Abstract
Blood groups of humans and great apes long have been considered similar although are not interchangeable between species. In this study, human monoclonal antibody technology was used to assign human ABO blood groups to whole blood samples from great apes housed in North American and European zoos and in situ managed populations, as a practical means to assist blood transfusion situations for these species. From a subset of each of the species (bonobo, common chimpanzee, gorilla, and orangutans), DNA sequence analysis was performed to determine blood group genotype. Bonobo and common chimpanzee populations were predominantly group A which concurred with historic literature and was confirmed by genotyping. In agreement with historic literature, a smaller number of the common chimpanzees sampled were group O although this O blood group was more often present in wild-origin animals as compared to zoo-born animals. Gorilla blood groups were inconclusive by monoclonal antibody techniques and by genetic studies were inconsistent with any known human blood group. As the genus and specifically the Bornean species, orangutans were identified with all human blood groups, including O, which had not been reported previously. Following this study, it was concluded that blood groups of bonobo, common chimpanzees, and some orangutans can be reliably assessed by human monoclonal antibody technology. However, this technique was not reliable for gorilla or orangutans other than those with blood group A. Even in those species with reliable blood group detection, blood transfusion preparation must include cross-matching to minimize adverse reactions for the patient.
Keywords: ABO, blood typing, cross-matching, great apes, transfusion
INTRODUCTION
Blood group determination, or “blood typing”, is performed within a species for management purposes of genetic variation, pedigree validation, forensics, and determination of taxonomic relationships [Socha, 1981; Crouse and Vincek, 1995; Saitou and Yamamoto, 1997; Penedo, 1993]. Medically, this technique is the cornerstone of effective blood transfusion as it categorizes potential donors to reduce likelihood for donor-recipient mistransfusion [Socha, 1981; Socha, et al., 1984; Feldman, 1999; Giger, 2000; Seltsam, et al., 2003; Stieger, et al., 2005]. Equally important, although often misunderstood, is cross-matching. This procedure refines the limited donor pool for a specific patient by assessing for the presence of circulating antibodies in the recipient to red blood cell antigens of the donor, including but not limited to ABO and Rh-factor [Feldman, 1999; Knottenbelt, 2002; Stieger, et al., 2005]. Even though these clinical procedures are readily available in human medicine, the technology for veterinarians has been focused largely in domestic dogs, cats, and horses, and to a lesser extent ferrets and llama [Penedo, 1993; Giger, 2000; Knottenbelt, 2002; Hohenhaus, 2004; Giger, et al., 2005; Stieger, et al., 2005]. For animals housed in zoos, it is not only the patients at risk but also the donors as these animals nearly always require sedation to even collect the blood sample for initial patient screening. Therefore to date for animals housed in zoos, transfusion procedures have been reliant on cross-matching [Greenberg, et al., 1999].
Blood groups in humans were identified in the early 1900s and subsequently in domestic animals by the 1950s and 60s, although the latter remained less specifically defined until the1980s [Eyquem, et al., 1962; Feldman, 1999; Giger, 2000; Knottenbelt, 2002]. Blood group investigation was limited in non-human primates - except in common chimpanzees until 1940, which follows the discovery of Rh-factor and its impact on human neonatal medicine [Socha, et al., 1984; Kendig, 2007]. The great apes were considered potential human models with substantial numbers of studies in laboratory-housed chimpanzees in 1925 and limited number of zoological or in situ samples for chimpanzees and other great ape species in the 1960s and 1970s [Weiner and Gordon, 1960; Eyquem, et al., 1962; Weiner, et al., 1963; Weiner and Moor-Jankowski, 1972; Socha, et al., 1973; Moor-Jankowski, et al., 1975; Weiner, et al., 1976; Socha, 1981; Socha, et al., 1984]. The great ape literature in this field then became quiescent, until DNA-based genotyping led to molecular description of the ABO blood group genes and interpretive phylogeny in non-human primate evolution during the last 20 years [Yamamoto, et al., 1990; Yazer, 2005]. In the last decade, these molecular techniques have entered mainstream clinical techniques for even domestic pet species [Feldman, 1999; Giger, et al., 2005; Stieger, et al., 2005]. In some of these studies, it was revealed that certain blood groups predominate within geographic human populations and domestic animal breeds, so knowledge of these patterns more quickly provides an appropriate donor selection pool [Weiner, et al., 1972; Giger, 2000; Knottenbelt, 2002; Hohenhaus, 2004; Stieger, et al., 2005].
Determination of ABO blood group is based on the carbohydrate-based antigen (H) on the surface of red blood cells (RBCs) [Yamamoto, et al., 1990; Stieger, et al., 2005; Yazer, 2005]. For primates, including humans, blood group studies focused largely on the ABO gene, which causes the conversion of the H antigen to either A- or B-antigen [Crouse and Vincek, 1995; Kermarrec, et al., 1999]. In primates, the blood group A is considered the ancestral phenotype [Saitou and Yamamoto, 1997; Kermarrec, et al., 1999] and blood group O is currently the most common phenotype in humans [www.bloodtyping.com, accessed February 2006 and March 2010]. Anti-A or Anti-B antibodies are produced against the converted carbohydrate in individuals that themselves do not have the A- or B-antigen on their RBCs. These antibodies circulate in the serum and destroy donor RBCs presenting with the converted antigen [Landsteiner, 1901; Socha, et al., 1984]. Misreading or malfunction in the ABO gene sequence produces no conversion of H; therefore no antibodies are produced and group O blood occurs [Yamamoto, et al., 1990; Kermarrec, 1999; Yazer, 2005]. In humans, antibodies to these carbohydrates are present innately, presumably arising from environmental or dietary exposure to gastrointestinal bacteria or plant epitopes which have structural components that resemble the RBC antigens [Knottenbelt, 2002; Kindt, et al., 2007]. Ongoing exposure to these sources is thought to induce individual sensitization and population changes in blood group profiles. Although primarily associated with RBCs, the ABO blood group antigens can be expressed in other cells and secreted through body fluids, such as saliva, which has been confirmed in humans and great apes [Weiner and Gordon, 1960; Weiner, et al., 1963; Moor-Jankowski, et al., 1964; Socha, et al., 1984; Crouse and Vincek, 1995]. Although blood groups are frequently named A, B, or O in great apes and other mammals, it is important to recognize that these antigens are not interchangeable with the human blood group A or B at the molecular level, but rather indicate reactivity to anti-A or anti-B antibodies for blood groups A and B respectively, or neither in blood group O [Eyquem, et al., 1962; Socha and Moor-Jankowski, 1978; Socha, et al., 1984; Giger, 2000; Knottenbelt, 2002; Hohenhaus, 2004; Steiger, et al., 2005].
When indicated, whole blood transfusions can be life-saving in any species. This technique is integral to human and veterinary medicine. However, routine blood transfusions in dogs and cats was not common until recently, and current veterinary literature has cited this procedure as handled primitively in all species but these two [Socha, et al., 1984; Feldman, 1999; Giger, 2000; Hohenhaus, 2004; Stieger, et al., 2005]. Clearly, the many exotic animals housed in zoos that could need transfusion have blood groups that remain minimally explored, even in the great apes that have medical care essentially modeled on humans. Poorly executed transfusions can produce immune reactions that result in the destruction of RBCs and compromise already weakened patients and can result in death of the recipient [Socha, et al., 1984; Feldman, 1999; Greenberg, et al., 1999]; Knottenbelt, 2002; Sheppard and Hillyer, 2007]. Naively, in veterinary medicine, it is often considered that a single transfusion can be performed without blood group determination or cross-matching, as generally the initial exposure does not immediately produce circulating antibodies in the recipient [Socha, et al., 1984; Feldman, 1999; Giger, et al., 2005]. In species where blood groups have not been defined, reliance on use of the single transfusion is especially invoked, even when use of simple cross-matching techniques could improve selection of a potential donor and minimize future blood mistransfusion. For example, although it may not be the immediate plan to transfuse an individual more than once, the clinical progression of the patient may warrant repeated whole blood infusions or pregnancy may be desired at a future date. Additionally, previously unknown medical history may have hidden prior sensitization episodes such as trauma; early pregnancy loss or prior multiple fetal gestations; or, in some species, natural antibodies are present without prior exposure [Wiener, et al., 1977; Feldman, 1999; Knottenbelt, 2002; Hohenhaus, 2004; Kendig, 2007]. In any of these situations, blood mistransfusion issues could occur with the first clinical blood transfusion for an animal.
Specifically, pregnancy may sensitize the dam or fetus through alloimunization [Wiener, et al., 1977; Kendig, 2007] due to incompatible blood groups of the dam and fetus. In domestic mammals which are strongly reliant upon colostral transfer of maternal antibodies, the maternal and fetal circulations are separated largely by placental barriers [Franks, 1962]. In these species, pregnancy itself will not be compromised by the production of maternal antibodies toward fetal antigens [Franks, 1962]. However, at colostral nursing, these neonates can be exposed to a marked concentration of hemoactive antibodies from the maternal circulation and rapidly present clinical signs consistent with transfusion of incompatible blood groups [Franks, 1962; Swisher, et al., 1962; Stieger, et al., 2005]. In primate species, the intimate contact of the maternal-fetal circulation by way of the hemochorial placenta can permit in utero fetal exposure to these blood antibodies from a sensitized dam [Franks, 1962]. As occurs in human neonatal isoerythrolysis [Weiner and Gordon, 1960; Socha, et al., 1984; Kendig, 2007], fetal death or birth of weakened neonates with infant mortality has been documented in common chimpanzees [Wiener, et al., 1977] and orangutans [Socha and van Foreest, 1981]. Prevention or prophylactic addressing of this problem relies upon accurate knowledge of blood groups and circulating antibodies, most notably ABO and Rh-factor, from both parents [Kendig, 2007].
In zoological mammalian species, and especially non-human primates, each transfusion episode should be handled consistently and include blood group determination whenever possible, and in all cases, major and minor cross-matching [Feldman, 1999; Knottenbelt, 2002]. As blood groups are not well defined in zoo species, reliance on cross-matching to detect circulating hemoactive antibodies has been the norm, although blood groups cannot be determined by this technique [Knottenbelt, 2002]. In positive major cross-matching, effects of the recipient serum against the donor RBCs are considered potentially more damaging than a positive minor cross-matching [Knottenbelt, 2002]. Because zoo species do not have dedicated donors and especially not ones that can be excluded from the breeding population and potentially sensitizing pregnancies, it is important to also evaluate donors at each transfusion regardless if they have previously successful donation history [Feldman, 1999].
In progressive domestic veterinary medicine, increased attention to transfusion medicine and concomitant success has been achieved with access to more reliable blood group determination techniques [Feldman, 1999; Giger, 2000; Giger, et al., 2005; Stieger, et al., 2005]. Similarly, “over-the-counter” blood group technology has been available for human medicine with cross-matching largely automated in hospital or commercial laboratories. This study was initiated to address the lack of simple, reliable, practical blood group determination that was considered to have negative effect on available great ape patient care in zoos. Previous studies have described blood groups for laboratory-housed common chimpanzees in sizeable numbers (~500 individuals), but did not use modern techniques for patient-side testing or have populations managed for genetic diversity as occurs in zoos. Furthermore, studies of blood groups of the other great ape species [bonobo (<10), gorilla (<30)] were documented to the literature in limited numbers or were unspecified to species [orangutans (~100)] so that descriptive blood types within populations of these animals was not possible previously [Weiner, et al., 1963; Wiener and Moor-Jankowski, 1972; Wiener, et al., 1976; Socha and Moor-Jankowski, 1978; Socha, et al. 1984]. If validated by molecular techniques and applied to both zoo and in situ populations, it was hypothesized that patient-side human blood group determination methods could be used to categorize blood groups for each great ape species. This information would then be useful to develop donor databases for each species and reduce the search time for critical patient care. Similarly, ongoing breeding management could incorporate this information to minimize maternal-fetal blood interactions.
METHODS
The study protocol was approved by Lincoln Park Zoo’s Research Committee and individual participating institutions as dictated by their research guidelines. Endorsement of the project was provided by the Species Survival Plan® (SSP) for each of the great ape species: bonobo (Pan paniscus), common chimpanzee (Pan troglodytes), gorilla (Gorilla gorilla), and orangutan (Pongo pygmaeus).
Animals
Zoo populations
Species Survival Plan® and European Endangered Species Program (EEP) participants were solicited through their veterinary staffs to opportunistically perform human monoclonal antibody blood group determination during routinely scheduled sedations for their collections. Sufficient individual identification was requested to positively identify the individual throughout and subsequent to the study, and included history on source (zoo-born versus wild-origin), age, gender, and studbook number assigned within the managed population (Table 1). Over the study period (May 2006 – February 2010), blood groups were recorded from bonobo (n=51; 69% of SSP population), common chimpanzee (n=135; 33% of SSP population), Western lowland gorilla (Gorilla gorilla gorilla) (n=154; 43% of SSP population), and Bornean (Pongo pygmaeus pygmaeus) (n=35; 26% of SSP population), Sumatran (Pongo pygmaeus abelli) (n=33; 38% of SSP population), and hybrid (Pongo pygmaeus sp.) (n=17; 39% of SSP population) orangutans.
Table 1.
Composition of Species Survival Program® (SSP) great ape populations (N=938) at time of study initiation (May 2006)a
| Bonobo | 72 |
| Common chimpanzee | 334 |
| Western lowland gorilla | 339 |
| Orangutan | 225 |
| Bornean orangutan | 99 |
| Sumatran orangutan | 88 |
| Hybrid orangutan | 38 |
Numbers provided by SSP coordinators for each species at time of project endorsement.
In situ populations
Sanctuaries within the home ranges of each great apes species were solicited through their veterinary staffs to opportunistically perform human monoclonal antibody blood group determination during routinely scheduled sedations for their collections. Sufficient individual identification was requested to positively identify the individual throughout and subsequent to the study, and included history on location of acquisition, age, gender, and name or number assigned within the managed population. Over the study period (May 2006 – February 2010), blood groups were recorded from bonobo (n=20), common chimpanzee (n=98), Eastern lowland gorilla (Gorilla berengei grauri) (n=4), mountain gorilla (Gorilla berengi berengi) (n=1), and Bornean (n=134) orangutan.
Blood group determination
Participating institutions were provided with monoclonal antibody testing materials. Whole blood (<0.25ml) was collected in EDTA (n=671) or heparin (n=7) anticoagulant according to the veterinarians’ direction. Patented technology of a card impregnated with human monoclonal antibodies for blood groups A and B and Rh-factor (ELDONCARD 2511 and ELDONCARD 2521, identical card technology but different packaging, Eldon Biologicals A/S, 2820 Gentofte, Denmark) was utilized for this study. According to manufacturer’s written directions, hydration of the monoclonal antibodies was performed with distilled water before application of a small volume (~0.02ml) of anti-coagulated whole blood to each of the three reagent sites and single control on the card [www.eldonbiologicals.com, Accessed: February 2006]. These instructions were provided to each institution as a summary from the package insert; additionally, when requested, or if card completion was determined unsuccessful, the instructions were supplemented as a video demonstration. Institutions in non-primary English speaking institutions were provided translations of the summarized written instructions as appropriate to their location; versions were made in Danish, Dutch, French, German, Italian, and Spanish by zoo veterinarians primarily fluent in the language.
Test results were compared to the manufacturer-provided chart to determine the individual’s blood group; if an animal was group A or B, visible agglutination, or cell clumping, was observed within the area labeled for anti-A- or anti-B- antibodies, respectively; group AB would produce visual agglutination in both areas; and group O was determined by a lack of reaction to either anti-A or anti-B antibodies. Similarly, Rh-factor was positive if visible agglutination was present at the site labeled D, indicating in humans the presence of Rh-positive RBCs. Control areas were not expected to agglutinate; if agglutination occurred, the card was considered invalid. The attending veterinarian marked the card with the determined blood group and confirmed the appropriate individual identification. Upon completion of the card, the whole blood applied to the card was permitted to dry entirely and the test area was sealed with the provided self-adhesive clear laminate to create a permanent record. Project coordinators (Gamble, Moyse) received these completed cards by mail for animals housed within the US or by digital image submitted electronically for animals housed outside the US. Once received by coordinators, appropriate completion of the card and result interpretation was confirmed independently by each coordinator before results were entered into a database for each species.
Blood group testing reliability and validity
Card
Reliability of card technology performance in each of the great ape species was assessed by side-by-side card repetition with an identical blood sample. This procedure was performed in bonobo (n=22), common chimpanzee (n=8), Western lowland gorilla (n=29), and Bornean (n=5), Sumatran (n=7), and hybrid (n=3) orangutan.
Genotyping
Results from monoclonal antibody testing were validated by direct sequencing of DNA from selected animals to determine a genotype. Individuals that were selected for these studies were limited to US SSP populations due to international shipping requirements impacting transfer of whole blood from great apes as both non-human primates and endangered species. This evaluation was not successful for all of the samples obtained for this procedure (n=118) due to low DNA yield but was conclusive for genotypes in 77 animals: bonobo (n=10), common chimpanzee (n=35), Western lowland gorilla (n=26), and Bornean (n=1), Sumatran (n=2), and hybrid (n=3) orangutan. Detailed description and analysis of the DNA sequencing data will be presented elsewhere (Thompson, et al., in preparation).
Initially, anticoagulated whole blood (~0.05ml) in cell lysis buffer [0.1 M Tris-HCl (pH 8.0), 0.1 M EDTA, 0.1 sodium chloride, and 2% (w/v) SDS (sodium dodecyl sulfate)] (n=49) was requested for this procedure. However, DNA harvest from this type of sample had insufficient quantity in 55% of the samples [Thompson, et al., in preparation]. Therefore, whole blood in EDTA (n=69) was requested subsequently. In this type of sample, DNA quality was insufficient due to primer failure in 20% of samples [Thompson, et al., in preparation].
EDTA-anticoagulated whole blood (~2.5ml) which had been sampled previously by monoclonal antibody technology was frozen in the collection tube and shipped on dry ice to Lincoln Park Zoo. These samples were maintained frozen at −80°C until analyses were performed in Department of Human Genetics at University of Chicago. Samples were thawed in a 37°C water bath at the time of processing for genotying. From the whole blood, DNA was extracted using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, Minnesota, USA) per manufacturer’s directions. For blood submitted in cell lysis buffer, samples were stored at room temperature. Proteinase K was added for DNA extraction.
Following DNA extraction, the two exons in the ABO gene that determine human ABO genotype, 6 and 7, were amplified by PCR in two separate amplicons using the human DNA sequence as the reference [Thompson, et al., in preparation]. Sequencing reactions were performed using a Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California, US). Chromatograms were aligned and analyzed using PolyPhred [Nickerson, et al., 1997] with the chimpanzee ABO gene as a reference sequence [http://www.ncbi.nlm.nih.gov/gene; accessed April 2007]. It was not possible to address the reliability of the monoclonal antibody technique for Rh-factor because the Rh-factor gene(s) in great apes were not successfully amplified in the common chimpanzees assessed so were not pursued in other species in this study.
RESULTS
Although human ABO blood groups are not identical at the DNA sequence level between or among great ape species, these label designations are maintained to be consistent with earlier reports [Socha and Moor-Jankowski, 1978].
Blood groups
Overall, 680 animals representing all great ape species were evaluated by Eldoncard with only two samples discarded for agglutinated control sites. All bonobos (n=71) of both zoo-born and wild-origin presented blood group A by monoclonal antibody testing (Table 2). The common chimpanzee samples (n=233) were predominantly blood group A, although a minority of the zoo-housed [SSP (n=3), EEP (n=4) (5.2%)] and in situ [(n=29) (29.6%)] animals were blood group O (Table 3). In SSP and EEP populations, wild-origin animals were more likely to have blood group O (11.4%) when compared to their overall group. Gorillas in SSP (n=147) and EEP (n=7) populations included in this study represented a single species (Western lowland gorilla) and were generally zoo-born, although 14.3% were wild-origin. The in situ gorilla samples had very limited representation of two species: Eastern lowland gorilla (n=4) and mountain gorilla (n=1). In all gorilla samples, the assigned blood group was O, except for two zoo-born SSP animals that were group A (Table 4). The SSP and EEP included Bornean, Sumatran, and Bornean-Sumatran hybrid orangutans, whereas the in situ population was comprised exclusively of Bornean orangutans (Table 5). Blood groups A, B, and AB were identified in all Bornean orangutan populations and blood group O also was identified in one EEP individual; blood groups A and AB were identified in Sumatran orangutans; blood groups A, AB, and O were identified in hybrid orangutans.
Table 2.
Blood groups of bonobo (Pan paniscus) (n=71) by monoclonal antibody analysis
Species Survival Program® (SSP), European Endangered Species Program (EEP); Lola ya Bonobo (in situ)
Zoo-born animals were found in SSP (n=43) and EEP (n=1) populations. Wild-origin animals were found in SSP (7) and in situ (n=20) populations.
Rh-factor was positive in all animals, except two zoo-born animals (SSP n=1, EEP n=1).
Rh-factor was positive (n=9) and negative (n=11) for this group.
Table 3.
Blood groups for chimpanzee (Pan troglodytes) (n=233) by monoclonal antibody analysis
| Populationa | Blood Group | |||
|---|---|---|---|---|
| A | B | AB | O | |
| SSP | 106c | 0 | 0 | 3f |
| EEP | 22d | 0 | 0 | 4g |
| in situ | 69e | 0 | 0 | 29h |
| Totalb | 197 | 0 | 0 | 36 |
Species Survival Program® (SSP), European Endangered Species Program (EEP); [Centre de Conservation pour Chimpanzees, Chimfunshi Wildlife Orphanage, Limbe Wildlife Centre, Ngamba Island Chimpanzee Sanctuary, Sanaga-Yong Chimpanzee Rescue Center, Tchimpounga Chimpanzee Rescue Centre] (in situ)
Zoo-born animals were found in SSP (n=80) and EEP (n=20) populations and one sanctuary-born animal occurred in the in situ population. Wild-origin animals were found in SSP (n=29), EEP (n=6) and in situ (n=97) populations.
Rh-factor was positive (n=56) and negative (n=23) in zoo-born animals. Rh-factor was positive (n=26) and negative (n=1) in wild-origin animals.
Rh-factor was positive in zoo-born (n=18) and wild-origin (n=4) animals.
Rh-factor was positive (n=53) and negative (n=16) for wild-origin animals.
Rh-factor was positive in zoo-born (n=1) and wild-origin (n=2) animals.
Rh-factor was positive in zoo-born (n=2) and wild-origin (n=2) animals.
Rh-factor was positive in wild-origin (n=23) and sanctuary born (n=1) animals. Rh-factor was negative in wild-origin (n=5) animals.
Table 4.
Blood groups of gorilla (Gorilla ssp.) (n=159) by monoclonal antibody analysis
Species Survival Program® (SSP), European Endangered Species Program (EEP), Mountain Gorilla Veterinary Program (in situ)
Western lowland gorilla (Gorilla gorilla gorilla)
Eastern lowland gorilla (Gorilla berengei grauri) (n=4), mountain gorilla (Gorilla berengei berengei) (n=1)
Zoo-born animals were found in SSP (n=127) and EEP (n=5) populations. Wild-origin animals were found in SSP (n=20), EEP (n=2), and in situ (n=5) populations.
Rh-factor was positive (n=1) and negative (n=1) in these zoo-born animals.
Rh-factor was positive in all wild-origin animals (n=20) and majority of zoo-born animals (n=116). Rh-factor was negative in a limited number of zoo-born animals (n=9).
Rh-factor was positive in zoo-born (n=5) and wild-origin (n=2) animals.
Rh-factor was positive in all individuals.
Table 5.
Blood groups of orangutan species (Pongo pygmaeus sp.) (n=219) by monoclonal antibody analysis.
| Populationa | Blood Group | |||
|---|---|---|---|---|
| A | B | AB | O | |
| Bornean orangutan | ||||
| SSP | 12c | 3c | 11g | 0 |
| EEP | 2c | 1c | 5h | 1c |
| in situ | 37d | 28c | 69i | 0 |
| Total b | 51 | 32 | 85 | 1 |
|
| ||||
| Sumatran orangutan | ||||
| SSP | 30e | 0 | 3j | 0 |
| EEP | 0 | 0 | 0 | 0 |
| in situ | 0 | 0 | 0 | 0 |
| Totalb | 30 | 0 | 3 | 0 |
|
| ||||
| Hybrid organutan | ||||
| SSP | 6c | 0 | 7c | 2k |
| EEP | 2f | 0 | 0 | 0 |
| in situ | 0 | 0 | 0 | 0 |
| Totalb | 8 | 0 | 7 | 2 |
|
| ||||
| Total all species | 89 | 32 | 95 | 3 |
Species Survival Program® (SSP), European Endangered Species Program (EEP); Nyaru Menen Orangutan Rehabilitation Center (in situ)
Zoo-born animals were found in SSP [Bornean (n=24, Sumatran (n=30), hybrid (n=15)] and EEP [Bornean (n=8), hybrid (n=2)] populations. Wild-origin animals were found in SSP [Bornean (n=2), Sumatran (n=3)], EEP [Bornean (n=1)], and in situ (n=134) populations.
Rh-factor was positive in all animals. With this footnote, all SSP animals were all zoo-born; all EEP animals were zoo-born except for one wild-origin animal; and all in situ animals were wild-origin.
Rh-factor was positive in all but one of these wild-origin animals.
Rh-factor was positive in zoo-born (n=12) and wild-origin animals (n=2). Rh-factor was negative for zoo-born (n=15) and wild-origin animals (n =1).
Rh-factor was negative in one and positive in one zoo born animal.
Rh-factor was positive in zoo-born (n=8) and wild-origin animals (n=2). Rh-factor was negative in zoo-born animals (n=1).
Rh-factor was positive (n=4) in zoo born animals and negative (n=1) in a wild-origin animal.
Rh-factor was positive in the majority (n=66) but negative (n=3) in a limited number of these wild-origin animals.
Rh-factor was positive (n=1) and negative (n=2) in these zoo-born animals.
Rh-factor was negative in all animals.
Rh-factor
Nearly all bonobo in SSP and EEP populations were Rh-positive while 55% of the in situ individuals were Rh-negative (Table 2). Similarly, common chimpanzees of blood group A were predominantly Rh-positive, although some animals in both SSP and EEP (19%) and in situ (23%) populations (Table 3) were Rh-negative; in blood group O chimpanzees, Rh-positive status predominated. Aside from a limited number of zoo-born individuals (n=6%) in SSP and EEP populations, gorilla were Rh-positive (Table 4). In orangutan species, animals were Rh-positive, except for 53% of the Sumatran orangutan of blood group A and all hybrid orangutan of blood group O (Table 5).
Test reliability
Card
Identical results were obtained from the same blood sample on duplicated cards in all animals.
Genotyping – Blood groups
Molecular technology confirmed at the genetic level that blood group A in bonobos, chimpanzees, and orangutans were identical to humans at the four sites within the ABO gene that determines human blood group A (Table 6); however, this determination does not indicate compatible blood group A between species because incompatibility could still result from the other differences in the DNA sequence that were observed. In fact, slight genetic diversity was present in some common chimpanzee blood group A (Table 6). No chimpanzees with blood group O were available for molecular genotyping. In Western lowland gorilla, only samples which were group O by Eldoncard were available for molecular genotyping. This blood group was found not comparable to any human blood group. Although it presented notable intraspecific diversity of genotype, some of the patterns were similar to the genotype-based blood groups of orangutans (Table 6). In addition to blood group A in orangutan, blood group AB (n=3) was evaluated by genotyping and these animals each presented a unique sequence that differed from one other (Table 6). Although each animal had sequences not observed in humans, they did have limited similarity to gorilla sequences of the current study [Thompson, et al., in preparation].
Table 6.
Genotype blood group verification against monoclonal antibody blood group in great apes [bonobo (n=10), common chimpanzee (n=35), Western lowland gorilla (n=26), orangutan (n=6)] [Ober, et al., in preparation]
| Monoclonal antibody blood group | Genotype | |
|---|---|---|
| Bonobo | A | A/A |
| Common chimpanzee | A (n=31) | A/A |
| A (n=4) | A/Non-A Non-Bd | |
| Western lowland gorilla | O (n=8) | Non-A Non-B |
| O (n=15) | Non-A Non-B | |
| O (n=3) | Non-A Non-B | |
| Orangutan | Aa (n=3) | A/A |
| ABb (n=1) | Non-A Non-B | |
| ABc (n=1) | Non-A Non-B | |
| ABb (n=1) | A/Non-A Non-B |
Sumatran orangutan (n=2), hybrid orangutan (n=1)
Hybrid orangutan (n=1)
Bornean orangutan (n=1)
Similar deletion in exon 7 [Kermerrac 1997]
Rh-factor
The Rh-gene could not be amplified in the DNA samples from the common chimpanzee samples attempted and other species were not evaluated. Therefore, the validity of the monoclonal antibody testing for this profile could not be determined in this study.
DISCUSSION
Access to great ape whole blood for blood group determination was variable, and widely spaced in time, during historic comparative human blood group literature [Socha, et al., 1984]. Notably, blood group studies in bonobo were not determined until 1970 and remain limited studies in this species [Moor-Jankowski, et al., 1972; Weiner and Moor-Jankowski, 1972; Moor-Jankowski, et al., 1975; Socha and Moor-Jankowski, 1978; Socha, et al., 1984]. The findings of the current study of exclusive blood group A for bonobo was in agreement with that reported in these citations.
In contrast, common chimpanzees were available routinely from laboratory situations so were assessed in large numbers (~500 individuals) from as early as 1911 [Socha and Moor-Jankowski, 1978; Socha, 1981; Socha, et al., 1984] and smaller in situ numbers (n=40) in the 1960s [Eyquem, et al., 1962]. Predominantly, blood group A was reported in common chimpanzees throughout this literature, with group O present comparably (2.5–12%) to that in SSP and EEP collections of the current study (5.2%) [Weiner and Gordon, 1960; Weiner, et al., 1963; Eyquem, et al., 1962; Moor-Jankowski, et al., 1964; Socha and Moor-Jankowski, 1978]. However, the strictly wild-origin animals of the in situ population of the current study demonstrated a larger presence (29.6%) of blood group O when compared to the overall group. When only wild-origin individuals are considered from the SSP and EEP population, blood group O (11.4%) was more common in the wild-origin SSP and EEP animals as compared to the overall group housed in zoos (5.2%). In review of the literature, studies documented earlier than 1940, when larger numbers of the captive chimpanzee population would have been wild-origin, a larger presence of blood group O (12–20%) was reported as compared to those studies conducted after 1960 (9.4–14.9%) [Wiener and Gordon, 1960; Wiener and Moor-Jankowski, 1972; Socha and Moor-Jankowski, 1978].
Given that laboratory populations are considered closed and conservatively maintained for purposes of study design, a trend towards reduced genetic diversity is not surprising [Weiner and Gordon, 1960; Socha, 1981]. Cooperative management programs, such as the SSP, are managed to slow the loss of genetic diversity from populations that are increasingly captive-born. Even so, it was considered probable that extinguishing of blood group O from the SSP chimpanzee population will occur [Penedo, 1993]. Interestingly, although the number of chimpanzees from the EEP (n= 26) was much smaller than that evaluated from the SSP (n=109) in the current study, it had a larger presence of blood group O, 18% versus 2.8%. This finding may be artificial from these fewer numbers assessed against the US population. However, it may be real due to different management of zoo great ape populations of Europe and North America.
As with bonobo, relatively few gorillas have been studied previously. In those studies, gorillas (n=23) were not assigned to species, so they could be either Western or Eastern lowland subspecies [Wiener, et al., 1976; Socha and Moor-Jankowski, 1978]. Regardless, gorilla blood groups were uniformly noted as non-reactive to anti-A or anti-B antibodies so were assumed to be group O [Weiner and Gordon, 1960; Socha, et al., 1972; Socha and Moor-Jankowski, 1978]. On the other hand, body fluids (saliva and urine) were weakly, but uniformly, assigned to group B [Weiner and Gordon, 1960; Weiner, et al., 1963; Moor-Jankowski, et al., 1964; 1976; Socha and Moor-Jankowski, 1978; Socha, et al., 1984; Crouse and Vincek, 1995]. In the current study, gorillas again were considered non-reactive, or blood group O, by monoclonal antibody technology. In comparison to lowland gorillas, access to clinical samples from mountain gorillas has never been widely available for blood group determination. Initially, no reported differences were noted between lowland and mountain gorilla (n=2) blood group assessment [Socha, et al. 1973]. However, one researcher – and a co-author in the lowland gorilla study - consistently remarked that mountain gorilla were similarly non-reactive as the lowland species when blood was utilized, but were group A on secretor status [Weiner and Gordon, 1960; Weiner, et al., 1963]. Sample sizes were quite small (n=4) and in a later report, it was commented that no differences existed in their interpretation between the lowland and mountain gorilla species [Weiner, et al., 1976].
For the current study, nearly uniform blood group O for the gorilla population is not a misinterpretation from this previous documentation, as no reactivity had been previously assessed in the literature from whole blood. Additionally, in humans, blood group O is produced by minor genotypic change on chromosome 9 which truncates the H antigen on the RBCs [Kermarrec, et al., 1999]. However, this same effect could be produced by any number of comparable genetic changes that abrogates the conversion of the H antigen to type A or B [Kermarrec, et al., 1999; Seltsam, 2003; Yazer, 2005]. In gorillas, no reaction occurred with the human anti-A and anti-B monoclonal antibodies, and would be assigned blood group O by this method. However by genotyping in the current study, gorillas had a novel sequence that differed from both human A and B groups, and would not necessarily abrogate the gene function, as occurs in human group O blood. The current study demonstrates that the species has a diverse blood group system that differs from the human ABO blood groups [Thompson, et al., in preparation].
As recently as 1999 [Kermarrec, et al., 1999], literature suggested that no ape species displayed all four of the human blood group phenotypes: A, B, AB, and O [Moor-Jankowski and Weiner, 1964; Weiner, et al., 1963; Socha and Moor-Jankowski, 1978]. The numbers of orangutans in the current study (n=219) is not only twice that of all previous studies combined, but also delineates between species and hybrid status [Socha and Moor-Jankowski, 1978; Socha, et al., 1984; Weiner, et al., 1963]. Previous studies reported blood groups of A, B, and AB in orangutans [Weiner and Gordon, 1960; Socha and Moor-Jankowski, 1978; Socha, et al., 1984], with the exception of one individual (species unspecified) that was blood group O [Weiner, et al., 1963]. In the current study, all four human blood phenotypes were present by monoclonal antibody technology. Although this diversity also was present specifically in Bornean orangutans (n=169), they represented the larger proportion of the samples evaluated; Sumatran orangutans were identified with blood groups A and AB and groups A, AB, and O were present in hybrids (Table 5).
The complete range of blood group diversity was documented in only Bornean orangutans and not in Sumatran orangutans. Therefore, the blood group distribution in the zoo-born orangutan hybrids could be simply a result of interspecies breeding. None of the individual hybrids (n=15) included in this study were younger than 23 years of age and born, therefore, before more effective contraception and population management for this species had been made available and hybridization was discouraged. Hybridization parentage by sire and dam may impact the overall hybrid distribution of blood groups as has been noted in domestic animal hybrids [Eyquem, et al., 1962] but it was not possible to assess this effect from the current study data.
Human and great ape blood group A as determined by monoclonal antibody technology and genotyping were identical at four sites within the ABO gene that determines human blood group A, but this does not mean they are interchangeable interspecifically (Table 6). However, this current study confirmation does support use of monoclonal antibody technology (Eldoncard) for patient-side typing in these animals. Despite this confirmation, it has been documented in the literature that a single transfusion from a human to a common chimpanzee could be tolerated although the converse was not true [Wiener and Moor-Jankowski, 1972] as common chimpanzees have been determined innately to not have agglutinating antibodies to human blood types while humans do possess such agglutins. In blood group O chimpanzees as with gorilla, while the blood was non-reactive with human anti-A and anti-B, and therefore group O, the gene controlling ABO group determination may not create a non-reactive protein. In fact, as no common chimpanzee blood group O individuals were available for genotyping, it is unclear if they themselves may be reactive with the species. Furthermore in the case of gorilla, genotyping determined that human blood groups are not detectable or comparable (Table 6) by human monoclonal antibody analysis. This finding represents a failure of the monoclonal antibody technology for this species. Orangutans with blood groups A and AB were available for genotyping; although A was quite consistent at the four sites evaluated, AB presented considerable diversity. As individuals with blood group B or O were not available for genotying, similar comments cannot be concluded. However, it is suggested that monoclonal antibody technology can be utilized cautiously in this species to assign blood groups.
In addition to blood group associated antibodies, other hemoactive proteins, such as Rh-factor, exist [Feldman, 1999; Knottenbelt, 2002]. This latter protein has become well described in human medicine literature as the predominant source for maternal-fetal blood interactions [Wiener, et al., 1977; Kendig, 2007]. Primate placental attachments are hemochorial and so permit close circulatory contact between the mother and fetus. When an Rh-negative mother carries a fetus with Rh-positive blood inherited from the father, close association of maternal and fetal blood presents exposure of the Rh-antigen to the mother with subsequent development of antibodies to this factor [Kendig, 2007]. Exposure of the Rh-positive fetus to these maternal antibodies presents essentially a mistransfusion where RBC destruction (neonatal isoerythrolysis) and its resultant systemic effects occur, resulting in in utero death, stillbirth, or delivery of weak neonates [Wiener, et al., 1977; Kendig, 2007]. Sensitization from prior pregnancies is the most common situation associated with this maternal-fetal incompatibility in humans. However, antepartum hemorrhage, spontaneous abortions, ectopic pregnancies, abdominal trauma during pregnancy – including clinical procedures of amniocentesis, chorionic villous sampling, or fetal surgery, blood mistransfusions, and twinning can similarly produce alloimmunization situations Wiener, et al., 1977; Kendig, 2007].
Rh-factor is clearly not the only circulating antigen and others would have potential to produce similar reactions in great ape gestations; in fact, this situation has been documented in common chimpanzees and orangutans [Wiener, et al., 1977; Socha and van Foreest, 1981]. Additionally, this issue could be associated with differing parent ABO blood groups [Weiner and Gordon, 1960; Wiener, et al., 1977]. Prevention of these responses can be accomplished by foreknowledge of blood groups of the parents which permits appropriate in utero fetal support or immediate neonatal intervention [Wiener, et al., 1977; Socha and van Foreest, 1981; Socha, et al., 1984; Kendig, 2007]. Therefore, parental analysis for breeding recommendations and evaluation of subsequent pairings will need cross-matching analysis as occurs with blood transfusion [Wiener, et al., 1977; Giger, 2000; Knottenbelt, 2002].
Blood group determination reveals potential donors from the population at large [Socha, et al., 1984]. For all great ape species, except gorilla, the individuals’ blood groups have been catalogued by species as a database to provide quick reference for those clinicians faced with clinical situations where transfusion is planned or has occurred. However for all great apes, as in humans, cross-matching will be necessary to confirm donor selections and, when properly completed, minimizes mistransfusion [Socha, et al., 1984]. This technique assesses for the presence of conflicting antibodies by separating the donor and recipient blood into its cellular (RBCs) and acellular (serum) components [Feldman, 1999; Knottenbelt, 2002]. In vitro exposure of donor RBCs to recipient serum (major cross-match) and donor serum to recipient RBCs (minor cross-match) is completed and evaluated for agglutination both grossly and microscopically. Although not providing a specific blood group, this determination can prevent clearly evident transfusion mismatch and is quick, easy, inexpensive, and requires little blood to complete [Feldman, 1999; Knottenbelt, 2002]. The addition of this information to the species-specific pedigree database will go towards increasing successful blood transfusions with reduced donor risk in zoological collections [Socha, 1981].
Blood transfusion complications typically arise as urgent clinical situations and include shock and hemodynamic collapse resulting from RBC destruction. Although cross-matching can assist the immediate problem, it does require whole blood collection from both donor and recipient which generally requires sedation, even in zoological collections. Knowledge of blood types and cross-matching status in advance of these problems would be the ideal. Because all great ape species are secretors (i.e., blood group antigens can be detected in other body fluids, such as saliva), this advance knowledge may some day be possible if appropriate testing can be developed [Weiner, et al., 1963; Socha, et al., 1984; Moor-Jankowski, 1964]. Finally, the current study has made clear that clinical transfusion interests aside, determination of great ape groups have other applications that warrant continued investigation of the great ape blood group genotypes.
CONCLUSIONS AND CLINICAL IMPLICATIONS
Bonobo are exclusively blood group A by monoclonal antibody technology. These animals as well as blood group A common chimpanzees and orangutans have the same genotype at the four sites that determine the human blood group A.
By monoclonal antibody technology, common chimpanzees are predominantly blood group A although limited portions of the SSP and EEP populations had blood group O. Wild-origin individuals were more likely to be group O than zoo-born animals. The latter blood group may be a true non-reactive O without expression on RBCs or may represent a novel, chimpanzee-specific allele.
Nearly all gorillas did not react to the antibodies of the monoclonal antibody technology which was consistent with blood group O phenotype. However, genetic studies revealed diverse blood groups that were not similar to human blood group A, B, or O.
Orangutans in the genus and Bornean species have all four human blood group phenotypes – A, B, AB, and O – identified by molecular antibody technology which suggested this species as the first non-human primate to present all four human blood group phenotypes, although only blood group A was similar at the genotype level to the human blood group A.
Bonobo and common chimpanzees had uniform or predominant blood groups throughout zoo populations. Although these blood transfusions can be conducted without much risk of adverse reactions, when such procedures involve wild-origin or founder common chimpanzees in SSP and EEP populations, higher frequency of blood group O may complicate identification of successful donors or present issues with maternal-fetal mismatch. In any transfusion situation, cross-matching of individuals is recommended strongly prior to transfusion and essential when wild-origin animals are involved.
Because gorillas do not have human A, B, or O blood groups, even single blood transfusions have risk of adverse reaction. Cross-matching of individuals is essential in this species prior to transfusion.
Orangutan blood groups were diverse, regardless of population. Because hybrids are present in zoo populations, ongoing blood group assessment is recommended to target appropriate transfusion candidates ahead of actual need, and cross-matching of individuals is essential prior to transfusion even in those individuals within a consistent blood group.
Figure 1.
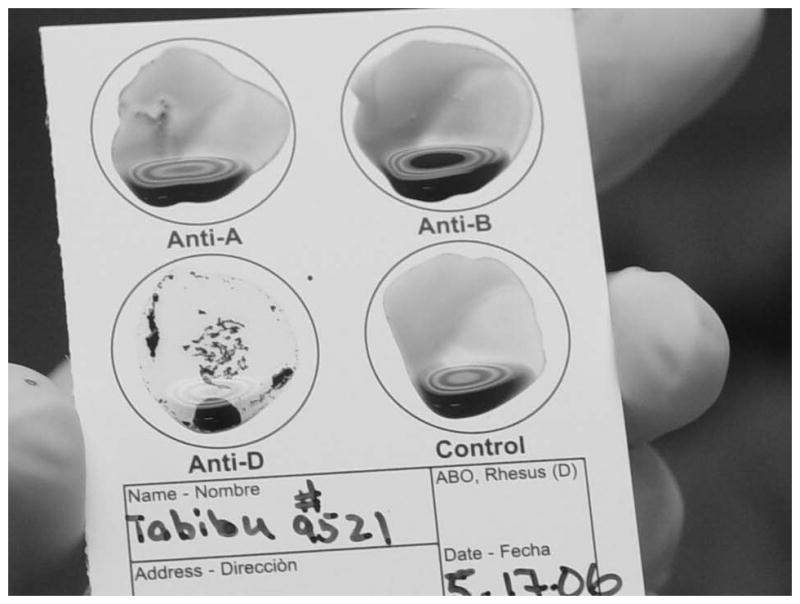
This ELDONCARD was completed for an adult gorilla (Gorilla gorilla). It is interpreted as blood group O as no agglutination was present at either the anti-A or anti-B sites. Rh-factor is interpreted as positive due to visible agglutination present at the anti-D site.
Acknowledgments
The authors greatly appreciate provision of materials from Eldon Biologicals, Inc., by Hans-Ole Hedegaard, General Manager; and project support and editorial review of Species Survival Plan® coordinators and veterinary advisors (Dr. Gay Reinartz and Dr. Victoria Clyde, Bonobo SSP; Steve Ross and Dr. Kay Backues, Common Chimpanzee SSP; Dr. Dan Wharton and Dr. Tom Meehan, Gorilla SSP; Lori Perkins and Dr. Rita McManamon and Dr. Chris Bonar, Orangutan SSP) and Dr. Kristin Lukas, Dr. Sue Margulis, Dr. Elizabeth Lonsdorf, and Maureen Leahy.
Contributing institutions are acknowledged for their provision of samples and animal identification: Species Survival Plan® Albuquerque Biological Park, Albuquerque, NM; Audubon Zoo, New Orleans, LA; Birmingham Zoo, Birmingham, AL; Bronx Zoo, Bronx, NY; Buffalo Zoological Gardens, Buffalo, NY; Busch Gardens Tampa Bay, Tampa, FL; The Calgary Zoo, Botanical Garden & Prehistoric Park, Calgary, Alberta, AB, Canada; Chicago Zoological Society-Brookfield Zoo, Brookfield, IL; Cincinnati Zoo & Botanical Garden, Cincinnati, OH; Cleveland Metroparks Zoo, Cleveland, OH; Columbus Zoo and Aquarium, Powell, OH; Dallas Zoo, Dallas, TX; Denver Zoological Gardens, Denver, CO; Detroit Zoological Park, Royal Oak, MI; Disney’s Animal Kingdom, Lake Buena Vista, FL; El Paso Zoo, El Paso, TX; Fort Wayne Children’s Zoo, Fort Wayne, IN; Fort Worth Zoo, Fort Worth, TX; Franklin Park Zoo, Boston, MA; Gladys Porter Zoo, Brownsville, TX; Gorilla Haven, Morganton, GA; Honolulu Zoo, Honolulu, HI; Houston Zoo, Inc., Houston, TX; Jackson Zoological Park, Jackson, MS; Jacksonville Zoo and Gardens, Jacksonville, FL; John Ball Zoological Garden, Grand Rapids, MI; Kansas City Zoo, Kansas City, MO; Knoxville Zoological Gardens, Knoxville, TN; Lincoln Park Zoo, Chicago, IL; Lion Country Safari, Loxahatchee, FL; Little Rock Zoo, Little Rock, AR; Los Angeles Zoo and Botanical Gardens, Los Angeles, CA; Louisville Zoological Garden, Louisville, KY; The Maryland Zoo in Baltimore, Baltimore, MD; Memphis Zoo, Memphis, TN; Miami Metrozoo, Miami, FL; Milwaukee County Zoological Gardens, Milwaukee, WI; North Carolina Zoological Park, Asheboro, NC; Oakland Zoo, Oakland, CA; Oklahoma City Zoological Park, Oklahoma City, OK; Omaha’s Henry Doorly Zoo, Omaha, NE; Oregon Zoo, Portland, OR; Philadelphia Zoo, Philadelphia, PA; Phoenix Zoo, Phoenix, AZ; Pittsburgh Zoo & PPG Aquarium, Pittsburgh, PA; Potawatomi Zoo, South Bend, IN; Riverbanks Zoo & Garden, Columbia, SC; Rolling Hills Wildlife Adventure, Salina, KS; Sacramento Zoo, Sacramento, CA; Saint Louis Zoo, Saint Louis, MO; San Diego Zoo, San Diego, CA; San Diego Zoo’s Wild Animal Park, Escondido, CA; San Francisco Zoological Gardens, San Francisco, CA; Santa Barbara Zoological Gardens, Santa Barbara, CA; Seattle Zoo, Seattle, WA; Sedgwick County Zoo, Wichita, KS; Smithsonian National Zoological Park, Washington, DC; Sunset Zoological Park, Manhattan, KS; Tampa’s Lowry Park Zoo, Tampa, FL; Toledo Zoological Gardens, Toledo, OH; Topeka Zoo, Topeka, KS; Toronto Zoo, Scarborough, ONT, Canada; Tulsa Zoo and Living Museum, Tulsa, OK; Utah’s Hogle Zoo, Salt Lake City, UT; Zoo Atlanta, Atlanta, GA; Zoo de Granby, Granby, QUE, Canada. European Endangered Species Program Artis Zoo, Amsterdam, Netherlands; Bristol Zoo, Bristol, England; Chester Zoo, Chester, England; Edinburgh Zoo, Edinburgh, Scotland; London Zoo, London, England; Rotterdam Zoo, Rotterdam, Netherlands; Twycross Zoo, Warwickshire, England; Whipsnade Zoo, Dunstable Bedfordshire, England; Zoo Aquarium Madrid, Madrid, Spain. In situ sanctuaries Center for Chimpanzee Conservation, Guinea; Chimfunshi Wildlife Orphanage, Zambia; Limbe Wildlife Center, Cameroon; Lola ya Bonobo, Democratic Republic of Congo; Mountain Gorilla Veterinary Project, Rwanda; Ngamba Island Chimpanzee Sanctuary, Uganda; Sanga-Yong Chimpanzee Rescue Center, Cameroon; Tchimpounga Chimpanzee Rehabilitation Center, Congo; Uganda Wildlife Education Center, Uganda; Nyaru Menteng Rehabilitation Center, Central Kalimantan, Borneo.
References
- Crouse C, Vincek V. Identification of ABO alleles on forensic-type specimens using rapid-ABO genotyping. Biotechniques. 1995;18:478–483. [PubMed] [Google Scholar]
- Eyquem A, Podliachouk L, Millot P. Blood groups in chimpanzees, horses, sheep, pigs, and other mammals. Ann N Y Acad Sci. 1962;97:320–328. doi: 10.1111/j.1749-6632.1962.tb34646.x. [DOI] [PubMed] [Google Scholar]
- Feldman BF. In-house canine and feline blood typing. J Am Anim Hosp Assoc. 1999;35:455–456. doi: 10.5326/15473317-35-6-455. [DOI] [PubMed] [Google Scholar]
- Franks D. Blood groups and hemolytic disease of the newborn foal. Ann N Y Acad Sci. 1962;97:235–250. doi: 10.1111/j.1749-6632.1962.tb34639.x. [DOI] [PubMed] [Google Scholar]
- Giger U. Blood typing and crossmatching to ensure compatible transfusions. In: Bonagura JD, editor. Kirk’s current veterinary therapy XIII small animal practice. 12. Philadelphia: W. B. Saunders Company; 2000. pp. 396–403. [Google Scholar]
- Giger U, Stieger K, Palos H. Comparison of various canine blood-typing methods. Am J Vet Res. 2005;66:1386–1392. doi: 10.2460/ajvr.2005.66.1386. [DOI] [PubMed] [Google Scholar]
- Greenberg JM, Janssen DL, Jamieson SW, Rothman A, Frankville DD, Cooper SD, Kriett JM, Adsit PK, Shima AL, Morris PJ, Sutherland-Smith M. Surgical repair of an atrial septal defect in a juvenile Sumatran orangutan (Pongo pygmaeus sumatraensis) J Zoo Wildl Med. 1999;30:256–261. [PubMed] [Google Scholar]
- Grehan JR, Schwartz JH. Evolution of the second orangutan: phylogeny and biogeography of hominid origins. J Biogeogr. 2009;36:1823–1844. [Google Scholar]
- Hohenhause AE. Importance of blood groups and blood group antibodies in companion animals. Trans Med Rev. 2004;18:117–126. doi: 10.1016/j.tmrv.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Kendig JW. Hemolytic disease of the newborn. In: Rakel RE, Bope ET, editors. Conn’s Current Therapy. Philadelphia: Saunders Elsevier; 2007. pp. 413–417. [Google Scholar]
- Kermarrec N, Roubinet F, Pol-Andre A, Blancher A. Comparison of allele O sequences of the human and non-human primate ABO system. Immunogenetics. 1999;49:517–526. doi: 10.1007/s002510050529. [DOI] [PubMed] [Google Scholar]
- Kindt T, Goldsby R, Osborne BA, Kuby J. Kuby Immunology. 6. New York: Macmillan; 2007. p. 574. [Google Scholar]
- Knottenbelt CM. The feline AB blood group system and its importance in transfusion medicine. J Feline Med Surg. 2002;4:69–76. doi: 10.1053/jfms.2001.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor-Jankowski J, Wiener AS. Human blood group factors in non-human primates. Nature. 1964;202:663–665. doi: 10.1038/202663a0. [DOI] [PubMed] [Google Scholar]
- Moor-Jankowski J, Wiener AS, Socha WW, Gordon EB, Mortelmans J. Blood groups of dwarf chimpanzees (Pan paniscus) J Med Primatol. 1972;1:90–101. doi: 10.1159/000460370. [DOI] [PubMed] [Google Scholar]
- Moor-Jankowski J, Wiener AS, Socha WW, Gordon EB, Mortelmans J, Sedgwick CJ. Blood groups of pygmy chimpanzees (Pan paniscus): human-type and simian-type. J Med Primatol. 1975;4:262–267. doi: 10.1159/000459862. [DOI] [PubMed] [Google Scholar]
- Nickerson DA, Tobe VO, Taylor SL. Polyphred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 1997;25:2745–2751. doi: 10.1093/nar/25.14.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penedo CMT. Blood typing in nondomestic animals. In: Fowler ME, editor. Zoo and wild animal medicine. 3. Philadelphia: WB Sanders Company; 1993. pp. 68–73. [Google Scholar]
- Saitou N, Yamamoto F. Evolution of ABO primate blood group genes and their homologous genes. Mol Biol Evol. 1997;14:399–411. doi: 10.1093/oxfordjournals.molbev.a025776. [DOI] [PubMed] [Google Scholar]
- Seltsam A, Hallensleben M, Kollmann A, Blasczyk R. The nature of diversity and diversification at the ABO locus. Blood. 2003;102:3035–3042. doi: 10.1182/blood-2003-03-0955. [DOI] [PubMed] [Google Scholar]
- Sheppard CA, Hillyer CD. Adverse effects of blood transfusion. In: Rakel RE, Bope ET, editors. Conn’s Current Therapy. Philadelphia: Saunders Elsevier; 2007. pp. 478–486. [Google Scholar]
- Socha WW. Blood groups as genetic markers in chimpanzees: their importance for the national chimpanzee breeding program. Am J Primatol. 1981;1:3–13. doi: 10.1002/ajp.1350010103. [DOI] [PubMed] [Google Scholar]
- Socha WW, Blancher A, Ruffie J. Comparative study of human monoclonal anti-D antibodies of IgG and IgM classes in tests with red cells of nonhuman primates. Rev Fr Transfu Hemobiol. 1993;36:485–497. doi: 10.1016/s1140-4639(05)80223-0. [DOI] [PubMed] [Google Scholar]
- Socha WW, Foreest van AW. Erythroblastosis fetalis in a family of captive orangutans. Am J Primatol. 1981;1:326. [Google Scholar]
- Socha WW, Moor-Jankowski J. Blood groups of anthropoid apes and their relationship to human blood groups. J Hum Evol. 1978;8:453–465. [Google Scholar]
- Socha WW, Moor-Jankowski J, Ruffie J. Blood groups of primates: present status, theoretical implications, and practical applications: a review. J Med Primatol. 1984;13:11–40. [PubMed] [Google Scholar]
- Socha WW, Wiener AS, Gordon EB, Moor-Jankowski J. Methodology of primate blood grouping. Transplant Proc. 1972;9:107–111. [PubMed] [Google Scholar]
- Socha WW, Weiner AS, Moor-Jankowski J, Mortelmans J. Blood groups of mountain gorillas (Gorilla gorilla beringei) J Med Primatol. 1973;2:364–368. doi: 10.1159/000460348. javascript:PopUpMenu2_Set(Menu6389882); [DOI] [PubMed] [Google Scholar]
- Stieger K, Palos H, Giger U. Comparison of various blood-typing methods for the feline AB blood group system. Am J Vet Res. 2005;66:1393–1399. doi: 10.2460/ajvr.2005.66.1393. [DOI] [PubMed] [Google Scholar]
- Weiner AS, Gordon EB. The blood groups of chimpanzees: A-B-O groups and M-N types. Amer J Phys Anthropol. 1960;18:301–311. doi: 10.1002/ajpa.1330180408. [DOI] [PubMed] [Google Scholar]
- Weiner AS, Moor-Jankowski J. Blood groups of chimpanzees. Primates Med. 1972;6:115–144. [PubMed] [Google Scholar]
- Wiener AS, Moor-Jankowski J, Gordon EB. Blood groups of apes and monkeys: II the A-B-O blood groups, secretor and Lewis types of apes. Amer J Phys Anthropol. 1963;21:271–281. doi: 10.1002/ajpa.1330210303. [DOI] [PubMed] [Google Scholar]
- Wiener AS, Socha WW, Arons EB, Mortelmans J, Moor-Jankowski Blood groups of gorillas: further observations. J Med Primatol. 1976;5:317–320. doi: 10.1159/000459977. [DOI] [PubMed] [Google Scholar]
- Weiner AS, Socha WW, Moor-Jankowski J. Erythroblastosis models: materno-fetal incompatibility in chimpanzee. Folia Primatol. 1977;27:68–74. doi: 10.1159/000155777. [DOI] [PubMed] [Google Scholar]
- Yamamoto F, Clausen H, White T, Marken J, Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- Yazer MH. What a difference 2 nucleotides make: a short review of ABO genetics. Transfus Med Rev. 2005;19:200–209. doi: 10.1016/j.tmrv.2005.02.003. [DOI] [PubMed] [Google Scholar]


