Abstract
Rationale
G protein-coupled receptor (GPCR) kinases (GRKs) acting in the cardiomyocyte regulate important signaling events that control cardiac function. Both GRK2 and GRK5, the predominant GRKs expressed in the heart, have been shown to be up-regulated in failing human myocardium. While the canonical role of GRKs is to desensitize GPCRs via phosphorylation, it has been demonstrated that GRK5, unlike GRK2, can reside in the nucleus of myocytes and exert GPCR-independent effects that promote maladaptive cardiac hypertrophy and heart failure (HF).
Objective
To explore novel mechanisms by which GRK5 acting in the nucleus of cardiomyocytes participates in pathological cardiac hypertrophy.
Methods and Results
In this study, we have found that GRK5-mediated pathological cardiac hypertrophy involves the activation of nuclear factor of activated T-cells (NFAT) as GRK5 causes enhancement of NFAT-mediated hypertrophic gene transcription. Transgenic mice with cardiomyocyte-specific GRK5 overexpression activate an NFAT-reporter in mice basally and after hypertrophic stimuli including transverse aortic constriction (TAC) and phenylephrine treatment. Complimentary to this, GRK5 null mice exhibit less NFAT transcriptional activity after TAC. Further, loss of NFATc3 expression in the heart protected GRK5 overexpressing transgenic mice from the exaggerated hypertrophy and early progression to HF seen after TAC. Molecular studies suggest that GRK5 acts in concert with NFAT to increase hypertrophic gene transcription in the nucleus via GRK5’s ability to bind DNA directly without a phosphorylation event.
Conclusions
GRK5, acting in a kinase-independent manner, is a facilitator of NFAT activity and part of a DNA binding complex responsible for pathological hypertrophic gene transcription.
Keywords: GRK, NFAT, heart failure
INTRODUCTION
Heart failure (HF) is a clinical end point defined by the heart’s inability to adequately perfuse the body with blood. This condition effects over 5 million Americans with 825,000 new cases annually 1. Although HF can be the result of many diverse etiologies there seems to be common underlying molecular mechanism including the dysfunction of the β-adrenergic receptor (β-AR) system, dysregulation of myocyte calcium handling and activation of the fetal gene program among which are genes that can lead to pathological cardiac hypertrophy 2. β-ARs act to drive the contractile function but become dysfunctional following chronic catecholamine stimulation, which occurs in HF. G protein-coupled receptor (GPCR) kinases (GRKs) phosphorylate these receptors leading to their desensitization and down-regulation 3. GRK2 and GRK5, the two major GRKs in the heart, are in fact up-regulated in HF leading to a loss of the heart’s inotropic reserve 4-6. In fact, GRK2 inhibition and the improved resensitization of β-AR signaling in the failing heart has led to HF reversal and a potential therapeutic strategy 7. The role of GRK5 on cardiac β-AR signaling is less understood although GRK5 can also desensitize these GPCRs 8.
Recently, many non-GPCR functions of GRKs have been discovered and some of these appear physiologically important. For example, GRK2 is a pro-death kinase in myocytes acting at the level of mitochondria in a non-GPCR fashion mediated by oxidative stress 9. While GRK5 is not found in the mitochondria it does contain a nuclear localization sequence (NLS) homologous to homeobox-containing transcription factors, which allows it to translocate to the nucleus where it has been shown to have DNA binding properties in some cells 10, 11. In cardiomyocytes, it has been demonstrated that GRK5 can accumulate in the nucleus in a Gq-dependent manner either through pharmacologic hypertrophic stimulation or left ventricular (LV) pressure-overload 12-14. Once in the nucleus GRK5 has been shown to phosphorylate histone deacetylase-5 (HDAC5) leading to derepression of MEF2-mediated hypertrophic gene transcription 14. This was best shown in transgenic mice with cardiac GRK5 overexpression as these mice, but not mice overexpressing a NLS-mutant GRK5 that cannot accumulate in the nucleus, displayed exaggerated cardiac hypertrophy and early-onset HF following pressure-overload 14. Not only did the NLS-GRK5 mutant mice not have maladaptive cardiac hypertrophy, but GRK5 knockout (KO) mice are also protected against pressure-overload stress 12.
In addition to the phosphorylation of HDAC5, nuclear GRK5 has been shown to activate the NF-κB pathway through phosphorylation of Iκ-Bα as well as regulate cell cycle progression through direct phosphorylation of nucleophosmin 15-17. Thus, there could be additional targets in the nucleus of hypertrophic cardiomyocytes. One of the key transcription factors involved with myocardial hypertrophy and subsequent HF is the nuclear factor of activated T cells (NFAT). NFAT resides in the cytoplasm in a hyper-phosphorylated state and then upon increased calcium entry, NFAT is de-phosphorylated by the calcium sensitive phosphatase calcineurin and then it can translocate to the nucleus where it controls transcription of several hypertrophic and maladaptive genes 18. The NFAT pathway is an interesting therapeutic target because it is involved in pathological, but not physiological hypertrophy and several drugs exist that can regulate this pathway including cyclosporine 19, 20.
Herein, we provide evidence that GRK5 is able to activate the NFAT transcriptional pathway following hypertrophic stress. Our data reveal that the GRK5 localization in the nucleus and not cytosol facilitates the transcriptional activity of NFAT in vitro and in vivo. Further, NFATc3 expression is needed for the induction of early pathology after hypertrophic stress with enhanced GRK5 expression. Molecular studies suggest that GRK5 acts in concert with NFAT at the level of chromatin to facilitate hypertrophic gene transcription in a kinase-independent manner. Thus, nuclear GRK5 appears to be a key player and determinant of pathological cardiac hypertrophy and limiting its accumulation in the nucleus may allow for the development of novel therapeutics and strategies to prevent and reverse the progression of maladaptive cardiac hypertrophy and HF.
METHODS
Cell culture
Neonatal rat ventricular myocytes (NRVMs) were isolated from 1- to 2-day old rats as previously described 21. H9c2 cells, a rat myoblast cell line, were cultured in DMEM from ATCC supplemented with 10% bovine calf serum and penicillin-streptomycin in a humidified chamber with 5% CO2 at 37°C.
Adenovirus, plasmids and transfection
NRVMs were infected with recombinant, replication-deficient adenoviruses at an MOI from 1-10 viral particles per cell. Plasmids encoding wild-type full length bovine GRK5, GRK5 K215R kinase dead and the GRK5 NES nuclear exclusion sequence mutant (a kind gift of Dr. Julie Pitcher at University College London) in the pRK5 vector were transiently transfected into H9c2 cells using Lipofectamine 2000 as described 11, 22-24.
Luciferase assay
Cells were harvested 48 hrs after infection in lysis buffer according to manufacturer’s protocol (Promega)14.
Real-time PCR
Total RNA was extracted by the TRIzol method and RT-PCR was performed as described 12.
Experimental animals
All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Temple University School of Medicine.
Echocardiography
To measure global cardiac function, echocardiography was performed with the VisualSonics VeVo 2100 imaging system in anesthetized animals as described 12.
Transverse aortic constriction
Transverse aortic constriction (TAC) was performed as described previously 14.
Mini-osmotic pumps
Chronic infusion of phenylephrine (PE, purchased from Sigma) was done using Alzet 3-day mini-osmotic pumps (model 1003D, DURECT Corporation) following the manufacturer’s specifications.
Cardiomyocyte cross-sectional area
Axial cut tissue sections were stained with Alexa Fluor 594 conjugated of wheat germ agglutinin. Cell borders were planimetered manually by an operator who was blinded to treatment group.
Electrophoretic Mobility Shift Assay (EMSA)
IRDye 700 end labeled NFAT consensus oligonucleotides were incubated with 2.5 μg NRVM nuclear extracts (NE). For antibody mediated super shift assay, 1 ug antibody was incubated with 5 μg NE in reaction mixture. Protein-DNA complexes were seperated on 4% non-denaturing polyacrylamide gel and visualized using an Odyssey infrared imaging system17.
Statistics
All the values in the text and figures are presented as mean +/− SEM. Statistical significance was determined by Student’s t-test or ANOVA. P values of <0.05 were considered significant.
RESULTS
GRK5 enhances cardiac NFAT transcriptional activity in vitro
Recent data from our laboratory have shown that GRK5 acting in the nucleus is a key mediator of pathological cardiac hypertrophy and this is due, in part, to its actions as a HDAC kinase 12-14. In this study we sought other actions of GRK5 in the nucleus since in addition to its kinase activity in the nucleus, GRK5 has been shown to have potential DNA binding capabilities 10, 11 and to interact with other nuclear proteins 25. It is well known that in addition to hypertrophic gene transcription occurring through the HDAC-regulated MEF2 transcription factor, NFAT is also a critical regulator of hypertrophic gene regulation 26. To investigate whether GRK5 may influence NFAT activity we first used an NFAT reporter assay in myocytes. In NRVMs expressing an NFAT-luciferase reporter adenovirus we overexpressed GRK5 and found significantly increased luciferase levels after stimulating the cells with the hypertrophic α1-adrenergic agonist, phenylephrine (PE) (Fig. 1A).
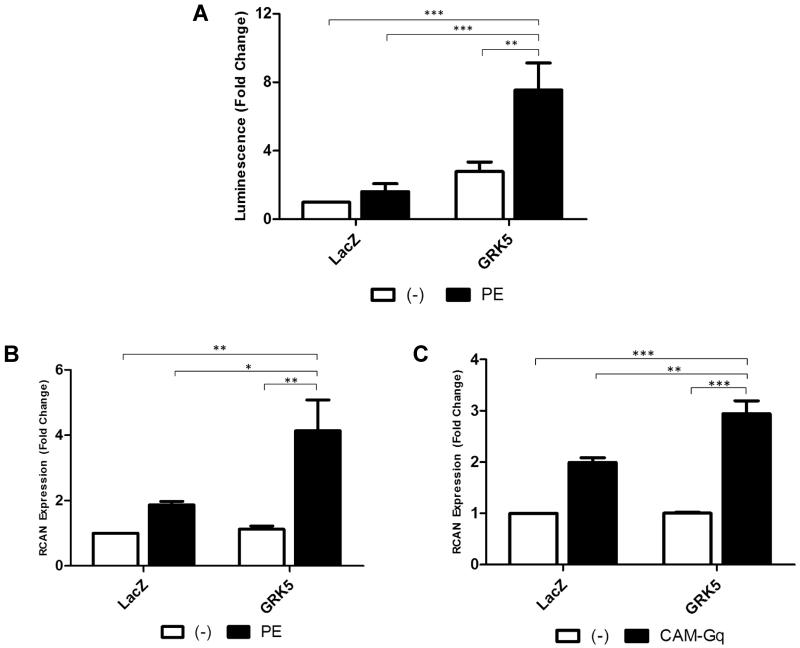
Figure 1. GRK5 Enhances Cardiac NFAT Transcriptional Activity In Vitro.
(A) NFAT luciferase activity in neonatal rat ventricular myocytes (NRVMs) infected with an NFAT luciferase reporter virus as well as a GRK5 or LacZ control virus. Cells were stimulated for 24 hours with 50μmol/L phenylephrine (PE). (n=6. **,p<0.01; ***, p<0.001 by ANOVA). (B) Quantitative RT-PCR for NFAT target gene RCAN1.4 (RCAN) in NRVMs overexpressing GRK5 or LacZ control for 48 hours and stimulated with 50μmol/L PE for 16 hours. (n=3. *,p<0.05; **, p<0.01 by ANOVA). (C) Quantitative RT-PCR for NFAT target gene RCAN in NRVMs overexpressing GRK5 or LacZ control adenovirus with or without CAM-Gq adenovirus 48 hours after adenoviral infection. (n=3. **, p<0.01; ***, p<0.001 by ANOVA).
In order to confirm that GRK5-mediated increases in NFAT luciferase activity post-PE was due to a true induction in NFAT transcriptional activity we performed an additional and complementary experiment where we measured expression levels of the NFAT target gene RCAN1.4 (henceforth referred to only as RCAN) in myocytes after PE stimulation with varying levels of GRK5. NRVMs were subjected to viral over-expression of GRK5 or LacZ as a control followed by stimulation with 50 μM PE for 16 hrs and RCAN levels were assessed by quantitative RT-PCR. While PE was able to increase RCAN expression in myocytes, we found a significant increase in RCAN mRNA levels when GRK5 levels were enhanced (Fig. 1B). This confirmed that GRK5 overexpression is able to induce increased NFAT transcriptional activity with RCAN expression serving as an endogenous reporter of NFAT activity.
Finally, PE is known to activate the α1-AR and hypertrophic signaling occurs down-stream of Gαq activation. In order to confirm that GRK5’s actions on NFAT after PE were due to Gαq activation, we performed RCAN expression assays in NRVMs expressing a constitutively active mutant of Gαq (CAM-Gq) and found that when GRK5 is elevated there is significant more RCAN expression induced with CAM-Gq (Fig. 1C).
In separate experiments using immunofluorescence and confocal microscopy we found that GRK5 overexpression in myocytes does not cause more NFAT to accumulate in the nucleus following PE stimulation. This indicates that the induction of NFAT activity by GRK5 is not due to more NFAT translocating to the nucleus, but rather somehow enhancing activity within the nucleus (Supplemental Fig. I). Supporting this are studies where overexpressing GRK5 had no effect on cytoplasmic calcineurin activity (Supplemental Fig. IC, ID).
GRK5 enhances NFAT transcriptional activity in vivo in models of cardiac pathology
After demonstrating in vitro that increased GRK5 expression in myocytes can enhanced NFAT activity and hypertrophic gene transcription we sought to confirm these findings in vivo. To do so we crossed cardiac-specific NFAT luciferase reporter mice (LUC) previously characterized 20 with mice that our lab previously created with cardiac-specific transgenic overexpression of GRK5 (TgGRK5) 14. Using in vivo bioluminescence imaging techniques we find that cardiac GRK5 overexpresssion causes increased NFAT activity in the upper thoracic cavity consistent with the heart (Supplemental Fig. II). Taking hearts from TgGRK5 mice, LUC mice and LUC/TgGRK5 hybrid mice we assessed the ex vivo luciferase activity and found approximately twice as much NFAT activity in the TgGRK5 mice compared to those with endogenous levels of GRK5 (Fig. 2A). Somewhat surprisingly, these results indicate that only by overexpressing GRK5 in the myocytes is NFAT activity enhanced. Overall, this activity, although significant, must not be robust enough to drive hypertrophy since the LUC/TgGRK5 mice do not exhibit altered cardiac mass at this age in the absence of hypertrophic stress.
Figure 2. GRK5 Enhances NFAT Transcriptional Activity In Vivo in Models of Cardiac Pathology.
(A) Ex vivo NFAT luciferase assay from whole heart of NFAT-luciferase reporter mice with concomitant GRK5 overexpression (TgGRK5/LUC) or littermates with endogenous levels of GRK5 (LUC). Hearts were removed from 8-12 week old mice. (LUC n=6, TgGRK5/LUC n=8. *,p<0.05 by t-test). (B) Ejection fraction was determined by echocardiography in TgGRK5/LUC or LUC littermates with endogenous levels of GRK5 2 weeks after transaortic constriction (TAC) or sham surgery (SHAM). (C) Ex vivo NFAT luciferase assay from whole heart of TgGRK5/LUC or LUC littermates after TAC. (D) Quantitative RT-PCR for NFAT target gene RCAN from whole heart of NFAT luciferase reporter mice. (LUC SHAM n=10, LUC TAC n=13, TgGRK5/LUC SHAM n=17, TgGRK5/LUC TAC n=17. **,p<0.01; ***,p<0.001 by ANOVA). (E) Ex vivo NFAT luciferase assay from whole heart of TgGRK5/LUC mice or LUC littermates after 24 hours of PE administration (35 mg/kg/day). (LUC PBS n=9, LUC PE n=7, TgGRK5/LUC PBS n=11, TgGRK5/LUC PE n=15. **,p<0.01; ***,p<0.001 by ANOVA).
To determine whether the increase in NFAT luciferase activity seen in TgGRK5 mice was relevant in the context of pathology, these mice were subjected to left ventricular (LV) pressure-overload through surgical transverse aortic constriction (TAC). Cardiac function in these animals was assessed 14 days after TAC by echocardiography. The ejection fraction (EF) in the TgGRK5/LUC mice that were subjected to TAC was significantly decreased (Fig. 2B), consistent with early post-TAC HF seen with TgGRK5 mice previously 14. Importantly, after 14 days of pressure-overload an ex vivo NFAT luciferase assay was performed on heart tissue and NFAT activity via luminescence readings was significantly higher in LUC mice as expected, however when GRK5 was elevated in these mice (LUC/TgGRK5) there was significantly more NFAT activity (Fig. 2C).
To confirm that this increase in luciferase activity was indeed a result of increased NFAT transcriptional activity we performed RT-PCR from the hearts of these mice for the NFAT target gene RCAN. Following TAC, animals with endogenous levels of GRK5 (LUC mice) showed an increase in RCAN expression while LUC/TgGRK5 hybrid animals demonstrated a significant increase in RCAN expression compared to all other groups (Fig. 2D). Therefore, using an endogenous gene targeted readout we were able to confirm that NFAT transcriptional activity is increased in TgGRK5 mice in a model of pathology.
It is known that TAC causes hypertrophy through the Gαq pathway and has been shown to drive GRK5 into the nucleus 14, 27; therefore, we wanted to test if GRK5 overexpression would enhance NFAT activity following stimulation of the Gαq pathway via the α1-adrenergic hypertrophic agonist PE in these animals. Mini-osmotic pumps were used to deliver a sub-pressor dose of PE subcutaneously for 24 hrs in LUC and LUC/TgGRK5 mice. As seen previously, TgGRK5 mice alone pumped with vehicle control have an increase in NFAT luciferase activity as compared to LUC-alone mice with endogenous levels of GRK5 (Fig 2E). After PE treatment, LUC/TgGRK5 mice demonstrate a significant increase in NFAT activity over all other groups (Fig. 2E). Thus, in this in vivo mouse model, we demonstrate that GRK5 is able to increase NFAT transcriptional activity basally and down-stream of the Gαq hypertrophic signaling pathway.
GRK5 KO mice demonstrate attenuated NFAT transcriptional activity following hypertrophic stress
In order to determine that GRK5 mediated activation of the NFAT pathway is physiologically relevant, we subjected GRK5 null (GRK5 KO) mice to LV pressure-overload via TAC. At baseline and 2 and 4 weeks post-TAC echocardiography was performed to assess LV posterior wall thickness (LVPWT) of these animals as a marker of hypertrophy. At baseline we saw no difference in wall thickness; however, at 4 weeks post-TAC wild-type mice showed a significant increase in wall thickness compared to all other groups (Fig. 3A). As expected from previous work in our laboratory 12, the increase in wall thickness seen following TAC was significantly attenuated in GRK5 KO mice (Fig. 3A). After 4 weeks hearts were harvested and RT-PCR was performed on heart tissue to determine the level of transcription of the NFAT target gene RCAN and we found a significant increase in RCAN transcription in wild-type mice subjected to TAC as compared to Sham groups (Fig. 3B). Interestingly, we found less RCAN transcription in GRK5 KO mice subjected to TAC as compared with wild-type littermates that also underwent TAC surgery (Fig. 3B) indicating that endogenous levels of GRK5 are required to get a normal hypertrophic response that includes NFAT-mediated RCAN expression. These results are consistent with GRK5 being relevant for normal NFAT activation following pressure-overload.
Figure 3. GRK5 KO Mice Demonstrate Attenuated NFAT Transcriptional Activity Following Hypertrophic Stress.
(A) Left ventricular wall thickness during systole (LVPWTs) as measured by echocardiography in GRK5 null mice (GRK5 KO) at baseline, 2 and 4 weeks after transaortic constriction (TAC) or sham surgery (SHAM) as compared to wildtype (WT) controls. (WT SHAM, closed circle, n=10; WT TAC, closed square, n=10; GRK5 KO SHAM, open circle, n=6; GRK5 KO TAC, open square, n=8. ***,p<0.001 by ANOVA). (B) Quantitative RT-PCR for NFAT target gene RCAN from whole heart of GRK5 KO mice 4 weeks after TAC or SHAM as compared to WT. (**,p<0.01; ***,p<0.001; #,p<0.05 vs WT TAC by ANOVA).
NFATc3 KO attenuates GRK5-mediated cardiac pathology after pressure-overload stress
To determine whether NFAT activity is required for GRK5-mediated cardiac pathology after TAC we bred TgGRK5 mice with mice null for NFATc3 (NFATc3 KO). We found significant differences in mice overexpressing GRK5 in the hearts with and without NFATc3 as early as 1 week post-TAC as determined initially by simple heart weight to body weight (HW:BW) ratio (Fig. 4A and Supplemental Fig. III). As expected, we saw a significant increase in HW:BW in TgGRK5 mice subjected to TAC compared to control non-transgenic, wild-type animals that have endogenous levels of GRK5 (Fig. 4A). Interestingly, NFATc3 deletion significantly attenuated the observed increase in heart weight seen in the TgGRK5 mice following TAC (Fig. 4A). We also assessed cardiac function in these animals by echocardiography at 1 week post-TAC and found EF% significantly decreased in the TgGRK5 mice compared to control mice supporting early onset HF (Fig. 4B and Supplemental Fig. IV). However, importantly, this LV dysfunction that was present in TgGRK5 mice at one week was not present in TgGRK5 mice where NFATc3 is deleted, including LV dilatation (Fig. 4B and 4C). Thus, the loss of NFATc3 can prevent early progression to HF after TAC when GRK5 is overexpressed in myocytes. Chronically, at 4 weeks post-TAC, these TgGRK5/NFATc3 KO mice were dysfunctional consistent with GRK5 overexpression exerting HDAC kinase effects (data not shown). We also assessed cardiomyocyte cross-sectional area in these animals to confirm that hypertrophy is occurring at the cellular level using wheat germ agglutinin staining and indeed TgGRK5 mice display a significantly increased cell size after pressure-overload compared to wild-type littermates while NFATc3 deletion leads to an attenuation of cardiomyocyte hypertrophy as shown by a significant decrease in cross-sectional area following TAC in TgGRK5 mice (Fig. 4D).
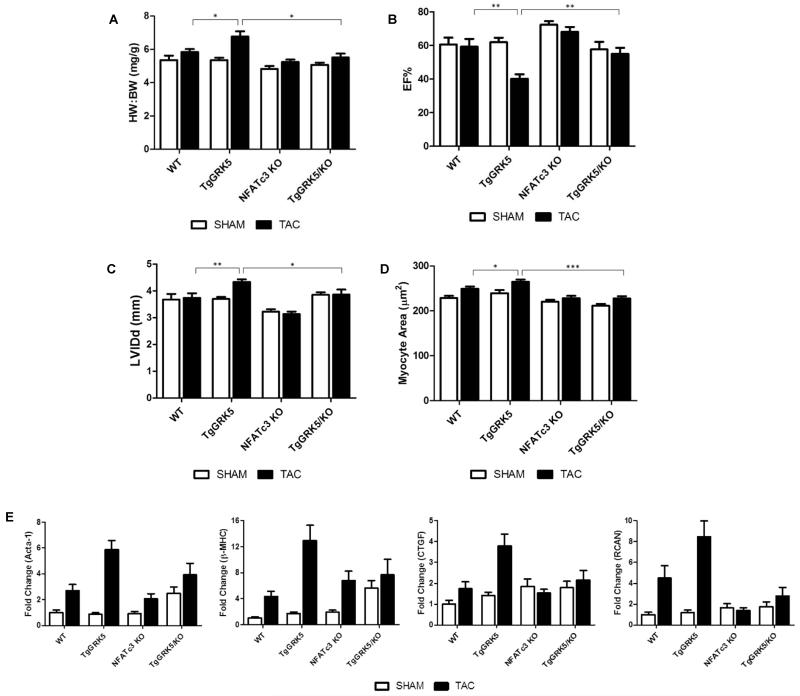
Figure 4. NFATc3 KO Attenuates GRK5-Mediated Cardiac Pathology After Pressure-Overload Stress.
(A) Heart weight to body weight ratio (B) ejection fraction as determine by echocardiography (C) left ventricular internal chamber dimension during diastole (LVIDd) as determined by echocardiography of WT, TgGRK5, NFATc3 KO, TgGRK5/NFATc3 KO mice 1 week after TAC or SHAM. (WT SHAM n=8, WT TAC n=9, TgGRK5 SHAM n=11, TgGRK5 TAC n=11, NFATc3 KO SHAM n=10, NFATc3 KO TAC n=9, TgGRK5xNFATc3 KO SHAM n=7, TgGRK5xNFATc3 KO TAC n=7. *, p<0.05; ** p<0.01; ***, p<0.001 by t test). (D) Cardiomyocyte cross-sectional area. (n=3 hearts per group with a minimum of 50 cells measured per heart. *, p<0.05; ***, p<0.001 by t test). (E) Quantitative RT-PCR for genes Acta-1, β-MHC, CTGF, RCAN from whole heart of the above double transgenic mice.
Finally, we assessed the transcription of select genes by quantitative RT-PCR to determine on a molecular level if NFATc3 deletion protects the TgGRK5 mice from transcriptional changes associated with the post-TAC GRK5-mediated HF phenotype (Fig. 4E). Induction of the fetal gene program was assessed by transcription of genes for the contractile proteins skeletal muscle actin (Acta-1) and β-myosin heavy chain (β-MHC). To assess remodeling and fibrosis of the heart transcription of the connective tissue growth factor (CTGF) gene was also determined. Finally, transcription of the NFAT target gene RCAN was analyzed to show specificity for NFAT. All of these transcripts were similarly up-regulated in TgGRK5 mice compared to wild-type mice following TAC, however expression of all these genes is attenuated when NFATc3 is deleted (Fig. 4E). Therefore, NFATc3 deletion in cardiac GRK5 overexpressing mice leads to a more favorable genetic profile following LV pressure-overload.
GRK5 interacts with NFAT in a kinase-independent manner through DNA binding
To assess the mechanism by which GRK5 activates the NFAT pathway, we used H9c2 myoblasts in order to introduce plasmids carrying wild-type (WT) GRK5 and mutants that render GRK5 kinase-dead (K215R) or incapable of being exported from the nucleus (NES) (Supplemental Fig. VII). All of these GRK5 proteins can accumulate in the nucleus after hypertrophic stimuli and we tested whether kinase activity and nuclear localization is required for NFAT activation via GRK5. These cells were also infected with the CAM-Gq mutant adenovirus to activate hypertrophic signaling and RT-PCR was performed for the NFAT target gene RCAN. We found that the NES mutant which becomes trapped in the nucleus caused the greatest activation of NFAT (Fig. 5A). This is not surprising as nuclear GRK5 is responsible for the exaggerated cardiac pathology seen in TgGRK5 mice after pressure-overload 14. Surprisingly, this experiment also demonstrates that the kinase dead K215R GRK5 was able to activate NFAT to a similar degree as WT GRK5 (Fig. 5A). This proves that the activation of NFAT by GRK5 does not involve a phosphorylation event and, therefore, segregates the activation of MEF2 by GRK5 via HDAC5 phosphorylation from the activation of the NFAT pathway.
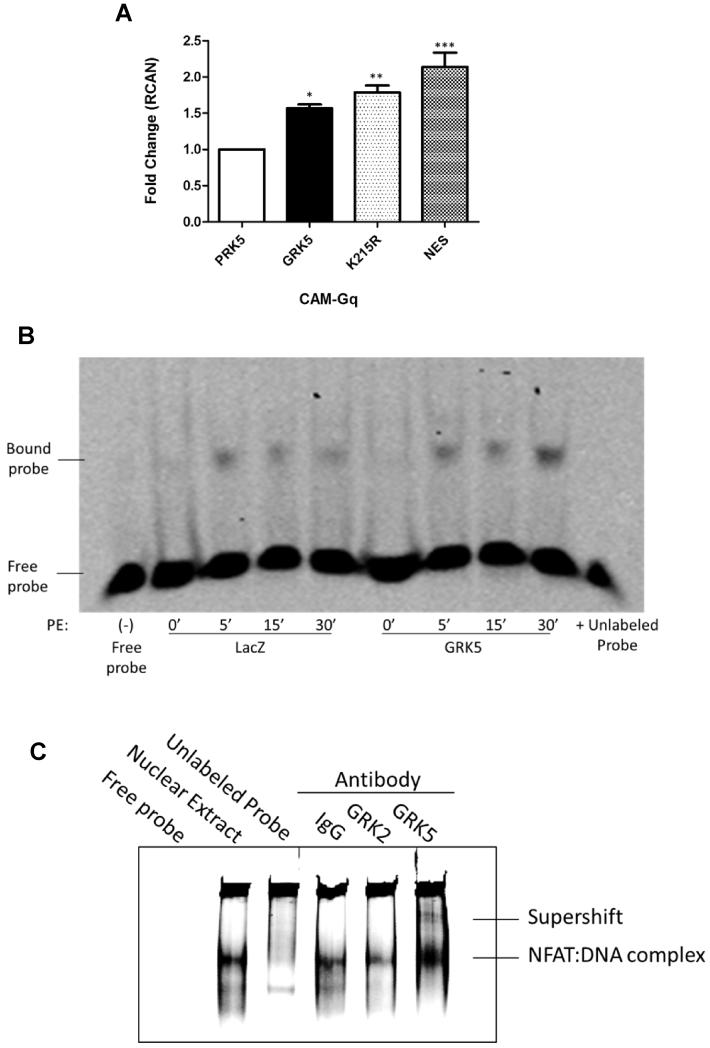
Figure 5. GRK5 Interacts with NFAT in a Kinase-independent Manner Through DNA Binding.
(A) Quantitative RT-PCR for NFAT target gene RCAN in H9c2 cells transfected with wild-type GRK5, kinase dead (K215R) GRK5 mutant, nuclear export sequence (NES) GRK5 mutant or vector (pRK5) control for 72 hours and infected with CAM-Gq virus for 24 hours. (n=3; *, p<0.05; ** p<0.01; ***, p<0.01 by ANOVA). (B) Electrophoretic mobility shift assay was carried out with 2 μg of nuclear extract (NE) from cultured NRVMs infected with GRK5 or LacZ control and stimulated with hypertrophic agonist phenylephrine (PE) for 0, 5, 15 or 30 minutes to analyze DNA binding activity of NFAT using IR dye-labeled oligonucleotides containing the consensus NFAT binding sequence. Lane 1 is free probe no NE; lanes 2 to 9 GRK5 overexpression and 50μmol/L PE treatment as indicated; lane 10 NE from GRK5 overexpressing myocytes stimulated with 50μmol/L PE for 30 minutes and 200-fold excess non-labeled consensus oligonucleotides. Shown is a representative image from 3 independent experiments. (C) Antibody-mediated super-shift EMSA using 5μg NE from AdGRK5 infected (NRVMs) stimulated with PE for 30 minutes. Samples were incubated with 1 μg specific antibodies to GRK2, GRK5 or rabbit IgG. Shown is a representative image from 3 independent experiments.
As the activation of NFAT by GRK5 appears to occur within the nucleus (Fig. 5A) we decided to perform an electrophoretic mobility shift assay (EMSA) to determine if GRK5 was able to alter DNA binding by NFAT. The assay was performed by incubating NFAT specific DNA probes with nuclear lysates from NRVMs overexpressing GRK5 or a LacZ control and stimulated with PE for 5, 15 or 30 min (Fig. 5B). We found that GRK5 was able to potentiate NFAT:DNA binding as GRK5 overexpression led to an increase in the amount of NFAT specific DNA probe that was bound following PE stimulation (Fig. 5B).
Next, we hypothesized that GRK5 may be interacting with NFAT at the level of DNA as GRK5 has previously been shown to bind DNA in a kinase-independent manner 10, 11. Accordingly, we performed an EMSA with antibody mediated supershift to determine if GRK5 was present in a complex with NFAT at the level of DNA (Fig. 5C). Nuclear lysates from NRVMs overexpressing GRK5 and stimulated with PE for 30 min were incubated with IgG, GRK2 or GRK5 antibodies. Importantly, a shift band was observed in the lysate incubated with GRK5 antibody, but not with IgG or GRK2 negative controls (Fig. 5C). This suggests that GRK5 is interacting with NFAT at the level of DNA in order to potentiate the binding of the NFAT:DNA complex and adds a new mechanism for GRK5 in the facilitation of hypertrophic gene transcription (Fig. 6).
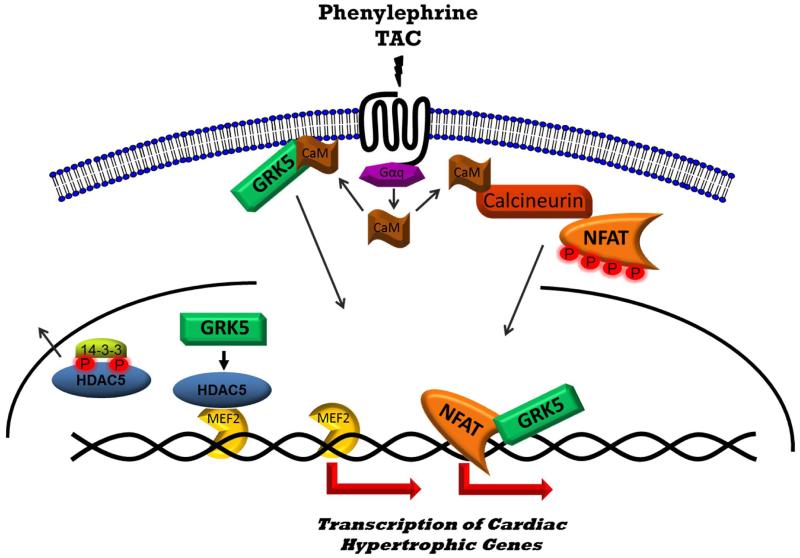
Figure 6. Schematic Depicting the Facilitation of Hypertrophic Transcription by GRK5.
Nuclear translocation of GRK5 occurs via stimulation of the Gq pathway following transaortic constriction (TAC) or phenylephrine stimulation via activated calmodulin (CaM) binding the N-terminus of GRK5. CaM binding causes GRK5 to dissociate from the plasma membrane and translocate to the nucleus 13. Once in the nucleus, GRK5 phosphorylates HDAC5 leading to its nuclear export and derepression of the transcription factor MEF2. In parallel, CaM also binds to and activates the phosphatase calcineurin which dephosphorylates NFAT leading to its nuclear translocation. At the level of the DNA, GRK5 potentiates NFAT:DNA binding and enhances the transcription of hypertrophic genes and subsequent maladaptive cardiac hypertrophy.
DISCUSSION
While the canonical role of GRKs is to phosphorylate activated seven transmembrane receptors leading to their desensitization and down-regulation, a growing non-GPCR ‘interactome’ is emerging 25. GRK5, as well as GRK6, have the unique properties among GRK family members to translocate and localize to the nucleus where in myocytes it has been shown GRK5 has the non-GPCR activity of acting as a Class II HDAC kinase facilitating maladaptive cardiac hypertrophy 12-14. This novel nuclear activity of GRK5 was confirmed as playing a role in the normal hypertrophic response of the heart as GRK5 KO mice (global as well as cardiomyocyte specific) have less growth after TAC as well delayed HF 12. Further, simply keeping overexpressed GRK5 out of the nucleus prevents the pathological growth of the heart after hypertrophic stimuli via lower HDAC kinase activity and less MEF2 activation 14. Since GRK5 has been shown to bind DNA in the nucleus of non-myocytes 10 and shown to interact with other nuclear proteins such as Iκ-Bα, p53 and nucleophosmin 15, 16, 28, we explored whether GRK5’s role in pathological cardiac hypertrophy, in addition to MEF2 regulation through phosphorylation of HDAC5, may involve other targets and mechanisms.
Here we identify the NFAT pathway as another target of GRK5 within the nucleus. While GRK5 is able to activate MEF2 in myocytes after hypertrophic stress, it appears that NFAT is a critical pathway through which GRK5 causes pathology after pressure-overload as NFATc3 deletion in GRK5 overexpressing mice is protective after TAC. The HDAC activity appears still in play after hypertrophic stress since chronic hypertrophy and HF still occurs in TgGRK5/NFATc3 KO hybrid mice following longer periods of TAC. This result could also be explained by an up-regulation in other NFAT isoforms which are able to partially compensate for the loss of the c3 isoform.
In this study we utilized mutant constructs of GRK5 to determine a possible mechanism for the regulation of the NFAT pathway by GRK5 and indeed confirmed a nuclear-dependent effect that happens to be kinase-independent. We found that the NES mutant of GRK5, which lacks a nuclear export sequence and is therefore trapped in the nucleus, leads to the greatest activation of NFAT activity. This is logical as in vivo experiments performed previously in our lab found nuclear GRK5 to be the cause of cardiac pathology after TAC 14. Surprisingly, the kinase-dead GRK5 K215R mutant was able to activate NFAT as well as WT GRK5 providing the first hint of kinase-independent regulation of this transcription factor by GRK5. Previously, GRKs have been known to exert kinase-independent effects including GRK5, which was shown recently to promote filamentous actin bundling through a kinase-independent manner by interacting with F-actin and PIP2 25, 29. This finding with GRK5-K215R limits the possibility of NFAT regulation through HDAC kinase activity or other phosphorylation events, which is different than how GRK5 can regulate MEF2 hypertrophic gene transcription. The kinase-independent activation of NFAT by GRK5 seems logical when one considers that NFAT is negatively regulated by phosphorylation yet GRK5, a kinase, is able to increase its activity. Other kinases such as ERK, casein kinase II, JNK, p38, PKA, and GSK3-β negatively regulate the NFAT pathway through phosphorylation of NFAT 26, 30-34. Additionally, CAMKII is able to oppose the NFAT pathway by direct phosphorylation of calcineurin 35.
Contrary to the above kinases which oppose NFAT activity, p90 ribosomal S6 kinase (RSK) has been shown to potentiate NFAT:DNA binding through interaction with the NFAT:DNA complex 36. This is of particular interest to this study as both RSK and GRK5 belong to the AGC protein kinase subfamily 37. Overall, our findings add significantly to the understanding of GRK5 in cardiac hypertrophic gene transcription through the two most important pathways (NFAT and MEF2). Importantly, these two transcriptional regulation pathways influenced by nuclear GRK5 occur due to either kinase-dependent effects (HDAC5 kinase) as well as kinase-independent actions (via NFAT:DNA binding and induction of NFAT transcriptional activity). These mechanisms are illustrated in Fig. 6.
While we are not the first to suggest that GRK5 can exert effects through its DNA binding property we are the first to show that this can lead to positive regulation of transcription 10, 11, 38. It has been demonstrated previously that GRK5 and GRK4 subfamily member GRK6 can bind directly to DNA in vitro 10, 11. In vivo Liu, et al. found that GRK5 binds directly to the Bcl-2 promoter and inhibits transcription of Bcl-2 38. As NFAT is known to have weak DNA binding ability and cooperates with other transcription factors in a complex 39, we believe our data are consistent with GRK5 being present in this transcriptional complex and is, therefore, able to enhance the transcription of NFAT target genes (Fig. 6). This is interesting because NF-κB, which shares the DNA binding Rel homology domain with NFAT, is also regulated by GRK5 although controversy surrounds the mechanism by which this occurs 40, 41. Sorriento, et al. believe that GRK5 is a negative regulator of NF-κB through forced nuclear accumulation of NF-κB inhibitor Iκ-Bα 42, 43. Islam, et al. and Patial, et al. find that GRK5 is a positive regulator of NF-κB signaling through either up-regulation of protein levels of NF-κB subunits p50 and p65 or phosphorylation of Iκ-Bα 17, 44. It is possible that GRK5 is able to further regulate the NF-κB pathway through interaction with p50 and p65 at the level of chromatin.
It remains to be seen if GRK5 is binding directly to DNA in order to potentiate NFAT:DNA binding and transcription or if GRK5 is simply present in a transcriptional complex with NFAT. While we cannot rule out a role for HDACs in the regulation of NFAT activity by GRK5 we find that GRK5 is acting in a kinase-independent fashion and therefore the phosphorylation of HDAC5 by GRK5 is not the mechanism at work (Fig. 6). Future experiments involving chromatin immunoprecipitation (ChIP) for GRK5 would allow for the identification of novel GRK5 targets and interactions. This data could then be used along with gene expression to determine which promoters are positively or negatively regulated by GRK5.
In summary, we provide evidence that GRK5 is able to activate the NFAT transcriptional pathway leading to the up-regulation of hypertrophic genes. We find that cardiac-specific NFAT luciferase reporter mice crossed with mice that overexpress wild-type GRK5 in a cardiomyocyte specific manner exhibit an increase in NFAT activity both in the basal state as well as after the hypertrophic stressors TAC and phenylephrine administration. Complimentary to this, GRK5 null mice exhibit less NFAT transcriptional activity after left ventricular pressure-overload as shown by the expression of the NFAT target gene RCAN. Importantly, loss of NFATc3 expression protected GRK5 over-expressing mice from the exaggerated hypertrophy and early progression to HF seen acutely after TAC. Molecular studies suggest that GRK5 acts in concert with NFAT to increase hypertrophic gene transcription in the nucleus and this is a kinase-independent action of GRK5 that involves its known property of DNA binding. The overall translational significance of these finding are substantial as simply finding a kinase inhibitor of GRK5 as a potential therapeutic for maladaptive cardiac hypertrophy would block its HDAC kinase activity in the nucleus, but it would not prevent the activation of the NFAT pathway as our data shows that GRK5 acts in a kinase-independent manner. We suggest that a more effective strategy would be to develop a therapy that is capable of inhibiting the nuclear accumulation of GRK5 allowing this enzyme to exert protective effects at the sarcolemma through transactivation of the β-AR and EGF receptor while preventing its activation of MEF2 and NFAT in the nucleus 45. By developing novel therapeutics to inhibit GRK5 nuclear accumulation it may be possible to prevent and reverse the progression of HF.
Supplementary Material
Novelty and Significance.
What Is Known?
G protein-coupled receptor kinase 5 (GRK5) is up-regulated in models of heart failure (HF), as well as in the failing human heart.
GRK5 enters the nucleus of cardiomyocytes via a functional nuclear localization sequence in response to hypertrophic stimuli where it can act in a non-GPCR manner as a histone deacetylase 5 (HDAC5) kinase enhancing MEF2-dependent hypertrophic gene transcription.
Increased nuclear GRK5 is pathological in the setting of chronic pressure-overload while GRK5 ablation significantly delays the onset of HF after pressure-overload.
What New Information Does This Article Contribute?
GRK5-mediated pathological cardiac hypertrophy involves the activation of nuclear factor of activated T-cells (NFAT) within the nucleus in a kinase-independent manner.
Molecular studies suggest that GRK5 acts in concert with NFAT to facilitate and increase hypertrophic gene transcription in the nucleus via GRK5’s ability to directly bind DNA.
GRK5-dependent cardiac maladaptive hypertrophy and early onset HF after pressure overload are dependent on NFAT activity as loss of NFATc3 expression in the heart protected GRK5 overexpressing transgenic mice from early progression to HF.
GRK5 can localize to the nucleus of cardiomyocytes and this localization increases under conditions of hypertrophic stress. Nuclear accumulation of GRK5 promotes maladaptive cardiac hypertrophy and HF at least in part due to novel HDAC kinase activity. However, in this study we sought additional mechanisms involved in GRK5-mediated cardiac pathology. We found that GRK5 can also facilitate activation of the hypertrophic transcription factor, NFAT. Studies in NFATc3 knockout mice with cardiac GRK5 overexpression demonstrate that GRK5-mediated cardiac pathology after pressure-overload requires NFAT expression. Molecular studies demonstrate that GRK5 acts in concert with NFAT to increase hypertrophic gene transcription in the nucleus in a non-canonical, kinase-independent manner via GRK5’s ability to directly bind DNA. The overall translational significance of these findings are substantial since GRK5 is elevated in the failing human heart, which would also lead to nuclear accumulation (has this been shown, or is it assumed? Clarify). Moreover, our results suggest that a kinase inhibitor of GRK5 would not prevent the activation of NFAT and not be effective in HF. We suggest that a more effective strategy would be to develop a therapy that is capable of inhibiting the nuclear accumulation of GRK5.
ACKNOWLEDGEMENTS
We thank Dr. Jeff Molkentin (Cincinnati) for the NFAT-LUC reporter mice and Dr. Julie Pitcher (London) for the GRK5 NES mutant construct. We also thank Zuping Qu for expert maintenance of our transgenic and KO mouse colony.
SOURCES OF FUNDING.
W.J.K is the W.W. Smith Chair in Cardiovascular Medicine at Temple University School of Medicine. This project was supported by National Institute of Health (NIH) grant P01 HL091799. W.J.K. is also supported by NIH grants R37 HL061690, R01 HL085503, P01 HL075443 (Project 2) and P01 HL108806 (Project 3). J.H. was supported by a Pre-Doctoral Fellowship from the Great Rivers Affiliate of the American Heart Association.
Nonstandard Abbreviations and Acronyms
- βAR
β-adrenergic receptor
- CaM
Calmodulin
- CAM-Gq
Constitutively active Gαq
- CaMK
Calmodulin-dependent kinase
- Cn
Calcineurin
- CTGF
Connective tissue growth factor
- EF
Ejection fraction
- EGFR
Epidermal growth factor receptor
- ERK
Extracellular signal-regulated kinase
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- GSK-3β
Glycogen synthase kinase 3β
- HDAC
Histone deacetylase
- HF
Heart failure
- HW/BW
Heart weight / Body weight
- JNK
c-Jun N-terminal kinase
- KO
Knockout
- LVID
Left ventricular internal diameter
- LVPWT
Left ventricular wall thickness
- PBS
Phosphate buffered saline
- PE
Phenylephrine
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PKA
Protein Kinase A
- MEF2
Myocyte enhancer factor 2
- MHC
Myosin heavy chain
- NE
Nuclear extract
- NES
Nuclear export signal
- NFAT
Nuclear factor of activated T cells
- NLC
Non-transgenic littermate control
- NLS
Nuclear localization signal
- NRVM
Neonatal rat ventricular myocyte
- RCAN
Regulator of calcineurin
- RSK
p90 ribosomal S6 kinase
- TAC
Transverse aortic constriction
- Tg
Transgenic
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Go ASMD, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunwald E, Bristow MR. Congestive heart failure: Fifty years of progress. Circulation. 2000;102:IV14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 3.Premont RT, Gainetdinov RR. Physiological roles of g protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 4.Dzimiri N, Basco C, Moorji A, Afrane B, Al-Halees Z. Characterization of lymphocyte beta 2-adrenoceptor signalling in patients with left ventricular volume overload disease. Clin Exp Pharmacol Physiol. 2002;29:181–188. doi: 10.1046/j.1440-1681.2002.03625.x. [DOI] [PubMed] [Google Scholar]
- 5.Dzimiri N, Muiya P, Andres E, Al-Halees Z. Differential functional expression of human myocardial g protein receptor kinases in left ventricular cardiac diseases. Eur J Pharmacol. 2004;489:167–177. doi: 10.1016/j.ejphar.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte grk2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 7.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the g protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu SS, Gao E, Koch WJ. Prodeath signaling of g protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ Res. 2013;112:1121–1134. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson LR, Scott MG, Pitcher JA. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol Cell Biol. 2004;24:10169–10179. doi: 10.1128/MCB.24.23.10169-10179.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LR, Robinson JD, Lester KN, Pitcher JA. Distinct structural features of g protein-coupled receptor kinase 5 (grk5) regulate its nuclear localization and DNA-binding ability. PLoS One. 2013;8:e62508. doi: 10.1371/journal.pone.0062508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold JI, Gao E, Shang X, Premont RT, Koch WJ. Determining the absolute requirement of g protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: Short communication. Circ Res. 2013;111:1048–1053. doi: 10.1161/CIRCRESAHA.112.273367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold JI, Martini JS, Hullmann J, Gao E, Chuprun JK, Lee L, Tilley DG, Rabinowitz JE, Bossuyt J, Bers DM, Koch WJ. Nuclear translocation of cardiac g protein-coupled receptor kinase 5 downstream of select gq-activating hypertrophic ligands is a calmodulin-dependent process. PLoS One. 2013;8:e57324. doi: 10.1371/journal.pone.0057324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martini JS, Raake P, Vinge LE, DeGeorge BR, Jr., Chuprun JK, Harris DM, Gao E, Eckhart AD, Pitcher JA, Koch WJ. Uncovering g protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc Natl Acad Sci U S A. 2008;105:12457–12462. doi: 10.1073/pnas.0803153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. G-protein-coupled-receptor kinases mediate tnfalpha-induced nfkappab signalling via direct interaction with and phosphorylation of ikappabalpha. Biochem J. 2009;425:169–178. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.So CH, Michal AM, Mashayekhi R, Benovic JL. G protein-coupled receptor kinase 5 phosphorylates nucleophosmin and regulates cell sensitivity to polo-like kinase 1 inhibition. J Biol Chem. 2012;287:17088–17099. doi: 10.1074/jbc.M112.353854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam KN, Bae JW, Gao E, Koch WJ. Regulation of nuclear factor kappab (nf-kappab) in the nucleus of cardiomyocytes by g protein-coupled receptor kinase 5 (grk5) J Biol Chem. 2013;288:35683–35689. doi: 10.1074/jbc.M113.529347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim HW, Molkentin JD. Calcineurin and human heart failure. Nat Med. 1999;5:246–247. doi: 10.1038/6430. [DOI] [PubMed] [Google Scholar]
- 19.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/nfat coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 21.Piper HM, Pinson A. Cell culture techniques in heart and vessel research. Springer; Berlin Heidelberg: 1990. Neonatal rat heart muscle cells; pp. 20–35. [Google Scholar]
- 22.Pitcher JA, Fredericks ZL, Stone WC, Premont RT, Stoffel RH, Koch WJ, Lefkowitz RJ. Phosphatidylinositol 4,5-bisphosphate (pip2)-enhanced g protein-coupled receptor kinase (grk) activity. Location, structure, and regulation of the pip2 binding site distinguishes the grk subfamilies. J Biol Chem. 1996;271:24907–24913. doi: 10.1074/jbc.271.40.24907. [DOI] [PubMed] [Google Scholar]
- 23.Premont RT, Koch WJ, Inglese J, Lefkowitz RJ. Identification, purification, and characterization of grk5, a member of the family of g protein-coupled receptor kinases. J Biol Chem. 1994;269:6832–6841. [PubMed] [Google Scholar]
- 24.Pronin AN, Benovic JL. Regulation of the g protein-coupled receptor kinase grk5 by protein kinase c. J Biol Chem. 1997;272:3806–3812. doi: 10.1074/jbc.272.6.3806. [DOI] [PubMed] [Google Scholar]
- 25.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: More than just kinases and not only for gpcrs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molkentin JD. Calcineurin-nfat signaling regulates the cardiac hypertrophic response in coordination with the mapks. Cardiovasc Res. 2004;63:467–475. doi: 10.1016/j.cardiores.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Zhu H, Yuan M, Fu J, Zhou Y, Ma L. G-protein-coupled receptor kinase 5 phosphorylates p53 and inhibits DNA damage-induced apoptosis. J Biol Chem. 2010;285:12823–12830. doi: 10.1074/jbc.M109.094243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Wang F, Long H, Chen Y, Wu Z, Ma L. Grk5 promotes f-actin bundling and targets bundles to membrane structures to control neuronal morphogenesis. J Cell Biol. 2011;194:905–920. doi: 10.1083/jcb.201104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow CW, Davis RJ. Integration of calcium and cyclic amp signaling pathways by 14-3-3. Mol Cell Biol. 2000;20:702–712. doi: 10.1128/mcb.20.2.702-712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow CW, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of nfat4 opposed by the jnk signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- 33.Porter CM, Havens MA, Clipstone NA. Identification of amino acid residues and protein kinases involved in the regulation of nfatc subcellular localization. J Biol Chem. 2000;275:3543–3551. doi: 10.1074/jbc.275.5.3543. [DOI] [PubMed] [Google Scholar]
- 34.Yang TT, Yu RY, Agadir A, Gao GJ, Campos-Gonzalez R, Tournier C, Chow CW. Integration of protein kinases mtor and extracellular signal-regulated kinase 5 in regulating nucleocytoplasmic localization of nfatc4. Mol Cell Biol. 2008;28:3489–3501. doi: 10.1128/MCB.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. Camkii negatively regulates calcineurin-nfat signaling in cardiac myocytes. Circ Res. 2009;105:316–325. doi: 10.1161/CIRCRESAHA.109.194035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang TT, Xiong Q, Graef IA, Crabtree GR, Chow CW. Recruitment of the extracellular signal-regulated kinase/ribosomal s6 kinase signaling pathway to the nfatc4 transcription activation complex. Mol Cell Biol. 2005;25:907–920. doi: 10.1128/MCB.25.3.907-920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce LR, Komander D, Alessi DR. The nuts and bolts of agc protein kinases. Nat Rev Mol Cell Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- 38.Liu P, Wang X, Gao N, Zhu H, Dai X, Xu Y, Ma C, Huang L, Liu Y, Qin C. G protein-coupled receptor kinase 5, overexpressed in the alpha-synuclein up-regulation model of parkinson’s disease, regulates bcl-2 expression. Brain Res. 2010;1307:134–141. doi: 10.1016/j.brainres.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Rao A, Luo C, Hogan PG. Transcription factors of the nfat family: Regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Glover JN, Hogan PG, Rao A, Harrison SC. Structure of the DNA-binding domains from nfat, fos and jun bound specifically to DNA. Nature. 1998;392:42–48. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- 41.Macian F. Nfat proteins: Key regulators of t-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 42.Sorriento D, Ciccarelli M, Santulli G, Campanile A, Altobelli GG, Cimini V, Galasso G, Astone D, Piscione F, Pastore L, Trimarco B, Iaccarino G. The g-protein-coupled receptor kinase 5 inhibits nfkappab transcriptional activity by inducing nuclear accumulation of ikappab alpha. Proc Natl Acad Sci U S A. 2008;105:17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorriento D, Santulli G, Fusco A, Anastasio A, Trimarco B, Iaccarino G. Intracardiac injection of adgrk5-nt reduces left ventricular hypertrophy by inhibiting nf-kappab-dependent hypertrophic gene expression. Hypertension. 2010;56:696–704. doi: 10.1161/HYPERTENSIONAHA.110.155960. [DOI] [PubMed] [Google Scholar]
- 44.Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. G-protein-coupled-receptor kinases mediate tnfalpha-induced nfkappab signalling via direct interaction with and phosphorylation of ikappabalpha. Biochem J. 2010;425:169–178. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the egfr confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.