Abstract
The aim of this work was to develop and validate an algorithm to monitor rates of, and response to, treatment of patients infected with hepatitis C virus (HCV) across England using routine laboratory HCV RNA testing data. HCV testing activity between January 2002 and December 2011 was extracted from the local laboratory information systems of a sentinel network of 23 laboratories across England. An algorithm based on frequency of HCV RNA testing within a defined time period was designed to identify treated patients. Validation of the algorithm was undertaken for one center by comparison with treatment data recorded in a clinical database managed by the Trent HCV Study Group. In total, 267,887 HCV RNA test results from 100,640 individuals were extracted. Of these, 78.9% (79,360) tested positive for viral RNA, indicating an active infection, 20.8% (16,538) of whom had a repeat pattern of HCV RNA testing suggestive of treatment monitoring. Annual numbers of individuals treated increased rapidly from 468 in 2002 to 3,295 in 2009, but decreased to 3,110 in 2010. Approximately two thirds (63.3%; 10,468) of those treated had results consistent with a sustained virological response, including 55.3% and 67.1% of those with a genotype 1 and non-1 virus, respectively. Validation against the Trent clinical database demonstrated that the algorithm was 95% sensitive and 93% specific in detecting treatment and 100% sensitive and 93% specific for detecting treatment outcome. Conclusions: Laboratory testing activity, collected through a sentinel surveillance program, has enabled the first country-wide analysis of treatment and response among HCV-infected individuals. Our approach provides a sensitive, robust, and sustainable method for monitoring service provision across England. (Hepatology 2014;59:1343-1350)
The Health Protection Agency (HPA) estimated that, in 2005, approximately 203,000 hepatitis C virus (HCV) antibody (Ab)-positive individuals 15-59 years of age were living in England; 161,000 were chronically infected.1 Treating these individuals represents a considerable challenge for the National Health Service (NHS), not least because for many, their infections remain undiagnosed. For those diagnosed, antiviral treatments are available that will successfully clear the virus in the majority of patients. Those who are treated and achieve a sustained virological response (SVR) will experience long-term disease remission and liver-related mortality rates comparable to the general population and are generally considered “cured”.2–4 However, despite well-disseminated guidelines on the management and treatment of HCV, service provision in England is variable, with low rates of onward referral and treatment.5,6
Primary diagnosis of HCV in England relies on testing for anti-HCV Ab, which, if positive, should be routinely followed by testing for the presence of HCV RNA, usually by polymerase chain reaction (PCR). Genotyping should also be routinely perfumed to guide treatment choice and is associated with treatment outcome. In the UK, as in Europe and North America, genotype 1 predominates,7–10 whereas genotype 4 is more prevalent in Africa and the Middle East.11,12 Genotypes of individuals diagnosed within the UK are therefore likely to reflect the countries within which they acquired their infection.
Treatment regimens described in the 2006 guidelines recommend a treatment duration of 48 weeks for those with a genotype 1 virus and 24 weeks for those with a genotype non-1 virus.13 For those who undergo treatment, response is monitored through repeat HCV RNA testing. Although frequency of HCV RNA testing varies between centers, testing is usually undertaken at the outset of treatment, at 4 weeks for detection of a rapid virological response, at 12 weeks for detection of an early virological response, if positive at 4 weeks, at the end of treatment, and again 3-6 months after the end of treatment to assess for SVR.13 Therefore, those on treatment would be expected to have had a minimum of three HCV RNA test results within 390 days of the treatment start date (because treatment monitoring extends beyond treatment administration), with additional HCV RNA test results beyond this time frame for those with a genotype 1 infection and longer treatment duration.
Previous attempts by the HPA and the British Association for the Study of the Liver (BASL) to collect national treatment data for HCV have been disappointing.14 In 2012, the HPA used national data from pharmaceutical companies, pharmacy purchasing data, and pharmacy prescribing data to estimate the total number of individuals who had been treated in England.15 However, as new drugs for treating hepatitis C are approved, these approaches are no longer valid and new methods are required to estimate treatment rates. As a result, there is currently no national surveillance system in place through which it is possible to monitor access to, and success of, treatment, without which the impact and implementation of national HCV strategies cannot be evaluated. The primary aim of this study was to use routine laboratory testing data to monitor the number of individuals undergoing patterns of repeat HCV RNA testing suggestive of referral and treatment and to thereby develop an easily applicable tool to monitor treatment uptake at the individual level. A secondary aim of the study was to explore the extent to which response to therapy could be assessed over time.
Materials and Methods
Data collection methods have been described elsewhere.16 In summary, demographic and testing data for all individuals tested for anti-HCV Ab and HCV RNA between January 2002 and December 2011 were extracted from 23 participating laboratory information systems in England. Individuals were identified using a unique reference number and linked to all related test results. Individuals were deduplicated, and test results for each individual were linked over time using a combination of soundex of surname, first initial, date of birth, and NHS number. Quality-control samples, children less than 1 year of age (because a positive test for anti-HCV in this group may be the result of passively acquired maternal Abs) those without a positive HCV RNA test result and individuals tested through renal units were excluded from this analysis. Participating laboratories are estimated to cover approximately 65% of the English population for primary and reference HCV testing and are broadly representative of most laboratories providing routine and reference HCV testing.17
Information on each individual and all their associated tests were run through a suite of algorithms written in the R statistical programming language on a Linux platform. Individuals with active infection indicated by a positive HCV RNA test result and three or more sequential HCV RNA test results within a 390-day period, suggestive of monitoring during treatment, were identified. The year of the first HCV RNA test result in this series was assumed to approximate to the year treatment was initiated. Results of qualitative and quantitative HCV RNA test results were combined to identify HCV RNA–positive individuals, and those treatment-experienced individuals with a final negative HCV RNA test result within the 390-window periods were considered to have responded to therapy and achieved an SVR. However, if an individual had a subsequent positive HCV RNA test result, they were reclassified as relapsed responders.
The algorithm was validated against treatment information contained within the Trent HCV Study Group database.6 This clinical database contains detailed information of patients followed up longitudinally as part of the Trent HCV cohort study based at Queens Medical Center in Nottingham and includes patients referred to one of the participating clinics in Derby, Leicester, Lincoln, Nottingham, and Sheffield. This cohort was established in 1991 with the objective of studying the epidemiology and natural history of HCV infection and facilitating research into the pathogenesis of HCV-associated disease. Laboratory testing information for centers in Nottingham and Derby are routinely collected through the sentinel surveillance system. Treatment information recorded in the Trent study database was compared to that inferred by patterns of repeat HCV RNA testing. Identifiers included NHS number, date of birth, and initials, all of which were used to match the individuals to the sentinel surveillance system.
Statistical Analysis
Data were stored in Oracle database (Oracle Corporation, 2001; Oracle9i Enterprise Edition Release; Redwood Sores, CA). Statistical analyses were performed in the R statistical environment; trends analyzed using chi-squared test for trend, and differences in ages were assessed using Wilcoxon's nonparametric test. The sensitivity and specificity of the algorithm was estimated against the Trent study database, and a Cohen's kappa statistic was calculated. Finally, multivariate logistic regression was performed to determine factors independently and significantly associated with being treated, and treatment response was expressed as adjusted odds ratios (aORs) with associated 95% confidence intervals (CIs).
Results
Individuals Treated in England
Between January 2002 and the end of December 2011, 267,887 HCV RNA test results among 100,640 individuals were received. Of these, 78.9% (79,360) tested positive for viral RNA, indicating an active infection. Overall, 20.8% (16,538) of individuals with an active infection had a repeat HCV RNA testing pattern suggestive of treatment (Table1); two thirds (65.6%; 10,842 of 16,538) of these were treated within 1 year of their first HCV RNA test, increasing to three quarters (74.1%; 12,250 of 16,538) within 2 years.
Table 1.
Characteristics of All Individuals Treated for HCV, England 2002-2011
| Treated | ||||||
|---|---|---|---|---|---|---|
| PCR Tested | Active HCV Infection (%) | Number (%)* | aOR† | CI | P Value | |
| Sex | ||||||
| Female | 31,615 | 23,091 (73.0) | 4,898 (21.2) | 0.99 | 0.95-1.03 | 0.590 |
| Male | 64,415 | 52,269 (81.1) | 11,145 (21.3) | 1 | — | — |
| Unknown | 4,610 | 4,000 (86.8) | 495 (12.4) | 0.52 | 0.47-0.57 | <0.001 |
| Age | ||||||
| <15 | 482 | 373 (77.4) | 136 (36.5) | 2.15 | 1.74-2.66 | <0.001 |
| 15-24 | 6,914 | 5,097 (73.7) | 998 (19.6) | 0.91 | 0.84-0.98 | 0.010 |
| 25-34 | 27,967 | 21,421 (76.6) | 4,052 (18.9) | 0.87 | 0.83-0.91 | <0.001 |
| 35-44 | 32,481 | 25,545 (78.6) | 5,401 (21.1) | 1 | — | — |
| 45-54 | 20,189 | 16,351 (81.0) | 4,183 (25.6) | 1.28 | 1.22-1.34 | <0.001 |
| 55-64 | 7,023 | 5,786 (82.4) | 1,395 (24.1) | 1.19 | 1.11-1.27 | <0.001 |
| >65 | 3,542 | 2,909 (82.1) | 341 (11.7) | 0.49 | 0.43-0.55 | <0.001 |
| Unknown | 2,042 | 1,878 (92.0) | 32 (1.7) | 0.06 | 0.04-0.09 | <0.001 |
| Ethnicity | ||||||
| White | 72,202 | 56,936 (78.9) | 12,943 (22.7) | 1 | — | — |
| Asian or Asian British | 9,539 | 7,848 (82.3) | 2,684 (34.2) | 1.78 | 1.69-1.87 | <0.001 |
| Black or black British | 602 | 465 (77.2) | 113 (24.3) | 1.1 | 0.87-1.34 | 0.431 |
| Mixed or other | 1,530 | 1,241 (81.1) | 367 (29.6) | 1.42 | 1.25-1.61 | <0.001 |
| Unknown | 16,767 | 12,870 (76.8) | 431 (3.3) | 0.11 | 0.11-0.13 | <0.001 |
| Genotype | ||||||
| 1 | 18,654 | 18,548 (99.4) | 5,460 (29.4) | 1 | — | — |
| 2 | 2,698 | 2,664 (98.7) | 826 (31.0) | 1.07 | 0.98-1.17 | 0.130 |
| 3 | 19,218 | 19,030 (99.0) | 5,835 (30.7) | 1.06 | 1.01-1.11 | 0.011 |
| 4 | 1,321 | 1,313 (99.4) | 475 (36.2) | 1.37 | 1.22-1.54 | <0.001 |
| 5 | 96 | 95 (99.0) | 17 (17.9) | 0.52 | 0.29-0.86 | 0.020 |
| 6 | 51 | 51 (100.0) | 16 (31.4) | 1.03 | 0.54-1.84 | 0.932 |
| Unknown | 58,602 | 37,659 (64.3) | 3,909 (10.4) | 0.28 | 0.26-0.29 | <0.001 |
| Grouped genotype | ||||||
| 1 | 18,654 | 18,548 (99.4) | 5,460 (29.4) | 1 | — | — |
| Non-1 | 23,384 | 23,153 (99.0) | 7,169 (31.0) | 1.1 | 1.09-0.14 | 0.001 |
| Unknown | 58,602 | 37,659 (64.3) | 3,909 (10.4) | 0.23 | 0.22-0.24 | <0.001 |
| Total | 100,640 | 79,360 (78.9) | 16,538 (20.8) | — | — | — |
Number of individuals treated, proportion of those with an active infection.
Odds ratios adjusted for age group, sex, ethnicity, and genotype.
A significantly higher proportion of males were found to have an active infection than females (81.0% vs. 72.8%; P < 0.001), and although there was no significant difference in proportion treated by gender (P = 0.58), the proportion treated was strongly associated with ethnicity, genotype, and age group (P < 0.001; Table1). Overall, individuals who were treated were older (Wilcoxon, P < 0.001) than those who were not, although the difference was small (median, 41 vs. 38 years).
The number of individuals with evidence of starting antiviral therapy has increased significantly (P < 0.001) since 2002 (Fig. 1), but declined between 2009 and 2010. When stratified by genotype, the increase in the numbers treated between 2007 and 2009 was less pronounced among those with a genotype 1 infection.
Fig 1.
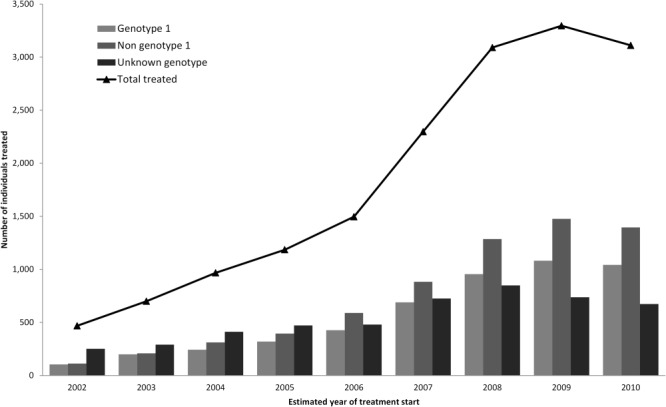
Estimated number and HCV genotype of individuals starting treatment each year in England between 2002 and 2010. Estimates rely on repeat PCR testing with a 390-day window period. Those tested later in the study period may have had a reduced opportunity of being detected as on treatment. This is particularly the case for those with a first PCR test result in 2011. The data for 2011 are therefore not plotted, but are included in the analysis of factors associated with treatment and response.
Overall, 63.3% (n = 10,468) of all individuals apparently treated had a final negative HCV RNA test result and were significantly younger than those without evidence of response to treatment (Wilcoxon, P < 0.001; median, 38 vs. 42). The odds of a treatment response (adjusted for age group, sex, ethnicity, and genotype) was significantly higher among females than males and among those of Asian or Asian British ethnicity, compared to those of white or white British ethnicity, and in those with a non–genotype 1 infection. Rates of treatment response varied by genotype, with 55.3% of those with a genotype 1 virus and 58.5% of those with a genotype 4 virus responding to treatment, compared to 69.2% and 67.4% among those with a genotype 2 and 3 virus, respectively (Table2).
Table 2.
Characteristics of All Individuals Treated for HCV Achieving an SVR, England 2002-2011
| Treatment Response | ||||
|---|---|---|---|---|
| Number (%)* | aOR† | CI | P Value | |
| Sex | ||||
| Female | 3,197 (65.3) | 1.12 | 1.05-1.20 | 0.002 |
| Male | 6,978 (62.6) | 1 | — | — |
| Unknown | 293 (59.2) | 0.86 | 0.72-1.04 | 0.125 |
| Age | ||||
| <15 | 54 (39.7) | 0.35 | 0.24-0.49 | <0.001 |
| 15-24 | 647 (64.8) | 0.97 | 0.84-1.12 | 0.693 |
| 25-34 | 2,671 (65.9) | 1.02 | 0.94-1.11 | 0.660 |
| 35-44 | 3,537 (65.5) | 1 | — | — |
| 45-54 | 2,609 (62.4) | 0.87 | 0.80-0.95 | 0.002 |
| 55-64 | 770 (55.2) | 0.65 | 0.57-0.73 | <0.001 |
| >65 | 156 (45.7) | 0.44 | 0.35-0.55 | <0.001 |
| Unknown | 24 (75.0) | 1.58 | 0.743.76 | 0.260 |
| Ethnicity | ||||
| White | 8,157 (63.0) | 1 | — | — |
| Asian or Asian British | 1,789 (66.7) | 1.17 | 1.07-1.28 | <0.001 |
| Black or black British | 66 (58.4) | 0.84 | 0.57-1.21 | 0.311 |
| Mixed or other | 227 (61.9) | 0.95 | 0.77-1.18 | 0.640 |
| Unknown | 229 (53.1) | 0.67 | 0.55-0.81 | <0.001 |
| Genotype | ||||
| 1 | 3,020 (55.3) | 1 | — | — |
| 2 | 572 (69.2) | 1.81 | 1.55-2.13 | <0.001 |
| 3 | 3,937 (67.5) | 1.68 | 1.55-1.81 | <0.001 |
| 4 | 278 (58.5) | 1.14 | 0.94-1.38 | 0.176 |
| 5 | 11 (64.7) | 1.48 | 0.56-4.31 | 0.440 |
| 6 | 14 (87.5) | 5.66 | 1.58-36.04 | 0.022 |
| Unknown | 2,636 (67.4) | 1.67 | 1.54-1.82 | <0.001 |
| Grouped genotype | ||||
| 1 | 3,020 (55.3) | 1 | — | — |
| Non-1 | 4,812 (67.1) | 1.65 | 1.54-1.78 | <0.001 |
| Unknown | 2,636 (67.4) | 1.67 | 1.53-1.82 | <0.001 |
| Total | 10,468 (63.3) | — | — | — |
Number of individuals achieving an SVR, proportion of those treated.
Odds ratios adjusted for age group, sex, ethnicity, and genotype.
Validation Against the Trent Clinical Database
The validation exercise used information on 889 HCV-positive individuals from the Trent clinical database who had attended either the Nottingham or Derby clinics. Overall, 711 (79.9%) individuals could be matched to the sentinel laboratory database using a combination of NHS number and date of birth. Of these, 55 (7.7%) had a treatment start date preceding the start of the sentinel surveillance system in 2002 and were excluded. Of the remaining 656 (93.3%), 198 (30.1%) did not match to an individual with a HCV RNA test result in the sentinel surveillance database and therefore could not be classified as having active or resolved infections, or as treatment experienced or naïve, and were therefore excluded. Of the remaining 458 (69.9%), the algorithm classified 163 (35.6%) individuals as treatment naïve, of whom 148 (90.8%) were listed as untreated in the Trent database, and 295 (64.4%) as treatment experienced, of whom 283 (95.9%) were listed as treated in the Trent database (Fig. 2). In this study population, the algorithm therefore demonstrated a sensitivity and specificity of 95% and 93%, respectively (positive predictive value [PPV]: 96%; negative predictive value [NPV]: 91%) and a Cohen's kappa score of agreement of 0.87.
Fig 2.
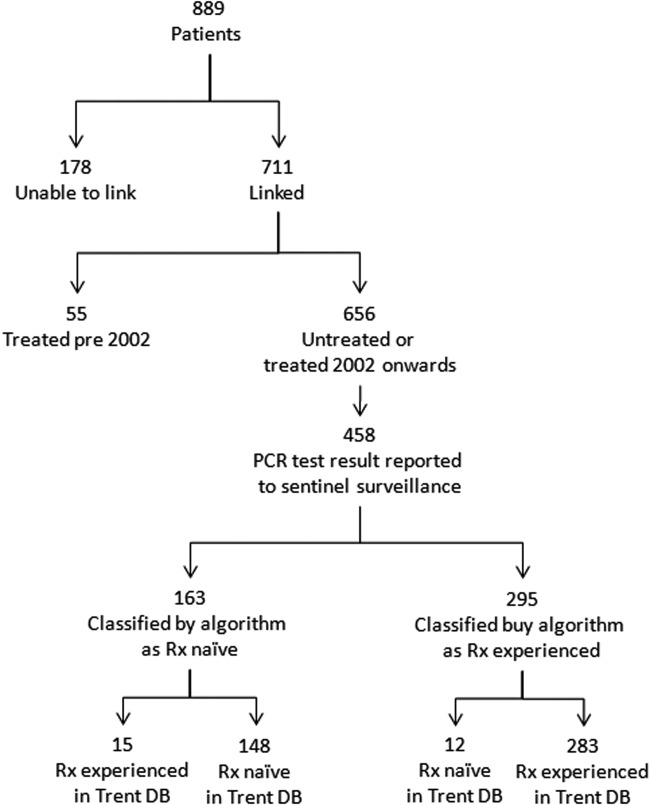
Validation of treatment algorithm against the Trent HCV database (DB). Treatment was attempted on individuals with no PCR test result in the sentinel surveillance database because they may not have had an active infection and therefore do not require treatment. Because the algorithm relies on sequential PCR test results, they could not have been appropriately classified.
The 15 individuals who were reported in the Trent database as treatment experienced, but were classified by the algorithm as treatment naïve, had an insufficient number of HCV RNA tests undertaken within a 390-day window period. After further checking against the Trent database, all 15 individuals had a number of HCV RNA tests results reported to the sentinel surveillance system, but these were unable to be linked to the patients because of insufficient identifiers.
Of the 12 individuals classified as treatment experienced, but recorded in the Trent database as naïve, 2 were shown to have been treated outside Trent (and therefore this information had not entered the Trent database); 1 had spontaneously cleared their infection, 4 were confirmed through their hospital records as never having been treated, and there was no additional information available for the remaining 5.
In total, 239 (84.5%) individuals who were correctly classified as treatment experienced had an outcome recorded in the Trent database. All 141 (100.0%) individuals with a documented SVR and 91 (93.0%) of the remaining 98 with no evidence of a treatment response were correctly classified by the algorithm. Therefore, as a result, in this study population, the algorithm demonstrated a sensitivity and specificity of 100% and 93% for identification of a successful outcome of therapy, respectively (PPV, 95%; NPV, 100%) and a Cohen's kappa score of agreement of 0.97.
HCV RNA test results held within the sentinel surveillance and the Trent database were reviewed to determine the likely cause of the seven discrepant results. In all cases, individuals in the sentinel surveillance database had a negative HCV RNA test result, which was likely to have been erroneously linked to that individual as a result of partially missing personally identifiable information and commonly occurring soundex codes.
Discussion
Data from laboratory information systems, collected as part of a sentinel surveillance program, are a rich and valuable information resource. These data have provided unique insights into changing testing practices, provided context for the interpretation of routine laboratory reports, and, for the first time, have enabled a country-wide analysis of treatment among HCV-infected individuals using a method that has been demonstrated to be robust. Here, we examined over 250,000 HCV RNA test results, among 100,809 individuals, of whom approximately four in five had an active infection, with only one in five having received antiviral therapy between 2002 and 2011. The number of individuals being treated in the catchment of the sentinel laboratories annually increased rapidly from under 500 in 2002 to well over 3,000 by 2008, but a more modest increase to 3,295 in 2009 and a decline to 3,110 by 2010. Even by 2010, not all individuals with an active infection were treated, and the majority of those who were treated had started therapy within 2 years of their first HCV RNA.
Similar trends in the estimated number of individuals treated for HCV in England have been reported using prescribing and drug sales.15 These show a similar trend with an increase in the numbers of people being treated from approximately 3,000 in 2006 to approximately 5,000 in 2008/2009 and then a plateau, with a potential fall in the overall numbers being treated in 2010/2011. Similar approaches using drug marketing data to estimate treatment uptake have been used for comparison across Europe, with the UK ranking below other European Union countries in the number of patients treated.18 Elsewhere in the UK, the number of people initiating therapy through HCV clinics in Scotland increased from 468 to 1,049 between 2007/2008 and 2010/2011,19 after the launch of the Scotland Hepatitis C Action Plan in 2006. Our data suggest that treatment in England increased to a similar extent after the 2004 Hepatitis C Action Plan for England.20
Differences in treatment rates between countries may reflect differences in overall prevalence or between exposure groups and their respective access to services. Studies exploring patient-level factors associated with treatment rates are therefore important.21–23 These studies provide a better understanding of barriers to treatment and on where to direct resources. However, analysis of factors associated with treatment and response are limited to those routinely collected through laboratory information systems and not the wider determinants of the offer and uptake of treatment.
Using sequential HCV RNA test results from sentinel laboratories across England will be an underestimate of those on treatment in England, but the method allows us to discern temporal trends using routinely available data, with the benefit of being able to explore individual-level characteristics in the future. Furthermore, with European and U.S. hepatitis treatment guidance recommending similar patterns of HCV RNA testing during therapy,24,25 this method could be employed more widely to obtain comparable data.
The standard of care for patients with HCV in the UK during this period of study was combination therapy with pegylated interferon (Peg-IFN) and ribavirin (RBV). These drugs, administered for either 48 weeks for those with a genotype 1 or for 24 weeks for those with non–genotype 1 infections, typically result in SVR rates of 40%-50% and 80%, respectively.26 We observed plausible SVR rates of approximately 55% among those with a genotype 1 virus and 67% among those with a non–genotype 1 virus. Therefore, routine laboratory data may also provide an approach by which response rates and patient-level factors can be explored. Over the coming years, with the introduction of new direct-acting antiviral (DAA) agents, the standard of care and virological monitoring of patients with chronic HCV infection will dramatically change. These new therapies and accompanying clinical guidelines will encompass more frequent and earlier HCV RNA testing, which should make these individuals easier to identify using the algorithm described here.
Stratifying the number of individuals starting treatment each year by genotype suggests a relative reluctance to treat individuals with genotype 1 virus on the regimens recommended in 2006.13 This reluctance may reflect the poorer response rates26 and/or a desire to wait for the new DAA agents. In 2012, these new agents (telaprevir and boceprevir) were recommended by the National Institute for Health and Clinical Excellence (NICE) for the treatment of previously untreated adults with compensated liver disease resulting from genotype 1 infection. This may result in increasing treatment numbers among patients with genotype 1 infection in the future.
Higher rates of treatment uptake among those under 15 years of age may reflect more frequent HCV RNA testing among this group not because they are on therapy, but as part of routine monitoring to better understand the natural history of infection in this patient group, where there may be a significant possibility of spontaneous clearance at any age in childhood. Furthermore, poor responses may reflect changes in drug licensing laws as the European Medicines Agency did not approve the use of Peg-IFN-α-2b and RBV until 2009, and NICE did not publish draft guidance recommending Peg-IFN in combination with RBV as an option for treating chronic hepatitis C in children and young people until 2013.
Information on the reason for testing and risk factors associated with HCV transmission is rarely recorded in laboratory information systems. This limits further exploration of the associations between genotype, ethnicity, treatment uptake, and outcome. As a result, it is plausible that treatment rates and outcomes are disproportionally affected by the overrepresentation of people who inject drugs among those of white ethnicity.
Comparison with the Trent HCV cohort data demonstrated that the algorithm based on testing patterns was both highly sensitive and specific at both identifying individuals on treatment, as well as treatment outcome. A small number of individuals were misclassified as treatment experienced when they were not (n = 12); however, these individuals had a clear pattern of HCV RNA testing indistinguishable from those on treatment. Some of these individuals were HCV RNA tested through another laboratory contributing to the sentinel surveillance database, suggesting that individuals were being treated through a service not captured by the Trent database. Conversely, the algorithm misclassified a small number of individuals (n = 15) as treatment naïve. All of these individuals had an insufficient number of HCV RNA test results identified within the sentinel surveillance database, with wide inter-test intervals. However, on further investigation, additional HCV RNA test results were identified for these individuals in the database, but were reported with insufficient patient identifiers and therefore could not be reliably linked to the individuals. Furthermore, the algorithm was able to identify all individuals who achieved an SVR, but misclassified a small number of nonresponders. This highlighted an additional source of error where the result of HCV RNA tests within the sentinel surveillance database might be ascribed to the wrong individual because of a lack of patient identifiers.
In England, the significant public health importance of HCV and the need for an intensified response was highlighted in the Hepatitis C Action Plan for England.20 Among other things, the plan called for improved surveillance activities and for high-quality services for the testing, assessment, and treatment of all patients with HCV. Surveillance and service delivery are also likely to be key components of the imminent National Liver Disease Outcomes Framework. However, the recent changes to the NHS have reorganized and redistributed commissioning and public health responsibilities27 and have potential to result in disruption to current HCV treatment pathways. We believe we have identified a sensitive, robust, sustainable, and efficient system to determine the effect of this transition and provide longer-term monitoring of treatment rates to drive improved, equitable service provision across England.
Acknowledgments
The authors thank all the staff in the testing laboratories, including the IT, medical, and scientific staff who supported this study on an ongoing basis. The sentinel surveillance of the hepatitis testing study was funded by the English Department of Health (study reference AIDB 2/30) until September 2009.
Glossary
- Ab
antibody
- aOR
adjusted odds ratio
- BASL
British Association for the Study of the Liver
- CI
confidence interval
- DAA
direct-acting antiviral
- HCV
hepatitis C virus
- HPA
Health Protection Agency
- NHS
National Health Service
- PCR
polymerase chain reaction
- SVR
sustained virological response
- NICE
National Institute for Health and Care Excellence
- NPV
negative predictive value
- Peg-IFN
pegylated interferon
- PPV
positive predictive value
- RBV
ribavirin
- SVR
sustained virological response.
Appendix 1: Laboratories Contributing Data to the Sentinel Surveillance Program During the Study Period
Hamid Jalal, Melanie Matthews, Rachael Smith, Addenbrookes Hospital, Cambridge; Chas Ashley, Peter Muir, Bristol Regional HPA Laboratory; Mark Atkins, Mark Green, Lesley Mayoh, Chelsea and Westminster Hospital, London; John Croall, Chester Microbiology Laboratory, Countess of Chester Hospital, Chester; Tony Vicca, Diana Princess of Wales Hospital, Grimsby; Julia Locklan, Sheila Waugh, Freeman General Hospital, Newcastle; Samreen Ijaz, Siew Lin Ngui, Richard Tedder, Sexually Transmitted and Blood Borne Virus Laboratory, Health Protection Agency, Center for Infections, London; Manoj Vallapil, Health Protection Agency, Newcastle Laboratory, Newcastle General Hospital, Newcastle; Elizabeth Boxall, Janet Mowbray, Health Protection Agency West Midlands Laboratory, Heart of England Foundation Trust, Birmingham; David Lewis, Antony Hale, Leeds Teaching Hospitals NHS Trust, Leeds; Ralph Henderson, David Johnson, Mark Zuckerman, Kings College Hospital, London; Alan Blackley, Paul Klapper, Manchester Medical Microbiology Partnership, Manchester Royal Infirmary, Manchester; Matthew Longbone, Mohammed Osman Hassan Ibrahim, Royal Sussex County Hospital, Brighton; Will Irving, Lisa Prichett, Queens Medical Center, Nottingham; Josephine Silles, HPA Collaborating Center, North Middlesex University Hospital, London; Louise Hesketh, Microbiology Laboratory, Royal Preston Hospital; Preston Lynne Ashton, Ian Hart, Royal Liverpool Hospital, Liverpool; Tony Oliver, Xose Couto-Parada, Barts and The London NHS Trust, London; Hasan Al-Ghusein, Phil Rice, St George's Hospital, London; Graham Hewitt, Gillian Underhill; St Mary's Hospital, Portsmouth; Mike Kidd, Peter Luton, University College Hospital, London; Mark Baker, James Nash, William Harvey Hospital, Ashford, Kent.
References
- 1.Harris RJ, Ramsay M, Hope VD, Brant L, Hickman M, Foster GR, et al. Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health. 2012;22:187–192. doi: 10.1093/eurpub/ckr083. [DOI] [PubMed] [Google Scholar]
- 2.Kasahara A, Tanaka H, Okanoue T, Imai Y, Tsubouchi H, Yoshioka K, et al. Interferon treatment improves survival in chronic hepatitis C patients showing biochemical as well as virological responses by preventing liver-related death. J Viral Hepat. 2004;11:148–156. doi: 10.1046/j.1365-2893.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 3.Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–684. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Arakawa Y, Sata M, Nishiguchi S, Yano M, Fujiyama S, et al. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology. 2002;123:483–491. doi: 10.1053/gast.2002.34785. [DOI] [PubMed] [Google Scholar]
- 5.Irving WL, Smith S, Cater R, Pugh S, Neal KR, Coupland CA, et al. Clinical pathways for patients with newly diagnosed hepatitis C—what actually happens. J Viral Hepat. 2006;13:264–271. doi: 10.1111/j.1365-2893.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohsen AH. Trent HCV. Study Group. The epidemiology of hepatitis C in a UK health regional population of 5.12 million. Gut. 2001;48:707–713. doi: 10.1136/gut.48.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zein NN, Rakela J, Krawitt EL, Reddy KR, Tominaga T, Persing DH. Hepatitis C virus genotypes in the United States: epidemiology, pathogenicity, and response to interferon therapy. Collaborative Study Group. Ann Intern Med. 1996;125:634–639. doi: 10.7326/0003-4819-125-8-199610150-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dusheiko G, Schmilovitz-Weiss H, Brown D, McOmish F, Yap PL, Sherlock S, et al. Hepatitis C virus genotypes: an investigation of type-specific differences in geographic origin and disease. Hepatology. 1994;19:13–18. [PubMed] [Google Scholar]
- 9.McOmish F, Yap PL, Dow BC, Follett EA, Seed C, Keller AJ, et al. Geographical distribution of hepatitis C virus genotypes in blood donors: an international collaborative survey. J Clin Microbiol. 1994;32:884–892. doi: 10.1128/jcm.32.4.884-892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nousbaum JB, Pol S, Nalpas B, Landais P, Berthelot P, Bréchot C. Hepatitis C virus type 1b (II) infection in France and Italy. Collaborative Study Group. Ann Intern Med. 1995;122:161–168. doi: 10.7326/0003-4819-122-3-199502010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Abdulkarim AS, Zein NN, Germer JJ, Kolbert CP, Kabbani L, Krajnik KL, et al. Hepatitis C virus genotypes and hepatitis G virus in hemodialysis patients from Syria: identification of two novel hepatitis C virus subtypes. Am J Trop Med Hyg. 1998;59:571–576. doi: 10.4269/ajtmh.1998.59.571. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain RW, Adams N, Saeed AA, Simmonds P, Elliott RM. Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. J Gen Virol. 1997;78(Pt 6):1341–1347. doi: 10.1099/0022-1317-78-6-1341. [DOI] [PubMed] [Google Scholar]
- 13.Booth JC, O'Grady J, Neuberger J. The Royal College of Physicians of London and the British Society of Gastroenterology. Clinical guidelines on the management of hepatitis C. Gut. 2001;49(Suppl. 1):I1–21. doi: 10.1136/gut.49.suppl_1.I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health Protection Agency. Hepatitis C in England: an update. London: Health Protection Agency; 2007. 2007. [Google Scholar]
- 15.Health Protection Agency. Hepatitis C in the UK. London: Health Protection Agency; 2012. 2012. [Google Scholar]
- 16.Brant LJ, Hurrelle M, Balogun MA, Klapper P, Ahmad F, Boxall E, et al. Sentinel laboratory surveillance of hepatitis C antibody testing in England: understanding the epidemiology of HCV infection. Epidemiol Infect. 2007;135:417–426. doi: 10.1017/S0950268806006832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brant LJ, Hurrelle M, Balogun MA, Klapper P, Ramsay ME. Where are people being tested for anti-HCV in England? Results from sentinel laboratory surveillance. J Viral Hepat. 2008;15:729–739. doi: 10.1111/j.1365-2893.2008.01000.x. [DOI] [PubMed] [Google Scholar]
- 18.Lettmeier B, Mühlberger N, Schwarzer R, Sroczynski G, Wright D, Zeuzem S, et al. Market uptake of new antiviral drugs for the treatment of hepatitis C. J Hepatol. 2008;49:528–536. doi: 10.1016/j.jhep.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Health Protection Agency. Hepatitis C in the UK. London: Health Protection Agency; 2011. 2011. [Google Scholar]
- 20.Department of Health. Hepatitis C Action Plan for England. London: Department of Health; 2004. [Google Scholar]
- 21.Bini EJ, Bräu N, Currie S, Shen H, Anand BS, Hu KQ, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100:1772–1779. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanwal F, Hoang T, Spiegel BMR, Eisen S, Dominitz JA, Gifford A, et al. Predictors of treatment in patients with chronic hepatitis C infection—role of patient versus nonpatient factors. Hepatology. 2007;46:1741–1749. doi: 10.1002/hep.21927. [DOI] [PubMed] [Google Scholar]
- 23.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut. 2007;56:385–389. doi: 10.1136/gut.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidelines CP. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 27.Department of Health. Healthy Lives. Healthy People: our strategy for public health in England. London: Department of Health; 2010. [Google Scholar]


