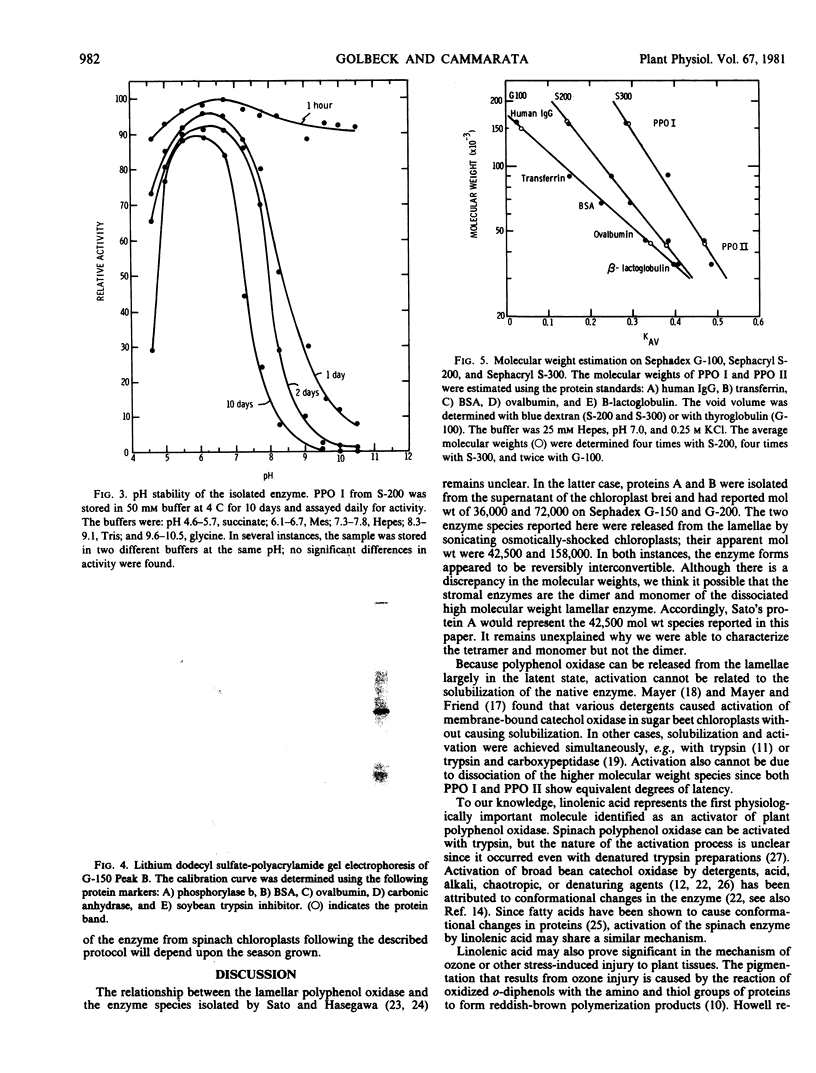
Abstract
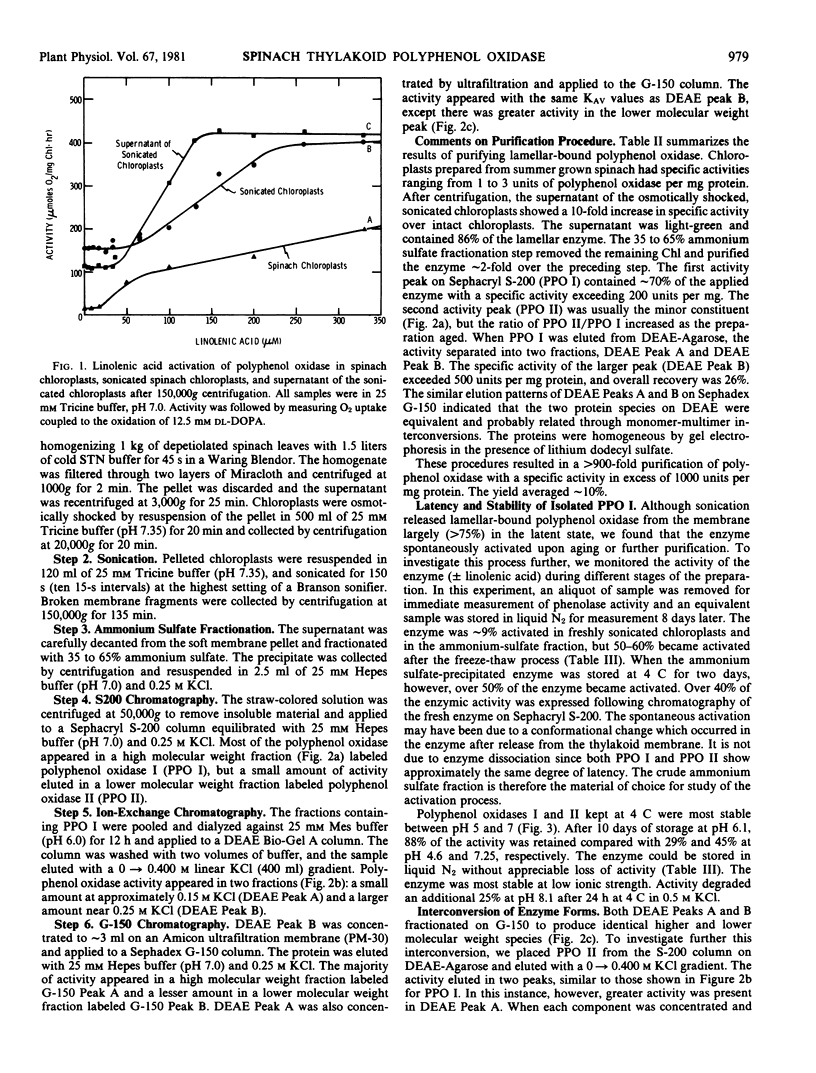
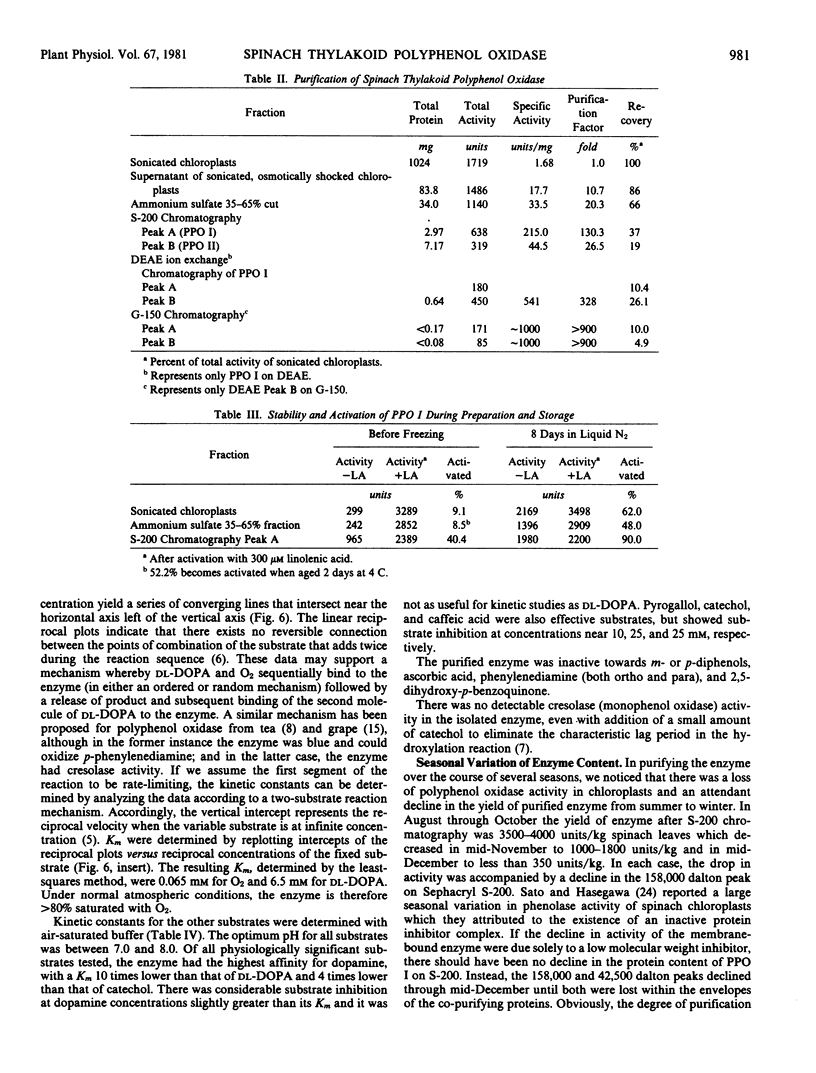
Polyphenol oxidase activity (E.C. 1.14.18.1) has been found in two enzyme species isolated from thylakoid membranes of spinach chloroplasts. The proteins were released from the membrane by sonication and purified >900-fold by ammonium sulfate precipitation, gel filtration, and ion-exchange chromatography. The enzymes appear to be the tetramer and monomer of a subunit with a molecular weight of 42,500 as determined by lithium dodecyl sulfate gel electrophoresis. The higher molecular weight enzyme is the predominant form in freshly isolated preparations but on aging or further purification, the amount of lower molecular weight enzyme increases at the expense of the higher.
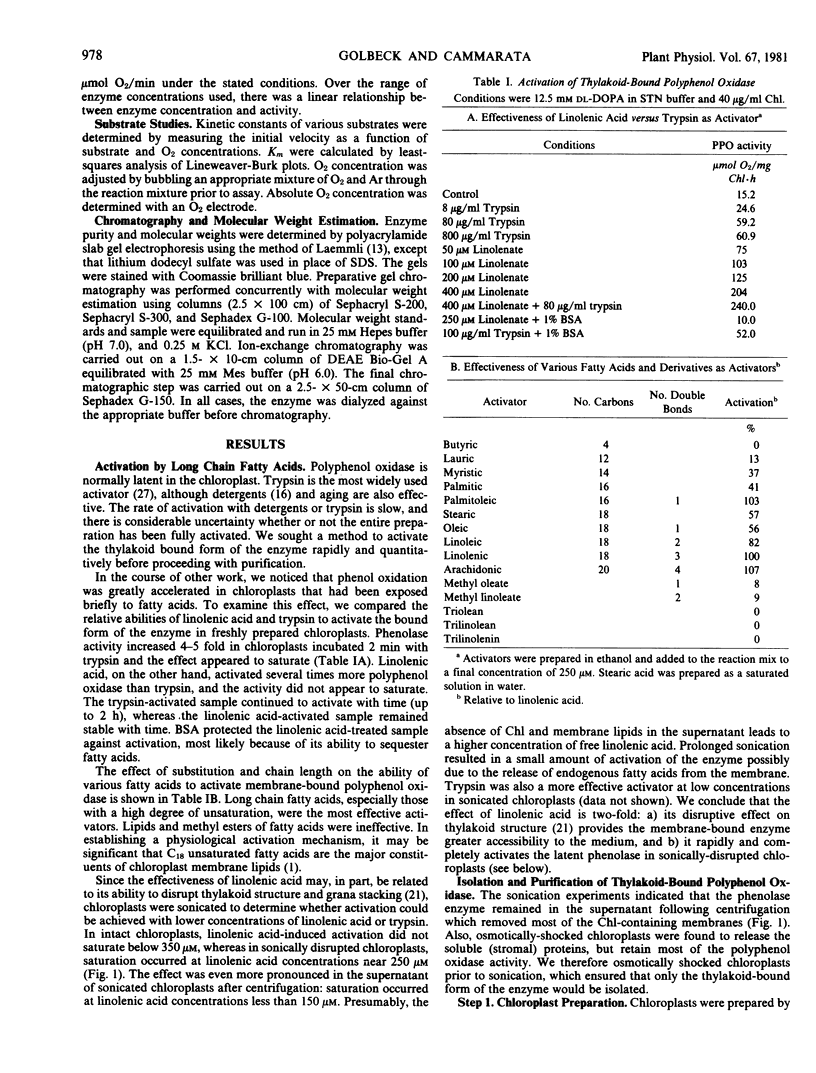
Sonication releases polyphenol oxidase from the membrane largely in the latent state. C18 fatty acids, especially linolenic acid, are potent activators of the enzymic activity. In the absence of added fatty acids, the isolated enzyme spontaneously, but slowly, activates with time.
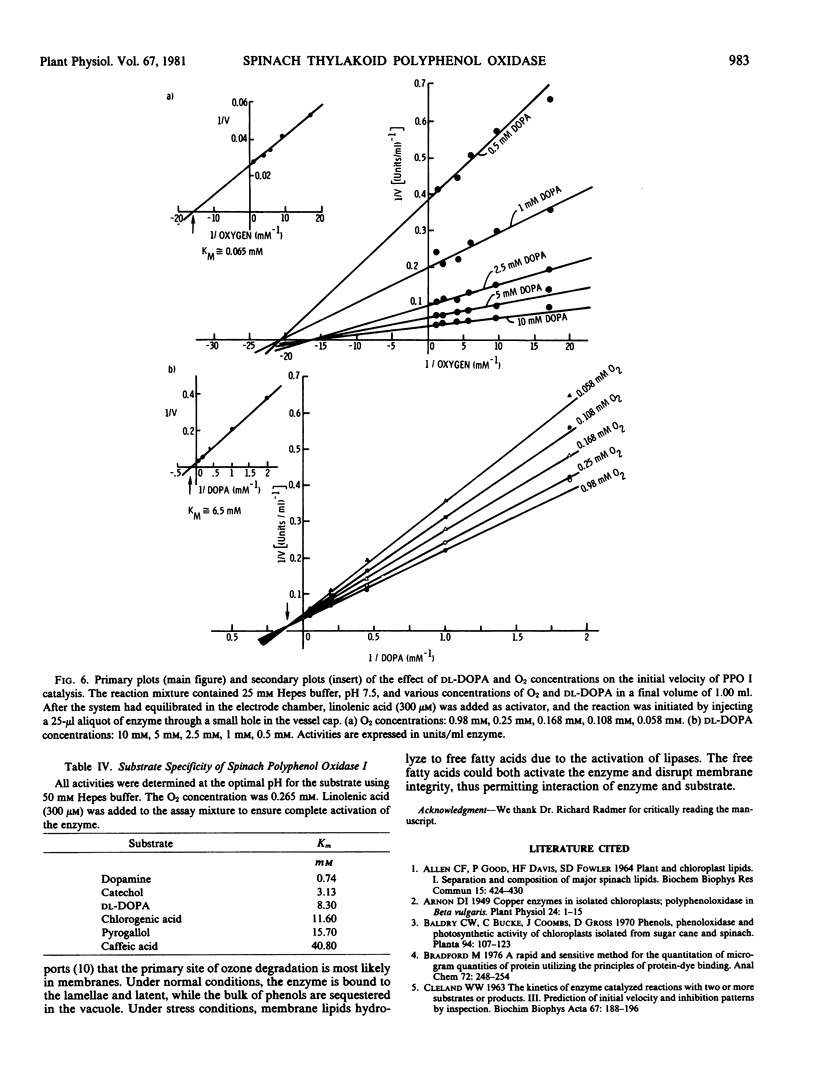
Purified polyphenol oxidase utilizes o-diphenols as substrates and shows no detectable levels of monophenol or p-diphenol oxidase activities. The Km values for 3,4-dihydroxyphenylalanine and O2 are 6.5 and 0.065 millimolar, respectively. Suitable substrates include chlorogenic acid, catechol, caffeic acid, pyrogallol, and dopamine; however, the enzyme is substrate-inhibited by the last four at concentrations near their Km A large seasonal variation in polyphenol oxidase activity may result from a decrease in enzyme content rather than inhibition of the enzyme present.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. F., Good P., Davis H. F., Fowler S. D. Plant and chloroplast lipids. I. Separation and composition of major spinach lipids. Biochem Biophys Res Commun. 1964 Apr 22;15(5):424–430. doi: 10.1016/0006-291x(64)90479-6. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Gregory R. P., Bendall D. S. The purification and some properties of the polyphenol oxidase from tea (Camellia sinensis L.). Biochem J. 1966 Dec;101(3):569–581. doi: 10.1042/bj1010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H. Latent phenolase in extracts of broad-bean (Vicia faba L.) leaves. I. Activation by acid and alkali. Biochem J. 1957 Oct;67(2):300–307. doi: 10.1042/bj0670300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Scanu A., Pollard H., Hirz R., Kothary K. On the conformational instability of human serum low-density lipoprotein: effect of temperature. Proc Natl Acad Sci U S A. 1969 Jan;62(1):171–178. doi: 10.1073/pnas.62.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E. Activation of polyphenol oxidase of chloroplasts. Plant Physiol. 1973 Feb;51(2):234–244. doi: 10.1104/pp.51.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]