Abstract
Individuals form first impressions of others all the time, which affects their social functioning. Typical adults form threat impressions in faces with neutral expressions quickly, requiring less than 40 ms. These impressions appear to be mediated by low spatial frequency (LSF) content in the images. Little is known, however, about mechanisms of first impression formation in schizophrenia. The current study investigated how quickly individuals with schizophrenia can form consistent impressions of threat compared with controls and explored the mechanisms involved. Patients and controls were presented intact, LSF- or high spatial frequency (HSF)-filtered faces with durations that varied from 39 – 1703 ms and were asked to rate how threatening each face was on a scale from 1 to 5. In order to assess the speed of impression formation for intact faces, correlations were calculated for ratings made at each duration compared to a reference duration of 1703 ms for each group. Controls demonstrated a significant relation for intact faces presented for 39 ms, whereas patients required 390 ms to demonstrate a significant relation with the reference duration. For controls, LSFs primarily contributed to the formation of consistent threat impressions at 39 ms, whereas patients showed a trend for utilizing both LSF and HSF information to form consistent threat impressions at 390 ms. Results indicate that individuals with schizophrenia require a greater integration time to form a stable “first impression” of threat, which may be related to the need to utilize compensatory mechanisms such as HSF, as well as LSF, information.
Keywords: schizophrenia, social cognition, spatial frequency, visual processing, first impressions, threat
1. Introduction
People with schizophrenia have deficits across a number of social cognitive domains including facial emotion recognition, theory of mind, and social perception (Green et al., 2008; Kohler et al., 2010; Savla et al., 2013). These impairments affect ability to engage in social interactions and are related to poor functional outcome (Couture et al., 2006; Fett et al., 2011; Irani et al., 2012). One important area of social functioning is ability to make spontaneous judgments about an individual's personality characteristics or perceived intent based on facial information. However, there is a paucity of studies examining mechanisms by which individuals with schizophrenia form first impressions.
Frequently, individuals form first impressions of others’ traits and characteristics to determine how threatening, trustworthy, intelligent, likeable, attractive, or competent they are. First impressions can be made based on emotional expressions in faces, facial structure, and even subtle expressions in neutral faces (Hassin and Trope, 2000; Oosterhof and Todorov, 2008; Said et al., 2009). This process is spontaneous, based on limited information, and, regardless of accuracy, can affect social interactions and behavior (Olivola and Todorov, 2010; Willis and Todorov, 2006).
Additionally, some judgments, particularly those of threat and trustworthiness, may be crucial for survival. Thus, one would expect these judgments to be made very quickly, which turns out to be the case. In two similar studies, ratings of threat (Bar et al., 2006) and trustworthiness (Todorov et al., 2009) made by healthy individuals after exposure to neutral faces in as short a duration as 33-39 ms agreed with ratings made at more leisure. Whether patients with schizophrenia need longer durations of viewing faces than controls to make a first impression remains an open question. Studies showing that patients need longer exposure durations to achieve configural processing of faces similar to that of controls (Butler et al., 2008) and have increased reaction time when making social appraisals (Taylor et al., 2011) suggest that this may be the case.
Little is known about mechanisms used to form first impressions, even in healthy individuals. Bar and colleagues (2006) assessed the role of spatial frequency content in first impression formation because low spatial frequency (LSF, low resolution) information is extracted much more rapidly than high spatial frequency (HSF, fine detail) information, providing coarse-to-fine processing of information (Bar, 2003), Furthermore, LSF information involves neural circuitry implicated in threat perception (Adolphs et al., 1999; Vuilleumier et al., 2003). As hypothesized, LSF processing played a role in first impression formation: a significant relationship was found between threat judgments made from LSF-filtered faces, but not HSF-filtered faces, shown for 39 ms and judgments made from unfiltered faces. Some studies show that patients with schizophrenia exhibit impairment in processing LSF information in objects, faces, and simple stimuli (Butler et al., 2005; Calderone et al., 2013; Martinez et al., 2011; Martinez et al., 2008; O'Donnell et al., 2002; Silverstein et al., 2010). Other studies found impairment in processing both LSF and HSF information (Keri et al., 2002; Slaghuis, 1998). Thus, patients may not utilize spatial frequency, particularly LSF information, similarly to controls in forming first impressions.
Given the impact of first impressions and difficulties in social cognition of patients with schizophrenia, it is important to understand the mechanisms of first impression formation. The present study utilized the paradigm of Bar et al. (2006) to investigate the possibility that patients need longer duration and utilize different mechanisms than healthy controls to form first impressions. Specifically, it was hypothesized that controls would be able to quickly form a consistent first impression that would be reliant on use of LSF information. It was hypothesized that patients with schizophrenia would take longer to make a stable first impression and require HSF as well as LSF information to do so.
2. Materials and Methods
2.1 Participants
Data were collected in two separate experiments. In Experiment 1, participants were 47 patients (39 male) meeting Diagnostic and Statistical Manual of Mental Disorder (Fourth Edition; DSM-IV) criteria for schizophrenia (n = 38) or schizoaffective disorder (n=9), and 43 controls (24 male) of similar age. In Experiment 2, participants were 40 patients (34 male) meeting criteria for schizophrenia (n=32) or schizoaffective disorder (n=8) and 38 controls (21 male) of similar age. Thirty-seven patients and 33 controls participated in both experiments, so that the total sample included 50 patients and 48 controls. Clinical and demographic information is presented in Table 1.
Table 1.
Demographics and clinical characteristics of patients with schizophrenia and healthy controls in each experiment.
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
| Patients (n=47) | Controls (n=43) | Patients (n=40) | Controls (n=38) | |
| Age, y | 38.9±10.8 | 37.4±12.1 | 39.0±10.4 | 38.4±12.0 |
| Gender (male/female) | 39/8 | 24/19* | 34/6 | 21/17* |
| Diagnosis | ||||
| Schizophrenia | 38 | -- | 32 | -- |
| Schizoaffective Disorder | 9 | -- | 8 | -- |
| Chlorpromazine daily equivalent (mg) | 733.4±588.7 (n=46)† | -- | 656.8±465.3 (n=39)† | -- |
| Antipsychotics | ||||
| Atypical | 35 | -- | 30 | -- |
| Typical | 1 | -- | 1 | -- |
| Both | 10 | -- | 8 | -- |
| None | 1 | -- | 1 | -- |
| Duration of illness (y) | 14.8±9.2 (n=46) | -- | 15.3±8.9 (n=39) | -- |
| Participant socioeconomic status | 28.2±12.9 | 45.7±8.8‡ | 26.0±10.5 | 45.9±9.1‡ |
| Parental socioeconomic status | 41.1±13.4 | 44.0±13.2 | 38.8±13.7 | 45.4±12.4 |
| PANSS total score | 70.4±12.7 (n=40) | -- | 70.0±12.5 (n=37) | -- |
| PANSS Positive Scale | 18.5±6.1 | -- | 18.4±5.9 | -- |
| PANSS Negative Scale | 17.2±3.9 | -- | 16.9±4.1 | -- |
| PANSS General Psychopathology Scale | 34.7±6.3 | -- | 34.5±6.3 | -- |
| SANS total score (including global scores) | 28.3±16.1 (n=39) | -- | 26.7±10.7 (n=34) | -- |
| Highest grade achieved | 12.3±2.2 (n=46) | 14.8±2.0 | 11.9±1.8 | 14.8±2.0 |
| IQ (Quick Test) | 96.3±9.1 | 108.4±10.9‡ | 95.8±9.3 | 107.5±10.4‡ |
Note: Values are M ± SD. Numbers of participants per group are noted when there are missing data. Socioeconomic status was measured by the four-factor Hollingshead Scale (Hollingshead, 1975). PANSS, Positive and Negative Symptom Schedule (Kay et al., 1987); SANS, Schedule for Assessment of Negative Symptoms (Andreasen, 1984).
Fisher's Exact Test, p < .01
Chlorpromazine equivalence mean is based on total amount of participants receiving medication at time of testing.
t-test, p < .001
Patients were recruited from inpatient and outpatient facilities associated with the Nathan Kline Institute for Psychiatric Research. Diagnoses were obtained using the Structured Clinical Interview for DSM-IV (SCID) and all available clinical information. Controls were recruited through the Volunteer Recruitment Pool at the Nathan Kline Institute and individuals with a history of SCID-defined Axis I psychiatric disorders were excluded. Participants were excluded if they had any neurological or ophthalmic disorders that might affect performance or met criteria for alcohol or substance dependence within the last six months or abuse within the last month. All participants provided informed consent according to the Declaration of Helsinki. This study was approved by the Nathan Kline Institute for Psychiatric Research/Rockland Psychiatric Center and Rockland County Department of Mental Health Institutional Review Boards.
Patients and controls did not differ significantly in age. However, there was a significant difference in gender between groups (Fisher's exact test, p=.006). All but one of the patients received antipsychotic medication at the time of testing. Chlorpromazine equivalents were calculated using conversion factors described previously (Hyman et al., 1995; Peuskens and Link, 1997; Woods, 2003). All participants had 20/32 or better corrected visual acuity on the Logarithmic Visual Acuity Chart (Precision Vision, LaSalle, IL, USA).
2.2 Stimuli
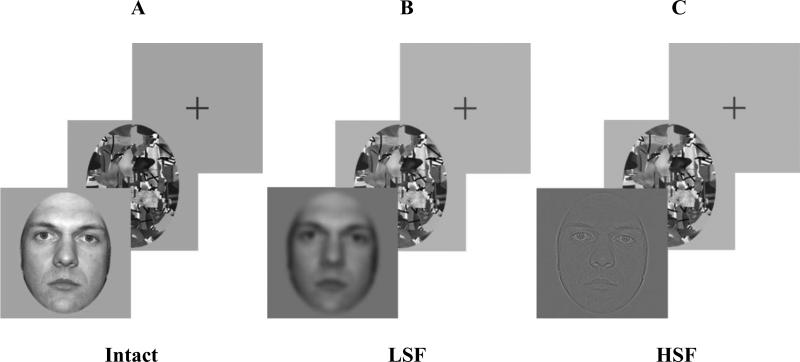
Stimuli for both experiments are shown in Figure 1 and consisted of 72 256 × 256 pixel grayscale images of faces, with each face 5° in the vertical dimension, presented on a gray background. The tasks were administered on an E5500 Dell Latitude Laptop, with a monitor resolution of 1024 x 768 pixels, and refresh rate of 75 Hz.
Figure 1.
Intact neutral faces (A) were shown for 39, 156, 390, and 1703 ms. Spatial frequency-filtered LSF (< 8 cycles/image; B) or HSF (> 24 cycles/image; C) neutral faces were shown for 39, 390, and 1703 ms. Faces were followed by a mask and then cross-hair fixation. Participants were asked to judge how threatening each face was on a scale of 1 (least threatening) to 5 (most threatening). Figure adapted with permission from Bar et al. (2006).
Faces were ones used by Bar et al. (2006) and obtained from sources that had previously rated the faces as neutral. Sources for the faces were: Ekman Pictures of Facial Affect (POFA; www.paulekman.com/), Cornell University database (www.macbrain.org/faces/), University of Texas, El Paso (Zarate et al., 2000), AR Face Database (Martinez and Benavente, 1998), University of Stirling (http://pics.psych.stir.ac.uk/), Database of Faces (http://www.uk.research.att.com/facedatabase.html), and Yale University (http://cvc.yale.edu/projects/yalefaces/yalefaces.html). For Experiment 1, 72 unfiltered faces were shown (Figure 1A). For Experiment 2, the 72 faces had a Gaussian filter applied to limit the spatial frequency content to either LSFs (≤ 8 cycles/image) (Figure 1B) or HSFs (≥ 24 cycles/image) (Figure 1C). In both experiments, each face was followed by one of 15 randomly presented masks, shown for 160 ms, consisting of black lines approximately 2 mm in diameter on an abstract background of gray and white. The mask was used to ensure that the faces were presented only for the intended exposure time (Todorov et al., 2009).
2.3 Procedure
In both experiments, participants were asked to rate the degree to which they perceived each face to belong to a threatening person on a 5-point scale from least threatening (1) to most threatening (5). Participants were instructed to follow their gut reaction. The participants made a verbal response and the experimenter recorded it with a button press. The next trial began 800 ms after the button press. In Experiment 1, full-spectrum unfiltered (intact) faces were presented for durations of 39, 156, 390, or 1703 ms. Durations were in the order from shortest to longest. The 72 faces were divided into four blocks of 18 with four counterbalanced orders of presentation. Each participant received one of the four orders of presentation so that each participant saw 18 faces at each duration and only saw each face once. Thus, all 72 faces were rated at each duration, with each face rated by a subset of participants. For Experiment 2, LSF and HSF faces were presented for durations of 39, 390, or 1703 ms, again from shortest to longest. All 72 faces were rated at each duration. The 72 faces were divided into six blocks of 12, with three durations for LSF and three for HSF. Participants received one of six counterbalanced orders of block presentations, with half of the participants receiving LSF first and half receiving HSF first. Thus, each participant saw 12 faces at each duration for LSF and 12 at each duration for HSF. A practice block consisting of 20 trials of face, mask, and fixation using faces not presented in actual experiments preceded the experiments.
2.4 Data Analyses
To evaluate whether the average threat ratings given by subjects depended on diagnosis, gender, duration or filter type (unfiltered, LSF, or HSF), repeated measures ANOVA-type of analysis was performed using mixed effects: participants’ ratings were modeled as a function of diagnostic group, gender (between subjects factors), duration and filter (as repeated within subject factors) and all 2-, 3- and 4-way interactions between them. The non-significant terms were removed one-by-one using backward elimination and preserving the hierarchical principle (i.e., if a higher order interaction term is in the model all lower order interaction terms and main effects that are contained in it are retained in the model, regardless of their statistical significance). Inferences about the effect of the four factors on the ratings are based on the final model.
To address the main research question, as was done previously (Bar et al., 2006; Todorov et al., 2009), for each face the mean threat ratings were calculated for each combination of duration and filtering, separately for the two diagnostic groups. The association between face ratings at shorter vs. the 1703 reference duration or between spatial frequency-filtered vs. non-filtered images was evaluated based on the Pearson's correlations coefficients. The significance of those correlations was judged at two-sided level α=0.05, with Bonferroni correction applied to estimates of similar correlations within a diagnostic group. For example, the three correlations for durations 39, 156 and 390 vs. the reference duration of 1703 ms for non-filtered faces in the schizophrenia group were judged statistically significant if their 2-sided p-values were less than 0.05/3=0.017. Everywhere the unadjusted p-values are reported. Effect sizes are reported either as η2p or as Cohen's d, as appropriate.
3. Results
3.1 Group, Duration, Filter, and Gender Comparisons
An ANOVA of group by filter type by duration with gender as a covariate did not show any significant interactions (η2p < 0.07 for all interactions) or main effects of diagnostic group (likelihood ratio test (LRT) χ2(1) = 0.15, p = 0.696; η2p = 0.0002), or gender (LRT χ2(1) = 2.64, p = 0.104; η2p = 0.003). The threat ratings were lower for 39 ms duration than for 390 ms (difference = 0.20, p < 0.001, Cohen's d = 0.25) and 1703 ms (difference = 0.26, p <0.001, Cohen's d = 0.32) durations (which did not differ between each other, Cohen's d = 0.07) and this result did not depend on diagnosis, gender or filter condition. The threat ratings for LSF faces were higher than those of HSF faces (difference = 0.17, p= 0.001, Cohen's d = 0.21), while the threat ratings of unfiltered faces fell in between and did not differ from either (Cohen's ds = 0.12 and 0.09); those results did not depend on diagnosis, gender or duration.
3.2 Experiment 1: Speed of Impression Formation of Full-Spectrum Unfiltered Faces
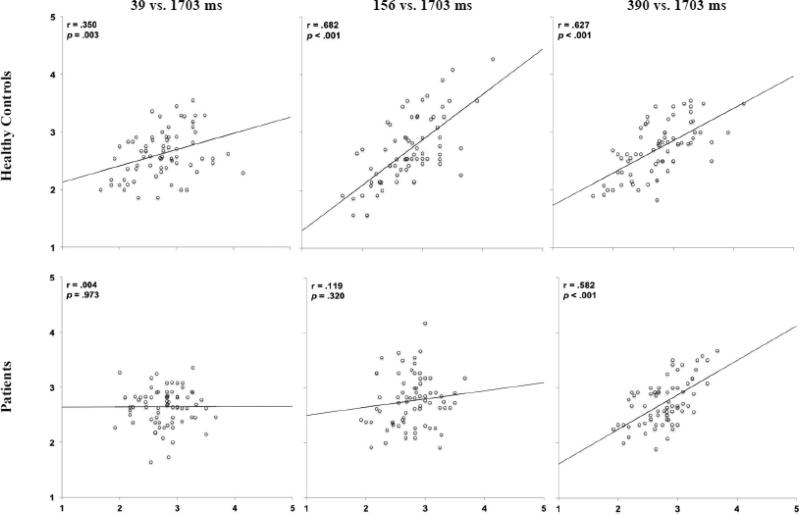
Threat ratings of neutral faces shown for 39 – 390 ms were compared to those made at 1703 ms (Bar et al., 2006; Figure 2). Healthy controls needed only 39 ms to demonstrate a significant linear relationship with ratings made at 1703 ms, as seen by a significant correlation (see plots for r values and significance levels). Stronger relationships were found as duration increased to 156 ms. Individuals with schizophrenia, however, needed 390 ms to yield a significant relationship. Correlations remained significant when Bonferroni corrections were applied.
Figure 2.
Scatterplots of relations between mean threat ratings of faces at 39, 156, and 390 ms (Y axis) compared to mean threat ratings of the same faces at 1703 ms (X axis) in healthy controls and patients in Experiment 1.
3.3 Experiment 2: Spatial Frequencies and Rapid Threat Impressions
3.3.1 LSF Results
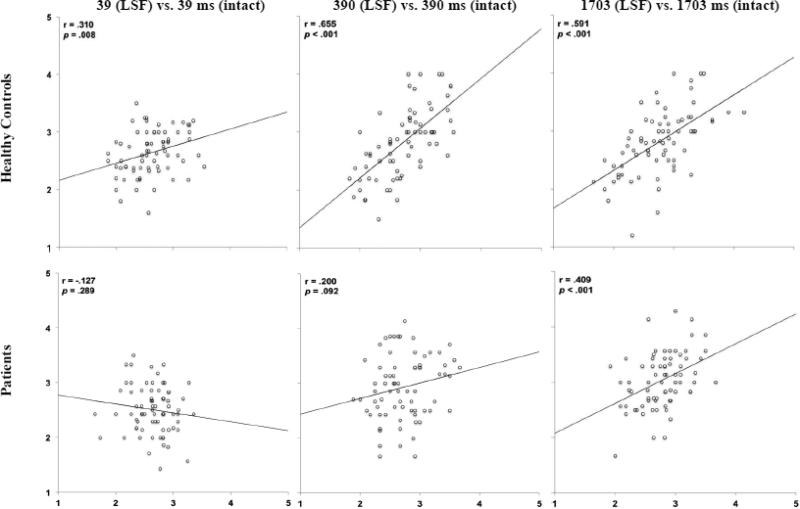
The156 ms duration was eliminated because controls showed correlations with ratings at 1703 ms as early as 39 ms and patients needed 390 ms in Experiment 1. How consistently LSF-filtered faces were ranked compared to intact faces was assessed at each duration (Figure 3). Controls showed a significant linear relationship between ratings of LSF-filtered faces shown for 39 ms and ratings of intact faces shown for 39 ms. Similar to Experiment 1, relationships were stronger at longer durations. As expected, due to needing longer than 39 ms to form a consistent first impression, patients with schizophrenia did not show a significant relationship between ratings of LSF-filtered faces made at 39 ms and ratings of intact faces shown for 39 ms. However, at 390 ms, the duration at which patients were able to form a consistent first impression of intact faces, they showed a trend for a significant correlation between LSF and intact faces. At 1703 ms, patients showed a significant correlation between LSF and intact faces. Correlations remained significant following Bonferroni correction.
Figure 3.
Scatterplots of relations between mean threat ratings of LSF faces (Y axis) and intact faces (X axis) across exposure durations and spatial frequencies in healthy controls and patients in Experiment 2.
3.3.2 HSF Results
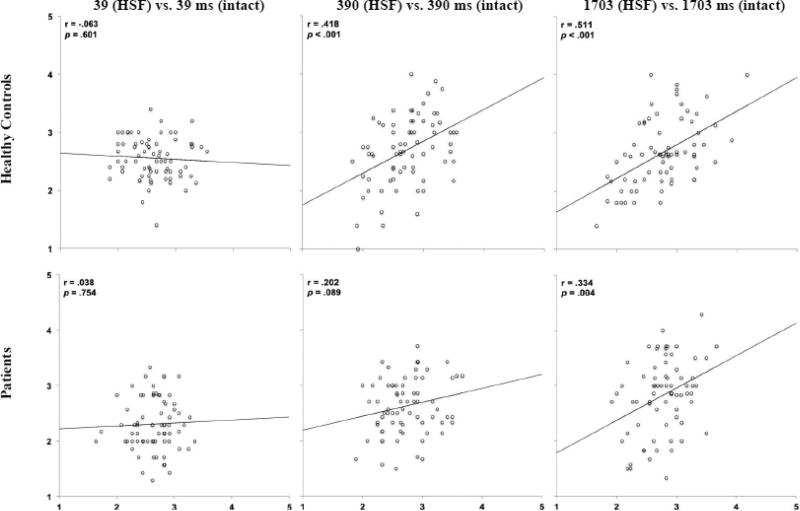
Figure 4 shows results of HSF-filtered vs. intact faces including r and p values. Unlike LSF results, controls did not show a significant linear relationship between ratings of HSF-filtered and intact faces shown for 39 ms. Controls did, however, show a significant relationship between HSF-filtered and intact faces shown for 390 and 1703 ms. Patients with schizophrenia did not show a significant relationship between ratings of HSF-filtered and intact faces made at 39 ms, but, like LSF results, showed a trend for a significant correlation between HSF and intact faces shown for 390 ms, the duration at which they were able to form a consistent first impression, and a significant correlation between HSF and intact faces at 1703 ms. Correlations remained significant following Bonferroni correction.
Figure 4.
Scatterplots of relations between threat ratings of HSF faces (Y axis) and intact faces (X axis) across exposure durations and spatial frequencies in healthy controls and patients in Experiment 2.
3.3 Correlations
For patients, no significant correlations were found between threat ratings at any condition and CPZ equivalents or PANSS rating scores.
4. Discussion
The purpose of this study was to explore the formation of first impressions in individuals with schizophrenia. Both the duration and effects of spatial frequency content of stimuli were assessed using the paradigm of Bar et al. (2006) to evaluate threat perception.
For controls, as hypothesized, consistent first impressions of threat in faces were formed rapidly, requiring only 39 ms to obtain significant agreement with faces shown for a longer duration (1703 ms). These results replicate Bar et al.'s (2006) findings that extraction of facial features necessary to form impressions of threatening and non-threatening neutral faces can be done even when faces are only shown for 39 ms. The current results are also in line with those of Todorov et al. (2009), who found that trustworthiness ratings of neutral faces made in 33 ms agreed with those made without time constraints. Our findings that improvements in agreement between ratings increased as duration increased from 39 to 156 ms are also consistent with those of Todorov et al. (2009), who found that agreements improved as duration of presentation increased from 33 to 100 ms, so that viewing times beyond ~150 ms do not appear to produce an advantage in forming a consistent first impression.
In agreement with our hypothesis, patients with schizophrenia needed longer exposure duration (i.e., 390 ms) to produce threat ratings consistent with ratings made when faces were shown for a considerably longer duration (i.e., 1703 ms). This is consistent with previous studies showing delays in processing configural aspects of faces (Butler et al., 2008), increased reaction time in making social appraisals (Taylor et al., 2011), impaired early-stage processing of faces in electrophysiological studies (Turetsky et al., 2007; Wynn et al., 2013) that may be related to impaired structural encoding of faces (Turetsky et al., 2007), and possibly impaired time continuity in schizophrenia (Giersch et al., 2013). Thus, impairments in temporal processing also extend to the ability to make a first impression.
Given that first impressions are formed so quickly in healthy individuals, Bar et al. (2006) assessed the role of LSFs, which are processed quickly, vs. HSFs, which are processed more slowly, in first impression formation. In their study, as well as in the current one, controls’ threat ratings of LSF-, but not HSF-, filtered faces shown for 39 ms correlated significantly with ratings made for unfiltered faces. Thus, the current study supports the hypothesis that LSF information is important in formation of first impressions for controls.
Patients, on the other hand, not only took longer than controls to form consistent first impressions, but utilized different mechanisms. At 390 ms, the duration at which patients formed a stable first impression, there was a trend for a significant correlation between both LSF- and HSF-filtered faces vs. intact faces. Thus, patients are utilizing both LSF and HSF information to some extent to form a first impression at 390 ms. In previous fMRI studies of face (Silverstein et al., 2010) and object (Calderone et al., 2013) recognition, patients had altered activation patterns to LSF and HSF stimuli depending on brain region, leading to the suggestion that there may be increased utilization of spatial frequency information in some brain regions in schizophrenia to compensate for such difficulties as decreased global processing in early visual areas. Our data indicate that a compensatory HSF activation may occur in first impression formation in schizophrenia. A recent study by Laprevote et al. (2010) found that patients demonstrated a bias towards LSF information in processing hybrid faces, but responded accurately when viewing HSF information in non-hybrid faces. They suggested that the time course of concurrently perceiving LSF and HSF information in patients with schizophrenia may be impaired, potentially due to longer durations of time needed to process LSF information. Impaired use of LSF information in the present study could contribute to the sluggish formation of first impressions, which might result from increased integration time needed for LSF information in the cortex. Indeed, increased P1 latency was found in response to LSF, but not HSF, sinusoidal gratings in a VEP study of people with schizophrenia (Butler et al., 2007). Further work is needed to better understand this phenomenon.
While the main objectives were to assess duration and mechanisms of first impression formation, differences between ratings of patients and controls were assessed as were effects of duration and filter type. Both groups rated faces as more threatening as duration increased from 39 to 390 ms. This is similar to Willis and Todorov's (2006) finding that, while controls formed trait judgments of attractiveness, likeability, trustworthiness, competence, and aggressiveness in 100 ms (a shorter duration was not examined), these judgments became more negative as duration of exposure to faces increased to 500 ms, but not from 500 to 1000 ms. Effects of duration have not, to our knowledge, been previously examined in first impression formation in patients with schizophrenia. Even though patients did not form stable threat judgments in 39 ms, like controls, they may show a “positivity” effect at the short duration. Alternatively, the lower ratings for patients at 39 ms could be due to difficulty detecting facial features that would cause them to give higher threat ratings. Further studies, such as those carried out by Bar et al., (2006) determining accuracy of face identification at short durations would help to answer this question. In addition, both groups showed more negative threat ratings in the LSF than HSF condition. This is consistent with LSF being used to make fast threat judgments, though patients may not be utilizing LSF information as effectively as controls to form consistent impressions.
No significant differences in ratings were found between groups and no significant relationships were found with clinical ratings. In previous studies of trait ratings of faces, patients gave similar ratings as controls for danger (Henry et al., 2010), friendliness (Taylor et al., 2011), and likeability (Kline et al., 1992) and gave similar (McIntosh and Park, 2014) or higher (Haut and MacDonald, 2010) ratings for attractiveness than controls. Trustworthiness ratings were more variable, though generally showed similar or more positive ratings for patients than controls (McIntosh and Park, 2014; Strauss et al., 2012; Haut and MacDonald 2010; Baas et al., 2008a,b; Couture et al., 2008; Pinkham et al., 2008: Hooker et al., 2011). Relationships between trait ratings and symptoms also vary between studies (e.g., Couture et al., 2008; Pinkham et al., 2008; Hooker et al., 2011; McIntosh and Park, 2014). Several studies suggest that while trait ratings of patients with schizophrenia may be normal (Haut and MacDonald 2010; Baas et al., 2008a), the mechanisms by which patients reach these ratings are different from those of controls. For instance, Baas et al. (2008a) found that patients had decreased amygdala activation during trustworthiness decision making, though behavioral ratings were similar between groups. The current results suggest that while behavioral ratings of threat were similar between groups, perceptual mechanisms including integration time differed. Consistent with this idea, previous studies have shown that misattribution of emotion in schizophrenia is related to visual perceptual properties of stimuli (Bedwell et al., 2013; McBain et al., 2010). The present results suggest that aberrant perceptual mechanisms may be utilized in social decision making in schizophrenia.
Of note, in schizophrenia patients, it seems that mechanisms used to make trait ratings are different from those used to recognize emotions. For instance, patients are more likely to misattribute neutral expressions as showing anger or disgust (Premkumar et al., 2008; Kohler et al., 2003; Pinkham et al., 2011), but conversely, make trait ratings of neutral faces, such as trustworthiness or, as in the present study – threat, that are similar to or more positive than those of controls (Baas et al., 2008a,b; Haut and MacDonald 2010). A recent study (McIntosh and Park 2014) sheds further light on this. When the same faces were used, patients showed impaired emotion recognition but intact trait judgments. However, further studies of neural, including perceptual mechanisms, involved in emotion versus trait ratings are needed to disentangle this issue.
Limitations of the study include all patients on antipsychotic medication, though no significant correlations were found between chlorpromazine equivalents and ratings of faces. In addition, there were more females in the control than patient group. Previous studies examining time to make a first impression did not evaluate males and females separately (Bar et al., 2006; Todorov et al., 2009). Previous studies of trait ratings in schizophrenia did not find an effect of gender (Hooker et al., 2011; Baas et al., 2008b). While there was not a gender effect in the present study, further studies need to be done to assess gender differences in formation of first impressions of traits.
In conclusion, the results of this study suggest that while the ratings of first impressions of threat in neutral faces do not differ for patients vs. controls, patients’ formation of first impressions is slower and does not appear to rely on coarse LSF information as it does in controls. First impressions have a huge impact on behavior (Todorov et al., 2005; Zebrowitz and McDonald, 1991). Further work is needed to determine whether sluggish formation as well as different mechanisms of first impression formation impact social function, which is frequently impaired in schizophrenia.
Acknowledgements
We are very grateful to all participants who donated their time and energy to this project.
Funding
This work was supported by the National Institutes of Health (RO1 MH084848 to PDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Authors PDB, MB, NGW, and IA designed the study. Authors JV, FT, and PDB managed the literature searches. Authors EP, ZS, JV, VZ, NGW, and PDB contributed to data analysis. Author JV wrote the first draft of the manuscript. All authors contributed to and approved the final version of the manuscript.
Conflict of Interest
No authors report financial relationships with commercial interests.
References
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, Anderson A, Lee GP, Damasio AR. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37(10):1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Baas D, Aleman A, Vink M, Ramsey NF, de Haan EHF, Kahn RS. Evidence of altered cortical and amygdala activation during social decision-making in schizophrenia. NeuroImage. 2008a;40:719–727. doi: 10.1016/j.neuroimage.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Baas D, van't Wout M, Aleman A, Kahn RS. Social judgement in clinically stable patients with schizophrenia and healthy relatives: behavioural evidence of social brain dysfunction. Psychol. Med. 2008b;38(5):747–754. doi: 10.1017/S0033291707001729. [DOI] [PubMed] [Google Scholar]
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J. Cogn. Neurosci. 2003;15:600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- Bar M, Neta M, Linz H. Very first impressions. Emotion. 2006;6(2):269–278. doi: 10.1037/1528-3542.6.2.269. [DOI] [PubMed] [Google Scholar]
- Bedwell JS, Chan CC, Cohen O, Karbi Y, Shamir E, Rassovsky Y. The magnocellular visual pathway and facial emotion misattribution errors in schizophrenia. Progress in Neuropsychopharmacology & Biological Psychiatry. 2013;44:88–93. doi: 10.1016/j.pnpbp.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Tambini A, Yovel G, Jalbrzikowski M, Ziwich R, Silipo G, Kanwisher N, Javitt DC. What's in a face? Effects of stimulus duration and inversion on face processing in schizophrenia. Schizophr. Res. 2008;103(1-3):283–292. doi: 10.1016/j.schres.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry. 2005;62(5):495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone DJ, Hoptman MJ, Martinez A, Nair-Collins S, Mauro CJ, Bar M, Javitt DC, Butler PD. Contributions of low and high spatial frequency processing to impaired object recognition circuitry in schizophrenia. Cereb. Cortex. 2013;23(8):1849–1858. doi: 10.1093/cercor/bhs169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Addington J, Woods SW, Perkins DO. Assessment of social judgments and complex mental states in the early phases of psychosis. Schizophr. Res. 2008;100(1-3):237–241. doi: 10.1016/j.schres.2007.12.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr. Bull. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Reviews. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Giersch A, Lalanne L, van Assche M, Elliott MA. On disturbed time continuity in schizophrenia: an elementary impairment in visual perception? Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00281. Article 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Penn DL, Bentall R, Carpenter WT, Gaebel W, Gur RC, Kring AM, Park S, Silverstein SM, Heinssen R. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr. Bull. 2008;34(6):1211–1220. doi: 10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassin R, Trope Y. Facing faces: studies on the cognitive aspects of physiognomy. J. Personality Social Psych. 2000;78(5):837–852. doi: 10.1037//0022-3514.78.5.837. [DOI] [PubMed] [Google Scholar]
- Haut KM, MacDonald AW., 3rd Persecutory delusions and the perception of trustworthiness in unfamiliar faces in schizophrenia. Psychiatry Res. 2010;178(3):456–460. doi: 10.1016/j.psychres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Henry JD, Von Hippel C, Ruffman T, Perry Y, Rendell PG. Threat perception in schizophrenia-spectrum disorders. J Int. Neuropsych.Society. 2010;16:805–812. doi: 10.1017/S1355617710000640. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- Hooker CI, Tully LM, Verosky SC, Fisher M, Holland C, Vinogradov S. Can I trust you? Negative affective priming influences social judgments in schizophrenia. J. Abnorm. Psychol. 2011;120(1):98–107. doi: 10.1037/a0020630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SS, Kanes SJ, Gur RE, Gur RC. Facial Emotion Recognition In Schizophrenia: Intensity Effects And Error Pattern. Am. J. Psychiatry. 2003;160:1768–1774. doi: 10.1176/appi.ajp.160.10.1768. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Arana GW, Rosenbaum JF. Handbook of psychiatric drug therapy. 3rd ed. Little, Brown, and Co, Boston; Boston: 1995. [Google Scholar]
- Irani F, Seligman S, Kamath V, Kohler C, Gur RC. A meta-analysis of emotion perception and functional outcomes in schizophrenia. Schizophr. Res. 2012;137(1-3):203–211. doi: 10.1016/j.schres.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Antal A, Szekeres G, Benedek G, Janka Z. Spatiotemporal visual processing in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2002;14(2):190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- Kline JS, Smith JE, Ellis HC. Paranoid and nonparanoid schizophrenic processing of facially displayed affect. J. Psychiatric Res. 1992;26(3):169–182. doi: 10.1016/0022-3956(92)90021-f. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr. Bull. 2010;36(5):1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprevote V, Oliva A, Delerue C, Thomas P, Boucart M. Patients with schizophrenia are biased toward low spatial frequency to decode facial expression at a glance. Neuropsychologia. 2010;48(14):4164–4168. doi: 10.1016/j.neuropsychologia.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of magnocellular dysfunction on processing attended information in schizophrenia. Cereb. Cortex. 2011;22(6):1282–1293. doi: 10.1093/cercor/bhr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from fMRI. J. Neurosci. 2008;28(30):7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AM, Benavente R. The AR face database. 1998.
- McBain R, Norton D, Chen Y. Differential roles of low and high spatial frequency content in abnormal facial emotion perception in schizophrenia. Schizophr. Res. 2010;122(1-3):151–155. doi: 10.1016/j.schres.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh LG, Park S. Social trait judgement and affect recognition from static faces and video vignettes in schizophrenia. Schizophr. Res. 2014 doi: 10.1016/j.schres.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell BF, Potts GF, Nestor PG, Stylianopoulos KC, Shenton ME, McCarley RW. Spatial frequency discrimination in schizophrenia. J. Abnorm. Psychol. 2002;111(4):620–625. doi: 10.1037//0021-843x.111.4.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivola CY, Todorov A. Elected in 100 milliseconds: Appearance-based trait inferences and voting. J. Nonverbal Behavior. 2010;34:83–110. [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. PNAS of the United States of America. 2008;105(32):11087–11092. doi: 10.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuskens J, Link CG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta. Psychiatr. Scand. 1997;96(4):265–273. doi: 10.1111/j.1600-0447.1997.tb10162.x. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Brensinger C, Kohler C, Gur RE, Gur RC. Actively paranoid patients with schizophrenia over attribute anger to neutral faces. Schizophr. Res. 2011;125:174–178. doi: 10.1016/j.schres.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr. Res. 2008;99(1-3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar P, Cooke MA, Fannon D, Peters E, Michel TM, Aasen I, Murray RM, Kuipers E, Kumari V. Misattribution bias of threat-related facial expressions is related to a longer duration of illness and poor executive function in schizophrenia and schizoaffective disorder. Eur. Psychiatry. 2008;23:14–19. doi: 10.1016/j.eurpsy.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said CP, Sebe N, Todorov A. Structural resemblance to emotional expressions predicts evaluation of emotionally neutral faces. Emotion. 2009;9(2):260–264. doi: 10.1037/a0014681. [DOI] [PubMed] [Google Scholar]
- Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr. Bull. 2013;39(5):979–992. doi: 10.1093/schbul/sbs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, All SD, Kasi R, Berten S, Essex B, Lathrop KL, Little DM. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol. Med. 2010;40(7):1159–1169. doi: 10.1017/S0033291709991735. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol. 1998;107(1):49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Lee BG, Waltz JA, Robinson BM, Brown JK, Gold JM. Cognition-emotion interactions are modulated by working memory capacity in individuals with schizophrenia. Schizophr. Res. 2012;141(2-3):257–261. doi: 10.1016/j.schres.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Chen AC, Tso IF, Liberzon I, Welsh RC. Social appraisal in chronic psychosis: role of medial frontal and occipital networks. J. Psychiatric Res. 2011;45(4):526–538. doi: 10.1016/j.jpsychires.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov A, Pakrashi M, Oosterhof NN. Evaluating faces on trustworthiness after miminal time exposure. Social Cognition. 2009;27:813–833. [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr. Res. 2007;94(1-3):253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat. Neurosci. 2003;6(6):624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Willis J, Todorov A. First impressions: making up your mind after a 100-ms exposure to a face. Psychol. Sci. 2006;17(7):592–598. doi: 10.1111/j.1467-9280.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clin. Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Jahshan C, Altshuler LL, Glahn DC, Green MF. Event-related potential examination of facial affect processing in bipolar disorder and schizophrenia. Psychol. Med. 2013;43(1):109–117. doi: 10.1017/S0033291712001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate MA, Sanders JD, Garza AA. Neurological dissociations of social perception processes. Social Cognition. 2000;18:223–251. [Google Scholar]
- Zebrowitz LA, McDonald SM. The impact of litigants’ baby-facedness and attractiveness on adjudications in small claims courts. Law Human Behavior. 1991;15(6):603–623. [Google Scholar]