Figure S1).
In vivo monitoring of drug delivery with non-invasive imaging has become a desirable tool for planning and evaluating the therapeutic strategy as well as optimizing individualized treatments. Most development and investigation of the intestinal drug delivery systems are dependent on the in vitro assessment/evaluation with conventional methods, such as cellular uptake[19] and mucus penetration.[34] Drug delivery systems combined with imaging probes for imaging strategies, such as QDs,[35] radioactive moieties[36, 37] and NIR dyes,[38] have recently been developed for image-guided assessment. In this study, we use magnetic nanoparticles as a core for the LBL structure, which provides a template for the LBL assembly and offers MRI contrast enhancement. As a result, the LBL nanoconstruct is expected to be used for MRI monitoring of drug delivery (Figure 7). Furthermore, magnetic iron oxide nanoparticles present potential capabilities of thermal-induced drug release and magnetic localization to improve the drug delivery with the reported LBL drug delivery system in the future exploration studies..
Lastly, although the utilization of DOX and ICG as model drug molecules was similar to many other earlier studies, we anticipate that hydrophobic drugs more relevant to specific diseases can be applied with the reported LBL construct.
5. Conclusion
A novel pH stable and enzymatic-responsive oral drug delivery nanoparticle system has been developed via a layer-by-layer design, using modified milk protein casein to form an outer layer that protected the hydrophobic drug loaded in the inner polymer coating layer which caps the magnetic iron oxide nanoparticle core. The casein outer layer is resistant to degradation by protease pepsin at low pH under gastric conditions, and can be disassembled by the small intestine enzyme trypsin at neutral pH. Therefore, small intestine targeted drug delivery can be achieved by reducing the pre-mature drug release in the acidic stomach and then conducting the enzymatic-responsive release in the small intestine. Furthermore, this nanoconstruct retains good MRI contrast enhancing effect, providing the potential capability of MRI monitored and/or magnetic directed drug delivery. Given high water solubility, pH stability and enzymatic responsiveness as well as excellent biocompatibility, the reported LBL CN-DOX-IO is a promising “smart” drug delivery system for oral delivery of hydrophobic drugs, capable of “bypassing” low stomach pH and enabling absorption in the lower GI tract with neutral pH.
Supplementary Material
Representative Prussian blue staining of GI tract sections from mice orally administrated with CN-ICG-IO and ICG-IO. The blue dots represent IONPs (pointed out in grey arrows) showed the delivery and absorption of CN-ICG-IO in small intestine, compared with the trap of ICG-IO in stomach due to the poor stability in gastric fluid.
Acknowledgments
This work is supported in part by NIH R01CA154846-02 (HM and LY). NCI’s Cancer Nanotechnology Platform Project (CNPP) grant U01CA151810-02 (HM and LY), and a seed grant from Center for Pediatric Nanomedicine of Children’s Healthcare of Atlanta (HM and TM). We thank Ms. Donna Kilcullen and Jessica Paulishen for their assistance in preparation and revision of the manuscript.
Abbreviations
- CN
casein
- CNIO
casein coated iron oxide nanoparticles
- CN-DOX-IO
casein coated doxorubicin-loaded iron oxide nanoparticles
- DAPI
4′,6-diamidino-2-phenylindole
- DLS
dynamic light scattering
- DOX
doxorubicin
- GI
gastrointestinal
- ICG
indocyanine green
- IONP
iron oxide nanoparticle
- LBL
layer-by-layer
- MRI
magnetic resonance imaging
- NIR
near infrared
- TEM
transmission electron microscope
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mei L, Zhang Z, Zhao L, Huang L, Yang X-L, Tang J, et al. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Delivery Rev. 2013;65:880–90. doi: 10.1016/j.addr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Leong KW, Sung HW. Nanoparticle- and biomaterials-mediated oral delivery for drug, gene, and immunotherapy Preface. Adv Drug Delivery Rev. 2013;65:757–8. doi: 10.1016/j.addr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Feng SS, Zhao L, Tang J. Nanomedicine for oral chemotherapy. Nanomedicine. 2011;6:407–10. doi: 10.2217/nnm.11.7. [DOI] [PubMed] [Google Scholar]
- 4.Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: Challenges and opportunities. J Controlled Release. 2013;170:15–40. doi: 10.1016/j.jconrel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Gamboa JM, Leong KW. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv Drug Delivery Rev. 2013;65:800–10. doi: 10.1016/j.addr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lautenschlaeger C, Schmidt C, Fischer D, Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Delivery Rev. 2014;71:58–76. doi: 10.1016/j.addr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Ma L, Jiang S, Liu Z, Huang J, Chen L, et al. A self-assembled nanocarrier loading teniposide improves the oral delivery and drug concentration in tumor. J Controlled Release. 2013;166:30–7. doi: 10.1016/j.jconrel.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Guo S, Zhu C, Zhu Q, Gan Y, Rantanen J, et al. Intestinal mucosa permeability following oral insulin delivery using core shell corona nanolipoparticles. Biomaterials. 2013;34:9678–87. doi: 10.1016/j.biomaterials.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Semo E, Kesselman E, Danino D, Livney YD. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocolloids. 2007;21:936–42. [Google Scholar]
- 10.Mohan MS, Jurat-Fuentes JL, Harte F. Binding of vitamin A by casein micelles in commercial skim milk. J Dairy Sci. 2013;96:790–8. doi: 10.3168/jds.2012-5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livney YD. Milk proteins as vehicles for bioactives. Curr Opin Colloid Interface Sci. 2010;15:73–83. [Google Scholar]
- 12.Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. J Controlled Release. 2012;161:38–49. doi: 10.1016/j.jconrel.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Elzoghby AO, El-Fotoh WSA, Elgindy NA. Casein-based formulations as promising controlled release drug delivery systems. J Controlled Release. 2011;153:206–16. doi: 10.1016/j.jconrel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Bachar M, Mandelbaum A, Portnaya I, Perlstein H, Even-Chen S, Barenholz Y, et al. Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled beta-casein micelles. J Controlled Release. 2012;160:164–71. doi: 10.1016/j.jconrel.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Wang L, Lin R, Wang AY, Yang L, Kuang M, et al. Casein-Coated Iron Oxide Nanoparticles for High MRI Contrast Enhancement and Efficient Cell Targeting. ACS Appl Mater Interfaces. 2013;5:4632–9. doi: 10.1021/am400713j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapira A, Davidson I, Avni N, Assaraf YG, Livney YD. beta-Casein nanoparticle-based oral drug delivery system for potential treatment of gastric carcinoma: Stability, target-activated release and cytotoxicity. Eur J Pharm Biopharm. 2012;80:298–305. doi: 10.1016/j.ejpb.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Chen K, Xie J, Xu H, Behera D, Michalski MH, Biswal S, et al. Triblock copolymer coated iron oxide nanoparticle conjugate for tumor integrin targeting. Biomaterials. 2009;30:6912–9. doi: 10.1016/j.biomaterials.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan H, Kuang M, Wang X, Wang YA, Mao H, Nie S. Reexamining the effects of particle size and surface chemistry on the magnetic properties of iron oxide nanocrystals: New insights into spin disorder and proton relaxivity. J Phys Chem C. 2008;112:8127–31. [Google Scholar]
- 19.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26:2713–22. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 20.Kenzaoui BH, Vila MR, Miquel JM, Cengelli F, Juillerat-Jeanneret L. Evaluation of uptake and transport of cationic and anionic ultrasmall iron oxide nanoparticles by human colon cells. Int J Nanomedicine. 2012;7:1275–86. doi: 10.2147/IJN.S26865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park MY, Kwon HJ, Sung MK. Intestinal absorption of aloin, aloe-emodin, and aloesin; A comparative study using two in vitro absorption models. Nutr Res Pract. 2009;3:9–14. doi: 10.4162/nrp.2009.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillman EMC, Moore A. All-optical anatomical co-registration for molecular imaging of small animals using dynamic contrast. Nature Photon. 2007;1:526–30. doi: 10.1038/nphoton.2007.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Sun X, Nakayama-Ratchford N, Dai H. Supramolecular chemistry on water-soluble carbon nanotubes for drug loading and delivery. ACS Nano. 2007;1:50–6. doi: 10.1021/nn700040t. [DOI] [PubMed] [Google Scholar]
- 24.Jin YH, Hu HY, Qiao MX, Zhu J, Qi JW, Hu CJ, et al. pH-sensitive chitosan-derived nanoparticles as doxorubicin carriers for effective anti-tumor activity: preparation and in vitro evaluation. Colloids Surf, B. 2012;94:184–91. doi: 10.1016/j.colsurfb.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 2000;6:1949–57. [PubMed] [Google Scholar]
- 26.Ren D, Kratz F, Wang S-W. Protein Nanocapsules Containing Doxorubicin as a pH-Responsive Delivery System. Small. 2011;7:1051–60. doi: 10.1002/smll.201002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Upadhyay KK, Bhatt AN, Mishra AK, Dwarakanath BS, Jain S, Schatz C, et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(gamma-benzyl L-glutamate)-b-hyaluronan polymersomes. Biomaterials. 2010;31:2882–92. doi: 10.1016/j.biomaterials.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi K, Ganguly K, Nadagouda MN, Aminabhavi TM. Polymeric hydrogels for oral insulin delivery. J Controlled Release. 2013;165:129–38. doi: 10.1016/j.jconrel.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Han L, Tang C, Yin C. Oral delivery of shRNA and siRNA via multifunctional polymeric nanoparticles for synergistic cancer therapy. Biomaterials. 2014;35:4589–600. doi: 10.1016/j.biomaterials.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Wang Xiaoying, Chen Yihang, Dahmani Fatima Zohra, Yin Lifang, Zhou Jianping, Yao J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials. 2014;35:7654–65. doi: 10.1016/j.biomaterials.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 31.Lin C, Chen F, Ye T, Zhang L, Zhang W, Liu D, et al. A novel oral delivery system consisting in “drug-in cyclodextrin-in nanostructured lipid carriers” for poorly water-soluble drug: Vinpocetine. Int J Pharm. 2014;465:90–6. doi: 10.1016/j.ijpharm.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Zuo Q, Lu J, Hong A, Zhong D, Xie S, Liu Q, et al. Preparation and characterization of PEM-coated alginate microgels for controlled release of protein. Biomed Mater. 2012:7. doi: 10.1088/1748-6041/7/3/035012. [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Yao W, Zhang L, Qian H, Wu W, Jiang X. Cell-penetrating hollow spheres based on milk protein. Chem Comm. 2010;46:7566–8. doi: 10.1039/c0cc02370a. [DOI] [PubMed] [Google Scholar]
- 34.Groo AC, Saulnier P, Gimel JC, Gravier J, Ailhas C, Benoit JP, et al. Fate of paclitaxel lipid nanocapsules in intestinal mucus in view of their oral delivery. Int J Nanomedicine. 2013;8:4291–302. doi: 10.2147/IJN.S51837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panthani MG, Khan TA, Reid DK, Hellebusch DJ, Rasch MR, Maynard JA, et al. In Vivo Whole Animal Fluorescence Imaging of a Microparticle-Based Oral Vaccine Containing (CuInSexS2-x)/ZnS Core/Shell Quantum Dots. Nano Lett. 2013;13:4294–8. doi: 10.1021/nl402054w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonaje K, Lin K-J, Wey S-P, Lin C-K, Yeh T-H, Nguyen H-N, et al. Biodistribution, pharmacodynamics and pharmacokinetics of insulin analogues in a rat model: Oral delivery using pH-Responsive nanoparticles vs. subcutaneous injection. Biomaterials. 2010;31:6849–58. doi: 10.1016/j.biomaterials.2010.05.042. [DOI] [PubMed] [Google Scholar]
- 37.Sakkinen M, Marvola J, Kanerva H, Lindevall K, Ahonen A, Marvola M. Are chitosan formulations mucoadhesive in the human small intestine? An evaluation based on gamma scintigraphy. Int J Pharm. 2006;307:285–91. doi: 10.1016/j.ijpharm.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Lee CM, Jeong HJ, Yun KN, Kim DW, Sohn MH, Lee JK, et al. Optical imaging to trace near infrared fluorescent zinc oxide nanoparticles following oral exposure. Int J Nanomedicine. 2012;7:3203–9. doi: 10.2147/IJN.S32828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative Prussian blue staining of GI tract sections from mice orally administrated with CN-ICG-IO and ICG-IO. The blue dots represent IONPs (pointed out in grey arrows) showed the delivery and absorption of CN-ICG-IO in small intestine, compared with the trap of ICG-IO in stomach due to the poor stability in gastric fluid.