Abstract
Objective
To study the effects of oromucosal detomidine gel administered sublingually to calves prior to disbudding, and to compare its efficacy with intravenously (IV) administered detomidine.
Study design
Randomised, prospective clinical study.
Animals
Twenty dairy calves aged 12.4 ± 4.4days (mean ± SD), weight 50.5 ± 9.0 kg.
Methods
Detomidine at 80 μg kg−1 was administered to ten calves sublingually (GEL) and at 30 μg kg−1 to ten control calves IV (V. jugularis). Meloxicam (0.5 mg kg−1) and local anaesthetic (lidocaine 3 mg kg−1) were administered before heat cauterization of horn buds. Heart rate (HR), body temperature and clinical sedation were monitored over 240 minutes. Blood was collected from the V. cephalica during the same period for drug concentration analysis. Pharmacokinetic variables were calculated from the plasma detomidine concentration-time data using non-compartmental methods. Statistical analyses compared routes of administration by Student's t-test and linear mixed models as relevant.
Results
The maximum plasma detomidine concentration after GEL was 2.1 ± 1.2 ng mL−1 (mean ±SD) and the time of maximum concentration was 66.0 ± 36.9 minutes. The bioavailability of detomidine was approximately 34% with GEL. Similar sedation scores were reached in both groups after administration of detomidine, but maximal sedation was reached earlier in the IV group (10 minutes) than in the GEL group (40 minutes). HR was lower after IV than GEL from 5 to 10 minutes after administration. All animals were adequately sedated, and we were able to administer local anaesthetic without resistance to all of the calves before disbudding.
Conclusions and clinical relevance
Oromucosally administered detomidine is an effective sedative agent for calves prior to disbudding.
Keywords: calves, detomidine, disbudding, oromucosal, sedatives, welfare
Introduction
Disbudding, the removal of calves' horn buds, is a common procedure in dairy animal husbandry, but the procedure causes severe acute pain, and is associated with behavioural and physiological responses (Morisse et al. 1995; McMeekan et al. 1998; Stafford & Mellor 2011). There is no EU legislation for pain management associated with disbudding. The European Council Directive 98/58/EC (1976) allows any skilled person to destroy or remove the horn-producing area of calves aged <4 weeks by chemical or heat cauterization.
Application of appropriate anaesthetics and analgesics is not compulsory in most countries but it is strongly recommended (AVA 2004; New Zealand Government 2005; AVMA 2012). Cornual nerve block and ring block around the horn buds with local anaesthetics effectively alleviates disbudding pain or delays its onset (Graf & Senn 1999; Fierheller et al. 2012). However infiltration of local anaesthetics to non-sedated calves is often difficult. Disbudding-related postoperative pain has been successfully alleviated by non-steroidal anti-inflammatory drugs, such as meloxicam (Stewart et al. 2009; Heinrich et al. 2010), ketoprofen (McMeekan et al. 1998; Faulkner & Weary 2000) and carprofen (Stilwell et al. 2012).
Despite recommendations, pain and distress related to disbudding of calves often remains untreated (Hoe & Ruegg 2006; Vasseur et al. 2010; Gottardo et al. 2011). According to the ALCASDE (2009), it is assumed that some kind of medical treatment is administered prior to or after calf disbudding only on 20% of European farms. In Italy, producers reported that 10% of their disbudded calves received local anaesthetics, 4% received a sedative and 5% received analgesics prior to disbudding (Gottardo et al. 2011). In Canada, use of sedatives or local anaesthetics was reported for 45% of herds, but no analgesics (NSAIDs) were mentioned (Vasseur et al. 2010). In the United States, sedatives or local anaesthetics were utilized by 12% and analgesics by 2% of dairy farmers (Fulwider et al. 2008).
Detomidine is a potent α2-adrenoceptor agonist that is used commonly for sedation or premedication in horses and cattle, including calves (Peshin et al. 1991). Sublingual administration of an oromucosal gel formulation of detomidine produced safe sedation in horses (DiMaio Knych & Stanley 2011; Kaukinen et al. 2011), and the bioavailability was approximately 22% (Kaukinen et al. 2011).
The primary objective of this study was to explore the use of oromucosal detomidine gel for sedation of calves prior to disbudding. Our hypothesis was that oromucosal detomidine would produce sufficient sedation to allow administration of local anaesthetics with minimum resistance. The secondary objective was to determine the plasma detomidine concentrations after sublingual administration and to compare level of sedation to intravenous (IV) administration of detomidine in calves undergoing disbudding.
Materials and methods
This study was conducted with permissions of the Animal Experiment Board of Finland and the Finnish Medicines Agency. Twenty clinically healthy dairy calves of both sexes were included. Their age was 12.4 ± 4.4 days (mean ± SD) and they weighed 50.5 ± 9.0 kg. The animals were disbudded as a routine measure of the farm. The calves were housed as other calves on the farm, and they were used to human handling. The calves were allocated randomly to one of two groups of 10 animals in each. One group was given sublingually (GEL) detomidine gel (Domosedan Gel 7.6 mg mL−1 oromucosal gel; Orion Pharma Ltd., Finland) 80 μg kg−1, the other group was given IV detomidine (Domosedan 10 mg mL−1 solution for injection; Orion Pharma Ltd.) 30 μg kg−1.
Prior to drug administration, the calves were examined clinically and the V. cephalica was cannulated (16 G Intraflon, 1.6 × 60 mm; Vygon, France) for blood sampling. In the IV group, the V. jugularis was also cannulated with a similar catheter for drug administration. Before placement of the catheters, local anaesthesia was provided by the subcutaneous infiltration of lidocaine (Lidocain 20 mg mL−1; Orion Pharma Ltd.).
The detomidine gel for sublingual administration was drawn into a 1 mL syringe to achieve an accurate dose, and was administered under the tongue of the calf. The IV detomidine was given as a bolus via the jugular catheter, which thereafter was flushed with 10 mL of isotonic saline. To alleviate postoperative pain, meloxicam (0.5 mg kg−1; Melovem 5 mg mL−1; Orion Pharma Ltd.) was administered subcutaneously. When clinical sedation was evident, the local anaesthetic, Lidocaine 20 mg mL−1, was injected subcutaneously around the cornual nerve and a ring block was inserted around both horn buds as previously described by Faulkner & Weary (2000). The total dose of lidocaine was 3 mg kg−1 per calf. The animal's response to administration of the local anaesthetic was recorded. The adequacies of the local blocks were tested with needle pricks, and once adequate, hot cauterization was used to destroy the horn buds.
Heart rate (HR) was measured by auscultation and the depth of sedation was recorded using a 17-point (0–16 where 0 is no sedation) behaviour-based clinical sedation score (CSS) which was modified from Kuusela et al. (2001). Details are presented as supporting information (Appendix S1). The CSS was recorded at 14 time points for 240 minutes after drug administration.
Rectal temperature was measured with a digital thermometer prior to treatment and every 30 minutes thereafter. The pen of the calf had a soft insulated floor with a mattress and adequate sawdust. Calves were covered with blankets to maintain the body temperature. The ambient temperature in the pens was 10.0–19.4 °C during the study.
Venous blood samples (3 mL) were collected from the catheter in the V. cephalica into EDTA tubes for analysis of plasma detomidine concentrations. Times of collection were, for the IV group at 5, 10, 20, 30, 60, 120, 180, 240 minutes, and for the GEL group at 30, 60, 90, 120, 150, 180, 210, 240 minutes after drug administration. After each sampling the catheter was flushed with 5 mL of isotonic saline with heparin (10 IU mL−1). Samples were stored at room temperature prior to centrifugation. Plasma was separated by centrifugation (3000 g for 15 minutes) within three hours after sampling, frozen and stored at −20 °C until analysis.
Quantitative analysis of detomidine concentrations in plasma was performed with reversed phase liquid chromatography (Shimadzu Prominence HPLC instrument; Shimadzu Corporation, Japan) combined with mass spectrometric detection (AB Sciex API4000 triple quadrupole mass spectrometer, Framingham, MA, USA) using an internal standard method. Details are presented in the supporting information, Appendix S2.
Pharmacokinetic variables were calculated from the plasma detomidine concentration-time data with the WinNonlin Professional software package, version 5.3 (Pharsight Corporation, CA, USA) using non-compartmental methods. The areas under the time plasma concentration curve (AUC) and time sedation score curve (AUCsed) were calculated with the trapezoidal method. Bioavailability of detomidine after sublingual administration was approximated by using the formula 100% * AUCSL(avg)/AUCIV(avg) * DIV/DSL, where D was the dose of detomidine. The AUCs and the maximum sedation scores detected for each animal were compared between administration routes using Student's t-test. The differences between administration routes in CSS and HR were compared with linear mixed models taking repeated samplings into account. Fixed factors were the sampling time, administration route and interaction between them. A calf was used as a random factor. Statistical analyses were performed using PASW 18.0.1 (SPSS Inc., IL, USA). p < 0.05 was considered statistically significant.
Results
Plasma detomidine concentrations are shown in Fig.1. The maximum plasma detomidine concentration (Cmax) after sublingual administration was 2.1 ± 1.2 ng mL−1 (mean ± SD), the time of maximum concentration (Tmax) was 66.0 ± 36.9 minutes, and the approximated bioavailability of detomidine was 34%. Pharmacokinetic parameters are shown in Table1.
Figure 1.
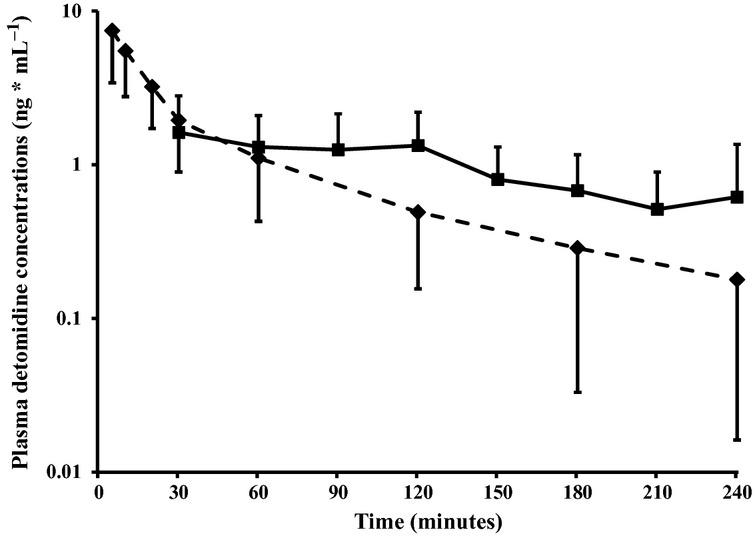
Mean ± SD plasma detomidine concentrations after sublingual (80 μg kg−1, solid line) and intravenous (30 μg kg−1, broken line) administration of detomidine to calves. N = 10 per group.
Table 1.
Pharmacokinetic parameters and sedation scores (scale 0–16 where 0 = no sedation) after sublingual (80 μg kg−1) or intravenous (30 μg kg−1) of detomidine administration
| Detomidine 80 μg kg−1 sublingually | Detomidine 30 μg kg−1 intravenously | p-value | |
|---|---|---|---|
| Cmax (ng mL−1) | 2.1 ± 1.2 | – | – |
| Tmax (minutes) | 66.0 ± 36.9 | – | – |
| AUClast | 226 ± 110 | 252 ± 127 | 0.62 |
| Maximum sedation score | 9.9 ± 2.1 | 10.7 ± 2.1 | 0.40 |
| AUCsed | 1026 ± 477 | 784 ± 209 | 0.16 |
All values mean ± SD. N = 10 animals per group.
The maximum sedation scores for each animal and the overall levels of sedation (AUCsed) did not differ between the treatments (Table1). Briefly, for the first 30 minutes after administration, CSS was higher in the IV than in the GEL group. Thereafter, CSS was higher in the GEL than in the IV group up to 210 minutes post administration (Fig.2). Heart rate decreased to a significantly lower level in the IV than in the GEL group (Fig.3).
Figure 2.
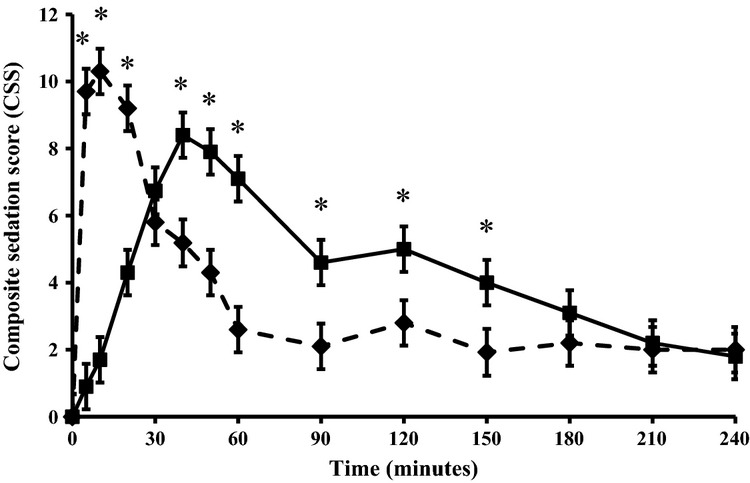
Mean ± SD composite sedation scores (0–16 where 0 is no sedation) after sublingual (80 μg kg−1, solid line) and intravenous (30 μg kg−1, broken line) administration of detomidine to calves. N = 10 per group. *Statistically significant difference (p < 0.05) between the groups.
Figure 3.
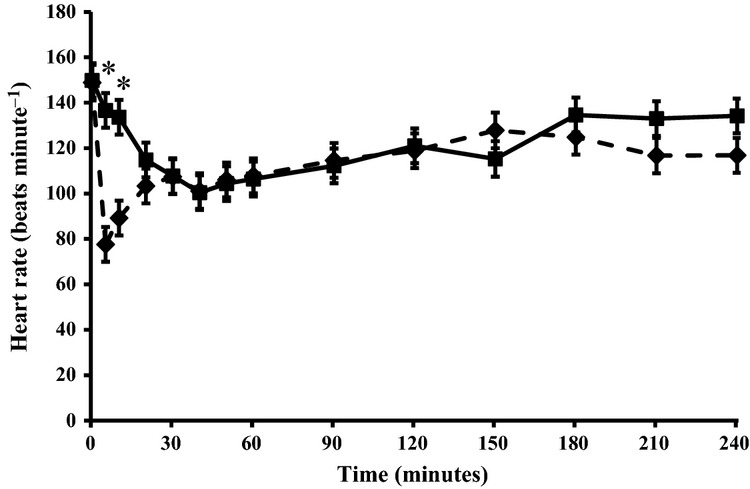
Heart rate (mean ± SD) after sublingual (80 μg kg−1, solid line) and intravenous (30 μg kg−1, broken line) administration of detomidine to calves. N = 10 per group. *Statistically significant difference (p < 0.05) between the groups.
Time from sedation to local anaesthetic administration was 11 minutes (range 5–20 minutes) in IV group and 38 minutes (range 25–55 minutes) in GEL group. None of the calves substantially resisted infiltration of the local anaesthetic nor reacted to hot cauterization of horn buds. All recoveries were eventless, and no adverse reactions were noted in any of the animals. All calves were able to stand up and walk at the end of the observation period. Rectal temperatures did not differ between the treatments, and hypothermia was not detected.
Discussion
Our results indicated that sublingual administration of detomidine gel prior to infiltration of local anaesthetics was an effective method to sedate healthy calves for disbudding.
Venous blood from the oral mucosal membrane drains via the jugular veins into the systemic circulation, and thus during the absorption phase of the drugs administered by this route, blood from jugular vein may have substantially higher drug concentrations than the mixed blood in systemic circulation (Messenger et al. 2011). Therefore plasma drug concentrations analyzed from the jugular vein following sublingual administration may not reflect systemic concentrations. We avoided this bias by collecting blood samples from the veins of extremities (V. cephalica).
In a previous study (Salonen et al. 1989), the pharmacokinetic profile of parenterally administered detomidine in milking cows was close to that of adult horses. In our study, the approximated bioavailability of sublingually administered detomidine in calves (34%) seemed to be relatively similar to the previously reported bioavailability in horses (22%) (Kaukinen et al. 2011). Our study was not designed to be a pharmacokinetic study, as our primary aim was to evaluate clinical sedation. We collected plasma samples up to 4 hours after drug administration, which probably affected the approximated bioavailability. The elimination phase (ß-phase) was not attained within the follow up time after sublingual administration, and thus we could not extrapolate the plasma concentration versus time curve to infinity.
As there was a risk that a fraction of the gel dose could be swallowed, we carefully administered the gel under the tongue. The volume of the gel was small (<1 mL per calf) and it seemed to remain easily in the oral cavity. Detomidine might undergo extensive first-pass metabolism if swallowed, but the amount of this metabolism is not known in calves. Thus further studies are still needed about the pharmacokinetics of detomidine in calves with this administration route.
In general, the detomidine induced sedation was equal in both treatment groups. However, maximum sedation was reached earlier in the IV group than in the GEL group. This suggested that 80 μg kg−1 of detomidine administered sublingually was equally potent as 30 μg kg−1 of IV administered detomidine. The decrease in heart rate was less intense with sublingual administration than with parenteral administration, as reported earlier also for horses (Malone & Clarke 1993; Kaukinen et al. 2011). Our findings imply that sublingual administration of detomidine would alleviate the decreases in heart rate observed with parenteral administration of detomidine. The efficacy and safety of detomidine after sublingual administration to calves needs further studies in various clinical indications.
In our study we measured regularly the rectal temperature of the animals. If needed, we used blankets to prevent hypothermia. Thus, hypothermia was not detected. It is important to monitor the body temperature and prevent its reduction in sedated animals as hypothermia affects drug metabolism and prolongs recovery.
We concluded that oromucosal detomidine gel is an effective sedative for calves prior to infiltration of local anaesthetics and to disbudding. This non-invasive and user-friendly oromucosal sedation method for calves could ease the use of local anaesthetics.
Acknowledgments
Funding for this study was provided by the Ministry of Agriculture and Forestry in Finland and by Orion Corporation, Orion Pharma Ltd. We warmly thank Juha Suomi, Pirjo Pursiainen, Simo Timonen and Pentti Korhonen for taking care of the animals during the study. We also thank Mari Palviainen, Merja Pöytäkangas and Vesa Raussi for their valuable help during this study.
Supporting Information
Additional Supporting Information may be found in the online version of this article
Components of the composite sedation scores (0–16 points), modified from Kuusela et al. (2001).
Measurement of detomidine concentrations.
References
- ALCASDE. Final report: Study on the improved methods for animal-friendly production, in particular on alternatives to the castration of pigs and on alternatives to the dehorning of cattle. 2009. SANCO/2008/D5/018 http://ec.europa.eu/food/animal/welfare/farm/alcasde_study_04122009_en.pdf (accessed January 9 2013)
- AVA. Policy Compendium on Cattle Health and Welfare. Australian Veterinary Association. 2004. http://www.ava.com.au/policy/84-dehorning-cattle (accessed January 9 2013)
- AVMA. Welfare implications of dehorning and disbudding of cattle. American Veterinary Medical Association. 2012. https://www.avma.org/KB/Policies/Pages/Castration-and-Dehorning-of-Cattle.aspx (accessed January 9 2013)
- DiMaio Knych HK, Stanley SD. Pharmacokinetics and pharmacodynamics of detomidine following sublingual administration to horses. Am J Vet Res. 2011;72:1378–1385. doi: 10.2460/ajvr.72.10.1378. [DOI] [PubMed] [Google Scholar]
- EC. The European Convention of 1976 for the Protection of Animals kept for Farming Purposes, with the Recommendation Concerning Cattle adopted by the Standing Committee on 21st of October 1988. 1976. http://www.coe.int/t/e/legal_affairs/legal_co-operation/biological_safety_and_use_of_animals/farming/Rec%20cattle%20E.asp (accessed January 9 2013)
- Faulkner PM, Weary DM. Reducing pain after dehorning in dairy calves. J Dairy Sci. 2000;83:2037–2041. doi: 10.3168/jds.S0022-0302(00)75084-3. [DOI] [PubMed] [Google Scholar]
- Fierheller EE, Caulkett NA, Haley DB, et al. Onset, duration and efficacy of four methods of local anesthesia of the horn bud in calves. Vet Anaesth Analg. 2012;39:431–435. doi: 10.1111/j.1467-2995.2012.00717.x. [DOI] [PubMed] [Google Scholar]
- Fulwider WK, Grandin T, Rollin BE, et al. Survey of dairy management practices on one hundred thirteen North Central and Northeastern United States dairies. J Dairy Sci. 2008;91:1686–1692. doi: 10.3168/jds.2007-0631. [DOI] [PubMed] [Google Scholar]
- Gottardo F, Nalon E, Contiero B, et al. The dehorning of dairy calves: practices and opinions of 639 farmers. J Dairy Sci. 2011;94:5724–5734. doi: 10.3168/jds.2011-4443. [DOI] [PubMed] [Google Scholar]
- Graf B, Senn M. Behavioural and physiological responses of calves to dehorning by heat cauterization with or without local anaesthesia. Appl Anim Behav Sci. 1999;62:153–171. [Google Scholar]
- Heinrich A, Duffield TF, Lissemore KD, et al. The effect of meloxicam on behavior and pain sensitivity of dairy calves following cautery dehorning with a local anesthetic. J Dairy Sci. 2010;93:2450–2457. doi: 10.3168/jds.2009-2813. [DOI] [PubMed] [Google Scholar]
- Hoe FGH, Ruegg PL. Opinions and practices of wisconsin dairy producers about biosecurity and animal well-being. J Dairy Sci. 2006;89:2297–2308. doi: 10.3168/jds.S0022-0302(06)72301-3. [DOI] [PubMed] [Google Scholar]
- Kaukinen H, Aspegrén J, Hyyppä S, et al. Bioavailability of detomidine administered sublingually to horses as an oromucosal gel. J Vet Pharmacol Ther. 2011;34:76–81. doi: 10.1111/j.1365-2885.2010.01193.x. [DOI] [PubMed] [Google Scholar]
- Kuusela E, Vainio O, Kaistinen A, et al. Sedative, analgesic, and cardiovascular effects of levomedetomidine alone and in combination with dexmedetomidine in dogs. Am J Vet Res. 2001;62:616–621. doi: 10.2460/ajvr.2001.62.616. [DOI] [PubMed] [Google Scholar]
- Malone JH, Clarke KW. A comparison of the efficacy of detornidine by sublingual and intramuscular administration in ponies. Vet Anaesth Analg. 1993;20:73–77. [Google Scholar]
- McMeekan CM, Stafford KJ, Mellor DJ, et al. Effects of regional analgesia and/or a non-steroidal anti-inflammatory analgesic on the acute cortisol response to dehorning in calves. Res Vet Sci. 1998;64:147–150. doi: 10.1016/s0034-5288(98)90010-8. [DOI] [PubMed] [Google Scholar]
- Messenger KM, Davis JL, LaFevers DH, et al. Intravenous and sublingual buprenorphine in horses: pharmacokinetics and influence of sampling site. Vet Anaesth Analg. 2011;38:374–384. doi: 10.1111/j.1467-2995.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- Morisse JP, Cotte JP, Huonnic D. Effect of dehorning on behaviour and plasma cortisol responses in young calves. Appl Anim Behav Sci. 1995;43:239–247. [Google Scholar]
- New Zealand Government. Animal Welfare (Painful Husbandry Procedures) Code of Welfare 2005. 2005. http://www.biosecurity.govt.nz/files/regs/animal-welfare/req/codes/painful-husbandry/painful-husbandry.pdf (accessed January 9 2013)
- Peshin PK, Singh AP, Singh J, et al. Sedative effect of detomidine in infant calves. Acta Vet Hung. 1991;39:103–107. [PubMed] [Google Scholar]
- Salonen JS, Vähä-Vahe T, Vainio O, et al. Single-dose pharmacokinetics of detomidine in the horse and cow. J Vet Pharmacol Ther. 1989;12:65–72. doi: 10.1111/j.1365-2885.1989.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Stafford KJ, Mellor DJ. Addressing the pain associated with disbudding and dehorning in cattle. Appl Anim Behav Sci. 2011;135:226–231. [Google Scholar]
- Stewart M, Stookey JM, Stafford KJ, et al. Effects of local anesthetic and a nonsteroidal antiinflammatory drug on pain responses of dairy calves to hot-iron dehorning. J Dairy Sci. 2009;92:1512–1519. doi: 10.3168/jds.2008-1578. [DOI] [PubMed] [Google Scholar]
- Stilwell G, Lima MS, Carvalho RC, et al. Effects of hot-iron disbudding, using regional anaesthesia with and without carprofen, on cortisol and behaviour of calves. Res Vet Sci. 2012;92:338–341. doi: 10.1016/j.rvsc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Vasseur E, Borderas F, Cue RI, et al. A survey of dairy calf management practices in Canada that affect animal welfare. J Dairy Sci. 2010;93:1307–1315. doi: 10.3168/jds.2009-2429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Components of the composite sedation scores (0–16 points), modified from Kuusela et al. (2001).
Measurement of detomidine concentrations.


