Abstract
Objective
To describe the relationship between altered white matter microstructure and neurodevelopment in children with d-transposition of the great arteries (d-TGA).
Study design
We report correlations between regional white matter microstructure as measured by fractional anisotropy (FA) and cognitive outcome in a homogeneous group of adolescents with d-TGA. Subjects with d-TGA (n=49) and controls (n=29) underwent diffusion tensor imaging and neurocognitive testing. In the group with d-TGA, we correlated neurocognitive scores with FA in 14 composite regions of interest in which subjects with d-TGA had lower FA than controls.
Results
Among the patients with d-TGA, mathematics achievement correlated with left parietal FA (r=0.39, p=0.006), inattention/hyperactivity symptoms with right precentral FA (r=−0.39, p=0.006) and left parietal FA (r=−0.30, p=0.04), executive function with right precentral FA (r=−0.30, p=0.04), and visual-spatial skills with right frontal FA(r=0.30, p=0.04). We also found an unanticipated correlation between memory and right posterior limb of the internal capsule FA (r=0.29, p=0.047).
Conclusion
Within the group with d-TGA, regions of reduced white matter microstructure are associated with cognitive performance in a pattern similar to healthy adolescents and adults. Diminished white matter microstructure may contribute to cognitive compromise in adolescents who underwent open-heart surgery in infancy.
Keywords: brain, neurodevelopment, fractional anisotropy, MRI, d-TGA
Although survival of infants with congenital heart disease (CHD) has dramatically increased in recent decades, many survivors manifest neurodevelopmental impairment. Studies of children with d-transposition of the great arteries (d-TGA) demonstrate mild reductions in intelligence quotient and more significant impairments in motor development, academic achievement, visual-spatial skills, attention, and executive function.1–4 These impairments persist into adolescence and translate to greater educational resource needs, reduced quality of life, and increased financial cost to society.5–8
A growing literature has explored macro- and microstructural changes on brain magnetic resonance imaging (MRI) that could underlie cognitive impairment in CHD patients. Most studies have focused on the perioperative period surrounding infant heart surgery.9–11 In the largest series of infants studied with conventional anatomic brain MRI before and after congenital heart surgery, 20% had preoperative white matter injury, and an additional 42% had new white matter injury in the postoperative period.12 Yet, perioperative white matter injury has been observed to resolve on conventional MRI obtained later in the first year of life.9 Moreover, a study of adolescents found MRI evidence of white matter abnormality in only 11% of patients who underwent cardiopulmonary bypass as infants.8
Quantitative MRI using diffusion tensor imaging (DTI) to measure fractional anisotropy (FA) provides information on white matter organization at the microstructural level that is not observed using conventional MRI techniques. One DTI study of infants with CHD showed diffuse reductions in FA in frontal, perirolandic, and posterior white matter.13 White matter microstructural changes are also apparent in adolescents born with d-TGA repaired in early infancy.14 In other populations ranging from premature infants to patients with dementia, DTI changes have been reported to relate to cognition.15, 16 Given the specific cognitive deficits in patients with d-TGA, quantitative imaging techniques may be more sensitive than conventional anatomic imaging for detection of subtle changes that correlate with cognitive impairment in this population.
We previously investigated the relationship between white matter microstructure and clinical risk factors in adolescents with d-TGA repaired in early infancy. We used whole brain DTI to measure white matter FA and identified regions of interest (ROI) that were reduced in adolescents with d-TGA compared with control adolescents. For the present study we correlated the FA for these ROIs with cognitive performance measured by standard neuropsychological tests in adolescents born with d-TGA to determine the relationship between reduced FA and cognition in this population.
Methods
We studied adolescents recruited originally to the Boston Circulatory Arrest Study that evaluated subjects with d-TGA undergoing the arterial switch operation before three months of age between April 1988 and February 1992. We have previously published trial methods and neurodevelopmental findings in the perioperative period and at ages 1, 4, 8, and 16 years.1, 4, 6, 17, 18 In addition, adolescents were recruited to serve as controls for this study and met criteria adapted from those used in the National Institutes of Health MRI study of normal brain development.19 Children with known risk factors for brain disorders (e.g., intra-uterine exposure to toxicants, history of closed head injury with loss of consciousness, language disorder or Axis 1 psychiatric disorder, first degree relative with a lifetime history of an Axis 1 psychiatric disorder, or abnormality on neurologic examination) were excluded. We also excluded subjects for whom MRI was contraindicated (eg, pacemaker, metal implants) and those with Trisomy 21, adolescents with other forms of CHD requiring surgical correction, and subjects whose primary language was not English. This study was approved by the Boston Children’s Hospital Institutional Review Board and adhered to both institutional guidelines and the Declaration of Helsinki. Parents provided written informed consent, and adolescents provided assent.
We evaluated regions previously found to differ between our sample of subjects with d-TGA and control adolescents using whole-brain DTI. Image acquisition and analysis, previously described in detail, are summarized herein.14 Both d-TGA and control adolescents each were scanned on a GE Twin 1.5T system (General Electric, Milwaukee, WI) at Boston Children’s Hospital using a quadrature head coil. Acquisition measurements were: voxel size=3.75×3.75×4mm3, TR/TE=13000ms/108ms, # of diffusion-weighted directions=6, b=750 s/mm2. Axial slices were acquired to cover the whole brain. Repeated acquisitions were obtained for each subject. Due to unacceptable artifact, some subjects contributed fewer acquisitions than others; an analysis of variance showed that there was no significant effect of series number (data not shown). All data were resampled to a resolution of 256×256.
SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/) was used.20 First, each subject’s reference image was partitioned into gray matter, white matter, and cerebrospinal fluid. Extra-brain tissue was removed, and the reference images were nonlinearly registered to standard space. A study-specific template was created using the normalized reference images from all study subjects. Next, FA images from each subject were nonlinearly registered to this template.20 Finally, the normalized FA images were smoothed, and voxels with FA < 0.15 were removed, to increase signal-to-noise ratio and exclude nonwhite matter voxels.
All FA images were entered into random effects analysis in SPM5 to identify regions in which control FA was significantly greater than d-TGA FA. The resulting statistical map was thresholded to indicate voxels surviving P<0.0001 (uncorrected) with a spatial cluster extent of 20 voxels or more. Eighteen voxel clusters demonstrated significantly lower FA in subjects with d-TGA relative to controls.14 However, in order to reduce the number of FA variables, we combined adjacent white matter clusters to generate the final 14 composite ROIs used for the correlation analysis: midbrain, right cerebellum, left cerebellum, right parietal, left parietal, right temporal isthmus, left temporal isthmus, right posterior limb of the internal capsule (PLIC), right anterior cingulate tract (ACT), right precentral, right frontal, right insula, anterior corpus callosum (ACC), and right basal ganglia (Figure 1; available at www.jpeds.com). Importantly, these 14 composite ROIs did not contain any structural abnormalities or punctate T1-weighted signal abnormalities consistent with white matter mineralization.
Figure 1.
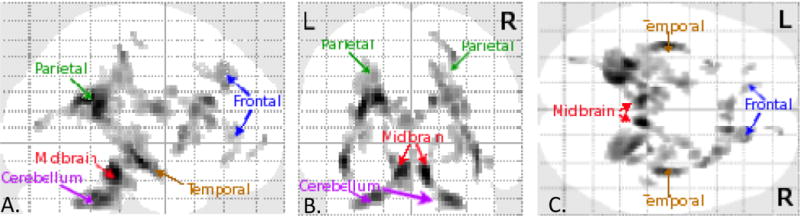
Orthogonal collapsed sagittal (A.), coronal (B.), and axial (C.) views of brain map for significant differences in FA for subjects with d-TGA versus controlss. Areas in which subjects with d-TGA white matter FA is significantly lower than that of control subjects is given by gray scale shading and is located in deep white matter of both hemispheres in frontal, parietal, and temporal lobes as well as cerebellum and midbrain. L=left; R=right.
Neuropsychological test scores of adolescents with d-TGA and controls were reported previously in detail.6 Briefly, to assess academic achievement we used the Wechsler Individual Achievement Test-Second Edition (WIAT) mathematics composite and reading composite scores.21 For memory we used the General Memory Index of the Children’s Memory Scale (CMS).22 To assess attentiveness and hyperactivity, we used the sex-specific attention deficit hyperactivity disorder (ADHD) Index T score from the Conners Rating Scales-Revised parent version (CADS-P).23 An executive function summary score was derived from the Delis-Kaplan Executive Function System (DKEFS) by averaging standard scores on the letter fluency and category fluency trials of verbal fluency, primary combined measure on design fluency, combined conditions score on sorting, total consecutively correct score on word context, and total achievement score on tower.24 Separately, executive function was assessed using the Behavior Rating Inventory of Executive Function-Parent Version (BRIEF-P) general executive composite score.25 Visual-spatial function was measured using the visual closure subscale of the Test of Visual-Perceptual Skills (TVPS) as this subscale was thought to be sensitive to subtle processing impairments.26 Finally, we assessed social cognition with the Adult Autism Spectrum Quotient (AQ).27 Intelligence quotient (IQ) had been previously measured at 8 years of age in the subjects with d-TGA using the Wechsler Intelligence Scale for Children-Third Edition.28
We collected demographic and medical variables thought to influence MRI findings or cognition including gestational age, age at MRI, sex, socioeconomic status at 16 years of age (defined as Hollingshead Four Factor Index of Social Status score),29 total cooling duration, presence of clinical or electrographic seizures, length of hospital stay, any open-heart surgery after the arterial switch operation, and high catheterization exposure (defined as ≥3 diagnostic catheterizations or ≥2 interventional catheterizations).
Statistical analyses
We compared demographic characteristics between the d-TGA and control groups using the Wilcoxon test for continuous variables and Fisher exact test for sex, and cognitive outcomes using linear regression adjusting for social class. Sex-adjusted Pearson correlation analysis was used to evaluate the relationship between regional FA in the 14 ROI and 8 cognitive outcome measures in the d-TGA group. We adjusted for sex but not age given evidence for a minimal effect of age on white matter microstructure within the study age range.30 The significance level was set at p<0.05. With a sample size of 49 subjects with d-TGA, we had 80% power to detect a correlation of 0.40 or higher at the two-sided 0.05 level. We did not adjust for multiple comparisons due to the exploratory nature of the analysis and interrelatedness of cognitive functions and white matter networks assessed.
We evaluated whether demographic and medical variables, including age, appreciably affected the relationship between FA and cognition in the subjects with d-TGA via multivariable stepwise linear regression models. Sex and regional FA were included in all models, and we employed a significance threshold of 0.05 for entry of demographic and medical variables into the models.
Finally, we performed two secondary analyses. First, we assessed sex-adjusted Pearson correlations between FA obtained at 16 years of age and IQ scores measured at 8 years in the group with d-TGA. IQ, measured at 8 years of age in the current cohort of subjects with d-TGA, was not repeated in adolescence to allow adequate time for cognitive testing in other domains with the expectation that Wechsler IQ scores measured at 8 and 16 years would demonstrate relative stability across this age range.31, 32 Second, we also performed sex-adjusted Pearson correlation analysis of regional FA with cognition including both subjects with d-TGA and controls while adjusting for the main effect of group in order to take advantage of increased sample size and a wider range of FA values and cognitive scores.
Results
Data from 49 adolescents with d-TGA and 29 control subjects were included in the final analysis. The scans of an additional 33 adolescents with d-TGA and 11 control subjects were excluded from analysis due to unacceptable signal artifact. Demographic characteristics of gestational age, sex, and age at MRI in the excluded subjects did not differ from those of subjects included in the final analysis. Adolescents with d-TGA, compared with controls, were older at MRI, more likely to be male, and lower in socioeconomic status, but had similar gestational age (Table I).
Table 1.
Demographic characteristics and cognitive outcomes of subjects with d-TGA and controls from whom DTI data were acquired at 16 years of age
| Variable | d-TGA (n=49) | Control (n=29) | p* |
|---|---|---|---|
| Gestational age, weeks | 40 (39.5–40) | 40 (39.5–40) | 0.42 |
| Age at MRI, years | 16.2 (16.0–16.4) | 14.9 (14.2–16.1) | <0.001 |
| Sex, % male | 84 | 52 | 0.004 |
| Social class at 16 y of age** | 48 (36–56) | 57 (53–61) | 0.001 |
| Wechsler Individual Achievement Test | |||
| Mathematics composite | 100.7 ± 18.6 | 110.1 ± 13.8 | 0.22 |
| Reading composite | 100.1 ± 15.4 | 111.2 ± 11.7 | 0.03 |
| General Memory Index | 92.9 ± 18.5 | 104.0 ± 15.8 | 0.15 |
| Parent Conners ADHD Index T-score | 52.7 ± 12.0 | 45.4 ± 4.7 | 0.003 |
| Executive function summary score | 9.2 ± 2.3 | 10.8 ± 1.5 | 0.02 |
| Behavior Rating Inventory of Executive Function-Parent | 55.6 ± 12.8 | 44.2 ± 8.5 | <0.001 |
| Test of Visual-Perceptual Skills | |||
| Visual closure | 83.1 ± 22.8 | 100.5 ± 23.9 | 0.02 |
| Autism-Spectrum Quotient | 17.9 ± 5.3 | 13.6 ± 5.3 | 0.003 |
Values are median (interquartile range) or mean ± standard deviation when appropriate.
P-values determined by Wilcoxon or Fisher exact tests for demographic characteristics or linear regression adjusted for social status for cognitive outcomes.
Score on Hollingshead Four Factor Index of Social Status, with higher scores indicating higher social status.
Among the subjects with d-TGA, median cooling duration was 15 minutes (interquartile range, 13–20 minutes), and median time in hospital was 8 days (interquartile range, 7–11). Four subjects had seizures (8%), 3 had high catheterization exposure (6%), and 7 had open-heart surgery after the arterial switch operation (14%). At 8 years of age, mean full-scale IQ was 100.6 (standard deviation, 15.9), mean verbal IQ was 103.7 (standard deviation, 16.6), and mean performance IQ was 97.1 (standard deviation, 15.1).
A table summarizing the complete sex-adjusted Pearson correlation analysis used to evaluate the relationship between regional FA in the 14 regions of interest and 8 cognitive outcome measures in the group with d-TGA is provided Table II (available at www.jpeds.com). Table III and Figure 2 present regions with statistically significant sex-adjusted Pearson correlations with cognitive outcomes in the subjects with d-TGA. Correlations that did not meet the significance threshold but are of particular interest are included in the text. Within the group with d-TGA, mathematics achievement correlated with left parietal FA (Figure 3, A). Seizures and socioeconomic status significantly contributed to the model using left parietal FA to predict WIAT mathematics composite score (p=0.047 and p=0.007, respectively); adjustment for these factors slightly strengthened the association (r=0.42, p=0.003). Reading achievement did not show any significant correlation with regional FA. Memory function measured with the General Memory Index score of the CMS correlated with FA in the right PLIC.
Table 2.
All correlations between regional FA and 16-year cognitive outcomes in subjects with d-TGA
| Composite ROI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| Cognitive Outcome | Midbrain | R cerebellum |
L cerebellum |
R parietal |
L parietal |
R temporal isthmus |
L temporal isthmus |
R PLIC |
R ACT |
R precentral |
R frontal |
R insula |
ACC | R basal ganglia |
| Wechsler Individual Achievement Test | ||||||||||||||
| Mathematics composite | 0.15 | 0.07 | −0.03 | 0.06 | 0.39‡ | −0.09 | −0.32† | 0.27* | 0.06 | 0.01 | 0.07 | −0.09 | −0.06 | 0.18 |
| Reading composite | 0.08 | 0.13 | 0.02 | 0.07 | 0.21 | 0.05 | −0.28* | 0.23 | 0.00 | −0.07 | 0.15 | 0.05 | −0.04 | 0.12 |
| General Memory Index | −0.03 | 0.04 | 0.16 | −0.02 | 0.08 | 0.11 | −0.04 | 0.29† | 0.00 | 0.08 | 0.08 | 0.19 | −0.03 | 0.05 |
| Parent Conners ADHD Index T score | −0.16 | 0.00 | −0.11 | −0.06 | −0.30† | 0.15 | 0.12 | 0.07 | −0.26* | −0.39‡ | −0.13 | 0.12 | 0.29† | −0.17 |
| Executive function summary score | 0.02 | 0.11 | 0.17 | 0.17 | 0.20 | 0.01 | −0.32† | 0.26* | −0.06 | −0.07 | −0.05 | −0.04 | −0.15 | 0.19 |
| Behavior Rating Inventory of Executive Function-Parent | −0.05 | −0.05 | −0.11 | −0.03 | −0.24 | 0.03 | 0.19 | 0.11 | −0.13 | −0.30† | −0.12 | 0.11 | 0.20 | −0.22 |
| Test of Visual-Perceptual Skills | ||||||||||||||
| Visual Closure | −0.01 | 0.19 | 0.10 | −0.14 | −0.01 | 0.04 | −0.09 | 0.11 | −0.12 | −0.07 | 0.30† | 0.08 | −0.16 | 0.13 |
| Autism-Spectrum Quotient | 0.02 | 0.00 | −0.17 | 0.09 | −0.15 | 0.08 | 0.17 | 0.12 | −0.15 | 0.29† | −0.08 | 0.04 | 0.20 | −0.20 |
Values are sex-adjusted Pearson r coefficients.
p<0.10;
p<0.05;
p<0.01
Table 3.
Correlations between regional FA and 16-year cognitive outcomes in subjects with d-TGA
| Cognitive Outcome | Composite ROI (Sex-adjusted Pearson r, p) |
|---|---|
| Wechsler Individual Achievement Test | |
| Mathematics composite | L parietal (0.39, 0.006); L temporal isthmus* (−0.32, 0.03) |
| Reading composite | – |
| General Memory Index | R PLIC (0.29, 0.047) |
| Parent Conners ADHD Index T score | R precentral (−0.39, 0.006); L parietal (−0.30, 0.04); ACC* (0.29, 0.04) |
| Executive function summary score | L temporal isthmus* (−0.32, 0.03) |
| Behavior Rating Inventory of Executive Function-Parent | R precentral (−0.30, 0.04) |
| Test of Visual-Perceptual Skills | |
| Visual Closure | R frontal (0.30, 0.04) |
| Autism-Spectrum Quotient | R precentral* (0.29, 0.047) |
For these associations, lower fractional anisotropy correlated with better cognitive performance.
Figure 2.
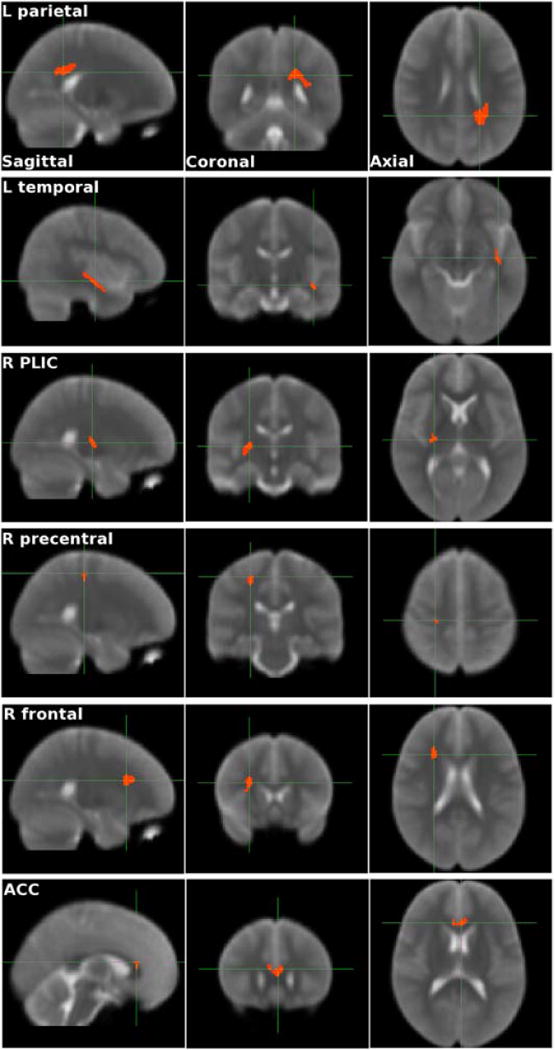
Sagittal, coronal, and axial views of the 6 regions of interest with statistically significant sex-adjusted Pearson correlations between FA and cognitive measures in the subjects with d-TGA.
Figure 3.
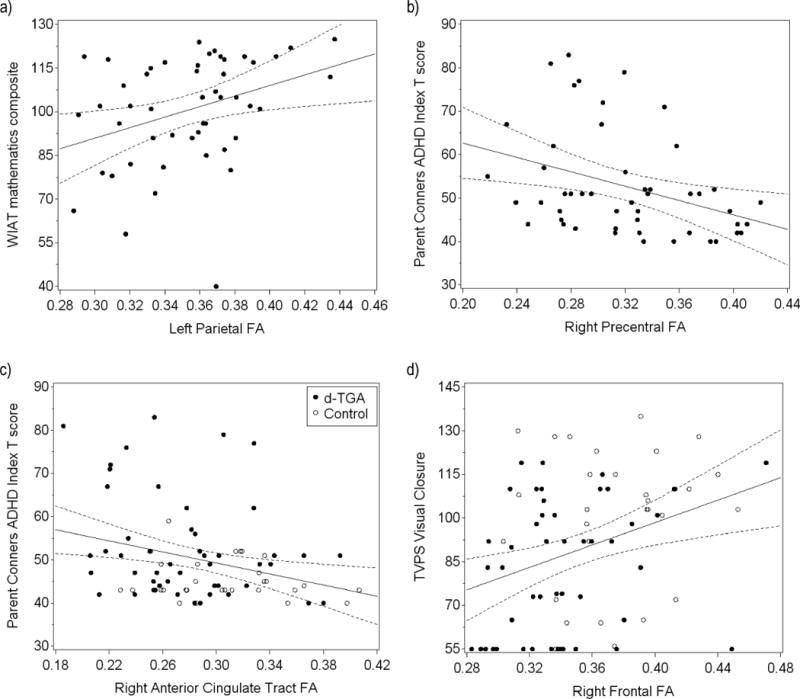
Regression lines (with 95% confidence intervals) demonstrating the correlation between cognitive outcomes and regional FA in d-TGA subjects (panels a and b) or subjects with d-TGA and controls combined (panels c and d), not adjusting for sex. a) WIAT mathematics composite score increases as left parietal FA increases (sex-adjusted Pearson r=0.39, p=0.006). b) Parent Conners ADHD Index T score decreases, indicating fewer ADHD symptoms, as right precentral FA increases (sex-adjusted Pearson r=−0.39, p=0.006). c) Parent Conners ADHD Index T score decreases, indicating fewer ADHD symptoms, as right ACT FA increases (sex-adjusted Pearson r=−0.23, p=0.04). d) TVPS visual closure score increases as right frontal FA increases (sex-adjusted Pearson r=0.23, p=0.04).
Inattentiveness and hyperactivity symptoms measured by the CADS-P sex-specific ADHD Index T score, which rises as parent-reported symptoms increase, inversely correlated with right precentral (Figure 3, B) and left parietal FA. Of interest, there was also a trend towards inverse correlation with FA in the right ACT (r=−0.26, p=0.07). High catheterization exposure significantly contributed to the model using right precentral FA to predict CADS-P score (p=0.03), but its inclusion in the model did not appreciably affect the strength of the association (r=−0.40, p=0.005). Executive function measured with the BRIEF-P general executive composite score, also a parent-report symptom scale in which higher numeric value corresponds to greater executive function impairment, correlated inversely with FA in the right precentral region. Visual-spatial skills assessed with the TVPS visual closure subscale correlated with FA in the right frontal region.
Within the d-TGA group, there were four instances in which lower FA predicted better cognitive scores. Both WIAT composite mathematics score and the DKEFS executive function summary score correlated inversely with FA in the left temporal isthmus. In addition, CADS-P ADHD Index T score correlated directly with FA in the ACC, and AQ score correlated directly with FA in the right precentral region; for both of these outcome measures higher scores indicate greater impairment, so increased symptoms of ADHD and reduced social cognition were associated with higher FA. High catheterization exposure significantly contributed to the model using ACC to predict CADS-P score (p=0.045), but its inclusion did not appreciably affect the strength of the association (r=0.29, p=0.046).
Two secondary analyses were performed. In the first exploratory analysis, in which regional FA was correlated with 8-year IQ scores in the subjects with d-TGA, verbal IQ correlated with FA in the right PLIC (r=0.31, p=0.03) and there was a marginally significant correlation between full-scale IQ and FA in the right PLIC (r=0.25, p=0.08). In the second exploratory analysis, both subjects with d-TGA and controls were combined to increase sample size and to expand the range of FA and cognitive score values. These sex-adjusted Pearson correlations were also adjusted for group because both groups were included. In this analysis, the trend towards inverse correlation between CADS-P score and right ACT FA became statistically significant (r=−0.23, p=0.04) and the correlation between visual closure and right frontal FA remained significant (r=0.23, p=0.04) (Figure 3, C and D).
Discussion
We found many correlations between FA and cognitive performance. These findings are consistent with the hypothesis that white matter injury resides in the causal pathway for neurocognitive impairment.
In the group with d-TGA, WIAT mathematics composite score, incorporating math problem solving and numerical operations, correlated with left parietal FA. Prior neuroimaging work indicates that a left fronto-parietal network mediates calculation, modulated by mathematical operation, size of calculation, and the child’s math proficiency.35 Indeed, a relationship between mathematics performance and left parietal FA has been found in healthy children and those with velocardiofacial syndrome.36, 37 The region of left parietal white matter in which FA correlates with mathematics achievement score in our cohort lies within these math-associated domains.
We also identified correlations between parent ratings of ADHD symptoms and executive function and fronto-parietal white matter microstructure. In our subjects with d-TGA, the CADS-P ADHD Index T score, reflecting parent perceptions of sustained attention, hyperactivity, and impulsivity, correlated with FA in right precentral and left parietal white matter. A trend for correlation between symptoms of ADHD and FA in the right ACT in the group with d-TGA became significant when control subjects were included in analyses. These correlations reflect well characterized, distributed systems mediating attention including fronto-parietal and -striatal regions connected via extensive white matter tracts.38, 39 Parent ratings of attention correlate with FA in deep posterior white matter among boys with and without ADHD, and continuous performance task scores correlate with right anterior cingulate FA in healthy adults.40, 41 ADHD often co-exists with executive function impairment, and similarly parent-reported symptoms of executive function impairment correlated with FA in right precentral white matter. As with attention, the cognitive functions comprising executive function are mediated by prefrontal cortex and fronto-parietal connections, and impaired executive function has been found to correlate with white matter abnormality, particularly when injury occurs early in life.34, 42–45 It is possible that diminished fronto-parietal connectivity adversely affects attentional control and executive function in children with d-TGA.
The visual closure subtest of the TVPS, thought to be sensitive for detecting subtle functional impairment in visual-spatial skills, correlated with FA in right frontal white matter. Visual-spatial information processing pathways integrate bilateral fronto-parietal regions of brain.46 Among healthy adolescents, higher FA in right fronto-parietal white matter correlates with better performance on visual-spatial information processing paradigms.47, 48 The correlation between visual-spatial performance and right frontal FA was significant in both d-TGA and combined group analyses, suggesting a particularly robust relationship between white matter microstructure and this cognitive measure in our cohort.
Although, in general, higher FA was associated with better cognitive scores, we identified four instances in which higher FA predicted poorer cognitive performance. As left temporal isthmus FA increased, mathematics and executive function scores declined. Similarly, higher ACC FA was associated with more ADHD symptoms, and higher right precentral FA predicted reduced social cognition. Although in many studies higher FA has correlated with better cognitive performance, the opposite has also been found. Adolescents with ADHD have been shown to have uniquely higher FA in the inferior frontal regions, and in William’s syndrome, visual-spatial skills correlate inversely with superior longitudinal fasciculus FA.49, 50 A study of adults with mild traumatic brain injury found increased FA in the genu of the corpus callosum, possibly related to shifts in extracellular fluid restricting perpendicular diffusion along tightly packed axons.51 These findings highlight the possibility that aberrant FA, both increased and decreased values, may reflect alteration of white matter organization that adversely affects cognition.
We observed correlations between FA of specific regions of white matter and individual cognitive measures. Importantly, correlation between regional white matter microstructure and cognition may reflect a multifocal alteration of function. As most higher cognitive function is mediated by more than one, interconnected gray matter locus, an alteration of white matter microstructure in a given region may adversely affect the cooperation of two or more cortical gray matter loci.
We found an unanticipated correlation between FA in deep white matter of the PLIC and memory. Similarly, exploratory analyses with 8-year IQ measures revealed a correlation between PLIC FA and verbal IQ, and a trend towards correlation with full-scale IQ. Although the IQ findings should be interpreted with caution given the discrepancy in age between FA and IQ measurements, these findings support a relationship between white matter microstructure in the PLIC and cognition in our cohort. The internal capsule harbors connections from frontal and parietal cortex to basal ganglia, thalamus, brainstem, and cerebellum via thalamocortical, corticopontine, and corticospinal tracts. Recently, spatial working memory has been found to correlate with putamenal volume and, similarly, IQ has been found to correlate with cerebellar, caudate, frontal, and parietal gray matter volumes in typically developing children.52 Accumulating evidence suggests that motor and cognitive functions not only interrelate but may also depend on development of identical cortical and subcortical structures.53, 54 Further, evidence supports a role for these tracts in cognition, with the posterior limb specifically involved in verbal and non-verbal fluency tasks.55 FA in the PLIC has been found to correlate with both reading ability in typically developing children and overall mental health functioning, as indicated by the Children’s Global Assessment Scale, in adolescents born at very low birth weight.56–58 Work in infants with CHD has also shown that cortical and subcortical injury can alter maturationally-expected increases in PLIC FA.59 We speculate that the correlations we identified between PLIC FA and cognition may relate to direct involvement of non-motor corticothalamic and thalamocortical white matter tracts carried in the PLIC or to reduced FA in the PLIC serving as a sensitive marker of more widespread brain injury.
Our study has some limitations. First, even though our sample size is substantial for a neuroimaging cohort, the study may be underpowered to detect some relationships. Second, this study focused on specific regions of difference in white matter FA between adolescents with d-TGA and controls. We hypothesized that the prenatal, perinatal, or perioperative course of patients with d-TGA exerted an adverse effect on brain white matter during infancy that persisted into adolescence and was detectable by measurement of white matter microstructure FA. White matter fiber tracts interconnect the cortical components of neural networks essential for higher cognitive function. Although white matter microstructure alone is unlikely to explain all of the cognitive differences between controls and adolescents with d-TGA, compromise of the long and short white matter tracts that interconnect cortical gray matter loci is known to impair cognitive processing.15, 16 Further studies will evaluate cortical thickness and gray matter volumes. Third, we used clinical instruments to assess functional implications of cognitive impairments. Use of clinically-based cognitive outcome tools improves the clinical applicability of our findings but sacrifices some specificity of cognitive correlation. Fourth, we correlated performance measures of IQ taken at 8 years of age with white matter microstructure measures obtained from the same patients at 16 years of age. Even though the IQ and anatomic white matter data were acquired at different ages, IQ generally remains stable during this period of development. Finally, our study population was limited to patients with d-TGA, who underwent reparative open-heart surgery in early infancy, potentially limiting generalizability of our findings to children with other cardiac lesions or those whose surgery was performed beyond early infancy.
The American Heart Association (AHA) and the American Academy of Pediatrics (AAP) recommend routine medical and developmental evaluation into adolescence for certain high-risk cardiac populations, particularly those children with abnormal neuroimaging findings.60 Our results in this sample of adolescents with d-TGA support this recommendation.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (RO1 HL77681), The Children’s Heart Foundation (Chicago), and the Farb Family Fund.
Abbreviations
- ACC
anterior corpus callosum
- ACT
anterior cingulate tract
- ADHD
attention deficit hyperactivity disorder
- AQ
Adult Autism-Spectrum Quotient
- BRIEF-P
Behavior Rating Inventory of Executive Function-Parent Version
- CADS-P
Conners Rating Scales-Revised parent version
- CHD
congenital heart disease
- CMS
Children’s Memory Scale
- DKEFS
Delis-Kaplan Executive Function System
- DTI
diffusion tensor imaging
- d-TGA
d-transposition of the great arteries
- FA
fractional anisotropy
- IQ
intelligence quotient
- MRI
magnetic resonance imaging
- PLIC
posterior limb of the internal capsule
- ROI
region of interest
- TVPS
Test of Visual-Perceptual Skills
- WIAT
Wechsler Individual Achievement Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Portions of the study were presented as a poster at the Child Neurology Society meeting, 2012.
References
- 1.Bellinger DC, Wypij D, Kuban KCK, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–32. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 2.Hovels-Gurich HH, Konrad K, Wiesner M, Minkenberg R, Herpertz-Dahlmann B, Messmer BJ, et al. Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child. 2002;87:506–10. doi: 10.1136/adc.87.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dittrich H, Buhrer C, Grimmer I, Dittrich S, Abdul-Khaliq H, Lange PE. Neurodevelopment at 1 year of age in infants with congenital heart disease. Heart. 2003;89:436–41. doi: 10.1136/heart.89.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellinger DC, Newburger JW, Wypij D, Kuban KC, Du Plessis AJ, Rappaport LA. Behavior at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–e67. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 6.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–9. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira FM, Coelho RM, Proenca C, Silva AM, Vieira D, Vaz C, et al. Quality of life experienced by adolescents and young adults with congenital heart disease. Pediatr Cardiol. 2011;32:1132–8. doi: 10.1007/s00246-011-0039-0. [DOI] [PubMed] [Google Scholar]
- 8.von Rhein M, Scheer I, Loenneker T, Huber R, Knirsch W, Latal B. Structural brain lesions in adolescents with congenital heart disease. J Pediatr. 2011;158:984–9. doi: 10.1016/j.jpeds.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I-109–I-14. [PubMed] [Google Scholar]
- 10.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 11.Beca J, Gunn J, Coleman L, Hope A, Whelan LC, Gentles T, et al. Pre-operative brain injury in newborn infants with transposition of the great arteries occurs at rates similar to other complex congenital heart disease and is not related to balloon atrial septostomy. J Am Coll Cardiol. 2009;53:1807–11. doi: 10.1016/j.jacc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 12.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–9. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 13.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 14.Rivkin MJ, Watson CG, Scoppettuolo LA, Wypij D, Vajapeyam S, Bellinger DC, et al. Adolescents with D-transposition of the great arteries repaired in early infancy demonstrate reduced white matter microstructure associated with clinical risk factors. J Thorac Cardiovasc Surg. 2013;146:543–9 e1. doi: 10.1016/j.jtcvs.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–8. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 16.Tartaglia MC, Zhang Y, Racine C, Laluz V, Neuhaus J, Chao L, et al. Executive dysfunction in frontotemporal dementia is related to abnormalities in frontal white matter tracts. J Neurol. 2012;259:1071–80. doi: 10.1007/s00415-011-6300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KCK, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–55. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 18.Bellinger DC, Wypij D, Du Plessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126:1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 19.Evans AC, Brain Development Cooperative Group The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 20.Ashburner J, Friston K. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Psychological Corp. The Wechsler Individual Achievement Test. 2. San Antonio, TX: Psychological Corp; 2002. [Google Scholar]
- 22.Cohen MJ. Children’s Memory Scale. San Antonio, TX: Psychological Corp; 1997. [Google Scholar]
- 23.Conners CK. Conners’ Rating Scales-Revised. North Tonawanda, NY: Multi-Health Systems Inc; 2001. [Google Scholar]
- 24.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corp; 2001. [Google Scholar]
- 25.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Resources Inc; 2000. [Google Scholar]
- 26.Gardner MF. Test of Visual-Perceptual Skills (Non-Motor) (Upper-Level) Revised. Hydesville, CA: Psychological and Educational Publications Inc; 1997. [Google Scholar]
- 27.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger’s syndrome/high-functioning autism, males and females. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler intelligence scale for children-third edition. San Antonio, TX: Psychological Corp; 1991. [Google Scholar]
- 29.Hollingshead AB. Unpublished working paper. 1975. Four factor index of social status. [Google Scholar]
- 30.Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Sternberg RJ, Grigorenko EL, Bundy DA. The predictive value of IQ. Merrill-Palmer Quarterly. 2001;47:1–41. [Google Scholar]
- 32.Mortensen EL, Andresen J, Kruuse E, Sanders SA, Reinisch JM. IQ stability: The relation between child and young adult intelligence scores in low-birthweight samples. Scandinavian Journal of Psychology. 2003;44:395–8. doi: 10.1111/1467-9450.00359. [DOI] [PubMed] [Google Scholar]
- 33.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. Journal of Cognitive Neuroscience. 2004;16:1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 34.Skranes J, Lohaugen GC, Martinussen M, Indredavik MS, Dale AM, Haraldseth O, et al. White matter abnormalities and executive function in children with very low birth weight. Neuroreport. 2009;20:263–6. doi: 10.1097/wnr.0b013e32832027fe. [DOI] [PubMed] [Google Scholar]
- 35.De Smedt B, Holloway ID, Ansari D. Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. Neuroimage. 2011;57:771–81. doi: 10.1016/j.neuroimage.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Barnea-Goraly N, Eliez S, Menon V, Bammer R, Reiss AL. Arithmetic ability and parietal alterations: a diffusion tensor imaging study in velocardiofacial syndrome. Cogn Brain Res. 2005;25:735–40. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Tsang JM, Dougherty RF, Deutsch GK, Wandell BA, Ben-Shachar M. Frontoparietal white matter diffusion properties predict mental arithmetic skills in children. PNAS. 2009;106:22546–51. doi: 10.1073/pnas.0906094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi M, Iwamoto K, Fukatsu H, Naganawa S, Iidaka T, Ozaki N. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neurosci Lett. 2010;477:72–6. doi: 10.1016/j.neulet.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 41.Qiu A, Rifkin-Graboi A, Tuan TA, Zhong J, Meaney MJ. Inattention and hyperactivity predict alterations in specific neural circuits among 6-year old boys. J Am Acad Child Adolesc Psychiatry. 2012;51:632–41. doi: 10.1016/j.jaac.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 43.Stevens MC, Skudlarski P, Pearlson GD, Calhoun VD. Age-related cognitive gains are mediated by the effects of white matter development on brain network integration. Neuroimage. 2009;48:738–46. doi: 10.1016/j.neuroimage.2009.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson V, Spencer-Smith M, Coleman L, Anderson P, Williams J, Greenham M, et al. Children’s executive functions: are they poorer after very early brain insult. Neuropsychologia. 2010;48:2041–50. doi: 10.1016/j.neuropsychologia.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 45.Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48:2496–508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 46.Watson CE, Chatterjee A. A bilateral frontoparietal network underlies visuospatial analogical reasoning. Neuroimage. 2012;59:2831–8. doi: 10.1016/j.neuroimage.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cognitive Brain Research. 2003;18:48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33:936–46. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 49.Hoeft F, Barnea-Goraly N, Haas BW, Golarai G, Ng D, Mills D, et al. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci. 2007;27:11960–5. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res. 2010;181:193–8. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ling JM, Pena A, Yeo RA, Merideth FL, Klimaj S, Gasparovic C, et al. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain. 2012;135:1281–92. doi: 10.1093/brain/aws073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pangelinan MM, Zhang G, VanMeter JW, Clark JE, Hatfield BD, Haufler AJ. Beyond age and gender: relationships between cortical and subcortical brain volume and cognitive-motor abilities in school-age children. Neuroimage. 2011;54:3093–100. doi: 10.1016/j.neuroimage.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diamond A. Close interrelation of motor development and cognitive development of the cerebellum and prefrontal cortex. Child Development. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 54.Wassenberg R, Kessels AGH, Kalff AC, Hurks J, Feron FJM, Hendriksen JGM, et al. Relation between cognitive and motor performance in 5- to 6-year old children: results from a large-scale cross-sectional study. Child Development. 2005;76:1092–103. doi: 10.1111/j.1467-8624.2005.00899.x. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan EV, Zahr NM, Rohlfing T, Pfefferbaum A. Fiber tracking functionally distinct components of the internal capsule. Neuropsychologia. 2010;48:4155–63. doi: 10.1016/j.neuropsychologia.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, et al. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–71. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–70. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–66. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 59.Partridge SC, Vigneron DB, Charlton NN, Berman JI, Henry RG, Mukherjee P, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640–51. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 60.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–72. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]


