Abstract
Background
Stopping smoking is associated with many important improvements in health and quality of life. The use of cessation medications is recommended to increase the likelihood of quitting. However, there is historical and renewed concern that smoking cessation therapies may increase the risk of cardiovascular disease events associated within the quitting period. We aimed to examine whether the 3 licensed smoking cessation therapies—nicotine replacement therapy, bupropion, and varenicline—were associated with an increased risk of cardiovascular disease events using a network meta-analysis.
Methods and Results
We searched 10 electronic databases, were in communication with authors of published randomized, clinical trials (RCTs), and accessed internal US Food and Drug Administration reports. We included any RCT of the 3 treatments that reported cardiovascular disease outcomes. Among 63 eligible RCTs involving 21 nicotine replacement therapy RCTs, 28 bupropion RCTs, and 18 varenicline RCTs, we found no increase in the risk of all cardiovascular disease events with bupropion (relative risk [RR], 0.98; 95% confidence interval [CI], 0.54–1.73) or varenicline (RR, 1.30; 95% CI, 0.79–2.23). There was an elevated risk associated with nicotine replacement therapy that was driven predominantly by less serious events (RR, 2.29; 95% CI, 1.39–3.82). When we examined major adverse cardiovascular events, we found a protective effect with bupropion (RR, 0.45; 95% CI, 0.21–0.85) and no clear evidence of harm with varenicline (RR, 1.34; 95% CI, 0.66–2.66) or nicotine replacement therapy (RR, 1.95; 95% CI, 0.26–4.30).
Conclusion
Smoking cessation therapies do not appear to raise the risk of serious cardiovascular disease events.
Keywords: bupropion, cardiovascular diseases, meta-analysis, smoking cessation, tobacco use cessation products, varenicline
Smoking is the leading preventable cause of death around the world.1 Approximately 50% of long-term smokers will die a smoking-related death.2 Early cessation of smoking is associated with important increases in life expectancy, improved quality of life, and reduced healthcare costs for smoking-associated conditions.2 Chief among the benefits of smoking cessation are improved cardiovascular health.3,4 For these reasons, clinical practice guidelines in the United States recommend the use of smoking cessation pharmacotherapies with all adult smokers interested in quitting unless contraindicated.5,6
In North America, there are 3 approved first-line classes of therapies: nicotine replacement therapy (NRT); bupropion, an antidepressant, and; varenicline, a nicotine receptor partial agonist. Many randomized, clinical trials (RCTs) and systematic reviews have demonstrated these agents to be effective in promoting smoking cessation.7,8 The medications have different mechanisms of action and side effect profiles. All underwent some scrutiny for potential cardiovascular effects when they came onto the market. When NRT first came onto the market, there were concerns in the literature and popular press about its safety profile with regard to cardiovascular events, particularly among users who continued to smoke.9 Clinical trials and laboratory research that followed indicated that NRT was safe even with a high-dose patch, combination NRT, and concurrent smoking.10–12 With bupropion, 3 trials consisting of 792 total smokers with cardiovascular disease (CVD) reported greater cardiovascular events among participants assigned to active versus placebo drug. The differences were not statistically significant; however, the trials were not powered for safety.13–15 Similar concerns have been raised about varenicline. In 2011, a meta-analysis by Singh et al16 involving 8216 participants reported that varenicline use may be associated with increased minor and major cardiovascular events (odds ratio, 1.72; 95% confidence interval [CI], 1.09–2.71), a finding at odds with the goal of smoking cessation that garnered a great deal of media attention. A follow-up meta-analysis found the difference between varenicline and placebo to be statistically and clinically nonsignificant.17
The large number of smokers attempting to quit by using pharmacotherapies and the widespread media reports of cardiovascular risks associated with pharmacotherapies make clear public health messages a priority. At the request of the Food and Drug Administration (FDA), the drug maker (Pfizer Inc) recently conducted a meta-analysis based on major adverse cardiovascular events (MACEs), defined as cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.18 With the use of individual patient data from industry-sponsored RCTs, the hazard ratio was not significant (hazard ratio, 1.95; 95% CI, 0.79–4.82). The most recent FDA safety communication on varenicline from December 2012 indicates that the events were uncommon in both active and placebo drug conditions and that the increased risk was not statistically significant. Similarly, an FDA mini-sentinel evaluation evaluating CVD events among 89 519 varenicline users and 113 378 bupropion users found no difference in CVD event risk between varenicline and bupro-pion (incidence rate ratio, 1.02; 95% CI, 0.71–1.47).19
The concern about varenicline has led investigators to more closely examine the other pharmacotherapies. A large cohort study found no difference in CVD events between varenicline and bupropion among a nationwide study in Denmark (hazard ratio, 0.96; 95% CI, 0.67–1.39).20 A meta-analysis examining only NRT found an increased risk for less serious cardiovascular events such as tachycardia and nonspecific chest pain but did not examine MACEs.21 Notably, few of the RCTs have been conducted within populations with secondary CVD risk profiles.15,22 Most trials have compared an active medication with a placebo, with few trials evaluating head-to-head comparisons of cessation medications. Using a statistical technique called network meta-analysis, we can examine both direct (head-to-head RCTs) and indirect evidence and thus increase the power and interpretability of a comparative analysis.23 We aimed to examine the comparative safety of NRT, bupropion, and varenicline, evaluating all CVD events and MACEs reported in published RCTs and FDA reports in smokers with and without preexisting CVD.23
Methods
Eligibility Criteria
We included any RCT of NRT at any marketed dose or combination, bupropion at licensed doses, or varenicline at licensed doses. Studies had to enroll smokers at the initiation of therapy and report whether any CVD events occurred. We included studies of any duration as long as they reported a complete trial, defined as having provided the pre-planned duration of study drug. For varenicline RCTs, we obtained the individual-level data via a request about the confidential FDA report.18
Study End Points
We considered 2 definitions of cardiovascular events: (1) all cardiovascular events, defined as clinical diagnoses of any cardiovascular event considered in previous systematic reviews on risk of cardiovascular events associated with smoking cessation therapies,16,17,24 and (2) MACEs using the same criteria as the FDA report.18 They included cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. In circumstances when an event is reported but not attributed to a group, we contacted the study authors for clarification.
Search Strategy
In consultation with a medical librarian, we established a previously published search strategy (available in the online-only Data Supplement).24 We searched independently, in duplicate, the following 10 databases (from inception to March 20, 2013): MEDLINE, EMBASE, Cochrane CENTRAL, AMED, CINAHL, TOXNET, Development and Reproductive Toxicology, Hazardous Substances Databank, Psych-info, and Web of Science. We also searched databases including the full text of journals (OVID, ScienceDirect, and Ingenta, which includes articles in full text from 1993). In addition, we searched the bibliographies of published systematic reviews and health technology assessments and contacted the authors of individual RCTs. Searches were not limited by language, sex, or age.
Study Selection
Two investigators (P.W., S.E.) independently and in duplicate scanned abstracts and then obtained the full-text reports of RCTs evaluating the interventions of interest. After obtaining full reports of the candidate trials, the same reviewers independently assessed eligibility from full-text articles.
Data Collection
Two reviewers (P.W., S.E.) conducted data extraction independently using a standardized prepiloted form with the categories of CVD (available from the authors on request). Reviewers collected information about the smoking intervention, the population studied (age, sex, underlying conditions), treatment doses and dosing schedules, CVD events, and loss to follow-up. Study evaluation included general methodological quality features using a modified Cochrane risk of bias tool.25
Data Analysis
We assessed inter-rater reliability on inclusion of articles using the φ statistic, which provides a measure of interobserver agreement that is independent of chance.26 Our analysis required 2 approaches: pairwise meta-analysis of all direct RCT evidence and a network meta-analysis that includes both the direct RCT evidence and indirect comparisons of those treatments. We evaluated the major outcomes as all CVD events and MACEs. For pairwise meta-analysis, we used the conventional DerSimonian-Laird approach to account for unexplained heterogeneity between studies.27 We calculated the relative risk (RR) and 95% CIs of outcomes according to the number of events reported in the original studies or substudies. We calculated the I2 statistic for each analysis as a measure of the proportion of the overall variation that is attributable to between-study heterogeneity. We considered an I2 value >30% to be important and investigated the cause of heterogeneity using subgroup analysis and random-effects meta-regression.
In the absence of many head-to-head trials evaluating all interventions, we conducted a bayesian random-effects network meta-analysis.28,29 A detailed description of the underlying statistical model is provided in the online-only Data Supplement.
Results
Study Characteristics
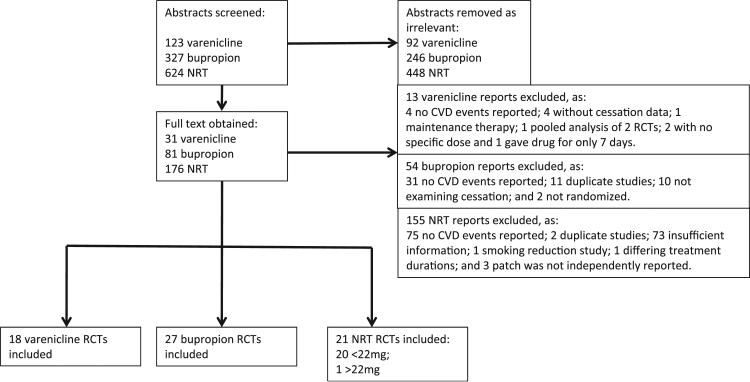
Figure 1 displays the flow diagram documenting the search and inclusion of relevant studies. Table I in the online-only Data Supplement lists the excluded studies that did not report on CVD events. Our review identified 63 eligible RCTs10,13–15,22,30–87 that reported cardiovascular events involving 30 508 patients. Table 1 displays the study characteristics. Of these 63 trials, there were 58 two-armed trials, 3 three-armed trials, and 2 four-armed trials. For trials that had multiple arms as a result of dose differences, we pooled those arms for each treatment. Nineteen RCTs evaluated NRT versus placebo10,30–34,36–38,40–46,49,53,68; 27 RCTs evaluated bupropion versus placebo13–15,47–49,51–71; 18 RCTs evaluated varenicline versus placebo22,54,55,72–79,81–87; 1 RCT evaluated high-dose NRT versus placebo39; 1 RCT evaluated combination NRT versus control35; 2 RCTs evaluated bupropion versus varenicline54,55; 3 RCTs evaluated bupropion versus NRT49,53,68; and 1 RCT evaluated varenicline versus NRT.80 Study quality was variable (Table II in the online-only Data Supplement).
Figure 1.
Flow diagram of randomized, controlled trials (RCT) selected for the meta-analysis of cardiovascular (CV) events associated with smoking cessation therapies. NRT indicates nicotine replacement therapy.
Table 1.
Characteristics of Included Trials of Nicotine Replacement Therapy, Bupropion, and Varenicline
| Trial | Participant Characteristics | Cigarettes per Day, mean (SD or range); median* | Years Smoking, mean (SD or range); median* | Treatment Duration, wk | Whole Study Duration, mo | Arm | Cotreatment | Age, mean (SD or range); median* | Male, % | n | Reported CV Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nicotine Replacement Therapy | |||||||||||
| Tønnesen et al,30 2012 |
Healthy | 22.7 (8.8) | NR | 52 | NR | Placebo | Counseling | 46.2 (11.3) | 54.7 | 161 | Myocardial infarction |
| Spray 1 mg | Counseling | 47.0 (10.9) | 56.9 | 318 | |||||||
| Thomsen et al,31 2010 |
Breast cancer surgery |
NR | NR | 2 | 12 | Placebo | Counseling | 56.5 (36–82) | 0.0 | 62 | CVD event |
| NRT | Counseling | 57.5 (35–79) | 0.0 | 58 | |||||||
| Shiffman et al,32 2009 |
Healthy | 25 (8) | 26 (12) | 12 | 6 | Placebo 2 mg | Counseling | 42.2 (13.3) | 34.5 | 817 | Heart rate |
| Gum 2 mg | Counseling | 42.1 (13.0) | 37.2 | 819 | |||||||
| Placebo 4 mg | Counseling | 46.3 (11.4) | 47.8 | 830 | |||||||
| Gum 4 mg | Counseling | 46.1 (11.3) | 52.4 | 830 | |||||||
| Oncken et al,33 2007 |
Postmenopausal women |
21 (8) | 33 (10) | 12 | 12 | Placebo | Group counseling |
56.6 (6.9) | 0.0 | 95 | Hospitalized chest pain |
| Patch 21 mg | Group counseling |
54.0 (6.9) | 0.0 | 57 | |||||||
| Wennike et al,34 2003 |
Healthy | 24 (7) | 29 (9) | 52 | 24 | Placebo 2 mg | 44.0 (10.0) | 41 | 68 | Heart palpitations |
|
| Gum 2 mg | 45.0 (10.0) | 35 | 65 | ||||||||
| Placebo 4 mg | 44.0 (10.0) | 41 | 138 | ||||||||
| Gum 4 mg | 45.0 (10.0) | 35 | 140 | ||||||||
| Etter et al,35 2002 |
Healthy | 30 (10) | ≥3 | 24 | 6 | Placebo | 41.7 | 49 | 269 | Stroke | |
| No treatment | 42.9 | 44 | 389 | ||||||||
| NRT 2, 15, 0.5 mg |
43.2 | 54 | 265 | ||||||||
| Glover et al,36 2002 |
Healthy | 29 (16) | 25 (11) | 12–24 | 12 | Placebo | 41.8 (11.6) | 44.6 | 121 | Atherosclerotic CVD | |
| Tablet 2 mg | 43.9 (10.0) | 47.5 | 120 | ||||||||
| Wallström et al,37 2000 |
Healthy | 19 (6) | 26 (10) | 12–24 | 12 | Placebo | 44.7 (11.4) | 45.2 | 124 | Atrial fibrillation | |
| Tablet 2 mg | 44.5 (11.6) | 36.6 | 123 | ||||||||
| Gum 4 mg | 41.4 (11.7) | 51.7 | 203 | ||||||||
| Hays et al,38 1999 |
Healthy | ≥15 | 26 (12) | 6 | 6 | Placebo | 44.1 (11.6) | 52.5 | 322 | Acute myocardial infarction |
|
| Patch 22 mg | 43.5 (11.2) | 48.6 | 321 | ||||||||
| Patch 10–15 mg |
28.2 (4.9) | 0.0 | 124 | ||||||||
| Tønnesen et al,39 1999 |
Healthy | 27 (10) | 23 (10) | 8 | 12 | Placebo | Advice brochure |
41.0 (10.0) | 52.0 | 714 | Heart palpitations, tachycardia, acute myocardial infarction |
| Patch 15 mg | Advice brochure |
41.0 (10.0) | 51.0 | 716 | |||||||
| Patch 25 mg | Advice brochure |
41.0 (10.0) | 53.0 | 715 | |||||||
| Blöndal et al,40 1997 |
Healthy | 25 (4–50) | 2.7 (1–5) | 12 | 24 | Placebo | 42 (21–67) | 38.5 | 78 | Heart palpitations | |
| Spray 1 mg | 42.0 (22–67) | 50.6 | 79 | ||||||||
| Sønderskov et al,41 1997 |
Healthy | ≥20 | 21 (11) | 12 | 6 | Placebo 14 mg |
38.9 (13.7) | 58.3 | 125 | Heart palpitations, chest pain |
|
| Patch 14 mg | 38.2 (12.9) | 41.7 | 119 | ||||||||
| Placebo 21 mg |
39.9 (10.9) | 49.2 | 142 | ||||||||
| Patch 21 mg | 39.1 (10.8) | 50.8 | 132 | ||||||||
| Joseph et al,10 1996 |
Cardiac disease | 28 | 44 | 10 | 6 | Placebo | Behaviour counseling |
60.0 | 98.6 | 290 | Stroke, acute myocardial infarction, atrial fibrillation, heart failure, CVD |
| Patch 7, 14, 21 mg |
Behaviour counseling |
61.0 | 98.6 | 294 | |||||||
| Gourlay et al,42 1995 |
Healthy | 27 (10) | 23 (10) | 12 | 6 | Placebo | Behavioral counseling |
41.0 (10.4) | 42.4 | 314 | Heart palpitations, cardiac arrhythmia |
| Patch 7–21 mg |
Behavioral counseling |
41.0 (10.4) | 42.4 | 315 | |||||||
| Schneider et al,43 1995 |
Healthy | 29 (10) | 22 (10) | 24 | 12 | Placebo | 39.7 (7.2) | 58.0 | 127 | Heart palpitations | |
| Spray 1 mg |
39.9 (7.7) | 52.0 | 128 | ||||||||
| Hjalmarson et al,44 1994 |
Healthy | 21 (10–70) | 26 (10) | 12 | 12 | Placebo | Group counseling |
44.9 (11.1) | 43.1 | 123 | Pounding heart |
| Spray 1 mg |
Group counseling |
44.9 (11.5) | 42.4 | 125 | |||||||
| Gum 2 mg |
Behavior modification program |
38.1 (8.8) | 76.0 | 76 | |||||||
| Sutherland et al,45 1992 |
Healthy | 26 (10) | 22 (10) | 4 | 12 | Placebo | 40.4 (9.4) | 34.2 | 111 | Pounding heart | |
| Spray 1 mg |
38.9 (9.4) | 37.1 | 116 | ||||||||
| Tønnesen et al,46 1988 |
Healthy plus chronic disease |
≥10 | NR | 6 | 24 | Placebo | Counseling | 45.5 (11.7) | 42.0 | 53 | Heart palpitations |
| Gum 2 mg |
Counseling | 44.9 (10.4) | 47.0 | 60 | |||||||
| Bupropion | |||||||||||
| Eisenberg et al,15 2013 |
Acute myocardial infarction |
23 (11) | 33 (12) | 9 | 12 | Placebo | Counseling | 53.4 (10.3) | 83.2 | 200 | Acute myocardial infarction, unstable angina, atrial fibrillation, cardiac arrest, tachycardia, cardiogenic shock, congestive heart failure, thrombo- endarterectomy |
| Bupropion 300 mg |
Counseling | 54.5 (10.4) | 83.8 | 192 | |||||||
| Planer et al,47 2011 |
Acute coronary syndrome |
31 (16) | NR | 8 | 12 | Placebo | Counseling | 51.5 (9) | 82.7 | 75 | Acute myocardial infarction, atrial fibrillation |
| Bupropion 300 mg |
Counseling | 52.4 (11) | 77 | 74 | |||||||
| McCarthy et al,48 2008 |
Healthy | 22 (10) | 25 (12) | 8 | 12 | Placebo | No counseling |
39.4 (11.3) | 46 | 116 | Stroke, aneurysm |
| Placebo | Counseling | 37.8 (12.8) | 47.9 | 121 | |||||||
| Bupropion 300 mg |
No counseling |
41.0 (12.6) | 50.9 | 116 | |||||||
| Bupropion 300 mg |
Counseling | 36.8 (11.4) | 54 | 113 | |||||||
| Covey et al,49 2007 |
Healthy | 21 (9) | NR | 20 | 12 | Placebo | Placebo gum | 42.5 (10.6) | 53.5 | 71 | Acute myocardial infarction |
| Placebo | Nicotine gum | 43.5 (10.8) | 54.2 | 72 | |||||||
| Bupropion 300 mg |
Placebo gum | 43.7 (10.8) | 53.4 | 73 | |||||||
| Bupropion 300 mg |
Nicotine gum | 40.3 (9.9) | 57.5 | 73 | |||||||
| Evins et al,50 2007 |
Schizophrenia | 26 (12) | 26 (11) | 12 | 6 | Placebo | Nicotine patch and gum |
43.6 (10.9) | NR | 26 | Heart palpitations |
| Bupropion 300 mg |
Nicotine patch and gum |
44.8 (9.2) | NR | 25 | |||||||
| Fossati et al,51 2007 |
Healthy | 23 (9) | ≥1 | 7 | 12 | Placebo | 48.5 (42–56) [IQR]* |
55.4 | 193 | Acute myocardial infarction |
|
| Bupropion 300 mg |
49.4 (40–57) [IQR]* |
62 | 400 | ||||||||
| Muramoto et al,52 2007 |
Adolescent | 11 (9) [IQR]* |
4* | 6 | 6 | Placebo | Counseling | 16* | 58.3 | 103 | Tachycardia |
| Bupropion 150 mg |
Counseling | 16* | 46.7 | 105 | |||||||
| Bupropion 300 mg |
Counseling | 16* | 57.7 | 104 | |||||||
| Uyar et al,53 2007 |
Pulmonary disease |
≥10 | ≥1 | 6 | 6 | Advice | 36.0 (10.6) | 70 | 31 | Tachycardia | |
| Bupropion 300 mg |
36.0 (10.5) | 88 | 50 | ||||||||
| Patch 7–21 mg |
36.3 (12.7) | 80.0 | 50 | ||||||||
| Gonzales et al, 54 |
Healthy | 21 (9) | 24 (12) | 12 | 12 | Placebo | Counseling | 42.6 (11.8) | 54.1 | 344 | Acute myocardial infarction, atrial fibrillation |
| Bupropion 300 mg |
Counseling | 42.0 (11.7) | 58.4 | 329 | |||||||
| Varenicline 2 mg/d |
Counseling | 42.5 (11.1) | 50 | 352 | |||||||
| Jorenby et al,55 2006 |
Healthy | 22 (12) | 25 (12) | 12 | 12 | Placebo | Counseling | 42.3 (11.6) | 58.1 | 341 | Acute myocardial infarction, coronary artery occlusion |
| Bupropion 300 mg |
Counseling | 42.9 (11.9) | 60.2 | 342 | |||||||
| Varenicline 2 mg/d |
Counseling | 44.6 (11.4) | 55.2 | 344 | |||||||
| Rigotti et al,13 2006 |
CVD | 22 (12) | 38 (11) | 12 | 12 | Placebo | Counseling | 54.9 (9.7) | 69 | 124 | Death in CVD |
| Bupropion 300 mg |
Counseling | 56.7 (9.7) | 69 | 124 | |||||||
| Puska et al,56 2005 |
Healthy | 23 (8) | ≥1 | 7 | 12 | Placebo | Motivational support |
40.3 (9.1) | 36 | 170 | Stroke |
| Bupropion 300 mg |
Motivational support |
40.3 (8.9) | 36 | 517 | |||||||
| Zellweger et al,57 2005 |
Healthy | 23 (8) | 26 (16) | 7 | 12 | Placebo | 40.3 (9.1) | 36 | 170 | Stroke | |
| Bupropion 300 mg |
40.3 (8.9) | 36 | 517 | ||||||||
| Dalsgareth et al,58 2004 |
Healthy | 19 (6) | 27 (13) | 7 | 6 | Placebo | 44.3 (9.4) | 25.4 | 114 | Tachycardia, acute myocardial infarction (death) |
|
| Bupropion 300 mg |
42.5 (9.9) | 25.3 | 221 | ||||||||
| Tonstad et al,14 2003 |
CVD | 25 (12) | 50 (25) | 7 | 12 | Placebo | 55.1 (9.0) | 79 | 313 | Angina pectoris, heart palpitations |
|
| Bupropion 300 mg |
55.6 (9.2) | 74 | 313 | ||||||||
| ZYB40030,59 2003 |
COPD | NR | NR | 9 | 9 wk | Placebo | 55 (9.5) | 63.4 | 159 | Acute myocardial infarction, angina |
|
| Bupropion 300 mg |
55 (9.5) | 63.4 | 155 | ||||||||
| George et al,60 2002 |
Schizophrenia | 24 (11) | NR | 10 | 6 | Placebo | 40.9 (9.4) | 50 | 16 | Irregular heartbeat | |
| Bupropion 300 mg |
45.4 (11.9) | 62.5 | 16 | ||||||||
| ZYB30011,61 2002 |
>1 CVD risk factor |
≥10 | ≥1 | 7 | 6 | Placebo | 49.2 (9.9) | 62.2 | 127 | Heart palpitations | |
| Bupropion 300 mg |
47.9 (9.7) | 69.3 | 127 | ||||||||
| Gonzales et al,62 2001 |
Healthy | ≥15 | NR | 12 | 6 | Placebo | 45.5 (11.2) | 45 | 224 | Stroke, acute myocardial infarction, atrial fibrillation, coronary artery disorder |
|
| Bupropion 300 mg |
44.5 (11.8) | 52 | 226 | ||||||||
| Hays et al,63 2001 |
Healthy | 27 (10) | ≥1 | 45 | 24 | Placebo | 45.4 (9.2) | 52.1 | 215 | Angina, stroke, acute myocardial infarction (death) |
|
| Bupropion 300 mg |
47.0 (9.7) | 45.3 | 214 | ||||||||
| Tashkin et al,64 2001 |
COPD | 28 (11) | 51 (24) | 12 | 6 | Placebo | 54.5 (9.5) | 55.1 | 205 | Stroke, cardiac arrest, myocardial infarction, |
|
| Bupropion 300 mg |
53.2 (9.0) | 54.9 | 206 | ||||||||
| ZYB40001,65 2001 |
Healthy | ≥15 | ≥1 month | 12 | 3 | Placebo | Behavioural support |
43.8 (22–68) | 50.3 | 143 | Stroke |
| Bupropion 300 mg |
Behavioural support |
43.7 (19–67) | 46.8 | 141 | |||||||
| ZYB40005,66 2001 |
NR | NR | NR | 24 | 12 | Placebo | 41.8 (18–71) | 53 | 304 | Acute myocardial infarction, congestive heart failure |
|
| Bupropion 300 mg |
42.4 (19–69) | 57.4 | 305 | ||||||||
| SMK20001,67 2000 |
Healthy | ≥15 | ≥1 | 7 | 12 | Placebo | 42.1 (10.2) | 51 | 143 | Stroke, acute myocardial infarction |
|
| Bupropion 300 mg |
42.9 (10.2) | 52.4 | 143 | ||||||||
| Jorenby et al,68 1999 |
Healthy | 26 (11) | 26 (11) | 9 | 12 | Placebo | None | 42.7 (10.2) | 41.2 | 160 | Acute myocardial infarction (death) |
| No treatment | Patch | 44.0 (10.9) | 48.4 | 244 | |||||||
| Bupropion 300 mg |
None | 42.3 (10.2) | 48.4 | 244 | |||||||
| Bupropion 300 mg |
Patch | 43.9 (11.6) | 50.6 | 245 | |||||||
| Hurt et al,69 1997 |
Healthy | 27 (10) | ≥1 | 7 | 12 | Placebo | 43.0 (10.7) | 40.5 | 153 | Cardiac arrest (death) | |
| Bupropion 100 mg |
44.1 (10.5) | 41.8 | 153 | ||||||||
| Bupropion 150 mg |
42.3 (11.3) | 49.7 | 153 | ||||||||
| Bupropion 300 mg |
45.0 (11.8) | 49.4 | 156 | ||||||||
| ZYBAK1A402,70 1994 |
Healthy | ≥20 | NR | 12 | 12 | Placebo | Counseling | 54 (11.3) | 86.3 | 95 | Tachycardia |
| Bupropion 300 mg |
Counseling | 51 (11.8) | 82.1 | 95 | |||||||
| AKIA401,71 1992 |
Healthy | ≥20 | NR | 12 | 12 | Placebo | Counseling | 58.0 (8.0) | 100 | 25 | Fatal hypotension (death) |
| Bupropion 300 mg |
Counseling | 55 (9.3) | 100 | 23 | |||||||
| Varenicline | |||||||||||
| Tønnesen et al,72 2013 |
Healthy | 23 (9) | NR | 12 | 52 | Placebo | Counseling | 55.6 (9.1) | 49.3 | 69 | Stroke, myocardial infarction |
| Varenicline 2 mg/d |
Counseling | 53.6 (8.2) | 42.9 | 70 | |||||||
| Rennard et al,73 2012 |
Healthy | 21 (10–70) | 25 (2–57) | 12 | 6 | Placebo | Counseling | 43.2 (12.2) | 59.6 | 166 | Carotid artery stenosis |
| Varenicline 2 mg/d |
Counseling | 43.9 (12.5) | 60 | 493 | |||||||
| Wong et al,74 2012 |
Perioperative | 17 (8) | ≥1 | 12 | 12 | Placebo | Counseling | 53.3 (11.4) | 50.4 | 135 | Myocardial infarction, ischemia, stroke, deep vein thrombosis, bradycardia |
| Varenicline 0.5–2 mg/d |
Counseling | 51.9 (11.8) | 55.0 | 151 | |||||||
| Garza et al,75 2011 |
Healthy | 22 (10–50) | 17 (3–49) | 12 | 3 | Placebo | Counseling | 33.8 (8.8) | 72.7 | 55 | Heart palpitations |
| Varenicline 2 mg/d |
Counseling | 33.4 (11.8) | 60 | 55 | |||||||
| Steinberg et al,76 2011 |
Hospitalized Patients |
≥10 | NR | 12 | 6 | Placebo | Counseling | 51 (22–78) | 60 | 40 | Heart palpitation, tachycardia, stroke, acute myocardial infarction |
| Varenicline 2 mg/d |
Counseling | 51 (22–78) | 59 | 39 | |||||||
| Tashkin et al,77 2011 |
Mild to moderate COPD |
24 (10–99) | 40 (11–67) | 12 | 12 | Placebo | Counseling | 57.1 (9.0) | 62.2 | 251 | Angina pectoris, stroke, acute myocardial infarction |
| Varenicline 2 mg/d |
Counseling | 57.2 (9.1) | 62.5 | 248 | |||||||
| Bolliger et al,78 2010 |
Healthy | 24 (10–90) | 26 (1–58) | 12 | 6 | Placebo | Counseling | 43.9 (10.8) | 65.7 | 198 | Tachycardia, atrial fibrillation |
| Varenicline 2 mg/d |
Counseling | 43.1 (10.8) | 57.7 | 390 | |||||||
| Fagerström et al,79 2010 |
Healthy | NR | 22 (11) | 12 | 6 | Placebo | Counseling | 43.9 (12.0) | 89.9 | 218 | Acute myocardial infarction |
| Varenicline 2 mg/d |
Counseling | 43.9 (12.0) | 88.7 | 214 | |||||||
| Rigotti et al,22 2010 |
CVD | 23 (10–60) | 40 (5–63) | 12 | 12 | Placebo | Counseling | 55.9 (8.3) | 82.2 | 359 | Hospitalized angina pectoris, coronary revascularization, acute myocardial infarction, stroke |
| Varenicline 2 mg/d |
Counseling | 57.0 (8.6) | 75.2 | 355 | |||||||
| Aubin et al,80 2008 |
Healthy | 23 (11–80) | 25 (1–62) | 12 | 9 | Varenicline 2 mg/d |
Counseling | 42.9 (10.5) | 48.4 | 376 | Myocardial infarction |
| Patch 7–21 mg |
Counseling | 42.9 (12.0) | 50 | 370 | |||||||
| Niaura et al,81 2008 |
Healthy | 22 (6–60) | 25 (2–50) | 12 | 12 | Placebo | Education booklet |
42.1 (11.7) | 53.5 | 160 | Acute myocardial infarction, atrial fibrillation, |
| Varenicline 0.5–2 mg/d |
Education booklet |
41.5 (11.3) | 50.3 | 160 | |||||||
| Nakamura et al,82 2007 |
Healthy | 24 (10) | 20 (11) | 12 | 12 | Placebo | Counseling | 39.9 (12.3) | 76 | 154 | Angina pectoris |
| Varenicline 0.5 mg/d |
Counseling | 40.2 (12.3) | 72.7 | 153 | |||||||
| Varenicline 1 mg/d |
Counseling | 39.0 (12.0) | 71.1 | 156 | |||||||
| Varenicline 2 mg/d |
Counseling | 40.1 (11.6) | 79.2 | 156 | |||||||
| Tsai et al,83 2007 |
Healthy | 23 (10–60) | 21 (3–52) | 12 | 6 | Placebo | Counseling | 40.9 (11.1) | 92.7 | 124 | Unstable angina |
| Varenicline 2 mg/d |
Counseling | 39.7 (9.3) | 84.9 | 126 | |||||||
| Williams et al,84 2007 |
Healthy | 23 (10–90) | 30 (4–57) | 52 | 12 | Placebo | Counseling | 46.6 (12.1) | 48.4 | 126 | CVD, acute myocardial infarction |
| Varenicline 2 mg/d |
Counseling | 48.2 (12.3) | 50.6 | 251 | |||||||
| Nides et al,85 2006 |
Healthy | 20 (8) | 24 (11) | 7 | 12 | Placebo | Counseling | 41.6 (10.4) | 52 | 127 | Stroke |
| Varenicline 0.3 mg/d |
Counseling | 41.9 (10.6) | 50 | 128 | |||||||
| Varenicline 1 mg/d |
Counseling | 42.9 (10.5) | 43.7 | 128 | |||||||
| Varenicline 2 mg/d |
Counseling | 41.9 (9.8) | 50.4 | 127 | |||||||
| Oncken et al,86 2006 |
Healthy | 21 (9) | 25 (10) | 12 | 12 | Placebo | Counseling | 43.0 (9.4) | 51.9 | 129 | Unstable angina, tachycardia |
| Varenicline 1 mg/d |
Counseling | 43.2 | 49.1 | 259 | |||||||
| Varenicline 2 mg/d |
Counseling | 43 | 48.6 | 259 | |||||||
| Tonstad et al,87 2006 |
Healthy | 21 (7) | 28 (10) | 12 | 12 | Placebo | 45.3 (10.4) | 48.3 | 607 | ||
| Varenicline 2 mg/d |
45.4 (10.4) | 50.2 | 603 | ||||||||
COPD indicates chronic obstructive pulmonary disease; CV, cardiovascular; CVD, cardiovascular disease; IQR, interquartile range; and NRT, nicotine replacement therapy.
Table 2.
Estimated RR and 95% CIs Produced by Random-Effects Pairwise Meta-Analysis for Cardiovascular Events in Smoking Cessation RCTs
| All CV Events | MACEs | ||||||
|---|---|---|---|---|---|---|---|
| Studies, n | Comparison | Events | RR (95% CI) | I2, % | Events | RR (95% CI) | I2, % |
| All trials | |||||||
| 21 RCTs10,30–46,49,53,68 | NRT vs placebo | 202/6329 vs 83/5318 | 1.81 (1.35–2.43) | 0 | 12/6329 vs 7/5318 | 1.38 (0.58–3.26) | 0 |
| 27 RCTs13-15,47–49,51–71 | Bupropion vs placebo | 50/5947 vs 42/4455 | 1.03 (0.71–1.50) | 0 | 15/5947 vs 25/4455 | 0.57 (0.31–1.04) | 0 |
| 18 RCTs22,54,55,72–79,81–87 | Varenicline vs placebo | 63/5469 vs 41/3603 | 1.24 (0.85–1.81) | 0 | 22/5469 vs 13/3603 | 1.44 (0.73–2.83) | 0 |
| 2 RCTs54,55 | Bupropion vs varenicline | 1/686 vs 2/696 | 0.74 (0.05–10.5) | 1/686 vs 0/696 | 3.07 (0.12–75.09) | ||
| 3 RCTs49,53,68 | Bupropion vs NRT | 4/367 vs 2/366 | 1.40 (0.25–7.82) | 2 | 0/367 vs 1/366 | 0.34 (0.01–7.94) | |
| 1 RCT80 | Varenicline vs NRT | 0/378 vs 2/379 | 0.20 (0.01–4.16) | 0/378 vs 2/379 | 0.20 (0.01–4.16) | ||
| High-risk patients only | k=13 | k=9 | |||||
| 3 RCTs10,46,53 | NRT vs placebo | 33/454 vs 26/374 | 1.24 (0.77–2.02) | 6/454 vs 4/374 | 1.48 (0.42–5.19) | NA | |
| 8 RCTs13–15,47,53,59,61,64 | Bupropion vs placebo | 27/1241 vs 25/1234 | 1.04 (0.59–1.83) | 0 | 9/1241 vs 15/1234 | 0.63 (0.28–1.41) | 0 |
| 3 RCTs22,74,77 | Varenicline vs placebo | 30/754 vs 26/745 | 1.15 (0.69–1.92) | 14/754 vs 11/745 | 1.35 (0.61–3.01) | 0 | |
| Bupropion vs varenicline | NA | NA | |||||
| 1 RCT53 | Bupropion vs NRT | 3/50 vs 0/50 | 7 (0.37–132.10) | 0/50 vs 0/50 | NA | ||
| Varenicline vs NRT | NA | NA | |||||
CI indicates confidence interval; CV, cardiovascular; MACE, major adverse cardiovascular event; NRT, nicotine replacement therapy; RCT, randomized, clinical trial; and RR, relative risk.
The 63 RCTs collectively included 30 508 participants. Among RCTs examining specific CVD risk groups, 8 trials included patients with CVD,10,13,15,22,46,47,61,87 4 trials included patients with chronic obstructive pulmonary disease,53,59,64,77 and 1 trial included perioperative patients.74 These RCTs were included in our analysis that was restricted to high-risk patients. The median duration of treatment across treatments was 12 weeks (interquartile range, 8–12 weeks), whereas the median duration of follow-up trial time was 12 months (interquartile range, 6–12 months). Attrition across the period of the trials was not importantly different by intervention or controls (NRT versus placebo, 23% versus 20%; bupropion versus placebo, 26% versus 31%; varenicline versus placebo, 28% versus 29%).
Pairwise Comparisons
We examined pairwise comparisons of all interventions with available head-to-head data. The results are reported in Table 2. We found no major evidence of heterogeneity because I2 values were equal or close to 0% at all times.
For NRT, the risk of any CVD event was statistically significantly increased compared with placebo (RR, 1.81; 95% credible interval [CrI], 1.35–2.43). When this was restricted to only MACEs, CIs became wide and thus did not suggest statistical evidence of harm (RR, 1.38; 95% CrI, 0.58–3.26). When this was restricted to high-risk patients, the RR decreased and CIs became wider.
For bupropion, the results suggested a direction of effect that is protective against MACEs for the entire study population (RR, 0.57; 95% CrI, 0.31–1.04). When the population was restricted to high-risk patients, the trend remained, but CIs became slightly wider. When only MACEs were considered, the RR became almost identical to 1.00.
For varenicline, the RR was slightly larger than 1.00 (ie, no difference) for both outcome definitions and population groups, but CIs were wide in all instances.
Network Meta-Analysis
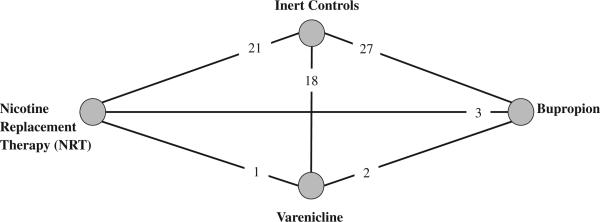
Figure 2 displays the trial network. The network meta-analysis results are reported in Table 3. The findings are similar to the pairwise findings and demonstrate that NRT was significantly associated with increased risk of all CVD events. In particular, risk of events with NRT was statistically increased compared with placebo and bupropion. However, when restricted to only MACE category of events, NRT was no longer significantly associated with harm.
Figure 2.
Geometric distribution of the mixed treatment comparison analysis, including randomized trials of nicotine replacement therapy (NRT), bupropion, and varenicline. Nodes represent the study therapies. Links between the nodes represent direct comparisons from randomized, clinical trials (RCTs). The numbers beside the nodes represent the number of RCTs.
Table 3.
Estimated RR and 95% CrI From Random-Effects Network Meta-Analysis for Cardiovascular Events in Smoking Cessation RCTs
| Comparison | All CVD events | MACEs |
|---|---|---|
| All trials | ||
| NRT vs placebo | 2.29 (1.39–3.82) | 1.95 (0.92–4.30) |
| Bupropion vs placebo | 0.98 (0.54–1.73) | 0.45 (0.21–0.85) |
| Varenicline vs placebo | 1.30 (0.79–2.23) | 1.34 (0.66–2.66) |
| Bupropion vs varenicline | 0.76 (0.33–1.73) | 0.33 (0.16–0.87) |
| Bupropion vs NRT | 0.43 (0.19–0.91) | 0.23 (0.08–0.63) |
| Varenicline vs NRT | 0.56 (0.25–1.27) | 0.67 (0.26–1.90) |
| High-risk populations (sensitivity analysis) | ||
| NRT vs placebo | 1.31 (0.58–3.32) | 1.53 (0.38–6.24) |
| Bupropion vs placebo | 1.06 (0.59–2.04) | 0.48 (0.18–1.21) |
| Varenicline vs placebo | 0.99 (0.45–1.88) | 1.22 (0.44–2.90) |
| Bupropion vs varenicline | 1.09 (0.46–2.92) | 0.39 (0.11–1.49) |
| Bupropion vs NRT | 0.81 (0.26–2.26) | 0.31 (0.05–1.68) |
| Varenicline vs NRT | 0.92 (0.34–2.19) | 0.81 (0.13–4.20) |
CrI indicates credibility interval; CVD, cardiovascular disease; MACE, major adverse cardiovascular event; NRT, nicotine replacement therapy; RCT, randomized, clinical trial; and RR, relative risk.
Bupropion appears to protect against the risk of MACEs relative to both NRT and varenicline. Varenicline was not associated with either benefit or harm in the network meta-analysis but had a significantly higher risk of harm compared with bupropion (Table 2).
High-Risk Populations
When we examined only RCTs that enrolled high-risk populations, the direction of effect was similar to the complete trials analysis, but none of the comparisons reached statistical significance (Table 2).
Sensitivity Analysis
We removed the MACEs from the NRT analysis to examine what end points were driving the harmful effect of NRT. When we removed all MACEs, the RR of NRT was 1.89 (95% CrI, 1.31–2.73). The most commonly reported NRT adverse events were heart palpitations. When we included only events we considered to be well-known lower-severity adverse events associated with NRT (ie, palpitations, bradycardia, and arrhythmia), the pooled RR was 2.08 (95% CrI, 1.35–3.19).
We also removed studies with <12 months’ duration to investigate potential effect modification by study duration. This analysis yielded results highly similar to the results of the main analysis for bupropion versus placebo (RR, 0.97; 95% CrI, 0.56–1.59) and for varenicline versus placebo (RR, 1.45; 95% CrI, 0.86–2.62). However, for NRT, the increased risk of all CVD was more pronounced and statistically evident 1 addressing all CVD events that included more minor events such as tachycardia, and 1 that followed FDA definitions of MACEs.18
Our study demonstrates that all 3 evaluated therapies were not harmful for MACEs. Bupropion appears to have a protective effect, whereas varenicline was not significantly associated with harm. NRT, the most widely used pharmacotherapy for smoking cessation, was associated with an increase in CVD events that was driven by lower-risk events, typically tachycardia, a well-known and largely benign effect of NRT.21 When our analysis was restricted to individuals with a higher-risk profile of having an event, because of a history of predisposing conditions, we did not find evidence of increased risk with any pharmacotherapy, although this was based on a smaller sample.
There are several strengths and limitations of this study to consider. Strengths include the comparative safety evaluation across pharmacotherapies, a strategy that, to the best of our knowledge, has not been applied previously. We evaluated 2 important definitions of CVD events, both all CVD events and the FDA definition of MACEs, considered to be a more stringent definition of patient important outcomes.18 Because we applied 2 different categories of events, our findings can inform where previous evaluations of safety may have been limited. Limitations of our review are driven predominantly by the necessity that trial reports or the FDA reports provided information on the outcomes of interest. Because concern about CVD risk with smoking cessation is a relatively new issue, many trials that reported effectiveness outcomes did not report CVD safety outcomes.24 Efforts to reduce this potential reporting bias by contacting study authors were hampered by nonresponse and the long period of time since the trials were published, particularly for NRT trials. Given the heterogeneous reporting of CVD events in RCTs, we used a composite outcome of MACEs, as used by the FDA.18 It is possible that individual components of the composite would find differing effects, but we acknowledge that any analysis of these would be hampered by lower power to detect a signal of harm. We found low rates of MACEs across the 3 interventions, resulting in wide CrIs. It is possible that with a vastly larger data set, treatment outcomes would change.18 However, we conducted post hoc power calculations to estimate the power of our comparisons for MACEs and found acceptable levels of power for all comparisons (see the online-only Data Supplement). Our varenicline analysis was hampered by lower power (online-only Data Supplement). For the most part, the findings are largely limited to smokers without preexisting heart disease. We found similar rates of attrition across interventions, ranging from 20% to 29%, yet it is possible that attrition reflects intolerability of the intervention and thus misses some events. We did not report the bayesian probability of risk because it are not widely understood and because the probability ranking can vary widely, depending on the sparseness of the data.88 Throughout this analysis, we present the point estimates with CrIs. Although some analyses did not reach statistical significance, the possibility of risk still exists when CrIs include an estimate that would be considered clinically important.
Our study found statistically significant evidence of all CVD events associated with NRT use. However, when we restricted this to MACEs, the finding was no longer statistically significant. When we examined these findings in a sensitivity analysis, we found that the treatment effects were driven predominantly by lower-level CVD events (RR, 1.91), including tachycardia and arrhythmia, both well-known adverse events of NRT use,9,21,89,90 and occurred primarily in studies with longer periods of follow-up.
There are several possible explanations why NRT use may increase some CVD events, and this has been recognized for some time, although it is not well understood or a major clinical concern.9,21,89,90 Chiefly, many smokers have a long history of smoking that may have established coronary artery disease. Those patients with unstable coronary syndrome may be exhibiting coronary vasoconstriction associated with plaque ruptures resulting from the increased strain of quitting and palpitations associated with NRT.89 Second, for those patients receiving NRT and continuing to smoke, high nicotine serum concentrations may stimulate the sympathetic nervous system response, thereby increasing blood pressure, stroke volume, and heart output.89 However, importantly, some research has documented more CVD events among patients with heart disease who smoked while on a placebo than on a nicotine patch.10 Furthermore, equivalent proportions of palpitations or chest pain were found among those who smoked and did not smoke during nicotine patch therapy.91
Only a few years on market, electronic cigarettes or e-cigarettes are a relatively new, and unregulated, approach to nicotine delivery. Consequently, the safety of these products and their use for quitting cigarette smoking have not been well evaluated. At this time, they are not considered cessation devices, and their contents and risk profiles are just beginning to be explored.92,93 Different guidelines and algorithms exist on the choice of cessation pharmacotherapy according to patient history of smoking, substance abuse, and chronic disease risk profiles. For example, both the Mayo Clinic and the Ottawa Model for Smoking Cessation recommend the use of NRT among at-risk CVD patients,94 whereas a US Surgeon General report (2010) advocates avoidance of NRT for 2 weeks after a major CVD event.95 Given the current findings of low risk of serious CVD events attributed to smoking cessation pharmacotherapies, combined with the well-established CVD and mortality risks of continued smoking, the benefits of use would seem to outweigh the risks; however, further study is needed, particularly investigation of the use of cessation medications in smokers hospitalized for ST-segment– elevation myocardial infarction.95
Our findings should be placed in the context of other available evidence. The concern about smoking cessation therapies increasing the risk of CVD events was most widely reported by Singh et al16 in 2011 in an evaluation of varenicline versus placebo RCTs. Using data from 14 RCTs, the study authors reported a Peto odds ratio for all CVD events of 1.72 (95% CI, 1.09–2.71). The Peto odds ratio is an artifact of a fixed-effects analysis and therefore has tighter CIs than random-effects models.96 Applying a random-effects analysis to their data set yields an RR of 1.43 (95% CI, 0.91–2.25), which is not very different from the findings in our analysis of 16 RCTs (RR, 1.24; 95% CI, 0.85–1.81). Much has been written about the choice of effect measure for RCTs, and it is well understood that odds ratios can be perceived as inflating the treatment effects.97 Prochaska and Hilton17,98 have demonstrated this with the varenicline and CVD risk data. As a result of the controversy about varenicline and CVD risk, the FDA conducted its own meta-analysis using individual patient data addressing its definition of MACEs on 30-day posttreatment outcomes and found a hazard ratio of 1.95 (95% CI, 0.79–4.82), which is not very different from the findings of our analysis based on additional aggregate data (RR, 1.57; 95% CI, 0.67–3.17). Our finding that less clinically concerning events drove the signifi-cant finding of NRT for all CVD events is consistent with findings from our previously published meta-analysis that is based on RCTs and observational data on the outcome of chest pain and palpitations (RR, 1.66; 95% CI, 1.22–2.28).21 Although the comparative effects of each therapy are, to the best of our knowledge, a new approach to evaluating the safety of smoking cessation therapies, a recent nationwide observational study in Denmark examined the comparative harms of bupro-pion and varenicline and did not demonstrate significant harm for either treatment.20 Similar findings were reported in the United States.19
The potential cardioprotective role of bupropion is not well understood. We did not find bupropion protective against all CVD events; however, we did find a statistically significant protective effect for MACEs. It is possible that the antidepressant origins of bupropion reduce vascular stress.99,100 However, at higher doses, bupropion also has sympathomimetic activity and can increase heart rate and blood pressure.99,100 On the basis of our present findings, bupropion may be cardioprotective, likely through its effects on increasing smoking cessation and alleviating depression, although closer investigation of the cardiovascular effects of bupropion are warranted.
Physicians often weigh the benefits and risks of available treatments, including cessation pharmacotherapies. Concerns about adverse events need to be balanced with the consistent evidence for the benefit of smoking cessation, and patients should be counseled about what adverse events may be associated with smoking cessation therapies, the symptoms associated with the withdrawal period from cigarettes, and the symptoms that may be attributable to existing diseases.
CLINICAL PERSPECTIVE.
Patients often use pharmacotherapies to aid in smoking cessation. Current licensed pharmacotherapies include nicotine replacement therapies, bupropion, and varenicline. Recently, there has been widespread public concern that varenicline may be associated with an increase in cardiovascular disease (CVD) events. Clinicians and the public are unsure about which smoking cessation therapies will offer the greatest likelihood of quitting with the safest adverse event profile. Using a statistical approach that permits the synthesis of direct and indirect randomized, clinical trial evidence, we compared the cardiovascular safety of nicotine replacement therapies, bupropion, and varenicline. We examined 2 categories of events: a composite of all CVD events that included both minor and major events and only major adverse CVD events. We included 63 randomized, clinical trials that reported CVD events. We found no increase in the risk of all CVD events with bupropion or varenicline. Nicotine replacement therapies had a statistically elevated risk that was driven predominantly by less serious events such as tachycardia. When the analysis was restricted to only major CVD events, we found a protective effect with bupropion and no clear evidence of harm with varenicline or nicotine replacement therapies. Our findings indicate that there is no clear evidence of major CVD events associated with smoking cessation. The increase in nicotine replacement therapy–associated CVD events was driven by well-known and largely benign events such as tachycardia and palpitations.
Acknowledgments
Drs Mills and Thorlund have consulted for Merck & Co Inc, Pfizer Ltd, Novartis, Takeda, or GlaxoSmithkline on network meta-analyses issues. However, no funding was received from any of these entities for this manuscript. Dr Prochaska has received an investigator-initiated research award from Pfizer Inc (WS981308). Pfizer Inc has had no role in this manuscript. Dr Mills receives salary support from the Canadian Institutes of Health Research through a Canada Research Chair. Dr Thorlund receives salary support from the Canadian Institutes of Health Research via the Drug and Safety Evaluation Network to develop methods for assessing harms using network meta-analysis. Dr Prochaska receives research and salary support from the National Institute on Drug Abuse (P50 DA09253 and R34DA030538), the National Institute of Mental Health (R01 MH083684), and the State of California Tobacco-Related Disease Research Program (17RT-0077 and 21BT-0018).
Footnotes
Disclosures The other authors report no conflicts.
References
- 1.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- 2.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-Century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 3.Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Arch Intern Med. 2000;160:939–944. doi: 10.1001/archinte.160.7.939. [DOI] [PubMed] [Google Scholar]
- 4.Glantz S, Gonzalez M. Effective tobacco control is key to rapid progress in reduction of non-communicable diseases. Lancet. 2012;379:1269–1271. doi: 10.1016/S0140-6736(11)60615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Health Consequences of Smoking: A Report of the Surgeon General. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2004. [Google Scholar]
- 6.Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence: 2008 update: clinical practice guideline. US Department of Health and Human Services, Public Health Service; 2008. http://www. surgeongeneral.gov/tobacco/treating_tobacco_use08. pdf. Accessed August 14, 2013. [Google Scholar]
- 7.Hartmann-Boyce J, Stead LF, Cahill K, Lancaster T. Efficacy of interventions to combat tobacco addiction: Cochrane update of 2012 reviews. Addiction. 2013;108:1711–1721. doi: 10.1111/add.12291. [DOI] [PubMed] [Google Scholar]
- 8.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dacosta A, Guy JM, Tardy B, Gonthier R, Denis L, Lamaud M, Cerisier A, Verneyre H. Myocardial infarction and nicotine patch: a contributing or causative factor? Eur Heart J. 1993;14:1709–1711. doi: 10.1093/eurheartj/14.12.1709. [DOI] [PubMed] [Google Scholar]
- 10.Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, Sherman SE, Cleveland M, Antonuccio DO, Antonnucio DO, Hartman N, McGovern PG. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335:1792–1798. doi: 10.1056/NEJM199612123352402. [DOI] [PubMed] [Google Scholar]
- 11.Haustein KO, Krause J, Haustein H, Rasmussen T, Cort N. Comparison of the effects of combined nicotine replacement therapy vs. cigarette smoking in males. Nicotine Tob Res. 2003;5:195–203. doi: 10.1080/146222003100073676. [DOI] [PubMed] [Google Scholar]
- 12.Zevin S, Jacob P, 3rd, Benowitz NL. Dose-related cardiovascular and endocrine effects of transdermal nicotine. Clin Pharmacol Ther. 1998;64:87–95. doi: 10.1016/S0009-9236(98)90026-1. [DOI] [PubMed] [Google Scholar]
- 13.Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y, Swartz S, Torres-Finnerty N, Emmons KM, Singer DE. Bupropion for smokers hospitalized with acute cardiovascular disease. Am J Med. 2006;119:1080–1087. doi: 10.1016/j.amjmed.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, Silagy C, van Spiegel PI, Astbury C, Hider A, Sweet R. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24:946–955. doi: 10.1016/s0195-668x(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg MJ, Grandi SM, Gervais A, O'Loughlin J, Paradis G, Rinfret S, Sarrafzadegan N, Sharma S, Lauzon C, Yadav R, Pilote L. ZESCA Investigators. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: a randomized, placebo-controlled trial. J Am Coll Cardiol. 2013;61:524–532. doi: 10.1016/j.jacc.2012.08.1030. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Loke YK, Spangler JG, Furberg CD. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183:1359–1366. doi: 10.1503/cmaj.110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856. doi: 10.1136/bmj.e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration FDA Drug Safety Communication: safety review update of Chantix (varenicline) and risk of cardiovascular adverse events. http://www.fda.gov/Drugs/DrugSafety/ucm330367.htm. Accessed December 12, 2012.
- 19.Toh S, Baker MA, Brown JS, Kornegay C, Platt R. Mini-Sentinel Investigators. Rapid assessment of cardiovascular risk among users of smoking cessation drugs within the US Food and Drug Administration's Mini-Sentinel program. JAMA Intern Med. 2013;173:817–819. doi: 10.1001/jamainternmed.2013.3004. [DOI] [PubMed] [Google Scholar]
- 20.Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176. doi: 10.1136/bmj.e7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation: a systematic review and meta-analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8:8. doi: 10.1186/1617-9625-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121:221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308:1246–1253. doi: 10.1001/2012.jama.11228. [DOI] [PubMed] [Google Scholar]
- 24.Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. 2012;44:588–597. doi: 10.3109/07853890.2012.705016. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meade MO, Cook RJ, Guyatt GH, Groll R, Kachura JR, Bedard M, Cook DJ, Slutsky AS, Stewart TE. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:85–90. doi: 10.1164/ajrccm.161.1.9809003. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 29.Thorlund K, Thabane L, Mills EJ. Modelling heterogeneity variances in multiple treatment comparison meta-analysis: are informative priors the better solution? BMC Med Res Meth. 2013:13. doi: 10.1186/1471-2288-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tønnesen P, Lauri H, Perfekt R, Mann K, Batra A. Efficacy of a nicotine mouth spray in smoking cessation: a randomised, double-blind trial. Eur Respir J. 2012;40:548–554. doi: 10.1183/09031936.00155811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen T, Tønnesen H, Okholm M, Kroman N, Maibom A, Sauerberg ML, Møller AM. Brief smoking cessation intervention in relation to breast cancer surgery: a randomized controlled trial. Nicotine Tob Res. 2010;12:1118–1124. doi: 10.1093/ntr/ntq158. [DOI] [PubMed] [Google Scholar]
- 32.Shiffman S, Ferguson SG, Strahs KR. Quitting by gradual smoking reduction using nicotine gum: a randomized controlled trial. Am J Prev Med. 2009;36:96–104.e1. doi: 10.1016/j.amepre.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 33.Oncken C, Cooney J, Feinn R, Lando H, Kranzler HR. Transdermal nicotine for smoking cessation in postmenopausal women. Addict Behav. 2007;32:296–309. doi: 10.1016/j.addbeh.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Wennike P, Danielsson T, Landfeldt B, Westin A, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98:1395–1402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- 35.Etter JF, Laszlo E, Zellweger JP, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: a randomized trial. J Clin Psychopharmacol. 2002;22:487–495. doi: 10.1097/00004714-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Glover ED, Glover PN, Franzon M, Sullivan CR, Cerullo CC, Howell RM, Keyes GG, Nilsson F, Hobbs GR. A comparison of a nicotine sublingual tablet and placebo for smoking cessation. Nicotine Tob Res. 2002;4:441–450. doi: 10.1080/1462220021000018443. [DOI] [PubMed] [Google Scholar]
- 37.Wallström M, Nilsson F, Hirsch JM. A randomized, double-blind, placebo-controlled clinical evaluation of a nicotine sublingual tablet in smoking cessation. Addiction. 2000;95:1161–1171. [PubMed] [Google Scholar]
- 38.Hays JT, Croghan IT, Schroeder DR, Offord KP, Hurt RD, Wolter TD, Nides MA, Davidson M. Over-the-counter nicotine patch therapy for smoking cessation: results from randomized, double-blind, placebo-controlled, and open label trials. Am J Public Health. 1999;89:1701–1707. doi: 10.2105/ajph.89.11.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, Rijcken B, Sawe U. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial: Collaborative European Anti-Smoking Evaluation: European Respiratory Society. Eur Respir J. 1999;13:238–246. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- 40.Blöndal T, Franzon M, Westin A. A double-blind randomized trial of nicotine nasal spray as an aid in smoking cessation. Eur Respir J. 1997;10:1585–1590. doi: 10.1183/09031936.97.10071585. [DOI] [PubMed] [Google Scholar]
- 41.Sønderskov J, Olsen J, Sabroe S, Meillier L, Overvad K. Nicotine patches in smoking cessation: a randomized trial among over-the-counter customers in Denmark. Am J Epidemiol. 1997;145:309–318. doi: 10.1093/oxfordjournals.aje.a009107. [DOI] [PubMed] [Google Scholar]
- 42.Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ. 1995;311:363–366. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider NG, Olmstead R, Mody FV, Doan K, Franzon M, Jarvik ME, Steinberg C. Efficacy of a nicotine nasal spray in smoking cessation: a placebo-controlled, double-blind trial. Addiction. 1995;90:1671–1682. doi: 10.1046/j.1360-0443.1995.901216719.x. [DOI] [PubMed] [Google Scholar]
- 44.Hjalmarson A, Franzon M, Westin A, Wiklund O. Effect of nicotine nasal spray on smoking cessation: a randomized, placebo-controlled, double-blind study. Arch Intern Med. 1994;154:2567–2572. [PubMed] [Google Scholar]
- 45.Sutherland G, Stapleton JA, Russell MA, Jarvis MJ, Hajek P, Belcher M, Feyerabend C. Randomised controlled trial of nasal nicotine spray in smoking cessation. Lancet. 1992;340:324–329. doi: 10.1016/0140-6736(92)91403-u. [DOI] [PubMed] [Google Scholar]
- 46.Tønnesen P, Fryd V, Hansen M, Helsted J, Gunnersen AB, Forchammer H, Stockner M. Effect of nicotine chewing gum in combination with group counseling on the cessation of smoking. N Engl J Med. 1988;318:15–18. doi: 10.1056/NEJM198801073180104. [DOI] [PubMed] [Google Scholar]
- 47.Planer D, Lev I, Elitzur Y, Sharon N, Ouzan E, Pugatsch T, Chasid M, Rom M, Lotan C. Bupropion for smoking cessation in patients with acute coronary syndrome. Arch Intern Med. 2011;171:1055–1060. doi: 10.1001/archinternmed.2011.72. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, Baker TB. A randomized controlled clinical trial of bupro-pion SR and individual smoking cessation counseling. Nicotine Tob Res. 2008;10:717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- 49.Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, LoDuca C, Petkova E, Rodriguez K. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction. 2007;102:1292–1302. doi: 10.1111/j.1360-0443.2007.01887.x. [DOI] [PubMed] [Google Scholar]
- 50.Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, Freudenreich O, Henderson DC, Schoenfeld DA, Rigotti NA, Goff DC. A 12-week double-blind, placebo-controlled study of bupropion SR added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27:380–386. doi: 10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- 51.Fossati R, Apolone G, Negri E, Compagnoni A, La Vecchia C, Mangano S, Clivio L, Garattini S. General Practice Tobacco Cessation Investigators Group. A double-blind, placebo-controlled, randomized trial of bupropion for smoking cessation in primary care. Arch Intern Med. 2007;167:1791–1797. doi: 10.1001/archinte.167.16.1791. [DOI] [PubMed] [Google Scholar]
- 52.Muramoto ML, Leischow SJ, Sherrill D, Matthews E, Strayer LJ. Randomized, double-blind, placebo-controlled trial of 2 dosages of sustained-release bupropion for adolescent smoking cessation. Arch Pediatr Adolesc Med. 2007;161:1068–1074. doi: 10.1001/archpedi.161.11.1068. [DOI] [PubMed] [Google Scholar]
- 53.Uyar M, Filiz A, Bayram N, Elbek O, Herken H, Topcu A, Dikensoy O, Ekinci E. A randomized trial of smoking cessation: medication versus motivation. Saudi Med J. 2007;28:922–926. [PubMed] [Google Scholar]
- 54.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 55.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 56.Puska PM, Barrueco M, Roussos C, Hider A, Hogue S. The participation of health professionals in a smoking-cessation programme positively influences the smoking cessation advice given to patients. Int J Clin Pract. 2005;59:447–452. doi: 10.1111/j.1368-5031.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 57.Zellweger JP, Boelcskei PL, Carrozzi L, Sepper R, Sweet R, Hider AZ. Bupropion SR vs placebo for smoking cessation in health care professionals. Am J Health Behav. 2005;29:240–249. doi: 10.5993/ajhb.29.3.5. [DOI] [PubMed] [Google Scholar]
- 58.Dalsgareth OJ, Hansen NC, Søes-Petersen U, Evald T, Høegholm A, Barber J, Vestbo J. A multicenter, randomized, double-blind, placebo-controlled, 6-month trial of bupropion hydrochloride sustained-release tablets as an aid to smoking cessation in hospital employees. Nicotine Tob Res. 2004;6:55–61. doi: 10.1080/14622200310001656867. [DOI] [PubMed] [Google Scholar]
- 59.ZYB40030: a multi-centre, randomised, double-blind, placebo controlled study to evaluate the efficacy and tolerability of bupropion hydrochloride (SR) sustained release versus placebo as an aid to smoking cessation in a population of smokers with chronic obstructive pulmonary disease. http://download.gsk-clinicalstudyregister.com/files/726.pdf; Accessed August 14, 2013.
- 60.George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, Kosten TR. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biol Psychiatry. 2002;52:53–61. doi: 10.1016/s0006-3223(02)01339-2. [DOI] [PubMed] [Google Scholar]
- 61.ZYB 30011:A multicentre, randomised, double-blind, placebo controlled study to evaluate the efficacy and tolerability of bupropion hydrochlo-ride (SR) sustained release (2 x 150mg per day) versus placebo as an aid to smoking cessation in smokers with at least one cardiovascular (CV) risk factor. http://download.gsk-clinicalstudyregister.com/files/730.pdf. Accessed August 14, 2013.
- 62.Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, Herrero LA, Krishen A, Sweeney A, Buaron K, Metz A. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin Pharmacol Ther. 2001;69:438–444. doi: 10.1067/mcp.2001.115750. [DOI] [PubMed] [Google Scholar]
- 63.Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, Sachs DP, Wolter TD, Buist AS, Johnston JA, White JD. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. a randomized, controlled trial. Ann Intern Med. 2001;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 64.Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, Gonzales D, Dozier G, Patel MK, Jamerson B. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomised trial. Lancet. 2001;357:1571–1575. doi: 10.1016/s0140-6736(00)04724-3. [DOI] [PubMed] [Google Scholar]
- 65.ZYB40001: A randomized, double-blind, placebo-controlled, 12-week smoking cessation trial of Zyban (150 mg bid) in adult smokers previously treated with Zyban. http://download.gsk-clinicalstudyregister.com/files/740.pdf; 2001. Accessed August 14, 2013.
- 66.ZYB40005: The effect of sustained-release bupropion HCl vs. placebo as an aid to smoking reduction leading to cessation among smokers unwilling and unable to quit smoking. http://download.gsk-clinicalstudyregister.com/files/729.pdf. Accessed August 14, 2013.
- 67.SMK20001:A Multi-Center, Double-Blind, Double-Dummy, Placebo-Controlled, Randomized, Parallel Group, Dose Response Evaluation of a New Chemical Entity (NCE) and ZYBAN (bupropion hydrochloride) Sustained Release (300mg/day) versus Placebo As Aids to Smoking Cessation. http://download.gsk-clinicalstudyregister.com/files/812.pdf 2000. Accessed August 14, 2013.
- 68.Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 69.Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 70.ZYBAKIA402: A single center evaluation of Wellbutrin (bupropion hydrochloride) versus placebo as an aid to smoking cessation (study 402) http://download.gsk-clinicalstudyregister.com/files/833.pdf; 1994. Accessed August 14, 2013.
- 71.AKIA401:A single-center evaluation of Wellbutrin (bupropion hydro-chloride) versus placebo as an aid to smoking cessation in heavy smokers (study 401) http://download.gsk-clinicalstudyregister.com/files/809.pdf; 1992. Accessed August 14, 2013.
- 72.Tønnesen P, Mikkelsen K. Varenicline to stop long-term nicotine replacement use: a double-blind, randomized, placebo-controlled trial. Nicotine Tob Res. 2013;15:419–427. doi: 10.1093/ntr/nts146. [DOI] [PubMed] [Google Scholar]
- 73.Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, St Aubin LB, Russ C. Flexible Quit Date Study Group. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2012;14:343–350. doi: 10.1093/ntr/ntr220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wong J, Abrishami A, Yang Y, Zaki A, Friedman Z, Selby P, Chapman KR, Chung F. A perioperative smoking cessation intervention with varenicline: a double-blind, randomized, placebo-controlled trial. Anesthesiology. 2012;117:755–764. doi: 10.1097/ALN.0b013e3182698b42. [DOI] [PubMed] [Google Scholar]
- 75.Garza D, Murphy M, Tseng LJ, Riordan HJ, Chatterjee A. A double-blind randomized placebo-controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biol Psychiatry. 2011;69:1075–1082. doi: 10.1016/j.biopsych.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 76.Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, Carson JL. Tobacco dependence treatment for hospitalized smokers: a randomized, controlled, pilot trial using varenicline. Addict Behav. 2011;36:1127–1132. doi: 10.1016/j.addbeh.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 77.Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139:591–599. doi: 10.1378/chest.10-0865. [DOI] [PubMed] [Google Scholar]
- 78.Bolliger CT, Issa JS, Posadas-Valay R, Safwat T, Abreu P, Correia EA, Park PW, Chopra P. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2011;33:465–477. doi: 10.1016/j.clinthera.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 79.Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi: 10.1136/bmj.c6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr, Gong J, Williams KE, Reeves KR. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niaura R, Hays JT, Jorenby DE, Leone FT, Pappas JE, Reeves KR, Williams KE, Billing CB., Jr The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr Med Res Opin. 2008;24:1931–1941. doi: 10.1185/03007990802177523. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Tsai ST, Cho HJ, Cheng HS, Kim CH, Hsueh KC, Billing CB, Jr, Williams KE. A randomized, placebo-controlled trial of varenicline, a selective alpha-4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther. 2007;29:1027–1039. doi: 10.1016/j.clinthera.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Williams KE, Reeves KR, Billing CB, Jr, Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr Med Res Opin. 2007;23:793–801. doi: 10.1185/030079907x182185. [DOI] [PubMed] [Google Scholar]
- 85.Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo-and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- 86.Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 87.Tonstad S, Tønnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Varenicline Phase 3 Study Group. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 88.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 89.Mathew TP, Herity NA. Acute myocardial infarction soon after nicotine replacement therapy. QJM. 2001;94:503–504. doi: 10.1093/qjmed/94.9.503. [DOI] [PubMed] [Google Scholar]
- 90.Ottervanger JP, Festen JM, de Vries AG, Stricker BH. Acute myocardial infarction while using the nicotine patch. Chest. 1995;107:1765–1766. doi: 10.1378/chest.107.6.1765. [DOI] [PubMed] [Google Scholar]
- 91.Gourlay SG, Forbes A, Marriner T, McNeil JJ. Predictors and timing of adverse experiences during transdermal nicotine therapy. Drug Saf. 1999;20:545–555. doi: 10.2165/00002018-199920060-00007. [DOI] [PubMed] [Google Scholar]
- 92.Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310:685–686. doi: 10.1001/jama.2013.109501. [DOI] [PubMed] [Google Scholar]
- 93.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2013 Mar 6; doi: 10.1136/tobaccocontrol-2012-050859. http://dx.doi.org/10.1136/tobaccocontrol-2012-050859. Accessed August 14, 2013. [DOI] [PMC free article] [PubMed]
- 94.University of Ottawa Heart Institute Ottawa Model for Smoking Cessation. http://www.ottawamodel.ca.
- 95.How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Department of Health and Human Services, Public Health Service, Office of Surgeon General; Rockville, MD: 2010. [PubMed] [Google Scholar]
- 96.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 97.Naylor CD, Chen E, Strauss B. Measured enthusiasm: does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med. 1992;117:916–921. doi: 10.7326/0003-4819-117-11-916. [DOI] [PubMed] [Google Scholar]
- 98.Prochaska JJ, Hilton JF. Varenicline's adverse events: choice of summary statistics: relative and absolute measures. BMJ. 2013;346:f1092. doi: 10.1136/bmj.f1092. [DOI] [PubMed] [Google Scholar]
- 99.Roose SP. Considerations for the use of antidepressants in patients with cardiovascular disease. Am Heart J. 2000;140(suppl):84–88. doi: 10.1067/mhj.2000.109977. [DOI] [PubMed] [Google Scholar]
- 100.Roose SP, Dalack GW, Glassman AH, Woodring S, Walsh BT, Giardina EG. Cardiovascular effects of bupropion in depressed patients with heart disease. Am J Psychiatry. 1991;148:512–516. doi: 10.1176/ajp.148.4.512. [DOI] [PubMed] [Google Scholar]