Abstract
Background
Psoriatic arthritis (PsA) and co-morbidities of psoriasis represent a significant clinical and economic burden for patients with moderate-to-severe psoriasis. Often these co-morbidities may go unrecognized or undertreated. While published data are available on the incidence and impact of some of them, practical guidance for dermatologists on detection and management of these co-morbidities is lacking.
Objective
To prepare expert recommendations to improve the detection and management of common co-morbidities in patients with moderate-to-severe psoriasis.
Methods
A systematic literature review was conducted on some common co-morbidities of psoriasis–cardiovascular (CV) diseases (including obesity, hypertension, hyperglycaemia and dyslipidaemia), psychological co-morbidities (including depression, alcohol abuse and smoking) and PsA–to establish the incidence and impact of each. Data gaps were identified and a Delphi survey was carried out to obtain consensus on the detection and management of each co-morbidity. The expert panel members for the Delphi survey comprised 10 dermatologists with substantial clinical expertise in managing moderate-to-severe psoriasis patients, as well as a cardiologist and a psychologist (see appendix) with an interest in dermatology. Agreement was defined using a Likert scale of 1–7. Consensus regarding agreement for each statement was defined as ≥75% of respondents scoring either 1 (strongly agree) or 2 (agree).
Results
The expert panel members addressed several topics including screening, intervention, monitoring frequency, and the effects of anti-psoriatic treatment on each co-morbidity. Consensus was achieved on 12 statements out of 22 (3 relating to PsA, 4 relating to psychological factors, 5 relating to CV factors). The panel members felt that dermatologists have an important role in screening their psoriasis patients for PsA and in assessing them for psychological and CV co-morbidities. In most cases, however, patients should be referred for specialist management if other co-morbidities are detected.
Conclusion
This article provides useful and practical guidance for the detection and management of common co-morbidities in patients with moderate-to-severe psoriasis.
Conflicts of interest
Prim. Associate Prof. Robert Strohal serves on speaker bureaus for Pfizer, Schülke and Mayer, Lohmann and Rauscher, Meda Pharmaceuticals, Menarini Pharmaceuticals, Stockhausen, and Smith and Nephew. He has consulting agreements with Pfizer, Astellas, Novartis, Lohmann and Rauscher, Urgo, Chemomedica, Schülke and Mayer, and Pantec Biotechnologies. He receives research and educational grants from Pfizer, Stockhausen,
3M-Woundcare, Smith and Nephew, Lohmann and Rauscher, Enjo Commercials, Urgo, Chemomedica, and Schülke and Mayer.
Prof. Brian Kirby receives research support/Principle Investigator (clinical trials) from Janssen, Abbvie, Serono and Pfizer. He acts as consultant for Merck Sharpe & Dohme (MSD), Pfizer, Janssen and Abbvie. He has received honoraria from Janssen, Pfizer, Abbvie, and has acted as a scientific advisory board member for Pfizer and Abbvie.
Prof. Lluís Puig has participated as Principal Investigator in clinical trials sponsored by Abbvie, Amgen, Janssen, Lilly, Novartis, Pfizer and VBL. He has received consultancy/speaker honoraria from Abbvie, Amgen, Celgene, Janssen, Eli Lilly, Merck, Merck-Serono, Novartis and Pfizer.
Prof. Giampiero Girolomoni has received honoraria for lectures, manuscript preparation, development of educational programmes and/or board membership, and has participated as Principal Investigator in clinical trials sponsored by Abbvie, Celgene, Galderma, Janssen, Eli Lilly, Merck-Serono, Otsuka, MSD, Novartis and Pfizer.
Prof. Knud Kragballe has acted as an advisor, investigator and/or speaker for Abbott, Amgen, Janssen-Cilag, Leo-Pharma, MSD and Pfizer.
Prof. Thomas Luger has participated as Principal Investigator in clinical trials sponsored by Novartis, Lilly, Pfizer and Janssen. He has received consultancy/speaker honoraria from Novartis, Abbvie, MEDA Pharma and Janssen, and has acted as scientific Advisory Board member for Abbvie, Celgene, Janssen, Pfizer, MEDA Pharma and Galderma. Prof. Frank Nestle has received consultancy/speaker honoraria from Abbvie, Celgene, Janssen, Novartis, Takeda and Pfizer.
Prof. Prinz has served as a consultant, investigator, speaker or advisory board member for Biogen-Idec (formerly Biogen), Novartis, Wyeth, Pfizer, Merck-Serono (formerly Serono), Essex Pharma, MSD, Galderma, Centocor, Abbott, Janssen-Cilag/Janssen-Ortho. Furthermore, he has received an unrestricted research grant from Biogen-Idec and Wyeth in the past.
Prof. Mona Ståhle has received consultancy/speaker honoraria from Janssen, Pfizer, Serono, Novartis and Abbvie. She has received unrestricted research support from Pfizer and Janssen.
Prof. Nikhil Yawalkar has participated as Principal or Co-Investigator in clinical trials sponsored by Abbvie, Amgen, Novartis, MSD and Pfizer. He has received consultancy/speaker honoraria from Abbvie, Amgen, Janssen, Eli Lilly, MSD, Novartis and Pfizer.
Funding source
This study was developed by an unrestricted grant from Pfizer Inc.
Introduction
Psoriasis is associated with psoriatic arthritis (PsA) and a range of co-morbid diseases and risk factors, including obesity, metabolic syndrome, cardiovascular (CV) disease, autoimmune disease, psychiatric illness, liver disease, smoking, chronic obstructive pulmonary disease, sleep apnoea, smoking and alcohol abuse.1 It should be noted that there is a greater degree of pathophysiological/genetic association between psoriasis and PsA than between psoriasis or PsA and their associated co-morbidities. Psoriasis patients with co-morbidities are more likely to need urgent care or hospitalisation, and incur greater total costs than those without co-morbidities. In the United States, compared with patients without co-morbidities, patients with co-morbidities incurred $2184 greater costs.2
A delay in the diagnosis of PsA is a significant contributor to poor patient outcomes, so early identification of PsA in patients with psoriasis is important.3 There is evidence that PsA is underdiagnosed, however, among psoriasis patients attending dermatology clinics.3,4 Psoriasis is linked with social stigmatisation, pain, discomfort, physical disability, psychological distress and financial hardship. These problems may be associated with anxiety, depression, smoking and alcohol abuse, which can all also have a direct negative impact on psoriasis and lead to poor compliance and treatment outcomes.5 As severe psoriasis is associated with increased mortality, and the most common cause of death is CV disease,6 detection and management of CV disease and its risk factors may be of great importance in patients with psoriasis.
While published data are available on the incidence and impact of PsA and some of these co-morbidities, practical guidance for dermatologists on their detection and management is lacking. The project reported here concentrated on PsA and certain common co-morbidities of psoriasis: psychological problems and CV diseases. A literature search was first carried out to establish the burden of PsA and each co-morbidity. The steering committee met to identify gaps in the literature on key topics and to develop statements to address these gaps. A survey was then conducted using Delphi methodology to obtain a consensus among an international expert panel regarding screening, intervention, monitoring frequency and effects of anti-psoriatic treatment for each co-morbidity. The aim was to develop recommendations to improve the detection and management of common co-morbidities in patients with moderate-to-severe psoriasis.
Methods
Literature search
A search of PubMed was carried out for articles relevant to the selected psoriasis co-morbidities in adults, published in English from 1 January 2005 to 31 December 2012. Search terms used were ‘Psoriasis’ plus the secondary terms listed in Box 1. Titles and abstracts of retrieved articles were scanned for relevance, and key information on the incidence and impact of each co-morbidity was extracted and collated. The full results of the literature search are described in a separate publication (L. Puig, unpublished data).
Box 1. Terms used in literature search.
“Psoriasis” plus:
Cardiovascular risk factors
Obesity/body mass index
Diabetes/insulin sensitivity/resistance
Metabolic syndrome
Hypertension/blood pressure
Dyslipidaemia/cholesterol/triglycerides/lipid profile/hyperlipidaemia
Major adverse cardiac events/myocardial infarction/stroke/coronary heart disease
Paediatric + each of above terms
Alcohol abuse/misuse/intake
Smoking
Depression/anxiety/psychiatric/psychosocial/mental health
Paediatric + depression/anxiety/psychiatric/psychosocial/mental health
Sexual dysfunction/function
Sleep/sleep apnoea
Survey participants
A steering committee, consisting of three expert dermatologists (R. Strohal, B. Kirby and L. Puig), met to define and develop the topics and statements for the Delphi survey. An international expert panel, comprising 10 dermatologists with substantial clinical expertise in managing patients with moderate-to-severe psoriasis, as well as a cardiologist with an interest in dermatology and a psychologist who works in psychodermatology, participated in the Delphi survey.
Delphi methodology
The Delphi method was developed to enable complex problems to be explored through structured group communication.7 It is an evidence-based approach that allows a group to explore the issues surrounding a problem, establishing the advantages and disadvantages of different arguments, and clarifying ideas and opinions.7,8 The original Delphi method has been modified to enable achievement of consensus, through the use of surveys and a final live meeting.9 The process involves the formation of a steering committee responsible for identifying the issues surrounding a complex problem, the development of relevant survey questions and the recruitment of a panel of experts to participate anonymously in the survey.
In this case, three rounds of anonymous completion of the survey were followed by live meetings during which individual survey responses remained anonymous to preserve objectivity. At the end of the process, and through relevant scientific discussion, consensus statements with regard to agreement and disagreement were collected whenever possible.
Survey questionnaire design application
After an initial discussion of topics between members of the expert panel, a core group developed a set of questions for the Delphi survey, which were then reviewed by the whole group. All of the expert panel members were then asked to complete the online questionnaire in English. Each participant answered questions related to screening, monitoring and treating PsA and psoriasis co-morbidities (Box 2), grouped into PsA (six questions), psychological factors (six questions) and CV risk factors (10 questions). These co-morbidities were chosen after a review of the literature indicated that these have a greater impact on the health of psoriasis patients. The questionnaire was designed to assess the strength of agreement with statements in the defined topic areas. This type of collaborative survey questionnaire is able to collect and synthesize opinions across a group and achieve a degree of consensus.
Box 2. Questions asked in the Delphi survey.
Psoriatic arthritis (PsA)
All patients with psoriasis should be examined initially and then at least annually for signs and symptoms of PsA.
In principle, the routine use of PsA screening questionnaires may be useful.
- Minimal standards of examination by the dermatologist should include:
- Examination of the hands and feet
- Questioning the patient about pain and stiffness in the joints, including peripheral and axial pain.
A definitive diagnosis of PsA should be made by a rheumatologist.
Treatment decisions for moderate-to-severe psoriatic joint symptoms can be made by the dermatologist alone.
Once a diagnosis of PsA has been made, the dermatologist can manage/treat the patient as per local PsA treatment guidelines.
Psychological factors
7 For psoriasis, all relevant psychological co-morbidities are covered by the Dermatology Life Quality Index (DLQI) or other quality of life scores.
8 Patients with moderate-to-severe psoriasis should be assessed regularly for anxiety, depression and addictive behaviour.
9 If psychological co-morbidities substantially impact the management of moderate-to-severe psoriasis, the patient should be referred to a psychiatrist.
10 If the dermatologist suspects severe depression, the patient should be referred to a psychiatrist.
11 Specific therapeutic interventions for psychological co-morbidities can be performed by the dermatologist.
Cardiovascular factors
- 12 Patients with moderate-to-severe psoriasis should have the following monitored initially and annually thereafter:
- Blood pressure
- Body mass index (BMI)
- Waist circumference
- Lipids
- (i) Cholesterol
- (ii) Triglycerides
- (iii) Low-density lipoprotein (LDL)/high-density lipoprotein (HDL)
- Fasting glucose
- Glycated haemoglobin (HbA1c)
- Smoking status
13 Moderate-to-severe psoriasis is an independent risk factor for cardiovascular disease; intervention thresholds should be adjusted accordingly (as they are for rheumatoid arthritis [RA]).
14 RA data suggest that aggressive management of cardiovascular risks is necessary. Cardiovascular risk management should also be adjusted in patients with moderate-to-severe psoriasis.
15 Dermatologists should use cardiovascular risk scores to assess patients with moderate-to-severe psoriasis.
16 Referral to a specialist should be based on an appropriate cardiovascular scoring system, as assessed by the dermatologist.
17 Referral to a specialist should be based solely on abnormal cardiovascular parameters, as assessed by the dermatologist.
18 Treatment choices for cardiovascular co-morbidities should be determined by a cardiologist, endocrinologist or family doctor.
19 Management of cardiovascular issues should be performed by the dermatologist, according to European guidelines.
20 In patients with increased cardiovascular risk factors, more intensive systemic treatment for moderate-to-severe psoriasis is appropriate.
21 Weight loss and lifestyle advice must be given by dermatologists during the routine management of patients with moderate-to-severe psoriasis.
Participants scored their opinion of each statement using a Likert scale from 1 to 7, where 1 = ‘Strongly agree’, 2 = ‘Agree’, 3 = ‘Agree somewhat’, 4 = ‘Neither agree nor disagree’, 5 = ‘Disagree somewhat’, 6 = ‘Disagree’ and 7 = ‘Strongly disagree’. A numerical identification system was used to ensure that participants remained anonymous.
After each round, the steering committee presented the survey results to the expert panel members and a discussion took place regarding the key issues identified and areas where consensus had and had not been reached. After the first survey round, the cardiologist answered only the questions relating to CV factors and the psychologist answered only those relating to psychological factors.
Data analysis
Following each round of completion of the questionnaires, responses were collected and analysed on a group basis. The percentage of respondents assigning each Likert score was calculated for each question. Summary statistics for each question included the minimum, median, mode and maximum scores, as well as an interquartile range.
To further establish the degree of agreement or disagreement, responses to statements were categorized as ‘Agree/strongly agree’ (score 1–2), ‘agree somewhat/neither agree nor disagree/disagree somewhat’ (score 3–5) or ‘Disagree/strongly disagree’ (score 6–7). Consensus regarding agreement for each statement was defined as ≥75% of respondents scoring either 1 (strongly agree) or 2 (agree).
Results
Survey rounds and consensus
For many of the statements, responses did not change greatly over the three rounds. For some statements, a consensus reached in round 1 became stronger over the following rounds (Fig. 1, top). For a few statements, the views of the experts changed considerably over the three rounds, with no consensus being reached (Fig. 1, bottom).
Figure 1.
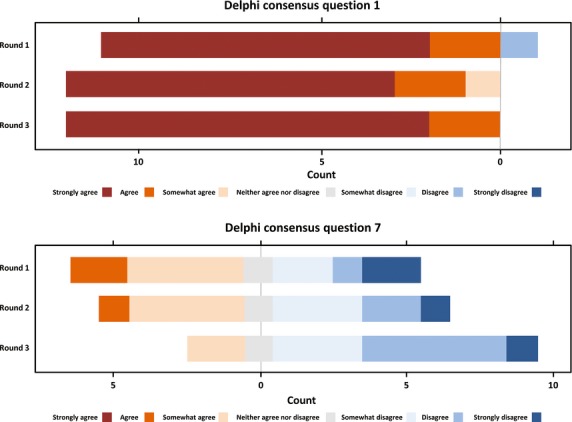
Horizontal bar charts of responses for questions 1 (top) and 7 (bottom) from first to third round Delphi questionnaire.
Following the third and final round of the survey, consensus agreement had been reached for 12 of the 22 statements (three relating to PsA, four relating to psychological factors and five relating to CV factors). These areas of consensus are listed in Box 3.
Box 3. Consensus statements on co-morbidities of moderate-to-severe psoriasis.
Psoriatic arthritis (PsA)
All patients with psoriasis should be examined initially and then at least annually for signs and symptoms of PsA
PsA screening questionnaires should be used routinely in dermatology clinical practice
- Minimal standards of examination by the dermatologist should include:
- Examination of the hands and feet
- Questioning the patient about pain and stiffness in the joints, including peripheral and axial pain
Psychological factors
Patients with moderate-to-severe psoriasis should be assessed regularly for anxiety, depression and addictive behaviour
If psychological co-morbidities substantially impact the management of moderate-to-severe psoriasis, the patient should be referred to a psychiatrist
If the dermatologist suspects (severe) depression, the patient should be referred to a psychiatrist
Cardiovascular factors
- Patients with moderate-to-severe psoriasis should have the following monitored initially and annually thereafter:
- Blood pressure, BMI, waist circumference, lipids (e.g., cholesterol, triglycerides, low-density/high-density lipoprotein), fasting glucose, glycated haemoglobin (HbA1c), smoking status
Moderate-to-severe psoriasis is an independent risk factor for cardiovascular disease; intervention thresholds should be adjusted accordingly (as they are for rheumatoid arthritis)
RA data suggest that aggressive management of cardiovascular risks is necessary. Cardiovascular risk management should also be adjusted in patients with moderate-to-severe psoriasis
Treatment choices for cardiovascular co-morbidities should be determined by a cardiologist, endocrinologist or family doctor
Weight loss and lifestyle advice must be given by dermatologists during the routine management of patients with moderate-to-severe psoriasis
Psoriatic arthritis
Understanding the burden
The estimated prevalence of PsA among patients with psoriasis varies widely, from 7.7% to 73% (Table 1),10,11 with several studies finding a prevalence between 20% and 30%.12–14 The costs associated with PsA are high and increase with disease severity and duration.15,16 Compared with psoriasis patients without arthritis, patients with PsA have more severe skin symptoms, a lower quality of life and greater impairment of productivity parameters.14 Increased rates of CV risk factors, metabolic syndrome and diabetes have been reported in PsA patients.17,18
Table 1.
Prevalence of co-morbidities in psoriasis
| Co-morbidity | Prevalence in psoriasis | Hazard*/odds ratio |
|---|---|---|
| Psoriatic arthritis | 8–73%10–14 | – |
| Psychological co-morbidities | ||
| Depression | 15–62%21,22 | 1.39*–1.4919,20 |
| Alcohol abuse | 15–30%26–29 | 3.10–3.6129,31 |
| Smoking | 30–51%12,30 | 1.31–2.9630–32 |
| Cardiovascular co-morbidities | ||
| Obesity | 8–41%12,30,50,51 | 1.18–5.4934 |
| Diabetes | 7–41%12,30,50 | 1.20–2.8034 |
| Metabolic syndrome | 16–40%12,52 | 1.30–5.9234 |
| Hypertension | 13–50%21,30,50,53,54 | 1.09–3.2734 |
| Dyslipidaemia | 6–61%2,30,53,54 | 1.00–2.0934 |
Hazard ratio.
Screening and diagnosis
The first four statements about PsA related to screening psoriasis patients for PsA. There was 100% agreement (Likert score 1–2) that all patients with psoriasis should be examined initially and then at least annually for signs and symptoms of PsA. Agreement that PsA screening questionnaires should be used routinely in dermatological practice was also strong (92%). In discussion, the expert panel did not come to a conclusion that any particular screening tool could be recommended. The third statement was that minimal standards of examination by the dermatologist should include: examination of the hands and feet; and questioning the patient about pain and stiffness in the joints, including peripheral and axial pain. All of the experts agreed with this statement. Regarding diagnosis, two-thirds of the panel members agreed that a definitive diagnosis of PsA should be made by a rheumatologist, but no consensus was reached on this question.
Management/treatment
The final two PsA statements related to patient management once a diagnosis of PsA has been made. No consensus was reached as to whether treatment decisions for moderate-to-severe psoriatic joint symptoms can be made by the dermatologist alone. Two-thirds of the panel members agreed that once a diagnosis of PsA has been made, the dermatologist can manage/treat the patient according to local PsA treatment guidelines, but no consensus was reached.
Psychological factors
Understanding the burden
The hazard ratio for depression in psoriasis patients vs. the general population is about 1.4–1.5 and increases with severity of psoriasis.20,21 Depression is reported in 15–62% of patients (Table 1),21,22 and suicidal ideation is reported in 5.5% to 9.7% of psoriasis patients.23 Other psychiatric diagnoses are also common.19 Psychiatric symptoms are associated with reduced quality of life in psoriasis patients.24,25 Alcohol abuse is reported in 15–30% of psoriasis patients.26–29 There is little evidence for an association between alcohol consumption and severity of psoriasis, but excess alcohol consumption appears to be associated with depression in psoriasis patients,26,28 and may adversely affect treatment outcomes, particularly regarding poor compliance.28 Psoriasis patients have an increased likelihood of smoking compared with the general population, with odds ratios from 1.31 to 2.96 reported.30–32 Smoking is also associated with psoriasis disease severity,29,32 and is correlated with impaired psoriasis-related quality of life.33 All of these addictive behaviours may be related to the emotional burden of the psoriasis patient having a stigmatising disease.
Assessment and referral
There was strong agreement (92% with Likert score 1–2) that patients with moderate-to-severe psoriasis should be assessed regularly for anxiety, depression and addictive behaviour (e.g. alcohol consumption, smoking). The panel members tended to disagree however (50% scored 6–7) with the statement, that for psoriasis, all relevant psychological co-morbidities are covered by the dermatology life quality index (DLQI). Consensus was reached on each of the next three statements regarding psychiatric referral: there was strong agreement (92% with Likert score 1–2) that the patient should be referred to a psychiatrist if psychological co-morbidities substantially impact the management of moderate-to-severe psoriasis or if the dermatologist suspects depression. Agreement was very strong (100% with Likert score 1–2) that if the dermatologist suspects severe depression, the patient should be referred to a psychiatrist.
Management/treatment
The final statement in this section was that specific therapeutic interventions for psychological co-morbidities can be performed by the dermatologist. Opinion was divided, with more than half of the panel members disagreeing with this statement.
Cardiovascular factors
Understanding the burden
Table 1 shows estimates from literature of the prevalence of various CV risk factors in patients with psoriasis. In a systematic review published in 2010, all but one of eight studies on obesity found an increased risk in psoriasis patients, with odds ratios ranging from 1.18 (95% CI 1.14–1.23) to 5.49 (3.09–9.74).34 The risk increased with severity of psoriasis. The same review found an increased risk of metabolic syndrome in three of three studies, with odds ratios ranging from 1.3 (1.1–1.4) to 5.92 (2.78–12.8).34 A significant association of psoriasis with diabetes was found in 11/14 studies (odds ratio 1.20 [1.14–1.25] to 2.80 [2.68–2.99]), with dyslipidaemia in 7/12 studies (1.0 [1.0–1.3] to 2.09 [1.23–3.54]) and with hypertension in 10/12 studies (1.09 [1.05–1.14] to 3.27 [2.41–4.43]).34 Two meta-analyses published since the literature search was performed found odds ratios of 1.58 (95% CI 1.42–1.76) for hypertension and 1.59 (1.38–1.83) for type 2 diabetes among patients with psoriasis compared with controls.35,36 Both found higher odds among patients with severe psoriasis.
A cross-sectional study found that the Framingham risk score was significantly higher in patients with psoriasis than in controls at 5 years (5.3 ± 4.4 [mean ± SD] vs. 3.4 ± 3.3, P < 0.001) and at 10 years (11.2 ± 8.1 vs. 7.3 ± 6.3, P < 0.001).37 Data on cardiac events in psoriasis patients are limited. One cohort study noted a significantly increased risk of major CV adverse events in patients with severe psoriasis.38 A recent meta-analysis of nine studies suggests that severe psoriasis significantly increases the risk of CV mortality (relative risk 1.39, 95% CI, 1.11–1.74), myocardial infarction (relative risk 1.70, 95% CI 1.32–2.18) and stroke (relative risk 1.56, 95% CI, 1.32–1.84).39 A large case–control study found an increased risk of CV death in psoriasis patients.6
Monitoring and assessment
The expert panel recognized the importance of assessing and reducing CV risk in patients with moderate-to-severe psoriasis. There was 100% agreement that patients with moderate-to-severe psoriasis should have the following monitored initially and annually thereafter: blood pressure, body mass index (BMI), waist circumference, lipids (e.g., cholesterol, triglycerides, low-density/high-density lipoprotein cholesterol), fasting glucose, glycosylated haemoglobin (HbA1c), smoking status. There was also good agreement (83% scored 1–2) that moderate-to-severe psoriasis is an independent risk factor for CV disease and that intervention thresholds should be adjusted accordingly (as recommended for patients with rheumatoid arthritis).40 The same proportion of the panel members agreed that aggressive management of CV risks is necessary in patients with moderate-to-severe psoriasis.
No consensus was reached on the next three statements:
Dermatologists should use CV risk scores to assess patients with moderate-to-severe psoriasis.
Referral to a specialist should be based on the European Society of Cardiology CV risk scoring charts, as assessed by the dermatologist.
Referral to a specialist should be based solely on abnormal CV parameters, as assessed by the dermatologist.
Management/treatment
According to the expert panel, weight loss and lifestyle advice should be given by dermatologists during the routine management of patients with moderate-to-severe psoriasis (83% scored 1–2). The panel felt that treatment choices for CV co-morbidities should be determined by a cardiologist, endocrinologist or family doctor (91% scored 1–2). Most of the panel members (58.4% [consensus not reached]) did not agree that management of CV issues should be performed by the dermatologist, according to ESC guidelines. Two-thirds of the experts considered that more intensive systemic treatment for moderate-to-severe psoriasis is needed in patients with increased CV risk factors, but consensus was not reached on this subject.
Discussion
Co-morbidities of psoriasis represent a significant burden for patients. A recent US population-based study found that the mean Carlson co-morbidity index was increasingly higher among patients with mild, moderate and severe psoriasis compared with matched controls.41 However, co-morbidities may often go unrecognized or undertreated. The Delphi survey reported here enabled consensus to be reached on 12 statements regarding the detection and management of PsA and of important psychological and CV co-morbidities in patients with moderate-to-severe psoriasis (Box 3).
The three consensus statements on PsA all related to screening and detection. The experts were unanimous in considering that all psoriasis patients should be examined at least annually for signs and symptoms of PsA, and that this should include examination of the hands and feet as well as questioning the patient about pain and stiffness in the joints. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) considers inflammation to include such features as pain involving joints, spine and/or entheses associated with erythema, warmth and swelling, and prominent morning and rest stiffness.43 The panel also strongly agreed that PsA screening questionnaires should be used routinely in dermatology clinical practice. Tools available include the Psoriatic and Arthritis Questionnaire (PAQ),43 the Psoriasis Epidemiology Screening Tool (PEST),44 the Psoriatic Arthritis Screening and Evaluation (PASE),45 the Toronto Psoriatic Arthritis Screening (ToPAS)46 and the Early ARthritis for Psoriatic patients (EARP) questionnaire.47 However, all of these instruments have deficiencies,3,48 and more work is needed before specific recommendations can be made about which one to use.
Group for Research and Assessment of Psoriasis and Psoriatic Arthritis recommends that diagnosis of PsA should follow the Classification Criteria for Psoriatic Arthritis (CASPAR) criteria.42,49 Whether the definitive diagnosis is made by the dermatologist or a rheumatologist will depend on the local situation and the expertise of the dermatologist. Some cases are easier to diagnose than others, and opinions will be influenced by the local availability of rheumatologists. The diagnosis of PsA requires a structured examination, and dermatologists can be competent in doing this. Many dermatologists will also be confident in treating PsA with a tumour necrosis factor inhibitor, while others may prefer to refer patients to a rheumatologist for consideration of treatment with a disease-modifying anti-rheumatic drug or a biological.
The expert panel recognized the importance of regularly assessing psoriasis patients for anxiety, depression and addictive behaviour. The frequency of assessment was not specified in the survey question, but the experts felt that annual assessment would be appropriate. They considered formal assessment to be necessary, as dermatologists are often not aware of psychological problems in their patients. The panel did not believe that dermatologists should be performing therapeutic interventions for psychological co-morbidities, recommending that patients with such co-morbidities, including depression, should be referred for specialist management. As smoking appears to be associated with psoriasis disease severity28,32 and is correlated with impaired psoriasis-related quality of life,33 smoking cessation should be considered a priority in patients with psoriasis.
Rheumatoid arthritis is considered an independent risk factor for CV disease, requiring intervention targets to be adjusted accordingly and aggressive management of CV risk factors.40 The expert panel agreed that the same approach proposed by the European League Against Rheumatism (EULAR) for CV disease risk management in patients with PsA should be taken in moderate-to-severe psoriasis. Psoriasis patients should have their blood pressure, BMI, waist circumference, lipids, fasting glucose, HbA1c and smoking status monitored on an annual basis. The experts felt that such monitoring is warranted, even in younger patients, given the high CV impact of psoriasis. It is less clear who should do the monitoring; it may be the dermatologist or the family doctor. If dermatologists are going to do this, they will need to become more familiar with the use of CV risk scores. The experts felt that dermatologists should give weight loss and other lifestyle advice to their psoriasis patients, but treatment choices for CV co-morbidities should be determined by other specialists or the family doctor.
This consensus article provides useful and practical guidance for the detection and management of common co-morbidities in patients with moderate-to-severe psoriasis. It also highlights areas where consensus was not reached and further research is clearly needed to optimize patient management.
Appendix
The following experts also took part in the Delphi survey: Prof. Peter Riis Hansen (Cardiologist), Gentofte Hospital, Copenhagen, Denmark Sandra Ros (Psychologist), Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
References
- 1.Kim N, Thrash B, Menter A. Comorbidities in psoriasis patients. Semin Cutan Med Surg. 2010;29:10–15. doi: 10.1016/j.sder.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Kimball AB, Guérin A, Tsaneva M, et al. Economic burden of comorbidities in patients with psoriasis is substantial. J Eur Acad Dermatol Venereol. 2011;25:157–163. doi: 10.1111/j.1468-3083.2010.03730.x. [DOI] [PubMed] [Google Scholar]
- 3.Haroon M, Kirby B, Fitzgerald O. High prevalence of psoriatic arthritis in patients with severe psoriasis with suboptimal performance of screening questionnaires. Ann Rheum Dis. 2013;72:736–740. doi: 10.1136/annrheumdis-2012-201706. [DOI] [PubMed] [Google Scholar]
- 4.Mease PJ, Papp KA, Gladman D, et al. 2012. The prevalence of rheumatologist-diagnosed psoriatic arthritis in psoriasis patients in European/North American dermatology clinics: Results of the PREPARE study. 3rd World Psoriasis & Psoriatic Arthritis Conference, Stockholm, Sweden, 27 June–3 July.
- 5.Hayes J, Koo J. Psoriasis: depression, anxiety, smoking, and drinking habits. Dermatol Ther. 2010;23:174–180. doi: 10.1111/j.1529-8019.2010.01312.x. [DOI] [PubMed] [Google Scholar]
- 6.Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the UK. Br J Dermatol. 2010;163:586–592. doi: 10.1111/j.1365-2133.2010.09941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linstone HA, Turoff M, editors. The Delphi Method: techniques and Applications. Reading, MA: Addison-Wesley; 1975. pp. 291–321. [Google Scholar]
- 8.Rauch W. The decision Delphi. Technol Forecast Soc Change. 1979;15:159–169. [Google Scholar]
- 9.Rowe HI. The ‘Collaborative’ Delphi symposium. 2005. Annual Sustainable Rangelands Roundtable Meeting, Phoenix, AZ, USA.
- 10.Sadek HA, Abdel-Nasser AM, El-Amawy TA, Hassan SZ. Rheumatic manifestations of psoriasis. Clin Rheumatol. 2007;26:488–498. doi: 10.1007/s10067-006-0307-1. [DOI] [PubMed] [Google Scholar]
- 11.Gisondi P, Girolomoni G, Sampogna F, Tabolli S, Abeni D. Prevalence of psoriatic arthritis and joint complaints in a large population of Italian patients hospitalised for psoriasis. Eur J Dermatol. 2005;15:279–283. [PubMed] [Google Scholar]
- 12.Al-Mutairi N, Al-Farag S, Al-Mutairi A, Al-Shiltawy M. Comorbidities associated with psoriasis: an experience from the Middle East. J Dermatol. 2010;37:146–155. doi: 10.1111/j.1346-8138.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- 13.Radtke MA, Reich K, Blome C, Rustenbach S, Augustin M. Prevalence and clinical features of psoriatic arthritis and joint complaints in 2009 patients with psoriasis: results of a German national survey. J Eur Acad Dermatol Venereol. 2009;23:683–691. doi: 10.1111/j.1468-3083.2009.03159.x. [DOI] [PubMed] [Google Scholar]
- 14.Reich K, Krüger K, Mössner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol. 2009;160:1040–1047. doi: 10.1111/j.1365-2133.2008.09023.x. [DOI] [PubMed] [Google Scholar]
- 15.Huscher D, Merkesdal S, Thiele K, et al. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis. 2006;65:1175–1183. doi: 10.1136/ard.2005.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poole CD, Lebmeier M, Ara R, Rafia R, Currie CJ. Estimation of health care costs as a function of disease severity in people with psoriatic arthritis in the UK. Rheumatology. 2010;49:1949–1956. doi: 10.1093/rheumatology/keq182. [DOI] [PubMed] [Google Scholar]
- 17.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–2172. [PubMed] [Google Scholar]
- 18.Husted JA, Thavaneswaran A, Chandran V, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken) 2011;63:1729–1735. doi: 10.1002/acr.20627. [DOI] [PubMed] [Google Scholar]
- 19.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmitt J, Ford DE. Psoriasis is independently associated with psychiatric morbidity and adverse cardiovascular risk factors, but not with cardiovascular events in a population-based sample. J Eur Acad Dermatol Venereol. 2010;24:885–892. doi: 10.1111/j.1468-3083.2009.03537.x. [DOI] [PubMed] [Google Scholar]
- 21.Altobelli E, Maccarone M, Petrocelli R, et al. Analysis of health care and actual needs of patients with psoriasis: a survey on the Italian population. BMC Public Health. 2007;7:59. doi: 10.1186/1471-2458-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito M, Saraceno R, Giunta A, Maccarone M, Chimenti S. An Italian study on psoriasis and depression. Dermatology. 2006;212:123–127. doi: 10.1159/000090652. [DOI] [PubMed] [Google Scholar]
- 23.Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol. 1998;139:846–850. doi: 10.1046/j.1365-2133.1998.02511.x. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt JM, Ford DE. Role of depression in quality of life for patients with psoriasis. Dermatology. 2007;215:17–27. doi: 10.1159/000102029. [DOI] [PubMed] [Google Scholar]
- 25.Sampogna F, Tabolli S, Söderfeldt B, et al. Measuring quality of life of patients with different clinical types of psoriasis using the SF-36. Br J Dermatol. 2006;154:844–849. doi: 10.1111/j.1365-2133.2005.07071.x. [DOI] [PubMed] [Google Scholar]
- 26.Aronson PJ, Malick F. Towards rational treatment of severe psoriasis in alcoholics: report of two cases. J Drugs Dermatol. 2010;9:405–408. [PubMed] [Google Scholar]
- 27.Biljan D, Laufer D, Filaković P, Situm M, Brataljenović T. Psoriasis, mental disorders and stress. Coll Antropol. 2009;33:889–892. [PubMed] [Google Scholar]
- 28.McAleer MA, Mason DL, Cunningham S, et al. Alcohol misuse in patients with psoriasis: identification and relationship to disease severity and psychological distress. Br J Dermatol. 2011;164:1256–1261. doi: 10.1111/j.1365-2133.2011.10345.x. [DOI] [PubMed] [Google Scholar]
- 29.Gerdes S, Zahl VA, Weichenthal M, Mrowietz U. Smoking and alcohol intake in severely affected patients with psoriasis in Germany. Dermatology. 2010;220:38–43. doi: 10.1159/000265557. [DOI] [PubMed] [Google Scholar]
- 30.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–328. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 32.Xiao J, Chen LH, Tu YT, Deng XH, Tao J. Prevalence of myocardial infarction in patients with psoriasis in central China. J Eur Acad Dermatol Venereol. 2009;23:1311–1315. doi: 10.1111/j.1468-3083.2009.03318.x. [DOI] [PubMed] [Google Scholar]
- 33.Davidsson S, Blomqvist K, Molin L, et al. Lifestyle of Nordic people with psoriasis. Int J Dermatol. 2005;44:378–383. doi: 10.1111/j.1365-4632.2005.01925.x. [DOI] [PubMed] [Google Scholar]
- 34.Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(Suppl 2):23–30. doi: 10.1111/j.1468-3083.2009.03564.x. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433–442. doi: 10.1097/HJH.0b013e32835bcce1. discussion 442–3. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 37.Gisondi P, Farina S, Giordano MV, Girolomoni G. Usefulness of the framingham risk score in patients with chronic psoriasis. Am J Cardiol. 2010;106:1754–1757. doi: 10.1016/j.amjcard.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 38.Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124:775 e1–775 e6. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systemic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2:e000062. doi: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696. [DOI] [PubMed] [Google Scholar]
- 41.Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchlin CT, Kavanaugh A, Gladman D, et al. Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009;68:1387–1394. doi: 10.1136/ard.2008.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alenius GM, Stenberg B, Stenlund H, Lundblad M, Dahlqvist SR. Inflammatory joint manifestations are prevalent in psoriasis: prevalence study of joint and axial involvement in psoriatic patients, and evaluation of a psoriatic and arthritic questionnaire. J Rheumatol. 2002;29:2577–2582. [PubMed] [Google Scholar]
- 44.Ibrahim GH, Buch MH, Lawson C, Waxman R, Helliwell PS. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469–474. [PubMed] [Google Scholar]
- 45.Husni ME, Meyer KH, Cohen DS, Mody E, Qureshi AA. The PASE questionnaire: pilot-testing a psoriatic arthritis screening and evaluation tool. J Am Acad Dermatol. 2007;57:581–587. doi: 10.1016/j.jaad.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Gladman DD, Schentag CT, Tom BD, et al. Development and initial validation of a screening questionnaire for psoriatic arthritis: the Toronto Psoriatic Arthritis Screen (ToPAS) Ann Rheum Dis. 2009;68:497–501. doi: 10.1136/ard.2008.089441. [DOI] [PubMed] [Google Scholar]
- 47.Tinazzi I, Adami S, Zanolin EM, et al. The early psoriatic arthritis screening questionnaire: a simple and fast method for the identification of arthritis in patients with psoriasis. Rheumatology (Oxford) 2012;51:2058–2063. doi: 10.1093/rheumatology/kes187. [DOI] [PubMed] [Google Scholar]
- 48.Walsh JA, Callis DuffinK, Krueger G, Clegg DO. Limitations of psoriatic arthritis screening instruments in patients with psoriasis. J Invest Dermatol. 2012;132:S39. abstract 229] [Google Scholar]
- 49.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 50.Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216:152–155. doi: 10.1159/000111512. [DOI] [PubMed] [Google Scholar]
- 51.Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527–1534. doi: 10.1001/archderm.141.12.1527. [DOI] [PubMed] [Google Scholar]
- 52.Love TJ, Qureshi AA, Karlson EW, Gelfand JM, Choi HK. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419–424. doi: 10.1001/archdermatol.2010.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Augustin M, Reich K, Glaeske G, Schaefer I, Radtke M. Co-morbidity and age-related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venereol. 2010;90:147–151. doi: 10.2340/00015555-0770. [DOI] [PubMed] [Google Scholar]
- 54.Mebazaa A, El Asmi M, Zidi W, et al. Metabolic syndrome in Tunisian psoriatic patients: prevalence and determinants. J Eur Acad Dermatol Venereol. 2011;25:705–709. doi: 10.1111/j.1468-3083.2010.03856.x. [DOI] [PubMed] [Google Scholar]


