Abstract
The mode of attachment of 70S ribosomes to thylakoid membranes from pea leaves was studied by determining the proportion of the bound RNA which was released by various incubation conditions. The results supported a model in which several classes of bound ribosomes could be distinguished: (a) very tightly bound, not released by any conditions yet tested (20% of the total); (b) monomeric ribosomes attached by electrostatic interaction with the membranes (30 to 40% of the total) and released by high salt; and (c) polysomes, with some of the ribosomes attached by a combination of electrostatic interactions and insertion of the nascent polypeptide chain into the membrane. These required a combination of puromycin and high salt for release. Other (“hanging”) ribosomes of the polysomes were inferred to be attached through mRNA but not actually attached to the membranes directly; they could be released by RNase under low salt conditions, as well as by puromycin plus high salt.
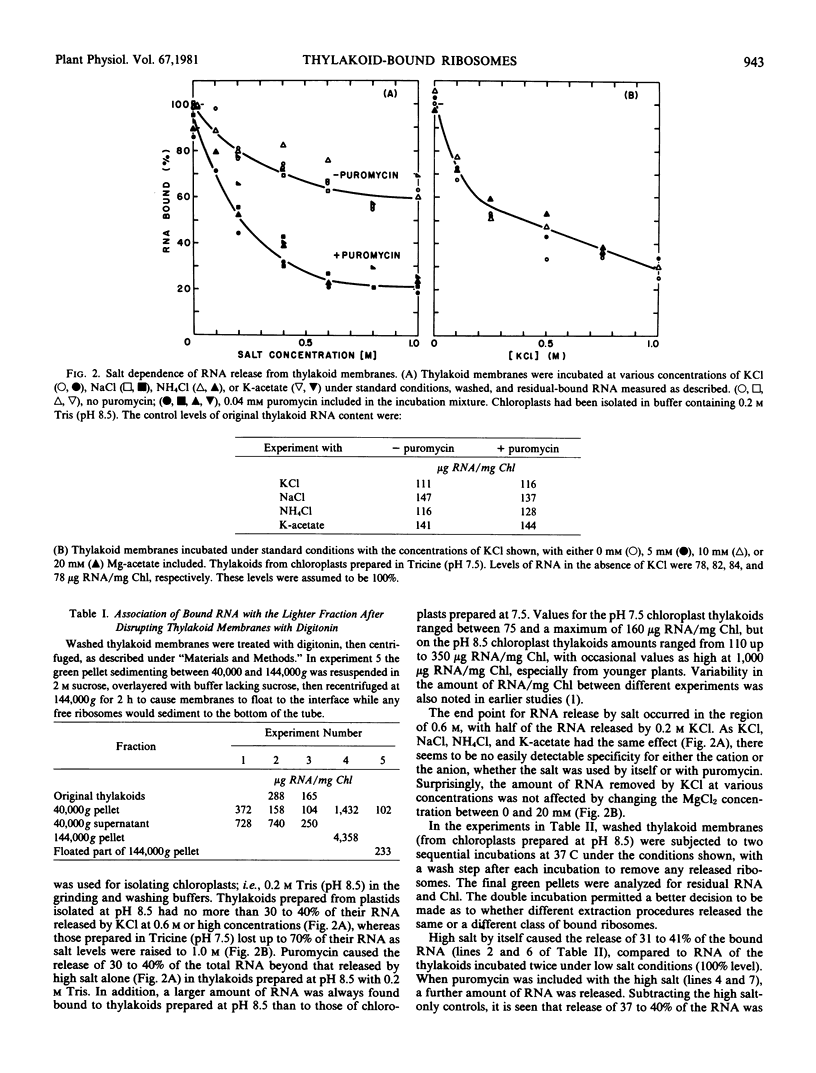
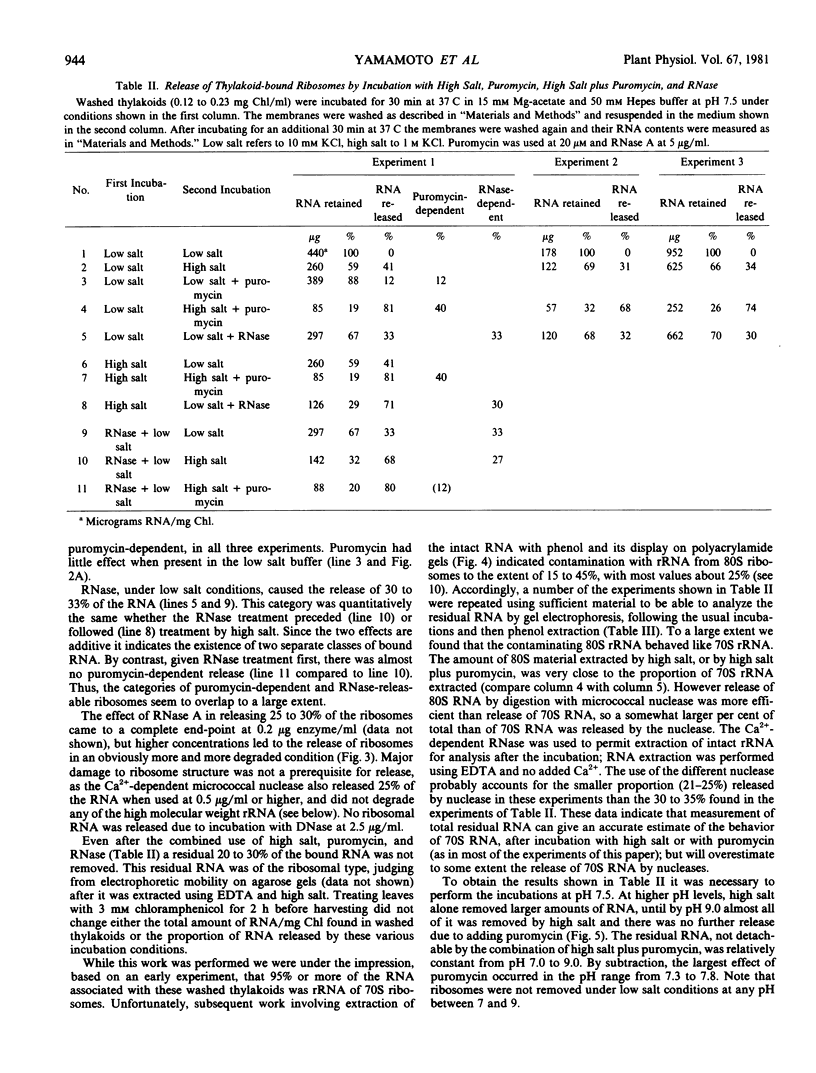
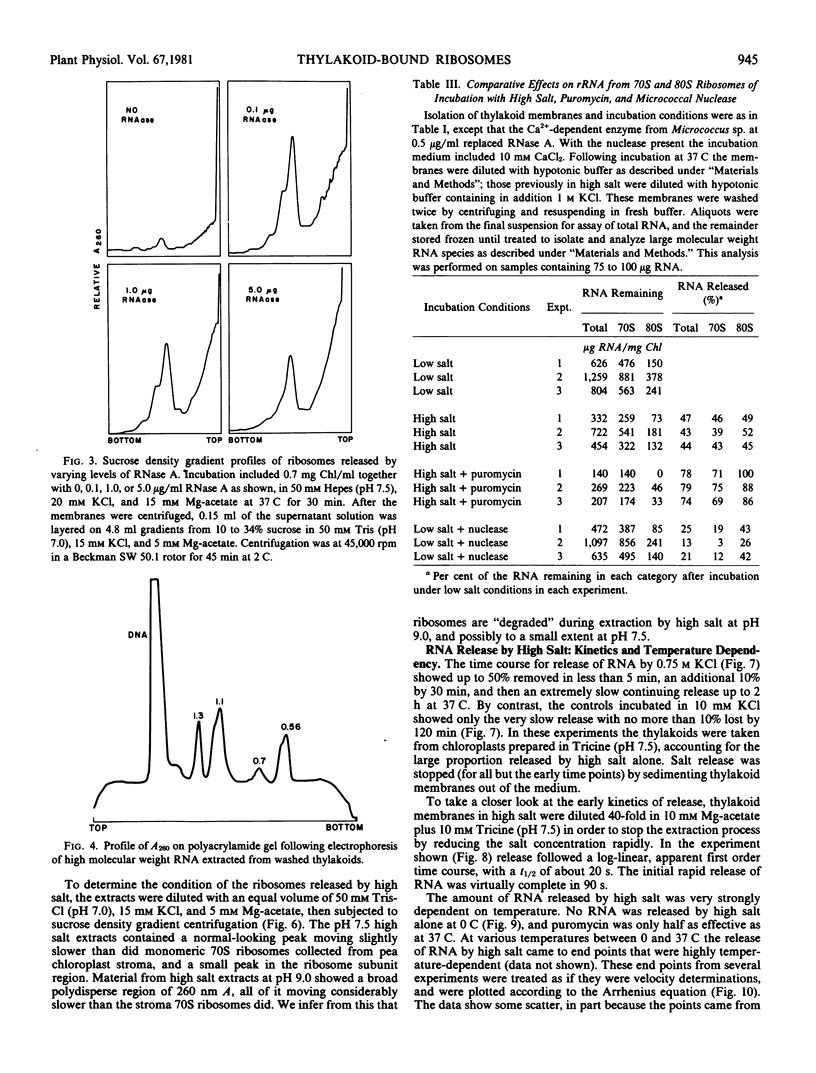
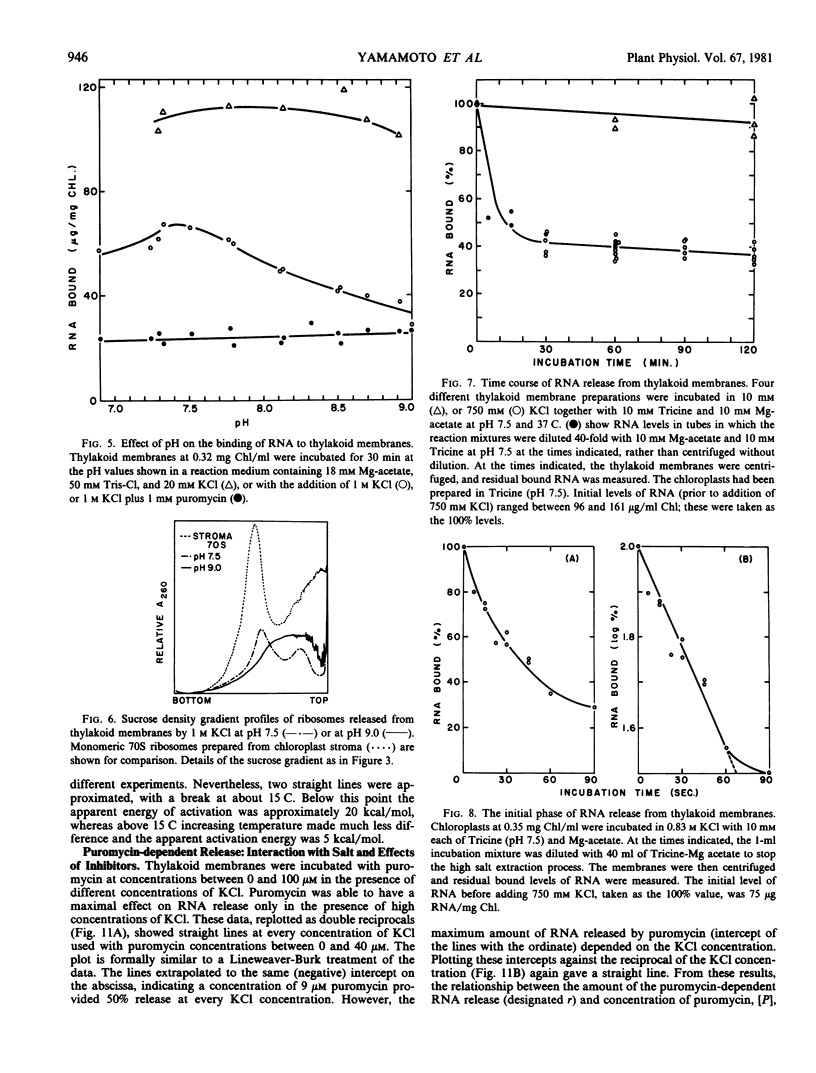
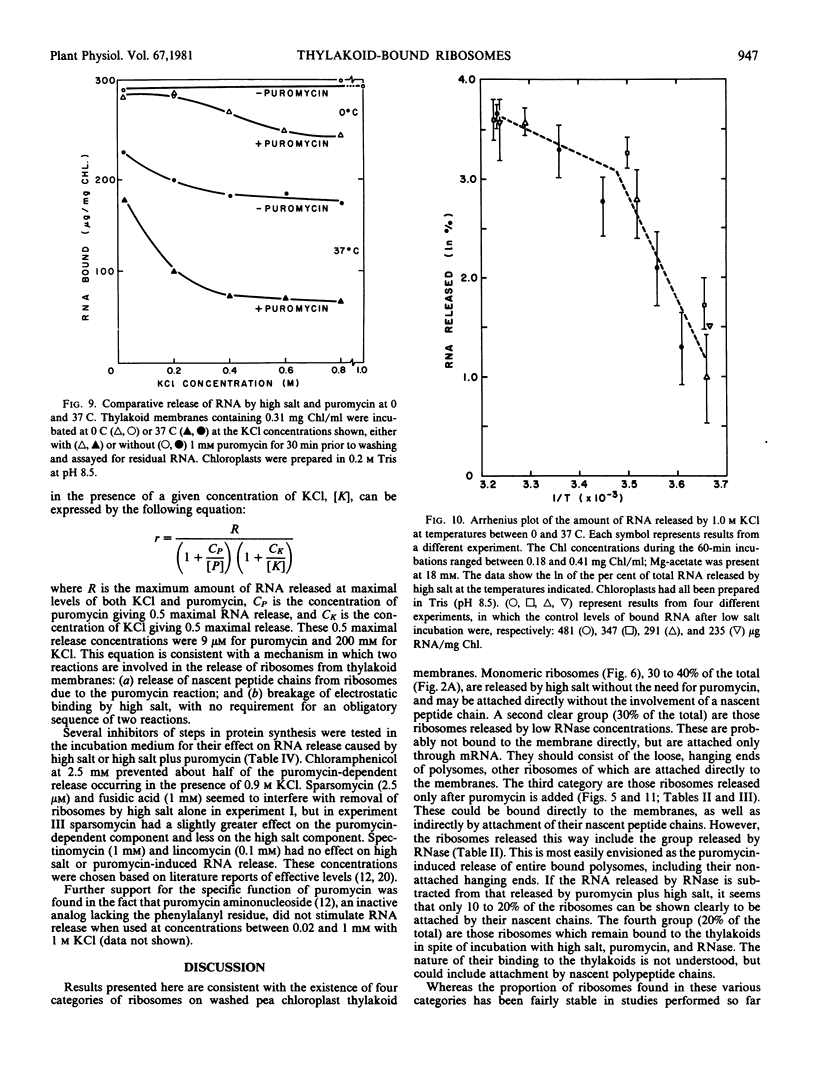
To obtain these results, chloroplasts had to be prepared in media containing 0.2 molar Tris at pH 8.5. Using Tricine buffers at pH 7.5 yielded thylakoid membranes whose ribosomes were removed almost completely by high salt alone; these showed no response to puromycin. However, pH 7.5 had to be used in all cases for ribosome dissociation in high salt media, as the ribosome structure appeared to be degraded by high salt at pH 8.5, and release then occurred without the need for puromycin.
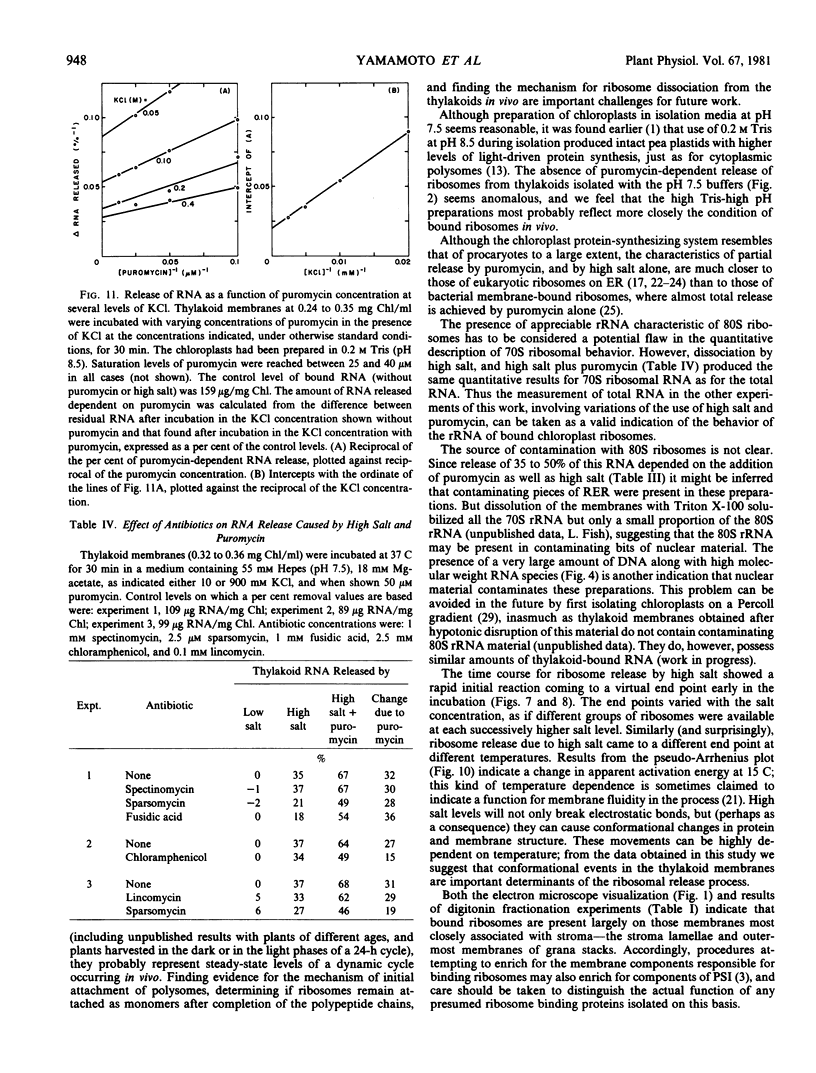
The kinetics of ribosome release by high salt showed a rapid initial phase with a half-life of 20 seconds. The extent of release by high salt was very dependent on the temperature of the incubation. Plotting the data according to the Arrhenius interpretation shows a significant break at about 15 C, with apparent activation energy of 20 kilocalories per mole below that temperature and 5 kilocalories per mole above that temperature. This result suggests that membrane fluidity might be an important factor permitting release of ribosomes under high salt conditions.
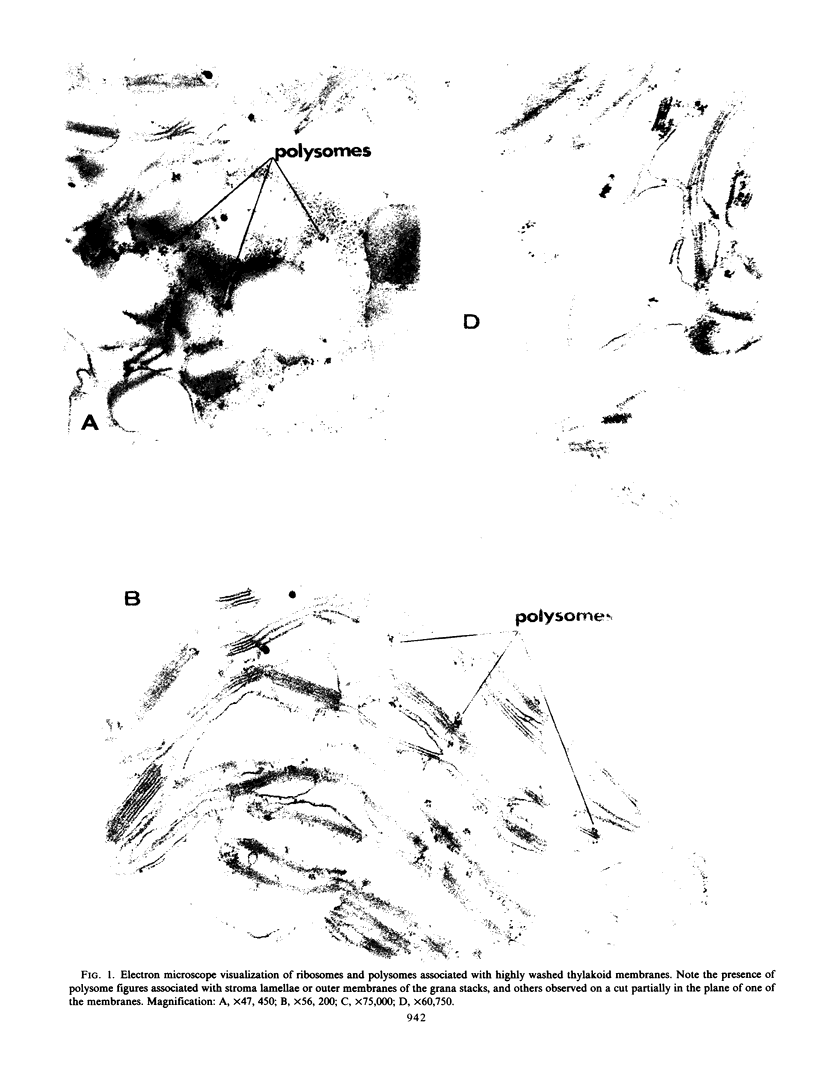
Electron microscope pictures of the washed thylakoids showed polysomes closely associated with the outer membranes of grana stacks, and with the stroma lamellae. Following digitonin treatment of the membranes and centrifugation, fractions enriched in Photosystem I and presumed stroma lamellae were also enriched in bound RNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alscher R., Patterson R., Jagendorf A. T. Activity of Thylakoid-bound Ribosomes in Pea Chloroplasts. Plant Physiol. 1978 Jul;62(1):88–93. doi: 10.1104/pp.62.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Wildman S. G. "Free" and membrane-bound ribosomes, and nature of products formed by isolated tobacco chloroplasts incubated for protein synthesis. Biochim Biophys Acta. 1970 May 21;209(1):207–219. doi: 10.1016/0005-2787(70)90677-5. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Blobel G., Siekevitz P., Palade G. E. Attachment of chloroplast polysomes to thylakoid membranes in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1973 May;70(5):1554–1558. doi: 10.1073/pnas.70.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blobel G., Siekevitz P., Palade G. E. Periodic variations in the ratio of free to thylakoid-bound chloroplast ribosomes during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1976 Nov;71(2):497–514. doi: 10.1083/jcb.71.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk H. Rough thylakoids: polysomes attached to chloroplast membranes. J Cell Biol. 1969 Aug;42(2):582–587. doi: 10.1083/jcb.42.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish L., Jagendorf A. T. A method for enzymic extraction and the measurement of chloroplast RNA. Plant Physiol. 1980 Apr;65(4):746–750. doi: 10.1104/pp.65.4.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Ingle J. The molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1971 Jun;123(2):235–243. doi: 10.1042/bj1230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies M. M., Michaels A. Free and membrane-bound chloroplast polyribosomes Chlamydomonas reinhardtii. Biochim Biophys Acta. 1975 Sep 1;402(3):297–308. doi: 10.1016/0005-2787(75)90267-1. [DOI] [PubMed] [Google Scholar]
- Margulies M. M., Michaels A. Ribosomes bound to chloroplast membranes in Chlamydomonas reinhardtii. J Cell Biol. 1974 Jan;60(1):65–77. doi: 10.1083/jcb.60.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh P. R., O'Toole K. The interaction of ribosomes and membranes in animal cells. Biochim Biophys Acta. 1976 Oct 26;457(2):171–212. doi: 10.1016/0304-4157(76)90010-1. [DOI] [PubMed] [Google Scholar]
- Mechler B., Vassalli P. Membrane-bound ribosomes of myeloma cells. I. Preparation of free and membrane-bound ribosomal fractions. Assessment of the methods and properties of the ribosomes. J Cell Biol. 1975 Oct;67(1):1–15. doi: 10.1083/jcb.67.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels A., Margulies M. M. Amino acid incorporation into protein by ribosomes bound to chloroplast thylakoid membranes: formation of discrete products. Biochim Biophys Acta. 1975 May 16;390(3):352–362. doi: 10.1016/0005-2787(75)90356-1. [DOI] [PubMed] [Google Scholar]
- Philippovich I. I., Tongur A. M., Alina B. A., Oparin A. I. Localization and conformation of polyribosomes bound to chloroplast lamellae. Exp Cell Res. 1970 Oct;62(2):399–406. doi: 10.1016/0014-4827(70)90571-9. [DOI] [PubMed] [Google Scholar]
- Raison J. K. Temperature-induced phase changes in membrane lipids and their influence on metabolic regulation. Symp Soc Exp Biol. 1973;27:485–512. [PubMed] [Google Scholar]
- Shore G. C., Tata J. R. Functions for polyribosome-membrane interactions in protein synthesis. Biochim Biophys Acta. 1977 Aug 9;472(2):197–236. doi: 10.1016/0304-4157(77)90017-x. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Nascent peptide as sole attachment of polysomes to membranes in bacteria. Proc Natl Acad Sci U S A. 1978 Feb;75(2):814–817. doi: 10.1073/pnas.75.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., Goodenough U. W. Morphological and Photosynthetic Properties of Digitonin-treated Chloroplast Membranes from the Wild-type and ac-5 Strains of Chlamydomonas reinhardi. Plant Physiol. 1975 May;55(5):864–869. doi: 10.1104/pp.55.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. B. A simplified method for preparing sucrose gradients. Biochem J. 1974 Jan;137(1):117–118. doi: 10.1042/bj1370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K. L., Jagendorf A. T. The ratio of free to membrane-bound chloroplast ribosomes. Biochim Biophys Acta. 1973 Nov 14;324(4):518–532. doi: 10.1016/0005-2787(73)90211-6. [DOI] [PubMed] [Google Scholar]