Abstract
PET with 18F-FDG allows for noninvasive assessment of regional lung metabolism reflective of neutrophilic inflammation. This study aimed at determining during early acute lung injury whether local 18F-FDG phosphorylation rate and volume of distribution were sensitive to the initial regional inflammatory response and whether they depended on the mechanism of injury: endotoxemia and surfactant depletion.
Methods
Twelve sheep underwent homogeneous unilateral surfactant depletion (alveolar lavage) and were mechanically ventilated for 4 h (positive end-expiratory pressure, 10 cm H2O; plateau pressure, 30 cm H2O) while receiving intravenous endotoxin (lipopolysaccharide-positive [LPS+] group; n = 6) or not (lipopolysaccharide-negative group; n = 6). 18F-FDG PET emission scans were then acquired. 18F-FDG phosphorylation rate and distribution volume were calculated with a 4-compartment model. Lung tissue expression of inflammatory cytokines was measured using real-time quantitative reverse transcription polymerase chain reaction.
Results
18F-FDG uptake increased in LPS+ (P = 0.012) and in surfactant-depleted sheep (P < 0.001). These increases were topographically heterogeneous, predominantly in dependent lung regions, and without interaction between alveolar lavage and LPS. The increase of 18F-FDG uptake in the LPS+ group was related both to increases in the 18F-FDG phosphorylation rate (P < 0.05) and to distribution volume (P < 0.01). 18F-FDG distribution volume increased with infiltrating neutrophils (P <0.001) and phosphorylation rate with the regional expression of IL-1β (P = 0.026), IL-8 (P = 0.011), and IL-10 (P = 0.023).
Conclusion
Non-invasive 18F-FDG PET-derived parameters represent histologic and gene expression markers of early lung injury. Pulmonary metabolism assessed with 18F-FDG PET depends on the mechanism of injury and appears to be additive for endotoxemia and surfactant depletion. 18F-FDG PET may be a valuable imaging biomarker of early lung injury.
Keywords: respiratory distress syndrome, adult, endotoxemia, pulmonary edema, positron emission tomography, fluorodeoxyglucose F18
Simon et al. recognized in 1947 that the injured lung is metabolically distinct from the normal lung (1). Lung neutrophilic infiltration, mediated by cytokines secreted at the site of inflammation by activated endothelial and epithelial cells and leukocytes, is a key feature of the acute respiratory distress syndrome (ARDS) (2) and a major component of that metabolic change (3–5).
We and others have explored the uptake of the glucose analog 18F-FDG as a biomarker of ARDS (5–7). For this, we applied a 4-compartment model to pulmonary 18F-FDG kinetics (8,9), which allows for the separation of the net uptake rate of the glucose analog 18F-FDG (Ki) into more specific and biologically relevant parameters: the cellular metabolic activity (phosphorylation rate constant, k3) and the distribution volumes of tracer available for phosphorylation (Fei) and in pulmonary edema (Fee) (8,9). Indeed, k3 has been associated with tissue hexokinase activity (10), tumor aggressiveness, and response to therapy (11) and Fee with lung water content (9). Yet, the relationship of these more specific measures of cellular metabolic activity with regional inflammation in early lung injury is currently unknown.
Human ARDS usually results from multiple injurious hits to the lung. These hits are expected to produce different degrees of cellular metabolic activity, pulmonary edema, and 18F-FDG tracer distribution. Indeed, endotoxemia produces marked tissue neutrophilic infiltration with substantial cellular activation (12) as well as hexokinase activation and translocation from the cytoplasm to the perimembrane region (13), whereas surfactant depletion by lung lavage produces discrete neutrophilic infiltration (7,14) with unknown effects on hexokinase. Knowledge is scant on the specific contributions of metabolic activity and tracer distribution to 18F-FDG uptake in different forms of acute lung injury, their topographic distribution in heterogeneous lungs of size comparable to the human, and the regional association of those metabolic changes with gene expression of inflammatory cytokines during early lung injury.
In this study, we aimed to investigate topographically the functional, metabolic, and inflammatory changes occurring in the early stages of lung injury produced by a combination of distinct injurious mechanisms including surfactant depletion, mechanical ventilation, and endotoxemia in a large-animal model with lung heterogeneity compatible with that of humans. We also correlated the metabolic changes assessed noninvasively by imaging and modeling of pulmonary 18F-FDG kinetics with regional RNA expression of cytokines usually associated with ARDS.
MATERIALS AND METHODS
Methods are further described in the supplemental material (available at http://jnm.snmjournals.org).
Experimental Protocol
The procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital. Twelve sheep (weight ± SD, 22.0 ± 1.8 kg) were anesthetized, intubated, and mechanically ventilated. A left-sided double-lumen endobronchial tube was placed through a tracheotomy and used to produce lung surfactant depletion by alveolar saline lavage. Warm saline (~400 mL) was instilled in the left bronchus (pressure, ~30 cm H2O) of initially supine sheep, followed by draining to gravity. After 3 aliquots, animals were turned prone for 3 additional aliquots. A regular endotracheal tube was then placed and double-lung ventilation resumed.
Animals were positioned supine in the PET scanner with the field of view immediately above the diaphragmatic dome and mechanically ventilated for 4 h using the following parameters: positive end-expiratory pressure, 10 cm H2O; fraction of inspired oxygen (FiO2), 0.6; inspiratory-to-expiratory ratio, 1:2; tidal volume adjusted to a plateau pressure of 30 cm H2O; and respiratory rate adjusted to normocapnia. Transmission and 13NN emission PET scans were obtained at baseline and at 4 h of mechanical ventilation. 18F-FDG PET scans were acquired after the 4-h 13NN scan. After baseline imaging, 6 sheep (lipopolysaccharide-positive [LPS+] group) received a continuous 10 ng·kg−1·min−1 intravenous infusion of endotoxin (Escherichia coli O55:B5; List Biologic Laboratories Inc.) whereas 6 did not (LPS-negative [LPS−] group).
PET Imaging Protocol and Processing
Images consisted of 15 transverse slices. Three scan modalities were performed.
Transmission scans were acquired for attenuation correction and calculation of the gas fraction (Fgas) from regional tissue density (Ftissue):
| Eq. 1 |
13NN emission scans were acquired after a bolus injection of 13NN-saline to measure regional pulmonary perfusion and shunt (15) and after inhalation of 13NN-gas to measure regional ventilation (16). 18F-FDG emission scans were obtained for quantification of regional 18F-FDG kinetics. Acquisition of sequential PET frames started simultaneously with the beginning of an intravenous constant-flow 60-s infusion of 18F-FDG (185–370 MBq [5–10 mCi]) and lasted 75 min. Each lung was divided for analysis into 3 adjacent isogravitational regions of interest (ROIs) of equal vertical height (nondependent, middle, and dependent).
18F-FDG Kinetics
A 4-compartment model designed to quantify 18F-FDG kinetics during lung injury was used to estimate kinetics parameters (Supplemental Fig. 1) (8). The rate constant k3 characterizes 18F-FDG phosphorylation (17). Two functionally distinct volumes of distribution were computed: Fei, containing 18F-FDG immediately available for phosphorylation, and Fee, containing 18F-FDG not immediately available for phosphorylation, presumably associated with lung edema (8). Parameters were estimated in each ROI by fitting the kinetics using an iterative optimization technique. The net uptake rate of 18F-FDG from plasma to tissue (Ki) was computed as:
| Eq. 2 |
A tissue fraction, blood fraction, and wet-to-dry ratio (w/d) normalized Ki (KiT) were calculated to account for the effects of regional lung aeration, blood volume, and water on 18F-FDG uptake (7).
Lung Neutrophil Counts, Histology, and Cytokines
Five-micrometer-thick tissue samples were stained with hematoxylin and eosin for light microscopy. Regional lung expression of tumor necrosis factor α (TNF-α), IL-1β, IL-6, IL-8, and IL-10 was measured using real-time quantitative reverse transcription polymerase chain reaction (Supplemental Table 1). Neutrophil counts, acute lung injury scores (18), and lung cytokine expressions were assessed in nondependent (ventral) and dependent (dorsal) regions of each lung.
Statistical Analysis
Data are expressed as mean ± SD if normally distributed or median (interquartile range [IQ], 25%–75%) otherwise. Two-way ANOVA with repeated measures was used to compare physiologic values at baseline and 4 h of mechanical ventilation and compare PET data at baseline and 4 h of mechanical ventilation and between lungs. Bonferroni-corrected post hoc tests were performed when the overall P value was less than 0.05 to identify statistically significant 2 × 2 comparisons. The relationship between 18F-FDG kinetics parameters and regional lung cytokine expression and neutrophil counts was assessed by linear regression.
RESULTS
Global Physiologic Variables
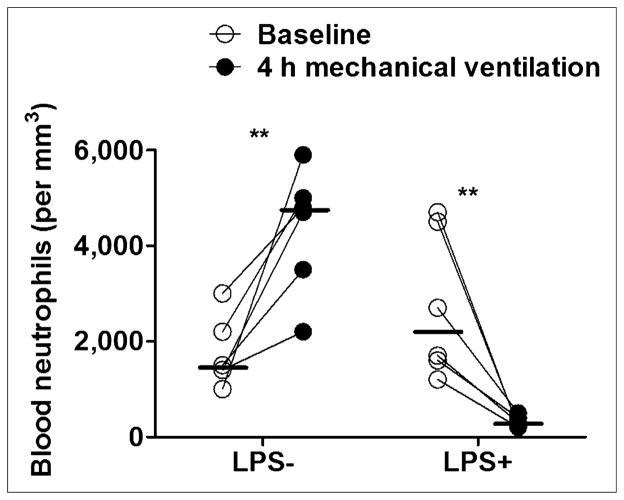
PaO2/FiO2 ratios were lower than 300 mm Hg in both groups at 4 h of mechanical ventilation. Systemic blood pressure remained stable (Table 1), whereas mean pulmonary artery pressure and pulmonary vascular resistances increased in the LPS+ group. At 4 h, the LPS+ group showed lower respiratory system compliance than the LPS− group. Blood neutrophil counts decreased markedly in the LPS+ group whereas they increased in the LPS− group (Fig. 1).
TABLE 1.
Global Physiologic Variables at Baseline (After Unilateral Lung Lavage) and After 4 Hours of Mechanical Ventilation
| Variable | LPS−
|
LPS +
|
P
|
|||
|---|---|---|---|---|---|---|
| Baseline (n = 6) | 4 h (n = 6) | Baseline (n = 6) | 4 h (n = 6) | Time | LPS | |
| VT (mL/kg) | 15.7 ± 5.6 | 16.6 ± 4.7 | 13.4 ± 3.5 | 11.8 ± 2.7 | 0.63 | 0.16 |
|
| ||||||
| PEEP (cm H2O) | 10 (10–11) | 10 (9–11) | 9 (8–10) | 9 (8–10) | 0.73 | 0.18 |
|
| ||||||
| Respiratory rate (/min) | 23 ± 4 | 23 ± 4 | 21 ± 5 | 21 ± 4 | 0.66 | 0.33 |
|
| ||||||
| PaO2/FiO2 (torr) | 149 (102–320) | 161 (123–173) | 109 (84–300) | 89 (64–211) | 0.92 | 0.37 |
|
| ||||||
| PaCO2 (torr) | 33 ± 5 | 37 ± 7 | 36 ± 10 | 39 ± 4 | 0.20 | 0.38 |
|
| ||||||
| Crs (mL/cm H2O) | 17.4 ± 6.3 | 18.6 ± 4.1 | 13.3 ± 3.5 | 11.2 ± 3.1* | 0.65 | 0.03 |
|
| ||||||
| Qs/Qt | 0.32 (0.18–0.48) | 0.35 (0.11–0.56) | 0.43 (0.23–0.53) | 0.56 (0.23–0.66) | 0.28 | 0.26 |
|
| ||||||
| PVR (dynes·s·cm−5) | 393 ± 99 | 314 ± 84 | 253 ± 82† | 584 ± 163‡ | <0.001 | 0.07 |
|
| ||||||
| MPAP (mm Hg) | 22 ± 2 | 22 ± 4 | 22 ± 5† | 34 ± 5‡ | <0.001 | 0.02 |
|
| ||||||
| MAP (mm Hg) | 102 ± 12 | 98 ± 18 | 84 ± 8 | 84 ± 23 | 0.77 | 0.04 |
|
| ||||||
| Heart rate (bpm) | 155 ± 43 | 157 ± 41 | 185 ± 29 | 167 ± 29 | 0.54 | 0.26 |
|
| ||||||
| Cardiac output (L/min) | 4.1 ± 1.3 | 3.3 ± 0.5 | 4.7 ± 0.8 | 3.7 ± 0.7 | 0.02 | 0.24 |
P < 0.05 vs. LPS− group at 4 h of mechanical ventilation and LPS (mechanical ventilation + LPS).
P < 0.001 vs. 4 h of mechanical ventilation + LPS for same group.
P < 0.001 vs. LPS− group at 4 h of mechanical ventilation and LPS (mechanical ventilation + LPS).
VT = tidal volume; PEEP = positive end-expiratory pressure; Crs = respiratory system compliance; Qs/Qt = global shunt fraction; PVR = pulmonary vascular resistances; MPAP = mean pulmonary arterial pressure; MAP = mean arterial pressure.
Data are mean ± SD or median, with IQ (25–75) in parentheses.
FIGURE 1.
Blood neutrophil counts at baseline and 4 h of mechanical ventilation. Counts increased significantly in LPS− group, in contrast to significant decrease in LPS+ group. Horizontal lines represent median values. **P < 0.01.
Global and Regional Gas Fraction, Shunt Fraction, and Perfusion
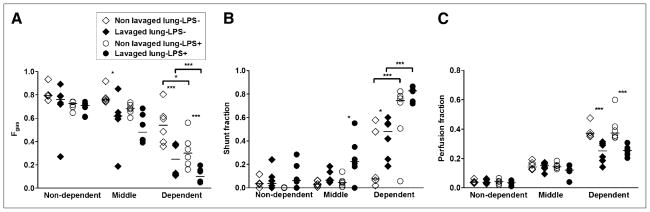
At 4 h of mechanical ventilation, whole-lung aeration (Fgas) was 30% lower in lavaged lungs and tended to be lower in the LPS+ group (Table 2). The LPS+ group presented lower Fgas in dependent regions than the LPS− group (P < 0.05), and lavaged lungs had lower Fgas than nonlavaged lungs (P < 0.001) (Fig. 2A).
TABLE 2.
Whole-Lung Variables at 4 Hours of Mechanical Ventilation
| Variable | LPS−
|
LPS+
|
P
|
||||
|---|---|---|---|---|---|---|---|
| Nonlavaged lung | Lavaged lung | Nonlavaged lung | Lavaged lung | Lavage | LPS | Interaction | |
| Ki (10−3/min) | 1.2 ± 1.0 | 2.3 ± 1.7* | 4.4 ± 1.9 | 7.2 ± 4.3† | <0.01 | 0.012 | 0.20 |
|
| |||||||
| Fei (10−2) | 7.6 ± 2.1 | 12.6 ± 1.4†,‡ | 14.7 ± 5.9 | 22.2 ± 8.6§ | <0.001 | 0.015 | 0.32 |
|
| |||||||
| k3 (10−2/min) | 1.6 ± 1.0 | 1.8 ± 1.3 | 3.1 ± 1.6 | 3.6 ± 2.5 | 0.45 | 0.10 | 0.78 |
|
| |||||||
| Fgas | 0.73 ± 0.08 | 0.50 ± 0.18§ | 0.59 ± 0.06 | 0.41 ± 0.08§ | <0.001 | 0.057 | 0.50 |
|
| |||||||
| Perfusion fraction | 0.52 ± 0.09 | 0.47 ± 0.09 | 0.56 ± 0.08 | 0.43 ± 0.08 | 0.12 | 0.37 | 0.47 |
|
| |||||||
| Shunt | 0.18 ± 0.06 | 0.27 ± 0.04‡ | 0.33 ± 0.09 | 0.58 ± 0.08† | 0.013 | 0.023 | 0.20 |
|
| |||||||
| Neutrophils/field | 3.3 ± 1.9 | 4.9 ± 2.2* | 4.3 ± 3.3 | 8.7 ± 4.5|| | <0.001 | 0.045 | 0.033 |
P < 0.01 vs. lavaged lung of LPS+ group.
P < 0.05 vs. nonlavaged lung of same group.
P < 0.05 vs. lavaged lung of LPS+ group.
P < 0.01 vs. nonlavaged lung of same group.
P < 0.001 vs. nonlavaged lung of same group.
FIGURE 2.
Fgas (A), shunt fraction (B), and perfusion fraction (C) for dependent, middle, and nondependent ROIs of lavaged and nonlavaged lungs of LPS− and LPS+ groups after 4 h of mechanical ventilation. Horizontal lines represent median values. *P < 0.05. ***P < 0.001.
LPS infusion and alveolar lavage increased whole-lung shunt fraction (Table 2). Shunt increased with LPS administration in both lavaged (P < 0.01) and nonlavaged lungs (P < 0.05) and from nondependent to dependent ROIs (P < 0.001) (Fig. 2B). Vertical shunt gradients were larger in the LPS+ than in the LPS− group (LPS infusion × ROI interaction, P < 0.001). Regional shunt was higher in lavaged than nonlavaged lungs in both groups (Fig. 2B).
Remarkably, the apparent effect of LPS on shunt contrasted with its lack of detectable effect on the distribution of lung perfusion to the whole lavaged versus nonlavaged single lungs (Table 2). At the regional level, perfusion fraction was also not affected by LPS (P = 0.61) (Fig. 2C) but was lower in dependent lavaged lungs in the LPS+ and LPS− groups and increased from nondependent to dependent regions (P < 0.001). Lavaged lungs presented lower vertical perfusion gradients than nonlavaged lungs (lavage × ROI interaction, P < 0.001), with dependent ROIs exhibiting approximately 30% lower perfusion fraction in lavaged than in nonlavaged lungs in both groups (P < 0.001).
Global and Regional Pulmonary 18F-FDG Kinetics
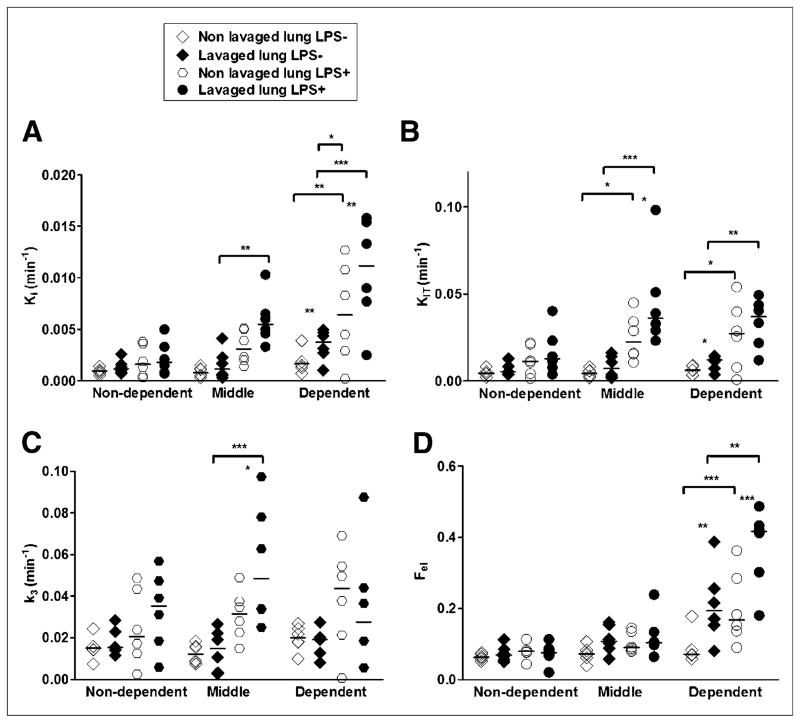
Metabolic activity quantified by the net 18F-FDG uptake rate Ki was higher in lavaged and LPS-exposed lungs after 4 h of mechanical ventilation (Table 2; Fig. 3). There was no statistically significant interaction between alveolar lavage and LPS, suggesting their additive rather than synergistic effect on Ki. Lavaged lungs exhibited 1.5 (IQ, 1.2–1.9) times higher Ki values than nonlavaged lungs. LPS+ lavaged lungs showed 2.6 (IQ, 1.1–5.6) times higher Ki values than LPS− lavaged lungs. Regional analysis revealed higher Ki in lavaged (P < 0.01) and LPS+ (P < 0.01) lungs (Fig. 4A). The effect of both LPS exposure and alveolar lavage on Ki interacted with ROI location (P < 0.001)—that is, both LPS+ and lavaged lungs presented larger 18F-FDG uptake vertical gradients than LPS− and nonlavaged lungs. In dependent and middle regions, LPS+ lavaged lung Ki values were 2.5 (IQ, 2.0–6.9) and 1.7 (IQ, 1.3–3.3) times higher than those of LPS− lavaged lungs.
FIGURE 3.
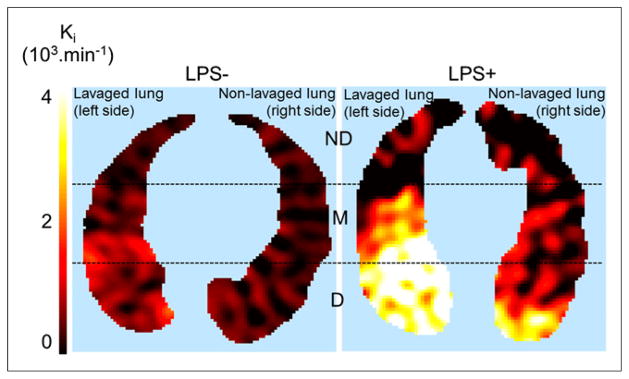
Transverse single slice of 18F-FDG uptake rate (Ki, computed voxel-by-voxel with Patlak method (28)) in LPS+ and LPS− animal. Color scale: black = lowest, white = highest values in image. Dashed lines indicate separation among 3 isogravitational ROIs: non-dependent (ND), middle (M), and dependent (D). There is higher 18F-FDG uptake in lavaged than in nonlavaged lung. LPS+ lungs exhibited markedly higher 18F-FDG uptake than LPS− lungs.
FIGURE 4.
18F-FDG kinetics parameters estimated with 4-compartment model in isogravitational ROIs of lavaged and nonlavaged lungs of LPS− and LPS+ groups. 18F-FDG net uptake rate (Ki) without (A) or with (KiT) (B) normalization for tissue density; blood volume; and w/d ratios (i.e., KiT = Ki·[w/d]/[Ftissue·(wN/dN)]) (where wN/dN is the normal w/d ratio of a sheep lung), rate constant k3 (associated with hexokinase activity) (C), and intracellular distribution volume of 18F-FDG (Fei) (D). *P < 0.05. **P < 0.01. ***P < 0.001.
Dependent regions of LPS+ and LPS− lavaged lungs had higher Ki values than those of nonlavaged lungs despite a 30% reduction in regional perfusion in these areas (Fig. 2C). Those significant effects of LPS administration (P < 0.001) and ROIs (P < 0.05) on 18F-FDG uptake were still present when regional aeration, blood volume, and water (Supplemental Fig. 2) were accounted for (KiT, Fig. 4B), indicating that they were not a mere consequence of regional changes in lung density. In contrast, there was no effect of lavage on KiT (P = 0.12).
To understand the factors determining regional Ki, we studied its components: the phosphorylation rate (k3) and the distribution volume of tracer immediately available for phosphorylation (Fei), where Ki = k3·Fei. This analysis revealed that k3 tended to increase with LPS but not with lavage (Table 2). In contrast, Fei was increased by both alveolar lavage and LPS exposure (Table 2). Regional k3 was higher in LPS+ lungs (P < 0.05) but not affected by lavage (P = 0.81) nor by ROI location (P = 0.20) (Fig. 4C). Regional Fei was higher with LPS (P < 0.05) and increased from nondependent to dependent regions (Fig. 4D; P < 0.001), with significant interaction (LPS × ROIs; P = 0.016), indicating that LPS magnified the vertical gradient of Fei. Regional Fei was also higher in lavaged lungs of the LPS− group (P < 0.001) and tended to be higher in the LPS+ group (P = 0.074).
Regional Lung Neutrophilic Infiltration and Lung Cytokine Messenger RNA Expression
Whole-lung neutrophil counts were higher with LPS and lung lavage (Table 2). In both groups, regional neutrophil counts were higher in dependent than nondependent regions and in dependent lavaged than nonlavaged regions (Supplemental Fig. 3A). Lavaged lungs of the LPS+ group exhibited 2.1 ± 1.3 times more lung neutrophils than those of the LPS− group. Lung injury score was increased by LPS both in lavaged (P = 0.056) and in nonlavaged (P < 0.001) lungs, with no effect of ROI position or lavage (Fig. 5; Supplemental Fig. 3B). LPS administration increased the pulmonary messenger RNA expression of all studied cytokines (Supplemental Fig. 4). In contrast, alveolar lavage increased only the expression of TNF-α.
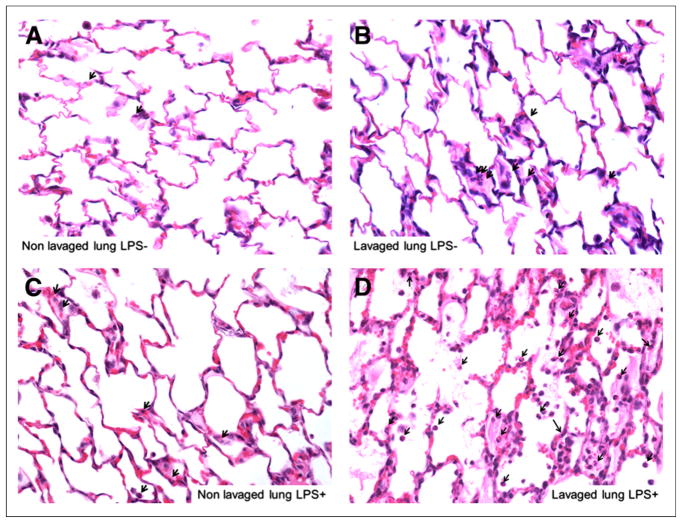
FIGURE 5.
Hematoxylin and eosin–stained histologic sections of dependent (dorsal) regions of nonlavaged (A and C) and lavaged (B and D) lungs from LPS− (A and B) and LPS+ animal (C and D) (magnification, ×400). Lavaged lungs exhibited neutrophilic infiltration (arrows) and mild thickening of alveolar walls but no other feature assessed in lung injury score (i.e., capillary congestion, alveolar edema, and alveolar hemorrhage). LPS exposure produced marked neutrophilic infiltration by neutrophils as well as capillary congestion and alveolar edema, particularly in lavaged lung (D).
The determinants of the 18F-FDG uptake rate, Fei and k3, were regionally associated with lung tissue neutrophil counts and cytokine expression. Fei increased with neutrophil counts in the LPS+ group (R2 = 0.31, P < 0.001) but not in the LPS− group (R2 = 0.08, P = 0.18) (Supplemental Fig. 5). The 18F-FDG phosphorylation rate k3 increased with the regional expression of IL-1β, IL-8, and IL-10 in the LPS+ but not in the LPS− group (Table 3; Supplemental Fig. 6).
TABLE 3.
Linear Regression Between Regional Lung Expression of Cytokines (TNF-α, IL-1β, IL-6, IL-8, and IL-10) and Regional Phosphorylation Rate k3 for LPS− and LPS+ Groups
| Cytokine | LPS−
|
LPS+
|
||
|---|---|---|---|---|
| R2 | P | R2 | P | |
| TNF-α | 0.02 | 0.45 | 0.01 | 0.58 |
|
| ||||
| IL-1β | 0.01 | 0.65 | 0.14 | 0.026* |
|
| ||||
| IL-6 | 0.01 | 0.80 | 0.04 | 0.22 |
|
| ||||
| IL-8 | 0.01 | 0.93 | 0.18 | 0.011 |
|
| ||||
| IL-10 | 0.07 | 0.14 | 0.14 | 0.023* |
Correlation was still significant after removing outlier point (IL-1β: R2 = 0.15, P = 0.019; IL-10: R2 = 0.10, P = 0.029).
Coefficients of determination (R2) and P values for correlation between k3 and regionally measured cytokines.
DISCUSSION
Our study showed that exposure to mild–moderate endotoxemia led to a substantially larger lung metabolic response, assessed with 18F-FDG uptake, than did surfactant depletion. The increase of 18F-FDG uptake in LPS-exposed lungs derived from increases in both the phosphorylation rate k3, a noninvasive measure of hexokinase activity, and the distribution volume of 18F-FDG available for phosphorylation. In contrast, increases in 18F-FDG uptake in lavaged lungs not exposed to LPS were predominantly related to increases in that distribution volume of 18F-FDG. In LPS-exposed lungs, the regional k3 was statistically albeit weakly associated with the local expression of key cytokines involved with neutrophil chemoattraction and the distribution volume of 18F-FDG with the number of infiltrating neutrophils.
We used mechanical ventilation pressures bound by accepted clinical limits and 2 well-defined experimental models of ARDS: endotoxemia, which induces pulmonary vascular sequestration of neutrophils within the first hour (19), and surfactant depletion by alveolar lavage, known to produce mild lung neutrophilic infiltration (7,14). The combination of these models allowed us to investigate regional pulmonary 18F-FDG kinetics and neutrophil recruitment in distinct injurious lung conditions. The contrasting response of blood neutrophil counts in LPS-exposed (decreased) versus nonexposed (increased) animals indicates that different levels of neutrophilic inflammation were triggered. Accordingly, lung neutrophilic infiltration was increased by both insults, to a greater extent with LPS than with surfactant depletion, consistent with the finding of higher 18F-FDG uptake with LPS.
Both surfactant depletion and LPS exposure increased the whole-lung net 18F-FDG uptake rate Ki. Ki distribution was markedly heterogeneous within lavaged and nonlavaged lungs, following a gradient along the ventral–dorsal axis. Importantly, this gradient was still present after accounting for regional lung density, blood volume, and water content (KiT), emphasizing that regional differences were not merely due to lung edema or collapse (7). Factors potentially accounting for larger inflammation in dependent regions are mechanical instabilities exacerbated by surfactant depletion with concentration of stresses (20), cyclic recruitment (7), and vertical perfusion gradients (6).
Ki derives from the product of 18F-FDG phosphorylation rate (k3), representing hexokinase activity (8,10,17,21,22), and the distribution volume of 18F-FDG immediately available for phosphorylation (Fei, Ki = k3·Fei). Regional quantification of these Ki components revealed that the effects of LPS exposure and lavage on 18F-FDG uptake differed not only by their magnitude, but also by the contribution of those components. Indeed, LPS increased 18F-FDG uptake through both Fei and k3. In contrast, alveolar lavage increased 18F-FDG uptake primarily through Fei. Considering that neutrophils are the main cells contributing to lung 18F-FDG uptake during lung injury (5,23,24), our in vivo data suggest that neutrophilic inflammation produced by endotoxemia is characterized not only by increased neutrophil counts but also importantly by more metabolically activated neutrophils than those during alveolar lavage. This finding is consistent with the higher injury scores found in LPS+ nonlavaged than in LPS− lavaged dependent regions, despite their similar neutrophil counts. The relevance of understanding and quantifying these components with a noninvasive technique translatable to humans is highlighted by the likely crucial role of neutrophil activation in the development of ARDS (2).
The value of k3 as a measure of cell phosphorylation rate (10), and thus as a direct noninvasive assessment of cellular metabolic activity, has been explored in oncology with large k3 values related to increased cancer aggressiveness and poor prognosis, whereas low k3 indicated effectiveness of therapy (10,11). Knowledge is scant on the value of k3 during ARDS. In sheep models of endotoxemic ARDS, higher k3 values were observed with an injurious versus a protective mechanical ventilation strategy in dependent lung regions (22) and related to the magnitude of regional lung strain (25).
Our data indicate a weak but statistically significant association between regional k3 and regional expression of IL-1β, IL-8, and IL-10, suggesting a relationship between the metabolic activity of neutrophils and the regional expression of neutrophil-chemoattracting cytokines. Interestingly, the cytokine with the strongest correlation with k3 was IL-8, presumably the main neutrophil-chemoattracting cytokine during ARDS (26). Such findings are consistent with our measurements of increased neutrophil counts and metabolic activity in endotoxin-exposed lung. The obtained r2 values for the k3-cytokine gene expression association were small, suggesting a relevant role for other biologic processes in determining regional k3. Potentially relevant biologic factors present between gene expression of cytokines and tissue phosphorylation rate (k3) include gene translation, presence of cytokine receptors and required enzymes in target areas, and time course of gene transcription and translation. Additional sources could contribute to variability such as coregistration between ROIs used to compute 18F-FDG kinetics parameters and lung tissue samples (cytokines) and precision of cytokines messenger RNA measurements.
In the LPS− group, however, regional Fei did not correlate with lung neutrophil counts and regional k3 did not correlate with regional lung cytokines. These findings are consistent with the mild neutrophilic infiltration produced by saline lavage (14) and its mild effect on cytokine expression, except for TNF-α (27). Interestingly, recent microautoradiography data in murine ARDS models not only confirmed predominant 18F-FDG uptake in neutrophils after LPS exposure, but also revealed that type 2 pneumocytes contributed significantly to 18F-FDG uptake in ventilator-induced injury (23). Thus, the lack of correlation between Fei and lung neutrophils in LPS− animals could imply the contribution of other cell types to 18F-FDG uptake (21,23).
Overall, our results indicate that regional 18F-FDG kinetics parameters during experimental ARDS depend on the mechanism of lung injury. Similar Ki values can derive from different combinations of k3 and Fei relevant for interpretation of 18F-FDG measurements. k3 and Fei allow for valuable assessment of inflammatory activity during early lung injury. In view of their noninvasiveness and human applicability, 18F-FDG kinetics measurements could be considered to study and manage patients at risk for ARDS and to evaluate therapeutic strategies. The time and logistic considerations currently necessary to scan these critically ill patients limit the potential use of these techniques to selected patients.
Perfusion is a key variable in the process of regional load of inflammatory cells and mediators and consequently a potential important determinant of regional 18F-FDG uptake and lung injury (6). However, we found that within isogravitational ROIs, perfusion fractions were similar irrespective of LPS exposure and lower in surfactant-depleted versus nondepleted lungs, implying that differences in regional perfusion could not account for the observed inter-ROI differences in 18F-FDG kinetics and regional inflammatory response. Such a result is unexpected, because in heterogeneously perfused lungs, a direct relationship between inflammation and blood flow would be anticipated (6). The lack of direct relationship suggests that differences in 18F-FDG kinetics parameters among groups, determined by glucose transport and metabolism processes, are not entirely limited by regional blood flow in the ranges of physiologic variables present in the study.
CONCLUSION
Our study shows that pulmonary metabolic activity measured in vivo and noninvasively by 18F-FDG PET increased globally and regionally in response to LPS, surfactant depletion, and mechanical ventilation. The increase in metabolic activity after 4 h of mechanical ventilation was higher after moderate endotoxemia than after alveolar lavage and predominated in dependent lung regions. Pulmonary 18F-FDG kinetics analysis showed that LPS increased lung metabolism through increases in both phosphorylation rate of lung tissue and the volume of distribution of 18F-FDG. That phosphorylation rate was associated with the regional gene expression of cytokines important to the regional control of neutrophil chemoattraction and the regional volume of distribution of 18F-FDG with the number of infiltrating neutrophils. These findings support the application of advanced modeling of 18F-FDG kinetics as an imaging-based biomarker of early lung injury.
Supplementary Material
Acknowledgments
We thank Dr. Florence Canoui-Poitrine for reviewing the statistical methods.
Footnotes
DISCLOSURE
This work was supported by NIH grant HL 5R01HL086827 and 1R01HL121228. GM was supported by 5R01HL094639. No other potential conflict of interest relevant to this article was reported.
References
- 1.Simon FP, Potts AM, Gerard RW. Metabolism of isolated lung tissue: normal and in phosgene poisoning. J Biol Chem. 1947;167:303–311. [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Jones HA, Cadwallader KA, White JF, Uddin M, Peters AM, Chilvers ER. Dissociation between respiratory burst activity and deoxyglucose uptake in human neutrophil granulocytes: implications for interpretation of 18F-FDG PET images. J Nucl Med. 2002;43:652–657. [PubMed] [Google Scholar]
- 4.Chen DL, Rosenbluth DB, Mintun MA, Schuster DP. FDG-PET imaging of pulmonary inflammation in healthy volunteers after airway instillation of endotoxin. J Appl Physiol. 2006;100:1602–1609. doi: 10.1152/japplphysiol.01429.2005. [DOI] [PubMed] [Google Scholar]
- 5.Musch G, Venegas JG, Bellani G, et al. Regional gas exchange and cellular metabolic activity in ventilator-induced lung injury. Anesthesiology. 2007;106:723–735. doi: 10.1097/01.anes.0000264748.86145.ac. [DOI] [PubMed] [Google Scholar]
- 6.Costa EL, Musch G, Winkler T, et al. Mild endotoxemia during mechanical ventilation produces spatially heterogeneous pulmonary neutrophilic inflammation in sheep. Anesthesiology. 2010;112:658–669. doi: 10.1097/ALN.0b013e3181cbd1d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Prost N, Costa EL, Wellman T, et al. Effects of surfactant depletion on regional pulmonary metabolic activity during mechanical ventilation. J Appl Physiol. 2011;111:1249–1258. doi: 10.1152/japplphysiol.00311.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder T, Vidal Melo MF, Musch G, Harris RS, Venegas JG, Winkler T. Modeling pulmonary kinetics of 2-deoxy-2-[18F]fluoro-D-glucose during acute lung injury. Acad Radiol. 2008;15:763–775. doi: 10.1016/j.acra.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dittrich AS, Winkler T, Wellman T, et al. Modeling 18F-FDG kinetics during acute lung injury: experimental data and estimation Errors. PLoS ONE. 2012;7:e47588. doi: 10.1371/journal.pone.0047588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okazumi S, Isono K, Enomoto K, et al. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992;33:333–339. [PubMed] [Google Scholar]
- 11.Dimitrakopoulou-Strauss A, Strauss LG, Burger C, et al. Prognostic aspects of 18F-FDG PET kinetics in patients with metastatic colorectal carcinoma receiving FOLFOX chemotherapy. J Nucl Med. 2004;45:1480–1487. [PubMed] [Google Scholar]
- 12.Markovic N, McCaig LA, Stephen J, et al. Mediators released from LPS-challenged lungs induce inflammatory responses in liver vascular endothelial cells and neutrophilic leukocytes. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1066–G1076. doi: 10.1152/ajpgi.00278.2009. [DOI] [PubMed] [Google Scholar]
- 13.Huang JB, Kindzelskii AL, Petty HR. Hexokinase translocation during neutrophil activation, chemotaxis, and phagocytosis: disruption by cytochalasin D, dexamethasone, and indomethacin. Cell Immunol. 2002;218:95–106. doi: 10.1016/s0008-8749(02)00582-8. [DOI] [PubMed] [Google Scholar]
- 14.Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand. 1980;24:231–236. doi: 10.1111/j.1399-6576.1980.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 15.Vidal Melo MF, Layfield D, Harris RS, et al. Quantification of regional ventilation-perfusion ratios with PET. J Nucl Med. 2003;44:1982–1991. [PubMed] [Google Scholar]
- 16.Vidal Melo MF, Harris RS, Layfield D, Musch G, Venegas JG. Changes in regional ventilation after autologous blood clot pulmonary embolism. Anesthesiology. 2002;97:671–681. doi: 10.1097/00000542-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 18.Mikawa K, Maekawa N, Nishina K, Takao Y, Yaku H, Obara H. Effect of lidocaine pretreatment on endotoxin-induced lung injury in rabbits. Anesthesiology. 1994;81:689–699. doi: 10.1097/00000542-199409000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Warner AE, DeCamp MM, Jr, Molina RM, Brain JD. Pulmonary removal of circulating endotoxin results in acute lung injury in sheep. Lab Invest. 1988;59:219–230. [PubMed] [Google Scholar]
- 20.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- 21.de Prost N, Tucci MR, Melo MF. Assessment of lung inflammation with 18F-FDG PET during acute lung injury. AJR. 2010;195:292–300. doi: 10.2214/AJR.10.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Prost N, Costa EL, Wellman T, et al. Effects of ventilation strategy on distribution of lung inflammatory cell activity. Crit Care. 2013;17:R175. doi: 10.1186/cc12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha D, Takahashi K, de Prost N, et al. Micro-autoradiographic assessment of cell types contributing to 2-deoxy-2-[18F]fluoro-D-glucose uptake during ventilator-induced and endotoxemic lung injury. Mol Imaging Biol. 2013;15:19–27. doi: 10.1007/s11307-012-0575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones HA, Clark RJ, Rhodes CG, Schofield JB, Krausz T, Haslett C. In vivo measurement of neutrophil activity in experimental lung inflammation. Am J Respir Crit Care Med. 1994;149:1635–1639. doi: 10.1164/ajrccm.149.6.7516252. [DOI] [PubMed] [Google Scholar]
- 25.Wellman TJ, Winkler T, Costa EL, et al. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep. Crit Care Med. 2014;42:e491–e500. doi: 10.1097/CCM.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnelly SC, Strieter RM, Kunkel SL, et al. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993;341:643–647. doi: 10.1016/0140-6736(93)90416-e. [DOI] [PubMed] [Google Scholar]
- 27.Imai Y, Kawano T, Iwamoto S, Nakagawa S, Takata M, Miyasaka K. Intratracheal anti-tumor necrosis factor-alpha antibody attenuates ventilator-induced lung injury in rabbits. J Appl Physiol. 1999;87:510–515. doi: 10.1152/jappl.1999.87.2.510. [DOI] [PubMed] [Google Scholar]
- 28.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.