Abstract
Rationale
Cardiac progenitor cells (CPCs) are believed to differentiate into the major cell types of the heart; cardiomyocytes, smooth muscle cells, and endothelial cells. We have recently identified Abi3bp as a protein important for mesenchymal stem cell (MSC) biology. Since CPCs share several characteristics with MSCs we hypothesized that Abi3bp would similarly affect CPC differentiation and proliferation.
Objective
To determine whether Abi3bp regulates CPC proliferation and differentiation.
Methods and Results
In vivo, genetic ablation of the Abi3bp gene inhibited CPC differentiation whereas CPC number and proliferative capacity was increased. This correlated with adverse recovery following myocardial infarction. In vitro, CPCs, either isolated from Abi3bp knockout mice or expressing an Abi3bp shRNA construct, displayed a higher proliferative capacity and, under differentiating conditions, reduced expression of both early and late cardiomyocyte markers. Abi3bp controlled CPC differentiation via integrin-β1, PKCζ, and Akt.
Conclusion
We have identified Abi3bp as a protein important for CPC differentiation and proliferation.
Keywords: Cardiac progenitor cells, extracellular matrix, integrin, cell signaling
INTRODUCTION
Cardiac progenitor cells (CPCs) are believed to give rise to the major cell types of the heart; these being cardiomyocytes, smooth muscle cells, and endothelial cells 1-3. c-Kit+/Sca-1+ CPCs have been the most heavily studied, however other proteins such as Nkx2.5, Wt1, Isl1, Tbx5 and eHand have also been used to define these cells 1, 4, 5. Endogenous CPCs are present in too low a number to completely regenerate a damaged heart following injury. However, recent reports have highlighted the potential therapeutic benefits of injected CPCs in the human heart following myocardial infarction 6, 7. Enhancing these beneficial effects requires characterization of the mechanisms by which CPCs differentiate and proliferate 8-10.
We have recently discovered that Abi3bp promotes mesenchymal stem cell (MSC) differentiation whilst simultaneously inhibiting proliferation 11. MSCs prepared from Abi3bp knockout mice were unable to differentiate into osteocytes and adipocytes. Significant impairment of chondrogenic and smooth muscle differentiation was also observed11. Conversely, knockout of Abi3bp increased MSC proliferation, with integrin-β1 and ERK being found to be necessary for the effect 11. Abi3bp is a protein of relatively unknown function; with roles in the olfactory system and tumorigenesis being ascribed to the protein 12, 13. Abi3bp was shown in vitro to reduce mitral cell dendritic complexity. This process is important in the developing brain as functional circuits are established by pruning immature connections12. Reduced Abi3bp expression has been observed in thyroid tumors. Re-expression of Abi3bp in thyroid cancer cells prevented tumor formation when the cells were injected into nude mice13.
C-Kit+ CPCs have been shown to possess mesenchymal markers 14 suggesting the possibility that Abi3bp may also similarly affect CPC differentiation and proliferation. Indeed, in this study we demonstrate both in vivo and in vitro that Abi3bp is important for the control of CPC proliferation and differentiation.
METHODS
Abi3bp knockout mice
Abi3bp−/+ mice, harboring a neoR replacement of the first exon, were originally purchased from Taconic. All experiments were performed with wild-type (Abi3bp+/+) and Abi3bp knockout (Abi3bp−/−) littermates in accordance with institutional guidelines (DLAR and IACUC).
Cardiac progenitor cell isolation
Enzymatic digestion
c-Kit+ CPCs were isolated from 8 week old male wild-type and Abi3bp knockout litter-mates. Minced ventricular tissue was digested in 100U of collagenase in Hank's Buffered Saline solution at 37° C for 15 minutes. Single cells were passed through a 100 μm sieve and low density cells were separated on a discontinuous Percoll gradient. Primary cells were cultured for 3 days in CPC-maintenance media (DMEM/F12-K 1:1, 20% ES cell qualified FBS, 10 ng/mL bFGF, 20 ng/mL EGF, 100U LIF, and 1x ITS (insulin-transferrin-selenium)). c-Kit+ cells were then selected by magnetic bead isolation (Miltenyi Biotech, Boston MA) and further cultured in CPC-maintenance media. Cells were differentiated at passage 3. At this passage the cells were positive for c-Kit and CD29 (Online Figure IA). Apoptosis and necrosis was not significantly different between c-Kit+ CPCs derived from wild-type and Abi3bp knockout mice (Online Figure IB). In CPC-maintenance media, Abi3bp knockout c-Kit+ CPCs expressed significantly lower levels of Abi3bp, Mef2C, and cardiac troponin-I (cTroponin-I) when compared to wild-type c-Kit+ CPCs, however, expression of Gata4 and Gata6 was not significantly different between wild-type and Abi3p knockout c-Kit+ CPCs (Online Figure IC). CPCs were not observed to beat during the experiments.
Explant from cardiac biopsies
c-Kit+ CPCs were isolated from the cardiac biopsies of 4 week old male wild-type and Abi3bp knockout litter-mates according to the method of Hatzistergos et al15 with minor modifications due to differences in organisms used in the two studies. A full method is provided in the Supplementary Methods. c-Kit+ CPCs isolated by this method were found to be weakly adherent15. Following expansion the c-Kit+ CPCs were used at passage 1. At this passage the cells were positive for c-Kit and CD29 (Online Figure IIA). Necrosis was not significantly different between c-Kit+ CPCs derived from wild-type and Abi3bp knockout mice (Online Figure IIB), though a slight elevation in apoptosis was noted in the Abi3bp knockout cells (Online Figure IIB). In CPC-maintenance media, Abi3bp knockout c-Kit+ CPCs expressed significantly lower levels of Abi3bp, Mef2C, and cardiac troponin-I (cTroponin-I) when compared to wild-type c-Kit+ CPCs, however, expression of Gata4 and Gata6 was not significantly different between wild-type and Abi3p knockout c-Kit+ CPCs (Online Figure IIC). CPCs were not observed to beat during the experiments.
CPC differentiation
CPCs were seeded at 25000 cells/cm2 in CPC-maintenance media. Twenty-four hours later the media was replaced with CPC-differentiation media (Advanced DMEM /F12, 0.2% w/v BSA, 2 mM L-glutamine, 1x ITS, 250 μmol/L ascorbic acid). Media was changed every two days.
Stable Abi3bp knockdown and re-expression
Full method described in Online Supplement. Significant knockdown of Abi3bp was observed (Online Figure IIIA). Scrambled shRNA had no effect on the basal expression of Abi3bp, Mef2C and Gata4 (Online Figure IIIB). Abi3bp expression levels in wild-type, Abi3bp knockout expressing cmyc, and Abi3bp knockout CPCs expressing cmyc-Abi3bp are shown in Online Figure IIIC.
Myocardial infarction (acute left anterior descending (LAD) coronary artery ligation)
Anesthetized (ketamine (100 mg/kg) and xylazine (5 mg/kg) by i.p. injection) 10-12 week old wild-type or Abi3bp knockout mice were intubated prior to left thoracotomy. Mice were ventilated at a tidal volume 0.7-1ml, respiratory rate 120 breaths per minute. Chest cavity was opened and a 7-0 nylon suture placed through the myocardium into the anterolateral LV wall, corresponding to the course of the left anterior descending artery. The suture was tied off (myocardial infarction), and apex of the LV observed for evidence of myocardial blanching indicating interruption in coronary flow. The wound was closed and following the resumption of spontaneous respiration the animal was allowed to recover. Post-operative analgesia was used for 5 days. Echocardiographic analysis was performed under minimal isofluorane anesthesia. The full method and procedure for staining is described in the Online Supplement.
Cardiac fibroblast and cardiomyocyte isolation
Cardiac fibroblasts and cardiomyocytes were isolated as previously described 16.
Images
Figures were prepared using CorelDraw. Microscopy images were exported from Axiovision Rel4.8 software.
Statistics
Statistical analysis was performed with GraphPad or R. Experiments containing two conditions a t-test was performed. ANOVA was used for experiments with three or more conditions followed by Bonferroni post-hoc tests for comparisons between individual groups. Mann-Whitney U tests, a non-parametric test which does not rely on assumptions of normality, was used for n=3 data.
RESULTS
To test the hypothesis that Abi3bp is important for CPC biology we isolated wild-type and Abi3bp knockout c-Kit+ CPCs from the non-cardiomyocyte fraction of the adult mouse heart. The characteristics of these isolated c-Kit+ CPCs, as described in the Methods Section, were consistent with resident CPCs reported in the literature5, 17. These cells also expressed integrin-β1/CD29 (Online Figure IA).
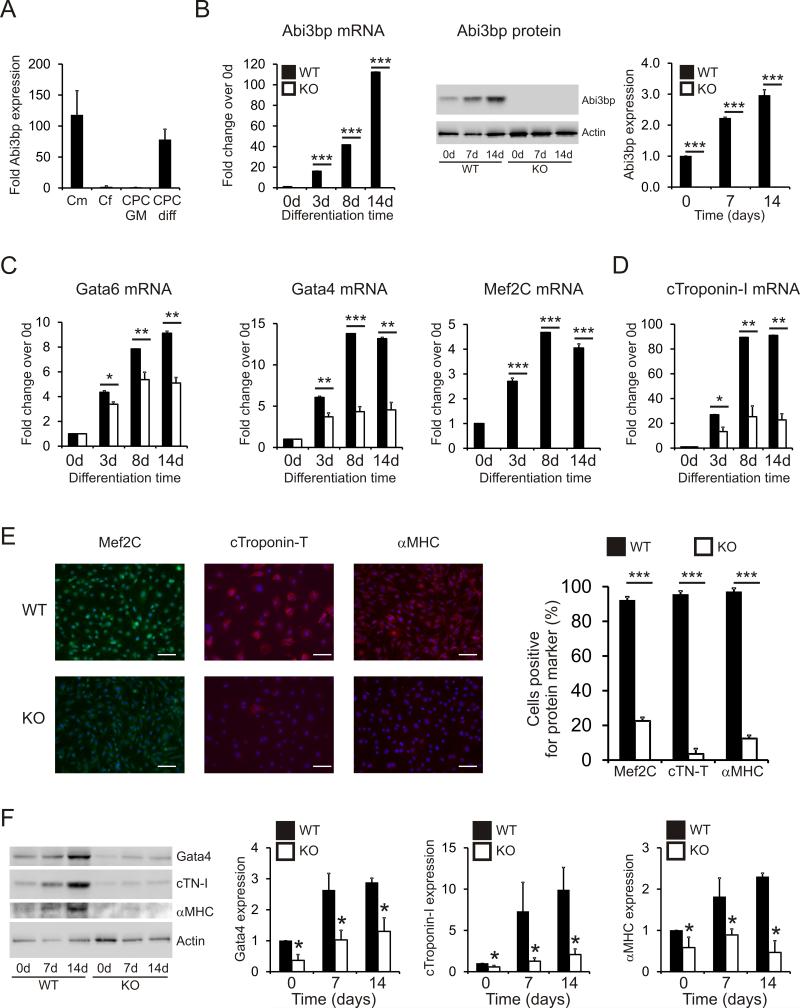
The heart contains many cell types, the majority being cardiomyocytes and cardiac fibroblasts. Abi3bp expression was easily detected in cardiomyocytes but was ~100-fold lower in cardiac fibroblasts and un-differentiated CPCs (Figure 1A). However, following 14 days culture in CPC-differentiation media, expression levels of Abi3bp in differentiated CPCs were comparable to cardiomyocytes (Figure 1A). This data suggested that Abi3bp may be important for the differentiation of CPCs and the maintenance of the differentiated phenotype in cardiomyocytes. To test this hypothesis we performed differentiation time-course experiments with wild-type and Abi3bp knockout CPCs. Expression of cardiac genes was assessed at 0, 3, 8 and 14 days following the addition of CPC-differentiation medium. In wild-type CPCs, Abi3bp mRNA increased dramatically during differentiation, reaching ~120-fold by day 14 (Figure 1B). As expected, no Abi3bp expression was observed in c-Kit+ CPCs prepared from Abi3bp knockout mice (Figure 1B). At the protein level, Abi3bp expression increased ~3-fold following 14 days of culture in differentiation media (Figure 1B).
Figure 1. Abi3bp knockout inhibits CPC differentiation.
(A) Abi3bp expression in cardiomyocytes [Cm], cardiac fibroblasts [Cf], and CPCs grown in either growth media [CPC GM] or differentiation media [CPC diff] was determined by qPCR. N=3. Data is shown as a fold expression with c-Kit+ CPCs grown in CPC-maintenance media taken to be 1. (B) Wild-type and Abi3bp knockout CPCs were cultured in CPC-differentiation media for up to 14 days. Expression of Abi3bp was determined by qPCR and immunoblotting. Expression in day 0 wild-type CPCs was taken to be 1. N=3. ***P≤0.001. (C-D) Wild-type and Abi3bp knockout CPCs were cultured in CPC-differentiation media for up to 14 days. Expression of Gata4, Gata6, Mef2C (C), and cardiac troponin-I (D) was determined by qPCR at the indicated time-points. Expression in day 0 wild-type CPCs was taken to be 1. N=3. ***P≤0.001. (E) Wild-type and Abi3bp knockout CPCs were cultured for 14 days in CPC-differentiation media. The cells were subsequently stained with Mef2C, cardiac troponin-T, or αMHC antibodies. DAPI was used to stain nuclei. Scale bar 100 microns. N=4. (F) Protein extracts (7.5μg) from wild-type and Abi3bp knockout CPCs cultured in CPC-differentiation media for 0, 7 and 14 days were probed for the indicated proteins. Actin was used as a loading control. Intensities were normalized to the loading control; normalized intensity of wild-type cells at day 0 was taken to be 1. N=3. *P≤0.05.
Markers of lineage commitment were then assessed in differentiating wild-type and Abi3bp knockout c-Kit+ CPCs. mRNA encoding Gata4, Mef2C, Nkx2-5, Gata6, and Ets1 are present in the early stages of CPC differentiation whilst cardiac troponins and α-myosin heavy chain mRNA is observed in the later stages of CPC differentiation towards a cardiomyocyte fate. In wild-type c-Kit+ CPCs, mRNA encoding the early markers Gata4, Mef2C, and Gata6 increased during differentiation (Figure 1C). However, Abi3bp knockout markedly inhibited the expression of these early markers at all the time-points tested (Figure 1C). Identical results were obtained with the late marker cardiac troponin-I. Wild-type c-Kit+ CPCs expressed cardiac troponin-I mRNA at increasing amounts when cultured in CPC-differentiation media. However, Abi3bp knockout c-Kit+ CPCs expressed cardiac troponin-I at a markedly lower level throughout the time-course of the experiment (Figure 1D).
To validate the qPCR experiments, c-Kit+ CPCs were cultured for 14 days with CPC-differentiation media and then stained for various cardiac markers. Differentiated wild-type CPCs showed robust staining for Mef2C, cardiac troponin-T and α-myosin heavy chain (αMHC) (Figure 1E). However, the number of Mef2C, cardiac troponin-T, and αMHC positive cells were severely reduced in differentiated Abi3bp knockout CPCs (Figure 1E). Immunoblotting for several cardiac markers was used to verify the immunostaining results. Wild-type c-Kit+ CPCs were found to express Gata4, cardiac troponin-I, and αMHC protein (Figure 1F). Expression increased in CPC differentiation media (Figure 1F). In contrast, Abi3bp knockout c-Kit+ CPCs expressed significantly lower amounts of Gata4, cardiac troponin-I, and αMHC both at baseline and during differentiation (Figure 1F).
To insure that the characteristics of the c-kit+ CPCs isolated from wild-type and Abi3bp knockout mice were not an artifact of the isolation procedure, we tested explanted c-Kit+ CPCs isolated from cardiac biopsies (see Methods). These cells yielded results identical to those from enzymatically dispersed cells. Compared to wild-type c-Kit+ CPCs, Abi3bp knockout c-Kit+ CPCs isolated by this method expressed lower basal levels of the Mef2C, Gata4, cardiac troponin-I, cardiac troponin-T, and αMHC protein (Online Figure IVA-C) and mRNA (Online Figure IVD) at baseline and following differentiation.
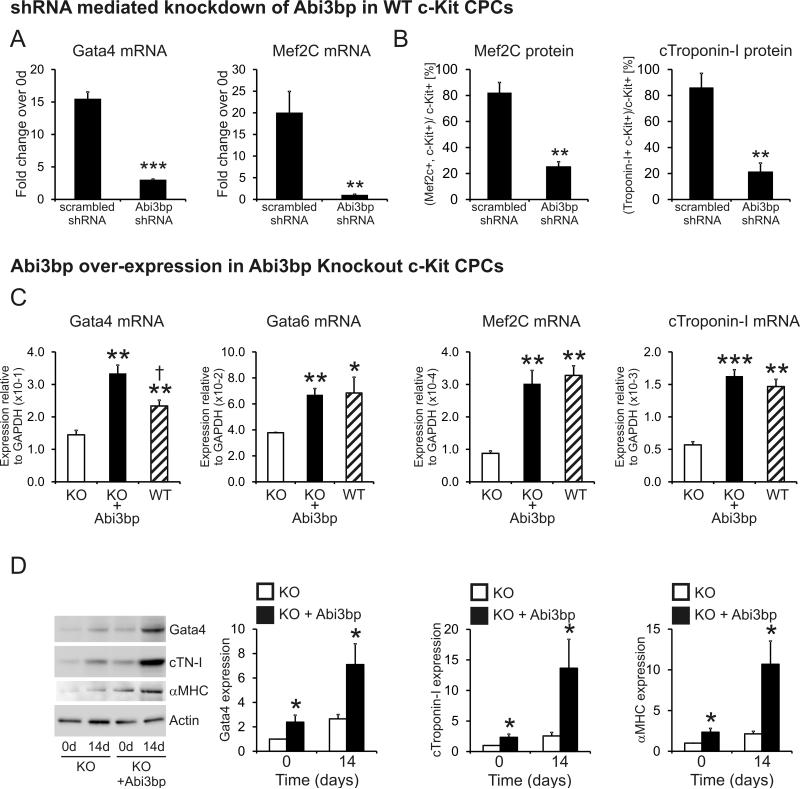
To verify that these findings were not due to a clonal effect, Abi3bp was stably knocked down in wild-type c-Kit+ CPCs by shRNA. Wild-type c-kit+ CPCs, expressing either a scrambled control or Abi3bp shRNA, were cultured for 14 days in CPC-differentiation media. Abi3bp expression increased ~170-fold in wild-type c-Kit+ CPCs expressing the scrambled control shRNA (data not shown). In contrast, as described in the Methods Section, no Abi3bp expression was observed in wild-type CPCs expressing the Abi3bp targeting shRNA. In c-Kit+ CPCs expressing the scrambled control shRNA, exposure to CPC differentiation media for 14 days increased expression of both Gata4 and Mef2C (Figure 2A). However, knockdown of Abi3bp had a significant inhibitory effect on the expression of both genes (Figure 2A). Following 14 days of culture in CPC-differentiation media the number c-Kit+ CPCs positive for either Mef2C or cardiac troponin-I was assessed by flow cytometry. Approximately 80% of wild-type c-Kit+ CPCs expressing the scrambled control shRNA were positive for either Mef2C or cardiac troponin-I following differentiation (Figure 2B). In contrast, Mef2C or cardiac troponin-I positive c-Kit+ CPCs were non-existent in Abi3bp-knockdown wild-type c-Kit+ CPCs (Figure 2B).
Figure 2. Re-expression of the Abi3bp in Abi3bp knockout CPCs recapitulates the wild-type phenotype.
(A) Wild-type CPCs, expressing either a scrambled control or an Abi3bp targeting shRNA, were cultured for 14 days in CPC-differentiation media. Gata4 and Mef2C expression was determined by qPCR. Expression of cells at day 0 was taken to be 1. N=3. Comparisons made between scrambled and Abi3bp shRNA expressing cells **P≤0.01, ***P≤0.001. (B) Following culturing in CPC-differentiation media for 14 days the percentage of CPCs positive for Mef2C or cardiac troponin-I was determined by flow cytometry. N=3. Comparisons made between scrambled and Abi3bp shRNA expressing cells, **P≤0.01, ***P≤0.001. (C) Wild-type and Abi3bp knockout CPCs, transiently transfected with either a myc or mycAbi3bp plasmid, were cultured for 7 days in CPC-differentiation media. Expression of Gata4, Gata6, Mef2C and cardiac troponin-T was determined by qPCR. Gene expression data is shown relative to GAPDH. N=3. ***P≤0.001, **P≤0.01, *P≤0.05. (D) Protein extracts (7.5μg) from Abi3bp knockout c-Kit+ CPC, expressing either the myc or mycAbi3bp plasmid, and cultured in CPC-differentiation media for either 0 or 14 days, were probed for the indicated proteins. Actin was used as a loading control. Intensities were normalized to the loading control; normalized intensity of wild-type cells at day 0 was taken to be 1. N=3. *P≤0.05.
Finally we verified our findings by re-expression of Abi3bp in knockout c-Kit+ CPCs. Abi3bp knockout c-Kit+ CPCs were transiently transfected with a vector encoding Abi3bp. Wild-type c-Kit+ CPCs were used as a positive control. Gata4, Gata6, Mef2C, and Tnni3 mRNA levels were significantly increased following transient re-expression of Abi3bp in Abi3bp knockout cells (Figure 2C). Interestingly, re-expression of Abi3bp in Abi3bp knockout c-Kit+ CPCs was sufficient to recover the phenotype. Gata4, Gata6, Mef2C and Tnni3 expression was identical in wild-type and Abi3bp knockout c-Kit+ CPCs transiently transfected with the Abi3bp vector (Figure 2C). Similarly, re-expression of Abi3bp in knockout CPCs increased Gata4, cardiac troponin-I, and αMHC protein levels at both baseline and following differentiation (Figure 2D).
To investigate whether Abi3bp affected CPC differentiation in vivo, we subjected wild-type and Abi3bp knockout mice to myocardial injury (MI). Abi3bp was found to be expressed in the normal and injured heart at similar levels (Figure 3A). Masson's Trichrome staining was performed to assess fibrosis. One week post MI fibrosis levels were similar between wild-type and Abi3bp knockout (Figure 3B). However one month post-MI fibrosis was significantly higher in the Abi3bp knockout animals (Figure 3C). Interestingly, at one month post MI, cardiac tissue from injured Abi3bp knockout mice appeared more fragile than that from the wildtype mice. Following embedding into paraffin, ~90% of the sections from Abi3bp knockout hearts showed breakage in the injured area as opposed to only about 10% in sections from wildtype hearts (Figure 3C).
Figure 3. Abi3bp knockout is associated with lower cardiac function and increased fibrosis following MI.
(A) Protein extracts (50μg) from sham and infarct hearts were immunoblotted with antibodies to the indicated proteins. β-tubulin was used as a loading control. Intensities were normalized to the loading control; normalized intensity of sham animals was taken to be 1. N=3. Serial sections from sham and MI mice were stained with Masson's trichrome. Fibrosis area is represented as a percentage of the left ventricle. (B) One-week post-infarct and (C) One month post-infarct. N=4-6 per group. Significance between MI groups is shown. ** P≤0.01. (D) One month following injury echocardiography was performed. Left and center panels: Ejection fraction and fractional shortening shown for wild-type and Abi3bp knockout mice subjected to MI. P values indicated. N=11 for wild-type, N=6 for Abi3bp knockout. Right panel: Sham ejection fraction and fractional shortening values (N=5 per group). Other parameters for pre-operative, MI and sham animals are shown in Supplementary Table I.
We next examined cardiac function by echocardiography. Knockout of the Abi3bp gene was associated with lower ejection fraction and fractional shortening one-month post MI (Figure 3D, see Online Table I for pre-injury, sham, and MI echocardiographic data).
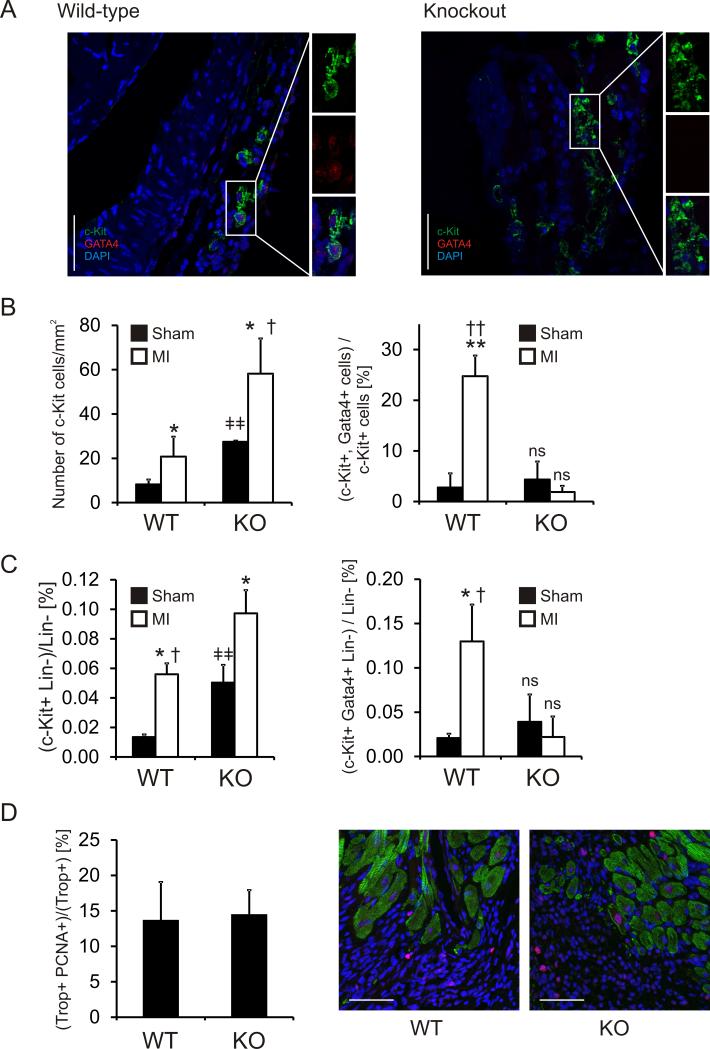
We used immunostaining to assess c-Kit+ CPC number and differentiation in the sham and MI hearts seven days following injury. Sequential sections through the entire infarct region of the heart were assayed for c-Kit and Gata4 expression. This allowed an assessment of the total number of c-Kit+ and Gata4+ cells in the peri-infarct region of each animal (Figure 4A; larger sections can be found in Online Figure V). Cardiac injury increased the number of c-Kit+ cells in both wild-type and Abi3bp knockout mice (Figure 4B). The number of c-Kit+ cells were significantly higher in Abi3bp knockout mice in both sham and MI animals (Figure 4B). Cardiac injury was associated with an increase in the percentage of double positive (c-Kit+/Gata4+) cells in wild-type mice (Figure 4B). In contrast, MI had no effect on the percentage of c-Kit+/Gata4+ cells in Abi3bp knockout mice (Figure 4B), suggesting that despite the increase in the numbers of c-Kit+ cells in these animals lineage commitment was being inhibited. The immunostaining results were verified by flow cytometry. The numbers of c-Kit+ cells was found to increase with injury in both wild-type and Abi3bp knockout mice; with significantly higher numbers observed in Abi3bp knockout animals (Figure 4C). Similarly, an increase in the percentage of double positive (c-Kit+/Gata4+) cells following MI was only observed in wild-type animals (Figure 4C). Cardiomyocyte proliferation in the border zone, one week following injury, was not significantly different between wild-type and Abi3bp knockout mice (Figure 4D).
Figure 4. Abi3bp knockout prevents CPC differentiation/commitment following cardiac injury.
Wild-type and Abi3bp knockout mice were subjected to either a sham operation or myocardial infarction [MI]. (A) Seven days following injury CPC differentiation was assessed by immunostaining. Peri-infarct regions were stained with c-Kit, Gata4, and DAPI. Scale bar 50 microns. Larger regions which contain these sections can be found in Online Figure V. (B) The entire peri-infarct region was visualized by serial sectioning through the heart tissue. Total numbers of c-Kit+ and Gata4+ cells were determined. The numbers of c-Kit+ cells are expressed per mm2 of the peri-infarct region.
*P≤0.05 comparing MI to sham,
†P≤0.05 comparing wild-type MI to Abi3bp knockout MI,
ǂǂ P≤0.01 comparing wild-type sham to Abi3bp knockout sham.
No other comparisons are significant.
The numbers of double positive (c-Kit+ Gata4+) cells are expressed as a percentage of the total c-Kit+ population in the peri-infarct region.
**P≤0.01 comparing wild-type MI to wild-type sham,
††P≤0.001 comparing wild-type MI to Abi3bp knockout MI.
No other comparisons are significant. N=4-6 per group.
(C) Hearts were collagenase digested and differentiating CPCs counted by flow cytometry. Differentiating CPCs were defined by the presence of c-Kit, expression of Gata4, and the absence of hematopoietic lineage markers. N=4-6 per group. Data is expressed as the percentage of c-Kit positive Gata4 positive cells in the total hematopoietic negative population. P-values indicated. (D) Serial sections of one-week post-MI tissue were stained for cardiac troponin-T (Trop) and the proliferation marker PCNA. Scale bar 50 microns. N=4-6 per group. No significance was observed between groups.
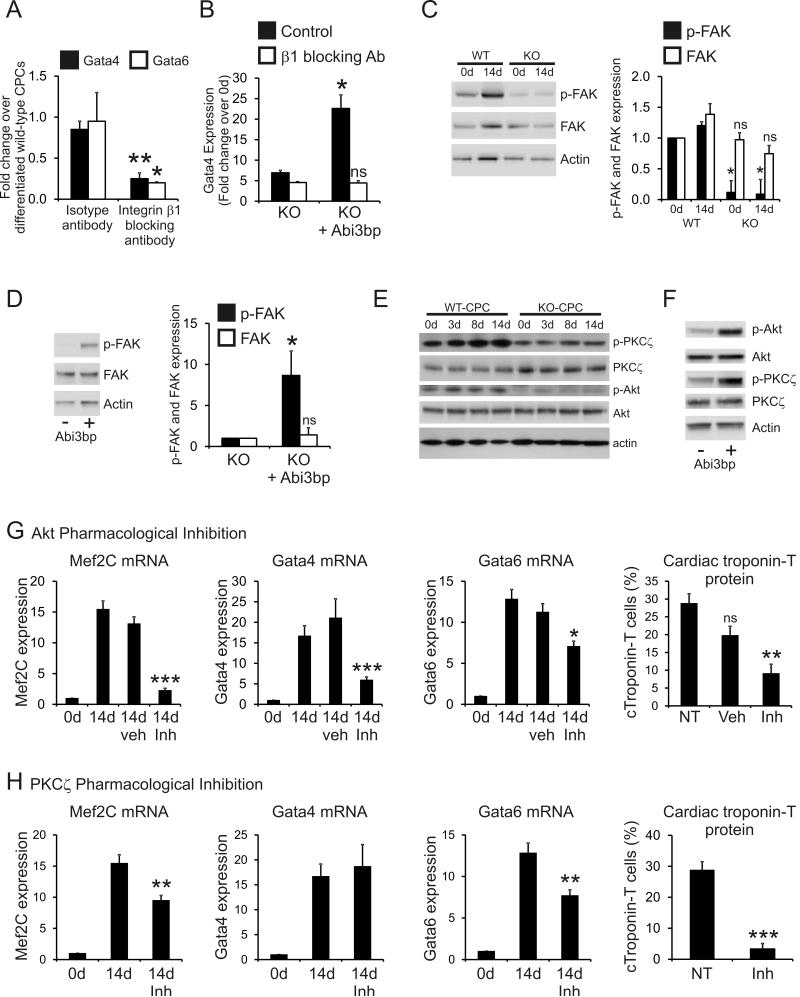
Taken together the above data indicated that Abi3bp is important for CPC cardiac differentiation. We have previously shown that integrin-β1 functions as the Abi3bp receptor 11. Moreover, c-Kit+ CPCs prepared by enzymatic dissociation as well as those prepared by explant, were found to express integrin-β1. Furthermore, c-Kit+ cells in vivo were found to express integrin-β1 (Online Figure VI). To examine the potential role of integrin-β1, isotype-control or integrin-β1 blocking antibodies were added to wild-type c-Kit+ CPCs cultured under differentiation conditions. Gata4 and Gata6 expression were evaluated by qPCR and compared to wild-type c-Kit+ CPCs cultured in CPC-differentiation media in the absence of either antibody. The isotype control antibody had no effect on CPC differentiation, Gata4 and Gata6 expression levels were comparable to cells differentiated in the absence of antibody (Figure 5A). In contrast, addition of the integrin-β1 blocking antibody markedly inhibited Gata4 and Gata6 expression (Figure 5A) indicating that the effects on CPC differentiation were mediated by integrin-β1. This experiment was also performed with Abi3bp knockout c-Kit+ CPCs transiently over-expressing either a control or Abi3bp expression plasmid. Isotype or integrin-β1 blocking antibodies were added to CPCs cultured under differentiation conditions and Gata4 expression evaluated by qPCR. Integrin-β1 blocking antibodies completely abrogated the positive effects of re-expression of Abi3bp (Figure 5B). Gata4 expression was not significantly different to cells expressing the control plasmid (Figure 5B). Phosphorylation of FAK is a key event following integrin activation. As expected, p-FAK levels were markedly lower in Abi3bp knockout c-Kit+ cells (Figure 5C). Furthermore re-expression of Abi3bp in Abi3bp knockout c-Kit+ CPCs increased FAK phosphorylation (Figure 5D). No significant differences in total FAK were observed.
Figure 5. Abi3bp controls CPC differentiation through integrin-β1, PKCζ and Akt.
(A) Wild-type c-Kit+ CPCs were cultured for 14 days in CPC-differentiation media. Where necessary isotype control or integrin-β1 blocking antibodies (10μg/ml) were added to the media for the duration of the experiment. Gata4 and Gata6 expression was determined by qPCR. Data is shown as a fold-change where expression values in control wild-type c-Kit+ CPCs were taken to be 1. Control wild-type c-Kit+ CPCs were differentiated for 14 days in the absence of antibody. N=3. Comparisons made between isotype and integrin-β1 blocking antibody treated cells, *P≤0.05, **P≤0.01. (B) Abi3bp knockout c-Kit+ CPCs were transiently transfected with either a myc or mycAbi3bp plasmid. Cells were cultured for 14 days in CPC-differentiation media with isotype control or integrin-β1 blocking antibodies (10μg/ml). Gata4 gene expression was determined by qPCR. Data is shown as a fold-change where expression values in day 0 cells were taken to be 1. N=3. No significant difference was observed between the integrin-β1 treated groups. (C) Wild-type and Abi3bp knockout c-Kit+ CPCs were cultured for 14 days with CPC-differentiation media. Protein extracts [5μg], taken at the indicated times, were immunoblotted for p-FAK, FAK and actin. N=3. *P≤0.05, ns not significant, comparisons made to WT day 0 cells. (D) Abi3bp knockout c-Kit+ CPCswere transiently transfected with either a myc or mycAbi3bp plasmid and cultured in growth media following transfection. Protein extracts [7.5μg] were immunoblotted for p-FAK, FAK and actin. N=3. *P≤0.05, ns not significant, comparisons made to the myc-expressing cells. (E) Wild-type and Abi3bp knockout c-Kit+ CPCs were cultured for 14 days with CPC-differentiation media. Protein extracts [5μg], taken at the indicated times, were immunoblotted for p-PKCζ, p-Akt, PKCζ, Akt and actin. N=3. Quantification supplied in Online Figure VIIA. (F) Abi3bp knockout c-Kit+ CPCs were transiently transfected with either a myc or mycAbi3bp plasmid and cultured in growth media following transfection. Protein extracts [7.5μg] were immunoblotted for p-PKCζ, p-Akt, PKCζ, Akt and actin. N=3. Quantification supplied in Online Figure VIIB. (G) Wild-type c-Kit+ CPCs were cultured for 14 days with CPC-differentiation media. Where appropriate c-Kit+ CPCs were treated with either vehicle [DMSO], or Akt inhibitor [DMSO soluble]. Mef2C, Gata4, and Gata6 expression was determined by qPCR. Expression at day 0 was taken to be 1. N=3. Comparisons made with day 14 CPCs exposed to neither vehicle nor inhibitor, *P≤0.05, **P≤0.01, ***P≤0.001. Flow cytometry was used to determine the number of cardiac troponin-T positive cells. N=3. Comparisons made with day 14 CPCs exposed to neither vehicle nor inhibitor, **P≤0.01. (H) Wild-type c-Kit+ CPCs were cultured for 14 days with CPC-differentiation media. Where appropriate c-Kit+ CPCs were treated with a PKCζ inhibitor [media soluble]. Mef2C, Gata4, and Gata6 expression was determined by qPCR. Expression at day 0 was taken to be 1. N=3. Comparisons made with day 14 CPCs exposed to neither vehicle nor inhibitor, *P≤0.05, **P≤0.01, ***P≤0.001. Flow cytometry was used to determine the number of cardiac troponin-T positive cells. N=3. Comparisons made with day 14 CPCs exposed to neither vehicle nor inhibitor, **P≤0.01.
Having ascertained that integrin-β1 was important for the effects of Abi3bp upon CPC differentiation we wished to identify the signaling proteins involved. Wild-type and Abi3bp knockout c-Kit+ CPCs were cultured for up to 14 days in CPC-differentiation media. Protein extracts from these cells were subsequently immunoblotted for PKCζ and Akt. Abi3bp knockout had no effect on the levels of total PKCζ and Akt in c-Kit+ CPCs (Figure 5E, with quantification provided in Online Figure VIIA). Both phospho-PKCζ (Thr410) and phospho-Akt (S473) were observed in wild-type c-Kit+ CPCs (Figure 5E). Phosphorylation levels of both kinases showed a modest, but significant increase, during differentiation (Figure 5E, with quantification shown in Online Figure VIIA). In contrast to wild-type c-Kit+ CPCs, Abi3bp knockout CPCs displayed markedly lower levels of both p-PKCζ and p-Akt at all time-points tested (Figure 5E, with quantification shown in Online Figure VIIA). Phosphorylation levels of PKCζ and Akt did not change when Abi3bp knockout CPCs were cultured in CPC-differentiation media (Figure 5E, with quantification shown in Online Figure VIIA). Re-expression of Abi3bp in Abi3bp knockout c-Kit+ CPCs increased phosphorylation of PKCζ and Akt (Figure 5F, with quantification shown in Online Figure VIIB).
The differences between wild-type and Abi3bp knockout c-Kit+ CPCs in the levels of p-PKCζ and p-Akt suggested that Abi3bp promotes CPC differentiation through these kinases. Pharmacological inhibition was employed to test this hypothesis. Wild-type c-Kit+ CPCs were cultured for 14 days in CPC differentiation media supplemented with either vehicle or pharmacological inhibitors. The concentrations employed for the pharmacological inhibitors were effective at inhibiting their respective kinases (Online Figure VIIC). Pharmacological inhibition of Akt significantly attenuated the increase in Mef2C, Gata4, and Gata6 mRNA levels and cardiac troponin-T protein expression observed during wild-type CPC differentiation (Figure 5G, FACS traces Online Figure VIID). The vehicle had no effect on cardiac gene expression (Figure 5G). The pharmacological inhibition of PKCζ was similar to that of Akt; increases in Mef2C, Gata6 and cardiac troponin-T expression that occur during wild-type CPC differentiation were significantly reduced by the inhibitor (Figure 5H, FACS traces Online Figure VIID). However, no effect was observed with Gata4 (Figure 5H) suggesting that the two kinases control different pathways.
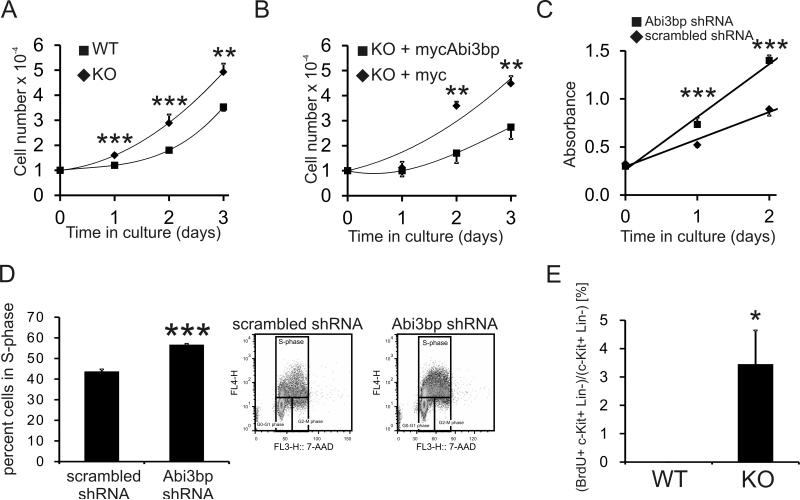
We hypothesized, based on our previous studies 11, that Abi3bp would affect CPC proliferation. Wild-type and Abi3bp knockout c-Kit+ CPCs were seeded at the same density, cultured for three days, and manually counted on a daily basis. Abi3bp knockout c-Kit+ CPC number was significantly higher that their wild-type counterparts 1, 2, and 3 days post-seeding (Figure 6A) with a doubling time of 1.3 days versus 1.7 days. Re-expression of Abi3bp in Abi3bp knockout cells decreased cell proliferation as determined by cell counting (Figure 6B). Re-expression of Abi3bp in Abi3bp knockout CPCs increased the doubling time to 2.3 days. Wild-type c-Kit+ CPCs were made to stably express either a scrambled-control or Abi3bp shRNA. C-Kit+ CPCs expressing the Abi3bp shRNA construct were found to have a higher growth rate when compared to control cells expressing a control scrambled shRNA (Figure 6C). Wild-type CPCs, expressing either the scrambled or Abi3bp shRNA construct, were incubated with the thymidine analogue BrdU to determine whether the changes in cell number arose from altered cell cycle kinetics. Flow cytometry was used to determine the number of BrdU-positive cells. Knockdown of Abi3bp increased the number of BrdU-positive CPCs indicating a greater proportion of cells in S-phase (Figure 6D). Knockdown and knockdown of Abi3bp removed contact inhibition, the exponential growth curves of Abi3bp knockout and knockdown CPCs continued beyond the time-frame of the experiment (data not shown).
Figure 6. Abi3bp inhibits CPC proliferation in vitro and in vivo.
(A) Wild-type and Abi3bp knockout c-Kit+ CPCs were seeded at the same density in growth media and manually counted for up to three days post-seeding. N=3. Comparisons between groups at the same time point *** P≤0.001. (B) Abi3bp knockout c-Kit+ CPCs, over-expressing either a control or Abi3bp plasmid, were seeded at the same density in growth media and manually counted for up to three days post-seeding. N=3. Comparisons between groups at the same time point ** P≤0.01. (C) MTS assay growth curves in growth media for wild-type CPCs expressing either scrambled or Abi3bp shRNA. N=6. Comparisons between groups at the same time point, *** P≤0.001. (D) Wild-type CPCs expressing scrambled or Abi3bp shRNA were incubated with in growth media supplemented with BrdU for 6 hours and analyzed by flow cytometry using 7-AAD to stain DNA. BrdU positive cells are in S-phase. N=3. *** P≤0.001. (E) Wild-type and Abi3bp knockout mice were injected with BrdU for four days, cells were collected by collagenase digestion and analyzed by FACS. Left panel, BrdU+/c-Kit+/lin- CPCs were counted and expressed as a percentage of the total c-Kit+/lin- CPC population. N=3. * P≤0.05.
Additional experiments were performed with c-Kit+ CPCs prepared from cardiac biopsies. In culture, Abi3bp knockout c-Kit+ CPC number was observed to be significantly higher 1, 2, 3, and 4 days post-seeding when compared to wild-type c-Kit+ CPCs (Online Figure VIIIA) with a doubling time of 0.95 days versus 1.3 days for the wild-type cells. Similarly, knockout of the Abi3bp gene increased BrdU incorporation in c-Kit+ CPCs (Online Figure VIIIB).
We then assessed the c-Kit+ CPC proliferation in wild-type and Abi3bp knockout mice. Consequently, we measured BrdU uptake in wild-type and Abi3bp knockout mice. Abi3bp knockout increased the proliferation of both c-Kit+ positive hematopoietic lineage-negative CPCs, as shown by the ~10-fold increase in BrdU incorporation (Figure 6E).
DISCUSSION
In this study we show that Abi3bp regulates critical aspects of CPC biology such as differentiation and proliferation.
Following injury to the heart the extracellular matrix undergoes a number of changes which affect cardiac function 18. The extracellular matrix (ECM) has been shown to affect the behavior of certain types of stem cells, such as mesenchymal stem cells (MSCs). ECM stiffness and composition has a strong effect on MSC differentiation 19, 20. ECM cross-linking proteoglycans of the heparan sulfate and chondroitin sulfate families are likely to be important in the lineage specification of MSCs, especially as they help to control matrix stiffness 21. In contrast, less is known about how the ECM regulates CPC behavior 22. ECM components such as fibronectin, laminin and vitronectin have been shown to augment Sca-1+ and Flk-1+ CPC proliferation 23, 24. In our study we show that Abi3bp, an ECM protein 11, is important for promoting CPC differentiation. Our study also suggests that like MSCs, ECM stiffness may have a role to play in CPC proliferation and differentiation. Abi3bp is itself a proteoglycan and we found previously that loss of the protein increased tensile stresses on MSCs 11. We found that Abi3bp promotes CPC differentiation via integrin-β1, Akt, and PKCζ. To our knowledge this is the first report linking these proteins to CPC differentiation. Akt helps to mediate IGF protein stimulation of embryonic stem cell differentiation to Nkx2-5+ CPCs 25 which agrees with our finding that Akt is important for CPC differentiation. The mechanisms involved in CPC proliferation have been more heavily characterized than those governing CPC differentiation. Activated β2-adrenergic receptors promote CPC proliferation in part through Akt 26. Our results suggest that other pathways exist that are important for CPC proliferation; as in our model higher Akt phosphorylation levels were found to correlate with reduced CPC proliferation. Indeed, fibronectin has been shown to increase CPC proliferation through a Akt independent pathway23 involving integrin-β1, focal adhesion kinase (FAK), Stat3, and PIM1 23. In our previous MSC study we found that Abi3bp inhibited proliferation by ERK sequestration at the plasma membrane by Src, an event mediated by integrin-β1 activation and paxillin phosphorylation11. Considering the similarities between MSCs and CPCs Abi3bp may inhibit CPC proliferation through the same pathway.
Our data has implications both for the therapeutic use of CPCs and the controversy surrounding these cells. c-Kit+ CPCs have been shown to be therapeutically beneficial7. Controversy exists regarding the differentiation potential of c-Kit+ CPCs. Using different models, two lineage tracing experiments arrived at a polar conclusion. One study found that c-Kit+ CPCs robustly differentiated to cardiomyocytes following injury27, the other study found that c-Kit+ cells only minimally contributed to the formation of cardiomyocytes28. In our study, c-Kit+ CPC numbers were elevated in the Abi3bp knockout mouse, similar results were obtained with Sca-1+ CPCs (data not shown). However, ablation of the Abi3bp gene inhibited CPC differentiation and adversely affected recovery following MI. Considering that cardiomyocyte proliferation was unaffected by Abi3bp knockdown this suggests that the critical determinant of the therapeutic potential of CPCs may not be cell number but rather their differentiation ability. It is worth noting that Abi3bp may have other effects on heart biology, independent of driving CPC differentiation. Lineage tracing experiments will be necessary to address this question. However, our model offers the potential to provide insight into the relative roles of cardiomyocyte proliferation versus CPC differentiation in the formation of new cardiomyocytes.
In conclusion we have identified Abi3bp as an ECM protein important for promoting CPC differentiation via integrin-β1, Akt, and PKCζ. The findings of this study are potentially important for the therapeutic uses of CPCs.
Supplementary Material
Novelty and Significance.
What Is Known?
Cardiac progenitor cells (CPCs) are believed to differentiate into cardiomyocytes, smooth muscle cells and endothelial cells.
CPCs have been shown to be therapeutically beneficial for the treatment of heart disease.
The mechanisms controlling CPC differentiation and proliferation are unclear.
What New Information Does This Article Contribute?
Removal of the Abi3bp gene inhibited CPC differentiation in vivo, whereas CPC proliferation and number were increased.
This correlated with adverse recovery following myocardial infarction.
Abi3bp promoted CPC differentiation via integrin-β1, PKCζ, and Akt activation.
CPCs are thought to differentiate into the major cell types of the heart and injection of CPCs into damaged heart tissue following injury promotes recovery. Despite their clinical relevance mechanisms controlling important aspects of CPC biology, such as differentiation and proliferation, are unclear. We found in vivo that removal of the Abi3bp gene inhibited CPC differentiation whereas CPC proliferation and number were increased. This correlated with adverse recovery following myocardial infarction. We also found that Abi3bp promoted CPC differentiation through the activation of an integrin-β1, PKCζ and Akt signaling pathway. These data show that Abi3bp is important for CPC differentiation and proliferation.
ACKNOWLEDGEMENTS
We would like to thank John Wong of the DHVI (Duke Human Vaccine Institute) for the use of flow cytometers. This work was supported by the NIH.
SOURCES OF FUNDING
Research conducted in these studies was supported by National Heart, Lung, and Blood Institute grants RO1 HL35610, HL81744, HL72010, and HL73219 and the Edna and Fred L. Mandel, Jr. Foundation
Nonstandard Abbreviations and Acronyms
- αMHC
Alpha myosin heavy chain
- Abi3bp
ABI family, member 3 (NESH) binding protein
- Akt
v-akt murine thymoma viral oncogene homolog
- BrdU
Bromodeoxyuridine
- BSA
Bovine serum albumin
- c-Kit
V-Kit Hardy-Zuckerman 4 Feline Sarcoma Viral Oncogene Homolog
- CPC
Cardiac progenitor cell
- DHVI
Duke Human Vaccine Institute
- DLAR
Division of Laboratory Animal Resources
- ECM
Extracellular matrix
- eHAND
Heart and neural crest derivatives expressed 1
- ES
Embryonic stem cell
- Ets1
v-ets avian erythroblastosis virus E26 oncogene homolog 1
- FAK
Focal adhesion kinase
- FGF
Fibroblast growth factor
- GATA
Gata binding protein
- IACUC
Institutional Animal Care & Use Committee
- Isl1
Islet-1
- ITS
Insulin-transferrin-selenium
- KO
Knockout
- LIF
Leukemia Inhibitory Factor
- Mef2C
Myocyte enhancer factor 2C
- MSC
Mesenchymal stem cell
- NeoR
Neomycin resistance gene
- Nkx2.5
Homeobox Protein Nkx-2.5
- PIM1
Pim-1 oncogene
- PKC
Protein kinase C
- Stat3
Signal transducer and activator of transcription 3
- Tbx5
T-box 5
- WT
Wild-type
- Wt1
Wilms tumor 1
Footnotes
CONTRIBUTIONS
Conrad P Hodgkinson: conception and design, collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript. Jose Gomez, Alan Payne, Lunan Zhang, Xiaowen Wang. Sophie Dal-Pra: collection and/or assembly of data. Richard Pratt: data analysis and interpretation. Victor J Dzau: conception and design, manuscript writing, data analysis and interpretation, final approval of manuscript.
DISCLOSURES
None
REFERENCES
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Bearzi C, Leri A, Lo Monaco F, Rota M, Gonzalez A, Hosoda T, Pepe M, Qanud K, Ojaimi C, Bardelli S, D'Amario D, D'Alessandro DA, Michler RE, Dimmeler S, Zeiher AM, Urbanek K, Hintze TH, Kajstura J, Anversa P. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci U S A. 2009;106:15885–15890. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: A paradigm shift in cardiac biology. Circulation. 2006;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 4.Bollini S, Smart N, Riley PR. Resident cardiac progenitor cells: At the heart of regeneration. J Mol Cell Cardiol. 2011;50:296–303. doi: 10.1016/j.yjmcc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 6.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: The scipio trial: Surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Noack C, Zafiriou MP, Schaeffer HJ, Renger A, Pavlova E, Dietz R, Zimmermann WH, Bergmann MW, Zelarayan LC. Krueppel-like factor 15 regulates wnt/beta-catenin transcription and controls cardiac progenitor cell fate in the postnatal heart. EMBO Mol Med. 2012;4:992–1007. doi: 10.1002/emmm.201101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, Sussman MA. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 2010;106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgkinson CP, Naidoo V, Patti KG, Gomez JA, Schmeckpeper J, Zhang Z, Davis B, Pratt RE, Mirotsou M, Dzau VJ. Abi3bp is a multifunctional autocrine/paracrine factor that regulates mesenchymal stem cell biology. Stem Cells. 2013;31:1669–1682. doi: 10.1002/stem.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng TW, Gong Q. Secreted tarsh regulates olfactory mitral cell dendritic complexity. Eur J Neurosci. 2009;29:1083–1095. doi: 10.1111/j.1460-9568.2009.06660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latini FR, Hemerly JP, Oler G, Riggins GJ, Cerutti JM. Re-expression of abi3-binding protein suppresses thyroid tumor growth by promoting senescence and inhibiting invasion. Endocr Relat Cancer. 2008;15:787–799. doi: 10.1677/ERC-08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambini E, Pompilio G, Biondi A, Alamanni F, Capogrossi MC, Agrifoglio M, Pesce M. C-kit+ cardiac progenitors exhibit mesenchymal markers and preferential cardiovascular commitment. Cardiovasc Res. 2011;89:362–373. doi: 10.1093/cvr/cvq292. [DOI] [PubMed] [Google Scholar]
- 15.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. Microrna-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jourdan-Lesaux C, Zhang J, Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci. 2010;87:391–400. doi: 10.1016/j.lfs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One. 2011;6:e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Sun A, Ma H, Yao K, Zhou N, Shen L, Zhang C, Zou Y, Ge J. Infarcted myocardium-like stiffness contributes to endothelial progenitor lineage commitment of bone marrow mononuclear cells. J Cell Mol Med. 2011;15:2245–2261. doi: 10.1111/j.1582-4934.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okolicsanyi RK, Griffiths LR, Haupt LM. Mesenchymal stem cells, neural lineage potential, heparan sulfate proteoglycans and the matrix. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Israeli-Rosenberg S, Manso AM, Okada H, Ross RS. Integrins and integrin-associated proteins in the cardiac myocyte. Circ Res. 2014;114:572–586. doi: 10.1161/CIRCRESAHA.114.301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstandin MH, Toko H, Gastelum GM, Quijada P, De La Torre A, Quintana M, Collins B, Din S, Avitabile D, Volkers M, Gude N, Fassler R, Sussman MA. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circ Res. 2013;113:115–125. doi: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heydarkhan-Hagvall S, Gluck JM, Delman C, Jung M, Ehsani N, Full S, Shemin RJ. The effect of vitronectin on the differentiation of embryonic stem cells in a 3d culture system. Biomaterials. 2012;33:2032–2040. doi: 10.1016/j.biomaterials.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engels MC, Rajarajan K, Feistritzer R, Sharma A, Nielsen UB, Schalij MJ, de Vries AA, Pijnappels DA, Wu SM. Igf promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem Cells. 2014 doi: 10.1002/stem.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan M, Mohsin S, Avitabile D, Siddiqi S, Nguyen J, Wallach K, Quijada P, McGregor M, Gude N, Alvarez R, Tilley DG, Koch WJ, Sussman MA. Beta-adrenergic regulation of cardiac progenitor cell death versus survival and proliferation. Circ Res. 2013;112:476–486. doi: 10.1161/CIRCRESAHA.112.280735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–842. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 28.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. C-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.