Abstract
The search for reliable early indicators of age-related cognitive decline represents a critical avenue for progress in aging research. Chronological age is a commonly used developmental index; however, it offers little insight into the mechanisms underlying cognitive decline. In contrast, biological age (BioAge), reflecting the vitality of essential biological systems, represents a promising operationalization of developmental time. Current BioAge models have successfully predicted age-related cognitive deficits. Research on aging-related cognitive function indicates that the interaction of multiple risk and protective factors across the human lifespan confers individual risk for late-life cognitive decline, implicating a multi-causal explanation. In this review, we explore current BioAge models, describe three broad yet pathologically relevant biological processes linked to cognitive decline, and propose a novel operationalization of BioAge accounting for both moderating and causal mechanisms of cognitive decline and dementia. We argue that a multivariate and mechanistic BioAge approach will lead to a greater understanding of disease pathology as well as more accurate prediction and early identification of late-life cognitive decline.
Keywords: Biological Age, Cognitive Aging, Inflammation, Oxidative Stress, Vascular Health, Early Identification
1. Introduction
The rapid growth of the older adult population is concomitant with an increased prevalence in age-related diseases, such as heart disease, cancer, and diabetes. Various age-related diseases, such as diabetes and atherosclerosis, have been linked to decreases in cognitive functioning. Indeed, multi-morbidity is known to accelerate cognitive decline in both normative and the more extreme pathological cognitive aging associated with Alzheimer’s disease (AD) (Bergman et al., 2007). It was estimated in 2005 that approximately 24.3 million people worldwide suffer from dementia, with a projected development of 4.6 million new cases every year (Ferri et al., 2005); such estimates underscore the staggering burden late-life cognitive decline has on individuals, families and society.
Most research on late-life development charts cognitive change as a function of chronological age (CA). Although chronicity is commonly used for late-life decision making (e.g., medical screening initiatives, employment, driving privileges), this approach represents a serious conceptual and practical issue for indexing developmental change. As described previously (Dixon, 2011), CA as an index of developmental time carries no causal implications and little explanatory power. CA represents a dimension along which causal processes operate (Birren, 1959; Salthouse, 1999), reflecting the accumulation of biological and environmental influences contributing to developmental change (MacDonald et al., 2004, 2011). The limitations of CA vis-à-vis informing mechanisms of cognitive change have been well documented (Anstey, 2008; Birren 1999; Birren and Cunningham, 1985; Ingram, 1983; MacDonald, et al., 2004; MacDonald et al., 2011). Consider two individuals; one who is 75 and has been diagnosed with Alzheimer’s disease and the other who is 75 and is not suffering from a neurodegenerative condition. In this scenario, using “age” to predict AD risk is not informative as it doesn’t take into consideration the neuropathological processes involved. Arguably, the utility of CA, particularly in isolation, may even impede efforts to identify specific biological mechanisms underlying age-related cognitive decline by placing a focus on a time metric rather than causal factors. As such, many other operationalizations have been constructed to index developmental change including: social age (Birren and Tenner, 1977), psychological age (Birren 1959), allostatic load (McEwen 2007), functional age (Anstey 1996) and biological age (BioAge) (Birren, 1959; MacDonald et al., 2004).
Advancing our understanding of the determinants and contributors of age-related cognitive decline requires that developmental time be operationalized using variables that index theoretical causes of cognitive aging (Birren, 1999; Dixon 2011; MacDonald et al., 2004). Markers of biological health may more accurately indicate cognitive changes in late life compared to CA (Birren and Cunningham, 1985), as they likely reflect both the genetic make-up and environmental exposures that combine to influence cognitive function (Spiro and Brady, 2008; Wohlwill, 1973).
In this review, we explore current BioAge models, describe evidence for three broad yet pathologically relevant biological processes linked to cognitive decline and neurodegeneration, and propose a novel operationalization of BioAge accounting for both moderating and causal mechanisms of late-life cognitive decline. Inflammation, oxidative stress and vascular integrity are key biological mechanisms that are definitively associated with many age-related diseases, and may underlie both normative (e.g., typical age-related) and pathological (e.g., dementia-related) cognitive decline. Targeting change in such potential mechanisms, rather than change in CA, may serve to strengthen the conceptual and practical framework of predictive models of change.
2. Extant Research on Biological Aging
The concept of BioAge was originally developed because of the inaccuracy of CA as an index of development in old age (Birren and Cunningham 1985). BioAge has frequently appeared in scientific literature since its formal introduction by J.E. Birren in 1959, who initially defined it as the estimation of a person’s present position with reference to their total potential lifespan. The BioAge concept has since expanded to reflect the functioning of essential physiological systems and processes in the body (Birren and Cunningham, 1985; MacDonald et al., 2004), to encompass a composite indicator of biological vitality (c.f. Dixon, 2011), physiological reserve (Goffaux et al., 2005), senescence (Nakamura and Miyao, 2007), organism viability (Comfort, 1969), or vigor (Nakamura and Miyao, 2003). Regardless of its formal definition, this area of research may provide insight into an individual’s lifespan more accurately compared to predictions based on chronology (Schroots and Birren 1990). Although the multitude of BioAge definitions and diverse methodology add to the controversy regarding its measurement, validity and usage (Bulpitt et al., 1995; Ingram, 1983; Ludwig and Smoke, 1980), considerable progress in honing the concept and operationalizing candidate markers has been made (c.f. Dixon, 2011) for both age-related health decrements (Goffaux et al., 2005; Spiro and Brady, 2008; Wahlin 2004) and cognitive decline (Anstey et al., 1997; Anstey and Smith, 1999; Elias et al., 1995; MacDonald et al., 2004, 2011). Certainly, the utility of non-cognitive variables to predict differences in age-related cognitive performance has been a coveted focus for cognitive aging research (c.f. Anstey, 2008), with an overarching goal to produce estimates that lead to an improved understanding of psychological aging and associated processes (c.f. Dixon, 2011). Although lacking direct mechanistic underpinnings, existing BioAge models have been successfully used to relate cognitive changes to physiological markers of development.
2.1 BioAge and Cognition
2.1.1 Indirect Markers Indexing Cognitive Change
The majority of BioAge markers used to date can be classified as being indirect, or rather targeting more distal processes (e.g., health factors as well as physiological and sensory function) (Dixon, 2011). Although indirect measures, such as physiological function (e.g., lung capacity, grip strength) are relatively accessible, inexpensive to measure and non-invasive, they also could be strong indicators of central nervous system (CNS) integrity and therefore may accurately predict age-related cognitive change (Anstey, 1999). Further, physiological functioning could indirectly index the functioning of underlying processes involved in cognitive functioning. For example, pulmonary capacity is likely related to physical activity levels, which have been associated with brain plasticity, brain-derived neurotrophic factor levels (Cotman and Berchtold, 2002) and inflammation (Kasapis and Thompson, 2005); blood pressure can be a marker for hypertension, in which oxidative stress likely plays a key pathological role (c.f. Rodrigo et al., 2011); and age-related declines in muscle strength can be indicative of sarcopenia, an age-related muscle degenerative disease, associated with high levels of inflammation and oxidative stress (c.f. Kamel 2003; Weindruch 1995;).
Conversely, physical health may play an equally important role of exposing those to risk factors linked to impaired function. Investigations into the link between frailty and cognition highlight the importance of overall health, spanning multiple domains (e.g., health status, history of diseases, sensory function) for the mitigation of neurodegenerative risk (Song et al., 2010, 2011). Conceivably, the link between overall health and cognitive decline is perhaps not simply a mechanistic one. Rather, it may represent a situation whereby compromised bodily health across multiple systems further predispose those to risk factors linked to compromised cognitive function, thereby influencing the neuropathological change-point of decline. Despite these associations, further research is needed to understand the true extent to which non-invasive biomarkers index or moderate causal processes.
Strong associations exist between physiological markers and cognitive functioning in older adults. Measures of sensory functioning, including visual and auditory acuity, have been associated with age-related cognitive decline (Anstey et al., 2001; Baltes and Lindenberger, 1997; Glass 2007; Lindenberger and Baltes, 1994; MacDonald et al., 2004; Tay et al., 2006), whereas measures of blood pressure (Anstey et al., 1996; Elias et al., 1995) and cerebrovascular function (Cherbuin et al., 2009) are negatively associated with cognitive performance. Further, physiological functioning, such as weakened grip strength (Christensen et al., 2000; Takata et al., 2008), lower limb strength (Anstey et al., 1997), muscle strength (Auyeung et al., 2008) and reduced peak expiratory flow (Albert et al., 1995) have been linked to cognitive decline. Combining BioAge indicators, such as grip strength, health status, forced expiratory volume, vibration sense, hearing and vision demonstrated that BioAge markers can explain most age-related variance in cognitive performance (Anstey et al., 1999; Wahlin et al., 2006) and can predict mortality in very old adults (Anstey et al., 2001). Further, a factor analytic approach, using biomarkers of visual and auditory acuity, grip strength, peak expiratory flow, blood pressure, and body mass index, predicted 12-year age-related cognitive decline in the elderly independent of CA (MacDonald et al., 2004). This investigation demonstrated that BioAge can uniquely explain considerable variance associated with between-group differences in intra-individual cognitive change over and above variance explained using CA.. Consistently, a BioAge/Physiological Reserve index, computed using physical fitness measures (6 minute walk, weight lifting, balance, one-foot stand, standing reach, physical activity), cognitive performance, and disease comorbidity status predicted 3-year adverse outcomes better than using CA (Goffaux et al., 2005). Furthermore, similar biological age indices have been employed in other studies assessing age-related changes (Nakamura and Miyao, 2003, 2007). Covariation between changes in biomarkers and changes in cognition within individuals indicates that biological status is an important predictor of age-related cognitive change (MacDonald et al., 2004). Recently, we observed significant time-varying covariation between theoretically-relevant biomarkers of cognition, such as muscle strength, body mass and pulmonary capacity, and declines in cognition, such as semantic memory and fluid reasoning within individuals (MacDonald et al., 2011). This research supports the interpretation that age-related changes in cognition are not due to CA, but they index multiple factors spanning a wide range of biological and physical health domains that simply arise along the CA continuum.
2.1.2 Direct Markers Indexing Cognitive Change
Despite the ability of indirect markers to account for age-related variance in cognitive performance, biological markers directly reflecting the functioning of key underlying processes will likely index cognitive function most accurately. It has been reasoned that more direct measures, which proximally target underlying biological processes theoretically relevant to cognitive decline and/or neurodegeneration (e.g., systemic oxidative stress or neuroinflammation), should be employed in future research on cognitive aging (MacDonald et al., 2011). Direct biomarkers symptomatic of the cellular processes involved in age-related cognitive change, such as hyperlipidemia, elevated levels of pro-inflammatory cytokines or byproducts of oxidative stress, could provide a more sensitive prediction of cognitive function while highlighting the contributing processes. Consistently, a primary goal of biomarker research is to target a biological index representing a proxy for a given behaviour (Biomarkers Definitions Working Group, 2011). In the field of cognitive aging, biomarkers of dementia have spanned a wide continuum of processes, ranging from genetic markers (Harris and Deary, 2011), CSF proteins (Blennow et al., 1995; Ewers et al., 2012) and brain beta-amyloid burden (Nordberg, 2010) to directly indexing brain atrophy (Spulber et al., 2010). Structurally indexing brain degeneration or beta amyloid accumulation (e.g., by using a PET amyloid ligand such as Pittsburgh compound B (PIB) (Klunk et al., 2004), or [18F]FDDNP PET (Small et al., 2012) is informative in terms of understanding cognitive symptoms and trajectories However, targeting progressive stages along the pathological continuum fails to highlight many of the causal processes implicated. Similarly, elevated levels of CSF tau (Blennow et al., 1995) and beta-amyloid 42 (c.f. Blennow et al., 2001) are consistently found in AD patients and are associated with the future development of AD in patients with mild cognitive impairment (MCI) (Hansson et al., 2006), with such proteins being increasingly used in clinical settings for disease detection and tracking. Despite some evidence that CSF tau and beta-amyloid can be used to predict future decline in cognitively normal older individuals (Fagan et al., 2007, Small et al., 2012), accumulation of such proteins may still be proximally related to symptom onset. That is, targeting the potential biological antecedents, such as neuroinflammation (Granic et al., 2009; Zotova et al., 2010), oxidative stress (Torres et al., 2011), neurovascular factors (Marchesi 2011) or brain-specific metabolic processes (Kaiser et al., 2010) that may underlie and/or exacerbate neurodegeneration or cognitive decline, may identify individuals at risk for future decline at the earliest stages. Furthermore, lumbar puncture poses substantial drawbacks in terms of expense and invasiveness, particularly in light of soaring dementia-related healthcare costs and frailty associated with the targeted demographic. Mechanism-based blood biomarkers (Kruman et al., 2002; Yagi et al., 2011) may fulfill the need for less invasive, inexpensive and efficient measures.
3. Toward an Emerging Concept of BioAge
Although multiple definitions of BioAge exist within the literature, a formal and universal operationalization of BioAge for indexing non-normative cognitive decline remains elusive. Despite increasing knowledge in the extant literature on the key biological processes involved in declining cognitive function with age, biological indicators are often selected based on convenience, rather than based on a priori, theory-driven mechanisms. That is, utilizing knowledge garnered by cross-disciplinary research as well as tailoring psychological research designs and methodological approaches to contemporary cognitive aging theories are critically important for the development of predictive models of cognitive change. Furthermore, environmental factors likely influence late-life cognitive function. Although this is an emergent area, there is much to be learned of the biological-by-environmental interactions that modulate the risk and/or acceleration of cognitive decline. Investigations into the cognitive implications of environmental influences are currently underway. For example, exposure to environmental stress has been linked to reactive oxygen species (ROS) accumulation and plaque pathology in AD-like brains (Seo et al., 2011), pesticide exposure has been linked to increased dementia risk (Hayden et al., 2010) and, although age-associated decline in general health status is a risk factor for AD (Song et al., 2011), higher physical activity levels are associated with the maintenance of cognitive and brain health in old age (c.f. Voss et al., 2011). Additionally, accumulating evidence suggests that environmental stressors may trigger epigenetic modifications (i.e., factors that influence gene expression but do not exert genomic alterations), such as DNA methylation (Wu et al., 2008) and histone acetylation (Fischer et al., 2007), that contribute to cognitive dysfunction. Although the mechanisms through which environmental contributions impact cognitive function remain unclear, altered risk/protective biological pathways are likely at the heart of such alterations (Sng and Meaney, 2009). Taken together, recent evidence indicates that late-life cognitive decline may be the result of a lifelong interaction of multiple environmental and genetic influences. Multivariate approaches that index, for example, key molecular (e.g., inflammatory cascades), physical (e.g., general physical health) and environmental (e.g., stress exposure) risk factors may more accurately index those at risk compared to considering any of these factors in isolation. Consistently, recent investigations into the link between health predictors and risk of cognitive decline reveal that a broad constellation of factors confer increased predictivity of dementia risk (Song et al., 2010, 2011) or cognitive maintenance (Yaffe et al., 2009). Combined with the need to index key biological processes directly underlying late-life cognitive function, implementing a multivariate BioAge approach represents a promising BioAge operationalization; one that more accurately indexes age-related cognitive change by capturing both aetiological causes and contributing factors.
In the following sections, we further explore three major biological processes (inflammation, oxidative stress and vascular health) and their use as biomarkers of cognitive decline. Considerable empirical evidence links these select processes with the neuro-aetiology of both AD-pathology and general age-related cognitive decline. The inclusion of process-specific markers will likely enhance the ability to identify cognitive decline at earlier phases by indexing the potential biological processes underlying the disease process.
4. Potential BioAge Mechanisms Underlying Cognitive Aging
BioAge conceptualizations have largely overlooked the direct biological contributions to functional changes with age. In modern conceptualizations, recent scientific gains in the area of molecular biological research should be integrated to reflect critical pathways underlying cognitive change. More specifically, it has been suggested that multiple biological processes, such as inflammation and oxidative stress, should be the focus for the pathophysiological progression of cognitive disorders, such as AD (Castellani et al., 2008). Further, vascular health is thought to be a crucial risk factor for cognitive decline. Not only is the apolipoprotein-4 (APOE ε4) allele, which is implicated in cholesterol transport and atherosclerosis, the most widely known genetic risk factor for late onset AD (Corder et al., 1993; Saunders et al., 1993;), it is also associated with MCI (Brainerd et al., 2013; Dixon et al., 2014), cognitive decline (Bretsky et al., 2003; Caselli et al., 2009) and cognitive deficits in old age (Small et al., 2004; Davies et al., 2014). However, evidence suggests that good episodic memory in young, healthy adults may be mediated by APOE ε4 (Mondadori et al., 2007). Indeed, greater beta-amyloid deposition has been found in cognitively healthy APOE ε4 carriers (Morris et al., 2010). Although APOE ε4 carriers have a greater risk for AD and cognitive decline (Seeman et al., 2005), its prevalence is low (e.g., approximately 50% of non-familial AD cases are APOE ε4 carriers), indicating that other susceptibility genes, likely in concert, are involved in the pathogenesis of age-related cognitive decline and late-onset AD. Consistently, several recent genome-wide association studies have identified multiple genetic AD risk loci, conferring significant population risk. Of note, principal biological processes implicated, among others, are; immune system function (e.g., clusterin (CLU), complement receptor (CRI), sialic acid-binding lg-like lectin (CD33)) (Harold et al., 2009; Hollingworth et al., 2011; Lambert et al., 2009; Naj et al., 2011), lipid and beta-amyloid metabolism (e.g., APOE, CLU, ATP-binding cassette transporter (ABCA7) (Harold et al., 2009; Hollingworth et al., 2011; Lambert et al., 2009;), and cell to cell communication (e.g., phosphatidylinositol binding clathrin assembly protein (PICALM), bridging integrator 1 (BIN1), CD33 and CD2-associated protein (CD2AP) (Harold et al., 2009; Hollingworth et al., 2011; Naj et al., 2011;). Although much evidence highlights the involvement of these particular processes, a recent meta-analysis utilizing the AlzGene database indicates that greater than a dozen genetic loci are implicated in AD susceptibility, including genes associated with vascular function (e.g., APOE, angiotensin converting enzyme (ACE), insulin degrading enzyme (IDE), oxidative stress (e.g., glyceraldehyde-3-phosphate dehydrogenase (GAPDH), mitochondrial transcription factor A (TFAM)) and inflammation (e.g., tumor necrosis factor alpha (TNF-α) (Bertram et al., 2007). These findings, along with a surfeit of studies associating these processes with cognitive decline and the more severe neurodegenerative disorders, indicate that inflammatory mediators, oxidative stress and vascular health indicators are critical BioAge targets for indexing the theoretical underlying causes of both normative and neuropathological cognitive decline. Although there are a multitude of indicators that can index these major biological processes, here we review evidence substantiating the need to incorporate such key biological processes in predictive models of aging-related cognitive change.
4.1 Inflammation
Markers of systemic and neuro-inflammation have been linked to both normative age-related cognitive change as well as non-normative decline associated with diseases of the aging process (MCI, AD, etc.). Inflammation is a complex immune response to harmful stimuli, such as infection, injury or irritation and is characterized by the infiltration and activation of the body’s immune cells and subsequent production of pro-inflammatory cytokines. The innate immune system, the first line of host defence which responds to stressors in a generic way, plays a major role in the generation of an immune response. Immune system activation is generally thought to be an adaptive mechanism which promotes organism survival. However, chronic levels of inflammation may be detrimental in late life (Franceschi et al., 2000) resulting in “Inflamm-aging,” the peculiar chronic inflammatory status characterizing aging (De Martinis et al., 2005).
Founded on the observations that peripheral blood mononuclear cells can produce higher amounts of pro-inflammatory cytokines in older compared to younger adults (Fagiolo et al., 1993), inflamm-aging highlights the critical importance and impact of a chronic, lifelong antigenic load on aging and longevity (Franceschi et al., 2007). Chronic inflammation is a major contributing factor in many age-related diseases such as cancer (c.f. Caruso et al., 2004; DeCarlo et al., 2010, 2012), diabetes (c.f. Granic et al., 2009), obesity (c.f. Dandona et al., 2004; Hotamisligil et al., 1993), arthritis, cardiovascular disease, osteoporosis, atherosclerosis, metabolic syndrome (Dik et al., 2007) as well as age-related cognitive decline (Weaver et al., 2002) and neurodegeneration (c.f. McGeer and McGeer, 2001). As such, the acute phase C-Reactive Protein (Jenny et al., 2012; Schram et al., 2007;) and interleukin-6 (IL-6) (Schram et al., 2007; Weaver et al., 2002), both sensitive markers of systemic inflammation, have been associated with an increased risk of age-related cognitive decline, and cognitive decline in high-functioning older adults (Yaffe et al., 2003).
The inflammatory underpinnings of neurodegenerative diseases have long since been recognized (McGeer et al., 1987). The link between brain inflammation and AD dates back to 1910 when Fischer described a foreign substance (now known as beta amyloid) in the cerebral cortex that induced both the formation of plaques and a local inflammatory response (Fischer, 1910). Correspondingly, inflammatory molecules have been associated with amyloid deposits in the brain (Eikelenboome and Stam, 1982). Further, inflammatory pathways likely play a role in disease susceptibility due to their implicit link to immune system function. Dementia frequently co-occurs with other conditions (Marengoni et al., 2009) and it has been found that individuals with multiple pathological disorders are significantly more likely to develop dementia than those diagnosed with just one disorder (Schneider et al., 2007), rendering disease comorbidity an essential factor to consider in aging models.
Microglia are the major contributors to brain inflammation, and are found co-localized with AD plaques within the neocortex (Griffin et al., 1995)). Both invading organisms and endogenous stimuli, such as damaged associated molecular patterns generated from injured cells or amyloid-beta oligomers, can trigger innate immune responses in the CNS by binding and activating Toll-like receptors (TLRs) (c.f. Salminen et al., 2009). TLRs are transmembrane receptors that recognize structurally conserved molecules and ligands. Once activated, TLRs are known to initiate host protective mechanisms by triggering the production of pro-inflammatory cascades; cascades which are thought to contribute to neuronal damage and dysregulation (c.f. Amor et al., 2010). TLR polymorphisms and expression levels have been implicated in AD susceptibility (Balistreri et al., 2007; Walter et al., 2007), and beta amyloid has been found to activate microglia through TLRs (Lotz et al., 2005). Additionally, a recent investigation into the involvement of innate immune pathways in AD revealed that TLR mediated nuclear-factor of kappa B (NF-κB), a downstream transcription factor responsible for the upregulation of proinflammatory genes, plays a critical role in the pathological effects of beta amyloid (Tan et al., 2008). Conditions such as pro-inflammatory cytokines, insulin receptor activation and oxidative stress can lead to chronic activation of NF-κB (c.f. Granic et al., 2009), which can influence beta-amyloid metabolism and inflammatory cascades; further perpetuating the activation and nuclear translocation of NF-κB (Granic et al., 2009). Inflammatory mediators induced by NF-κB are strongly associated with neurodegenerative diseases. Polymorphisms ofIL-6, a pro-inflammatory cytokine, have been linked to AD susceptibility (Combarros et al., 2009), particularly in the presence of TNF-α polymorphism (Vural et al., 2009), and elevated plasma IL-6 levels have been found in AD patients (Bermejo et al., 2008; Engelhart et al., 2004; Licastro et al., 2000; Singh and Guthikonda, 1997; ) with protein levels contributing to a 6-protein AD signature (Gomez Ravetti and Moscato, 2008). Polymorphisms in the acute phase (TNF-α) have been linked to AD risk (Gnjec et al., 2008; Randall et al, 2009;), TNF-α over-expression has been associated with a decline in learning capabilities (Aloe et al., 1999) and elevated plasma TNF-α levels have been found in AD patients (Bermejo et al., 2008) with protein levels contributing to several recent AD protein signatures (Ray et al., 2007; Ravetti and Moscato, 2008; Soares et al., 2009). Further, elevated levels of C Reactive Protein have been associated with an increased risk of AD (Engelhart et al., 2004; Schmidt et al., 2002;).
Therapeutic use of non-steroidal anti-inflammatory drugs (NSAID) to stop the progression of AD is a controversial topic. Although NSAID use has been found to decrease beta amyloid aggregation (Thomas et al., 2001) and slow MCI conversion to dementia (Gomez-Isla et al., 2008), the inability of clinical trials to arrest the progression of the disease (Aisen et al., 2003; Feldman et al., 2007) indicates the multi-factorial nature of the disease and/or the need for interventions to target neurodegeneration at its earliest stages (Marchesi 2011). Recently, however, promising progress has been made on the use of immune-based treatments for cognitive decline associated with neurological conditions (c.f. Hughes et al., 2009). As described, substantial evidence implicates the role of inflammatory mediators in age-related cognitive impairment and AD, although the precise mechanisms remain unresolved.
4.2 Oxidative Stress
Akin to inflammatory processes, oxidative stress pathways and markers have been associated with both age-related cognitive decline and neurodegeneration associated with non-normative age-related changes. A popular molecular hypothesis for age-related changes is the oxidative stress theory of aging, which stems from the Free Radical Theory of Aging, proposed by D. Harman in 1956. The oxidative stress theory posits that the balance between the production and scavenging of ROS determines the state of oxidative stress within a cell. It is now understood that mitochondria are major intracellular sources of damaging ROS, which promote damage to cellular proteins, lipids and DNA (c.f. Bokov et al., 2004) as well as mitochondrial DNA and oxidative phosphorylation machinery. If left unrepaired, oxidative stress may have pervasive detrimental effects on aging-related mechanisms, such as cell senescence, inflammation and mitochondrial dysfunction (c.f. Vitale et al., 2013). Furthermore, extant literature identifies a potential causal link between ROS-mediated impaired mitochondrial respiration and cognitive decline (c.f., Chakrabarti et al., 2011). t The brain, in particular, is highly susceptible to oxidative damage due to high oxygen consumption rates, a high proportion of polyunsaturated fatty acids that are targeted for lipid peroxidation, and naturally low levels of antioxidants. Consequently, the link between oxygen radicals, such as superoxide and hydrogen peroxide, and both age-related normative cognitive change and non-normative neurodegeneration has long since been recognized.
Like inflammation, much literature exists which link oxidative stress to age-related ailments, such as diabetes and cardiovascular disease, and associate heightened oxidative stress with age-related cognitive decline. Oxidative stress levels have been found to increase with age, corresponding to an age-related decrease in antioxidant capacity (Rodrigues Siqueira et al., 2005). This highlights oxidative stress as a likely process involved in the initiation of cognitive decline. As such, oxidative stress pathways and markers have been widely associated with age-related cognitive decline. Age-related memory and cognitive decline has been associated with a decrease in brain and plasma anti-oxidant levels (Akbaraly et al., 2007; Rinaldi et al., 2003; ) and an increase in oxidative stress levels (Torres et al., 2011). Furthermore, neuroproteomic analysis of the aging hippocampus indicates that there is a decreased expression of anti-oxidant proteins, which likely aids in the increase in hippocampal oxidative stress and associated inflammation often found in both normative aging and age-related neurodegeneration (c.f. VanGuilder and Freeman, 2011).
The link between oxidative stress and neurodegeneration has plenty of empirical support. It has been found that oxidatively damaged lipid membranes in the brain promote the aggregation of amyloid beta proteins, which in turn promote lipid oxidation and perpetuates beta amyloid misfolding and aggregation in AD (Murray et al., 2005). Moreover, plasma levels of antioxidant enzymes have been found to be decreased in both AD and MCI patients compared to controls (Guidi et al., 2006) and levels of oxidized proteins, such as malondialdehyde and protein carbonyls, have been found at increased levels in AD patients (Keller et al., 2005). Although ROS can potentially affect all classes of biomolecules, their detriment to nucleic acids is particularly worrisome, as this may impart long-term changes in the host (Marchesi, 2011). As such, pronounced increases in DNA damage (Mullaart et al., 1990; Su et al., 1997), a lower capacity for DNA repair (c.f. Boerrigter et al., 1992) and increased RNA oxidation (Nunomura et al., 1999) have all been found in patients suffering from AD. Further, levels of oxidative stress by-products are highly associated with neurodegeneration. Paraoxonase1 (PON1), an antioxidant enzyme which is responsible for the protective effects of HDL on LDL peroxidation, has been implicated in the pathogenesis of AD (Dantoine et al., 2002; Erlich et al., 2006) and PON1 polymorphisms are associated with the progression of white matter lesions in the elderly (Schmidt et al., 2000). Furthermore, isoprostanes, which are potent inflammatory mediators induced by oxidative stress-mediated lipid peroxidation, are present at high levels in the CSF of MCI (Brys et al., 2009) and AD patients (Pratico et al., 1998), are increased in many brain regions of patients with MCI and AD (Marksbery et al., 2005) and are associated with MCI to AD conversion (Brys et al., 2009).
Evidence from investigations into the implications of a redox imbalance during aging indicates that NF-κB is activated by ROS and finely balanced by both inflammatory and antioxidant pathways (c.f. Lavrovsky et al., 2000). The NF-κB transcription factor, responsible for regulating the transcription of genes involved in inflammatory responses, is altered in response to oxidative stress (Adcock et al., 1994) as well as age-related increases in ROS production (Kim et al., 2000). Oxidative stress may therefore be involved in the initiation and/or propagation of inflammatory responses associated with age-related cognitive decline or AD-related neurodegeneration.
4.3 Vascular Health
Like inflammatory and oxidative stress pathways, the detrimental effects of poor vascular health is empirically linked to both normative and neurodegenerative cognitive decline. Aging is associated with changes in the vascular system. Vascular health factors play a critical role in many age-related ailments, such as cardiovascular disease, obesity, diabetes, chronic kidney disease and cancer. Epidemiological studies strongly associate deteriorating vascular health with cognitive deficits. High blood pressure, particularly systolic blood pressure, at midlife (Knecht et al., 2009; Swan et al., 1998;), plasma aldosterone (Yagi et al., 2011), a risk factor for cardio- and cerebrovascular disease, hyperlipidemia, and metabolic syndrome (Dik et al., 2007; Raffaitin et al., 2011), have all been associated with cognitive decline in late life. High blood levels of homocystein, a risk factor for cardiovascular disease and contributor to impaired DNA repair associated with beta amyloid-induced oxidative stress (Kruman et al., 2002), have been linked with dementia risk (Seshadri et al., 2002). Increasing evidence points towards a link between cholesterol homeostasis and cognitive decline. High mid-life plasma cholesterol and low density lipoprotein levels have been associated with late-life cognitive decline (Helzner et al., 2009), and research suggests a link between cholesterol-related genes, such as 3-hydroxy-3-methylglutaryl1-coenzyme A synthase 2 (HMGCS2), an enzyme involved in sterol biosynthesis, and farnesyl diphosphate synthase (FDPS), an enzyme involved in providing cholesterol to cells, and risk for AD (Wollmer et al., 2007). Further, even the presence of subclinical vascular disease, indexed using measures such as arm-ankle systolic blood pressure ratio and internal carotid artery thickness, is predictive of incident cardiovascular disease (Newman et al., 1993), stroke, frailty, mortality as well as physical and cognitive decline (Chaves et al., 2004).
APOE ε4, a protein which coordinates cholesterol transport in nerves (Boyles et al., 1989) and plays a critical role in hippocampal cell membrane remodelling following injury (Poirier et al., 1991), is associated with diseases involving the transport or metabolism of lipids, such as diabetes mellitus, high cholesterol and cardiovascular disease (c.f. Martins et al., 2006) as well as age-related cognitive impairment (Caselli et al., 2009; Dixon et al., 2014), vascular dementia (Hebert et al., 2000) and AD (Corder et al., 1993; Saunders et al., 1993;). In AD, APOE ε4 is involved in many of the pathological events, such as beta amyloid generation, deposition and elimination, neurofibrillary tangle formation, APP expression and production, and inflammation (c.f. Leoni, 2011) with AD risk in particular linked to allele phase (Lescai et al., 2011).
Cerebrovascular abnormalities are concomitant with presence of cognitive deficits and dementia. Correspondingly, individuals with a history of cerebrovascular infarcts are at risk for subsequent declines in memory and AD (Snowdon et al., 1997). Changes in vascular density have been found in the hippocampus and basal forebrain regions of AD brains (Fischer et al., 1990). Vascular changes have been associated with a reduced threshold for AD diagnosis amongst non-symptomatic individuals with AD pathology, and cerebral infarctions have been associated with greater dementia severity (Heyman et al., 1998). White matter hyperintensities (WMHs), associated with a history of hypertension, stroke, and diabetes (Fazekas et al., 1993), have been specifically linked with declines in frontal lobe functioning (Burton et al., 2004). In addition, WMHs may be the result of chronic kidney disease (Takahashi et al., 2010), recently found to be a risk factor for cognitive impairment (Etgen et al., 2009); further demonstrating the likely co-morbid nature of age-related cognitive decline. Correspondingly, it was recently suggested that oxidative stress, along with neuroinflammation and somatic mutations of APP, contributes to the neurovascular origin of AD (Marchesi, 2011). It is conceivable that genetic susceptibilities, such as APOE status, interact with oxidative stress and inflammation to initiate and propagate neurodegeneration.
5. Conclusions
A primary objective in this review is to foster a significant theoretical step forward in identifying early markers and development of multivariate models that increase our ability to detect those at risk of cognitive decline and impairment at early disease phases. Although there are a wealth of indicators that can index inflammation, oxidative stress and vascular health, we reviewed evidence substantiating the need to incorporate such key biological processes in predictive models of aging-related cognitive change. To advance our understanding of the causes of and contributors to age-related cognitive deficits, operationalizations of developmental time must move away from using CA as a sole predictor. Increased knowledge gleaned from basic science research outlining critical biological processes associated with neuropathology, such as inflammation, oxidative stress or vascular health, should be incorporated into predictive models of cognitive change. Although heuristically useful for indexing age-related cognitive and functional change, CA is but an indirect reflection of accumulated biological and environmental influences. As such, CA is not a causal mechanism underlying cognitive and functional decline, but rather a temporal dimension along which causal factors operate. Arguably, supplementation and perhaps supplantation of chronological age with theoretically linked biological and health variables will help advance our understanding of the mechanisms underlying age-related cognitive change.
Despite the focus on aetiological mechanisms, moderators of cognitive health are of importance. Exposure to environmental stressors, epigenetic modifications, disease comorbidities or general health ailments, for example, likely play an important role in late-life cognitive function. Frailty research highlights that a diverse range of health deficits, themselves not known for being risk factors for dementia, can be combined as a multivariate frailty index to identify those at risk of not only death (due to all causes) but dementia as well (Song et al., 2011). The fact that a related but novel frailty index comprised of 19 “nontraditional” risk factors can predict dementia status, independent of traditional risk factors such as cognitive deficit, suggests that accumulating deficits in general bodily health warrants further attention. Overall health may predispose those already at risk for dementia to an accelerated disease course, and as such, may represent an early precipitating factor whereby none of the individual components play a particularly central role. That is, declines in general health or increased frailty may be a harbinger of cognitive deterioration. Likewise, in a recent dementia review (Grand et al., 2011), it was argued that until the precise underlying mechanisms are known, attention should be placed on the maintenance of general health. Although the issue of mechanism(s) of cognitive decline remains important, recent theorizing underscores the importance of improving the general health of those at greatest risk and suggests that such a focus may be as beneficial as targeting specific etiologic mechanisms (Dartigues and Feart, 2011; Song et al., 2011).
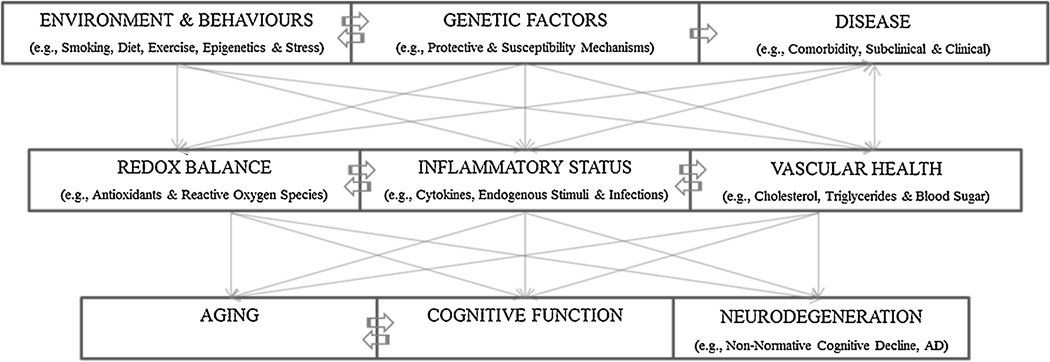
An emergent area in cognitive aging research concerns the implementation of multivariate and interactive approaches. . As such, an individual’s level of cognitive function could be best understood as a result of a complex interaction between host genetic, biological and environmental risk and protective factors across the lifespan (Figure 1). Given that a diversity of biomarker predictors have been shown to exert differential influences on cognitive performance, as well as the many specific relations documented between cognition and brain structure and function, the search for single causal mechanisms of age-related declines in cognition appears less tenable than multi-causal, interactive explanations. As such, many investigations into age-related changes have utilized multivariate models, such as the construction of a biological aging score (Nakamura et al., 2003, 2007) and prediction of dementia risk (Kivipelto et al., 2006; Song et al., 2010, 2011), and death (Goffaux et al., 2005).
Figure 1. An interaction model of possible moderating and causal mechanisms contributing to aging, cognitive function and brain neurodegeneration.
Both moderators (i.e., epigenetics, disease comorbidity, DNA repair mechanisms, etc.) and theoretical causal influences (i.e., inflammation, oxidative stress, etc.) are thought to interact with each other over the lifespan, resulting in a unique individual susceptibility to age-related diseases such as cognitive decline or more severe neurodegenerative disorders.
To the extent that BioAge models index health moderators (e.g., general health) as well as causal factors (e.g., anti-oxidant levels, inflammatory status, vascular health), BioAge may more accurately index the true multi-determined, interactive nature of cognitive function; an approach congruent with systems biology, which focuses on the simultaneous interaction between various components or systems and the resulting function/behaviour. To be sure, as part of a targeted, theory-driven, multi-factorial approach, the research design and methodologies used are also particularly critical components. The utility and predictivity of any marker or group of risk-factors rests on the relevance of the indexed biological process(s) (e.g., vascular, redox) to the cognitive outcome under study (MacDonald et al., 2011). In addition, adopting a multivariate approach entails identifying latent factors of biological aging in a structural equation modelling framework. Further, as most biological processes are dynamic and change at different rates across individuals, appropriate longitudinal analytic approaches are required for the accurate measurement of such processes across time, settings and individuals (Hofer and Sliwinski, 2001; Mendes de Leon et al., 2007;). As such, using appropriate methodology, an a priori-driven, multivariate BioAge approach may improve upon existing knowledge of cognitive aging and associated processes, relate cognitive function to biomarkers of development, and, thereby, more accurately predict cognitive change at an early stage by indexing theoretical causes and contributors rather than indexing the continuum upon which these factors operate.
The process of decline likely begins years, if not decades, prior to the onset of detectable symptoms, but detection usually occurs at the point when irreversible neuronal damage has already been established. For example, recent evidence suggests that the early prodromal stage of cognitive impairment and dementia can onset 10 years or more prior to the first clinical signs of cognitive deficit and subsequent diagnosis (Thorvaldsson et al., 2011), but that the onset of such decline can be delayed by select factors (e.g., Hall et al., 2009). Recent data from the U.S. indicate that delaying the clinical manifestation of dementia by 2 years would reduce the total number of AD cases by 600,000 or more. As such, early identification of high-risk populations may be essential for the implementation of early prevention strategies which could slow disease progression, lead to better disease outcomes and lessen the impact of cognitive impairment or decline on patients and their families. Implementing a multivariate, mechanism-based approach to the early identification of at-risk individuals is an exciting and promising enterprise.
Highlights.
A theoretical step forward in identifying early markers of cognitive decline
Emphasizes multivariate models for predicting cognitive impairment in late life
Identifies theoretically relevant biological processes of cognitive decline
Proposition of a novel BioAge operationalization of late-life cognitive decline
Acknowledgements
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (418676–2012) and a Scholar Career Investigator Award from the Michael Smith Foundation for Health Research to S.W.S. MacDonald, the National Institutes of Health/National Institute on Aging (R01 AG 008235) to R.A. Dixon, and a doctoral training award from the Alzheimer’s Society of Canada to C. A. DeCarlo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock IM, Brown CR, Kwon O, Barnes PJ. Oxidative stress induces NF kappa B DNA binding and inducible NOS mRNA in the human epithelial cell line A549. Biochemical Society Transactions. 1994;22(2):186S. doi: 10.1042/bst022186s. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL Alzheimer's Disease Cooperative Study. Effects of rofecoxib or naproxen vs placebo on alzheimer disease progression: A randomized controlled trial. JAMA : The Journal of the American Medical Association. 2003;289(21):2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Akbaraly NT, Faure H, Gourlet V, Favier A, Berr C. Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA study. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2007;62(3):308–316. doi: 10.1093/gerona/62.3.308. [DOI] [PubMed] [Google Scholar]
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychology and Aging. 1995;10(4):578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- Aloe L, Properzi F, Probert L, Akassoglou K, Kassiotis G, Micera A, Fiore M. Learning abilities, NGF and BDNF brain levels in two lines of TNF-alpha transgenic mice, one characterized by neurological disorders, the other phenotypically normal. Brain Research. 1999;840(1–2):125–137. doi: 10.1016/s0006-8993(99)01748-5. [DOI] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ. Alcohol exposure and cognitive development: An example of why we need a contextualized, dynamic life course approach to cognitive aging--a mini-review. Gerontology. 2008;54(5):283–291. doi: 10.1159/000161735. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, Giles LC, Andrews GR. Demographic, health, cognitive, and sensory variables as predictors of mortality in very old adults. Psychology and Aging. 2001;16(1):3–11. doi: 10.1037/0882-7974.16.1.3. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Smith GA. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychology and Aging. 1999;14(4):605–618. doi: 10.1037//0882-7974.14.4.605. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Lord SR, Williams P. Strength in the lower limbs, visual contrast sensitivity, and simple reaction time predict cognition in older women. Psychology and Aging. 1997;12(1):137–144. doi: 10.1037//0882-7974.12.1.137. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Lord SR, Smith GA. Measuring human functional age: A review of empirical findings. Experimental Aging Research. 1996;22(3):245–266. doi: 10.1080/03610739608254010. [DOI] [PubMed] [Google Scholar]
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment--the relationship between physical and cognitive function. Neuroepidemiology. 2008;31(3):167–173. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistreri CR, Candore G, Listi F, Fazio T, Gangi S, Incalcaterra E, Caruso C. Role of TLR4 polymorphisms in inflammatory responses: Implications for unsuccessful aging. Annals of the New York Academy of Sciences. 2007;1119:203–207. doi: 10.1196/annals.1404.003. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Villar AM. Differences of peripheral inflammatory markers between mild cognitive impairment and alzheimer's disease. Immunology Letters. 2008;117(2):198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of alzheimer disease genetic association studies: The AlzGene database. Nature Genetics. 2007;39(1):17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clinical Pharmacology and Therapeutics. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Birren JE. Theories of aging: A personal perspective. In: Bengtson VL, Schaie KW, editors. Handbook of theories of aging. New York: Springer; 1999. [Google Scholar]
- Birren JE, Cunningham W. Research on the psychology of aging: Principles, concepts and theory. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. New York: Van Nostrand Reinhold; 1985. pp. 3–34. [Google Scholar]
- Birren JE, Tenner VJ. Research on the psychology of aging: Principles and experimentation. In: Birren JE, Smith GA, editors. Handbook of the psychology of aging. N.Y: Van Nostrand Reinhold Company; 1977. pp. 3–38. [Google Scholar]
- Birren JE. Principles of research on aging. In: Birren JE, editor. Handbook of aging and the individual. xii ed. Oxford: Chicago Press; 1959. pp. 3–42. [Google Scholar]
- Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for alzheimer's disease. Molecular Neurobiology. 2001;24(1–3):87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in alzheimer disease? Molecular and Chemical Neuropathology / Sponsored by the International Society for Neurochemistry and the World Federation of Neurology and Research Groups on Neurochemistry and Cerebrospinal Fluid. 1995;26(3):231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- Boerrigter ME, Wei JY, Vijg J. DNA repair and alzheimer's disease. Journal of Gerontology. 1992;47(6):B177–B184. doi: 10.1093/geronj/47.6.b177. [DOI] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mechanisms of Aging and Development. 2004;125(10–11):811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Zoellner CD, Anderson LJ, Kosik LM, Pitas RE, Weisgraber KH, Ignatius MJ. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. The Journal of Clinical Investigation. 1989;83(3):1015–1031. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Petersen RC, Smith GE, Kenney AE, Gross CJ, Taub ES. Neuropsychology. 2013;27(1):86–94. doi: 10.1037/a0030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretsky P, Guralnik JM, Launer L, Albert M, Seeman TE MacArthur Studies of Successful Aging. The role of APOE-epsilon4 in longitudinal cognitive decline: MacArthur studies of successful aging. Neurology. 2003;60(7):1077–1081. doi: 10.1212/01.wnl.0000055875.26908.24. [DOI] [PubMed] [Google Scholar]
- Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, de Leon MJ. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiology of Aging. 2009;30(5):682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulpitt CJ. Assessing biological age: Practicality? Gerontology. 1995;41(6):315–321. doi: 10.1159/000213701. [DOI] [PubMed] [Google Scholar]
- Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Annals of the New York Academy of Sciences. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. The New England Journal of Medicine. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. Journal of Neuropathology and Experimental Neurology. 2008;67(6):523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves PH, Kuller LH, O'Leary DH, Manolio TA, Newman AB Cardiovascular Health Study. Subclinical cardiovascular disease in older adults: Insights from the cardiovascular health study. The American Journal of Geriatric Cardiology. 2004;13(3):137–151. doi: 10.1111/j.1076-7460.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Munshi S, Banerjee K, Thakurta IG, Sinha M, Bagh MB. Mitochondrial dysfunction during brain aging: role of oxidative stress and modulation by antioxidant supplementation. Aging and Disease. 2011;2(3):242–256. [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Reglade-Meslin C, Kumar R, Jacomb P, Easteal S, Christensen H, Anstey KJ. Risk factors of transition from normal cognition to mild cognitive disorder: The PATH through life study. Dementia and Geriatric Cognitive Disorders. 2009;28(1):47–55. doi: 10.1159/000229025. [DOI] [PubMed] [Google Scholar]
- Christensen H, Korten AE, Mackinnon AJ, Jorm AF, Henderson AS, Rodgers B. Are changes in sensory disability, reaction time, and grip strength associated with changes in memory and crystallized intelligence? A longitudinal analysis in an elderly community sample. Gerontology. 2000;46(5):276–292. doi: 10.1159/000022172. [DOI] [PubMed] [Google Scholar]
- Combarros O, van Duijn CM, Hammond N, Belbin O, Arias-Vasquez A, Cortina-Borja M, Lehmann DJ. Replication by the epistasis project of the interaction between the genes for IL-6 and IL-10 in the risk of alzheimer's disease. Journal of Neuroinflammation. 2009;6:22. doi: 10.1186/1742-2094-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort A. Test-battery to measure aging-rate in man. Lancet. 1969;2(7635):1411–1414. doi: 10.1016/s0140-6736(69)90950-7. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of alzheimer's disease in late onset families. Science (New York, N.Y. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends in Immunology. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Dantoine TF, Drouet M, Debord J, Merle L, Cogne M, Charmes JP. Paraoxonase 1 192/55 gene polymorphisms in alzheimer's disease. Annals of the New York Academy of Sciences. 2002;977:239–244. doi: 10.1111/j.1749-6632.2002.tb04821.x. [DOI] [PubMed] [Google Scholar]
- Dartigues JF, Feart C. Risk factors for alzheimer disease: Aging beyond age? Neurology. 2011;77(3):206–207. doi: 10.1212/WNL.0b013e31822550af. [DOI] [PubMed] [Google Scholar]
- Davies G, Harris SE, Reynolds CA, Payton A, Knight HM, Liewalk DC, Lopez LM. A genome-wide association study implicates the APOE locus in non-pathological cognitive aging. Mol Psychiatry. 2014;19(1):76–87. doi: 10.1038/mp.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-aging and lifelong antigenic load as major determinants of aging rate and longevity. FEBS Letters. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- DeCarlo CA, Rosa B, Jackson RJ, Niccoli S, Escott NG, Zehbe I. Toll-like receptor transcriptome in the HPV-positive cervical cancer microenvironment. Clinical and Developmental Immunology. 2012 doi: 10.1155/2012/785825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo CA, Severini A, Edler L, Escott NG, Lambert PF, Ulanova M, Zehbe I. IFN-kappa, a novel type I IFN, is undetectable in HPV-positive human cervical keratinocytes. Laboratory Investigation; a Journal of Technical Methods and Pathology. 2010;90(10):1482–1491. doi: 10.1038/labinvest.2010.95. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Comijs HC, Deeg DJ, Kok A, Yaffe K, Penninx BW. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007;30(10):2655–2660. doi: 10.2337/dc06-1190. [DOI] [PubMed] [Google Scholar]
- Dixon RA, DeCarlo CA, MacDonald SWS, Vergote D, Jhamandas J, Westaway D. APOE and COMT polymorphisms are complementary biomarkers of status, stability, and transitions in normal aging and early mild cognitive impairment. Front. Aging Neurosci. 2014 doi: 10.3389/fnagi.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA. Enduring theoretical themes in psychological aging: Derivation, functions, perspectives, and opportunities. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 7th Edition ed. New York: Academic Press; 2011. [Google Scholar]
- Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. an immunoperoxidase study. Acta Neuropathologica. 1982;57(2–3):239–242. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- Elias MF, D'Agostino RB, Elias PK, Wolf PA. Neuropsychological test performance, cognitive functioning, blood pressure, and age: The framingham heart study. Experimental Aging Research. 1995;21(4):369–391. doi: 10.1080/03610739508253991. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: The rotterdam study. Archives of Neurology. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Erlich PM, Lunetta KL, Cupples LA, Huyck M, Green RC, Baldwin CT MIRAGE Study Group. Polymorphisms in the PON gene cluster are associated with alzheimer disease. Human Molecular Genetics. 2006;15(1):77–85. doi: 10.1093/hmg/ddi428. [DOI] [PubMed] [Google Scholar]
- Etgen T, Sander D, Chonchol M, Briesenick C, Poppert H, Forstl H, Bickel H. Chronic kidney disease is associated with incident cognitive impairment in the elderly: The INVADE study. Nephrology, Dialysis, Transplantation : Official Publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(10):3144–3150. doi: 10.1093/ndt/gfp230. [DOI] [PubMed] [Google Scholar]
- Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR., Jr Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiology of Aging. 2012;33(7):1203–1214. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Archives of Neurology. 2007;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, Cozzi E, Paganelli R. Increased cytokine production in mononuclear cells of healthy elderly people. European Journal of Immunology. 1993;23(9):2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, Lane R. Effect of rivastigmine on delay to diagnosis of alzheimer's disease from mild cognitive impairment: The InDDEx study. Lancet Neurology. 2007;6(6):501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M Alzheimer's Disease International. Global prevalence of dementia: A delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- Fischer O. Die presbyophrene demenz, deren anatomische grundlage und klinische abgrenzung. Z Ges Neurol u Psychiat. 1910;3:371–471. [Google Scholar]
- Fischer VW, Siddiqi A, Yusufaly Y. Altered angioarchitecture in selected areas of brains with alzheimer's disease. Acta Neuropathologica. 1990;79(6):672–679. doi: 10.1007/BF00294246. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Salvioli S. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of Aging and Development. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. an evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Glass JM. Visual function and cognitive aging: Differential role of contrast sensitivity in verbal versus spatial tasks. Psychology and Aging. 2007;22(2):233–238. doi: 10.1037/0882-7974.22.2.233. [DOI] [PubMed] [Google Scholar]
- Gnjec A, D'Costa KJ, Laws SM, Hedley R, Balakrishnan K, Taddei K, Martins RN. Association of alleles carried at TNFA-850 and BAT1 -22 with alzheimer's disease. Journal of Neuroinflammation. 2008;5:36. doi: 10.1186/1742-2094-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffaux J, Friesinger GC, Lambert W, Shroyer LW, Moritz TE, McCarthy M, Hammermeister KE., Jr Biological age--a concept whose time has come: A preliminary study. Southern Medical Journal. 2005;98(10):985–993. doi: 10.1097/01.smj.0000182178.22607.47. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Blesa R, Boada M, Clarimon J, Del Ser T, Domenech G TRIMCI Study Group. A randomized, double-blind, placebo controlled-trial of triflusal in mild cognitive impairment: The TRIMCI study. Alzheimer Disease and Associated Disorders. 2008;22(1):21–29. doi: 10.1097/WAD.0b013e3181611024. [DOI] [PubMed] [Google Scholar]
- Grand JH, Caspar S, Macdonald SW. Clinical features and multidisciplinary approaches to dementia care. Journal of Multidisciplinary Healthcare. 2011;4:125–147. doi: 10.2147/JMDH.S17773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Ravetti M, Moscato P. Identification of a 5-protein biomarker molecular signature for predicting alzheimer's disease. PloS One. 2008;3(9):e3111. doi: 10.1371/journal.pone.0003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granic I, Dolga AM, Nijholt IM, van Dijk G, Eisel UL. Inflammation and NF-kappaB in alzheimer's disease and diabetes. Journal of Alzheimer's Disease : JAD. 2009;16(4):809–821. doi: 10.3233/JAD-2009-0976. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in alzheimer's disease: Significance in plaque evolution. Journal of Neuropathology and Experimental Neurology. 1995;54(2):276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Guidi I, Galimberti D, Lonati S, Novembrino C, Bamonti F, Tiriticco M, Scarpini E. Oxidative imbalance in patients with mild cognitive impairment and alzheimer's disease. Neurobiology of Aging. 2006;27(2):262–269. doi: 10.1016/j.neurobiolaging.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73(5):356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Aging: A theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with alzheimer's disease. Nature Genetics. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends in Cognitive Sciences. 2011;15(9):388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Norton MC, Darcey D, Ostbye T, Zandi PP, Breitner JC Cache County Study Investigators. Occupational exposure to pesticides increases the risk of incident AD: The cache county study. Neurology. 2010;74(19):1524–1530. doi: 10.1212/WNL.0b013e3181dd4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Contribution of vascular risk factors to the progression in alzheimer disease. Archives of Neurology. 2009;66(3):343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, Pieper CF. Cerebral infarcts in patients with autopsy-proven alzheimer's disease: CERAD, part XVIII. consortium to establish a registry for alzheimer's disease. Neurology. 1998;51(1):159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Sliwinski MJ. Understanding aging. an evaluation of research designs for assessing the interdependence of aging-related changes. Gerontology. 2001;47(6):341–352. doi: 10.1159/000052825. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, Williams J. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with alzheimer's disease. Nature Genetics. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science (New York, N.Y. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Dalakas MC, Cornblath DR, Latov N, Weksler ME, Relkin N. Clinical applications of intravenous immunoglobulins in neurology. Clinical and Experimental Immunology. 2009;158(Suppl 1):34–42. doi: 10.1111/j.1365-2249.2009.04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK. Toward the behavioral assessment of biological aging in the laboratory mouse: Concepts, terminology, and objectives. Experimental Aging Research. 1983;9(4):p225–p238. doi: 10.1080/03610738308258457. [DOI] [PubMed] [Google Scholar]
- Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, Newman AB. Long-term assessment of inflammation and healthy aging in late life: The cardiovascular health study all stars. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2012;67(9):970–976. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel HK. Sarcopenia and aging. Nutrition Reviews. 2003;61(5 Pt 1):157–167. doi: 10.1301/nr.2003.may.157-167. [DOI] [PubMed] [Google Scholar]
- Kaiser E, Schoenknecht P, Kassner S, Hildebrandt W, Kinscherf R, Schroeder J. Cerebrospinal fluid concentrations of functionally important amino acids and metabolic compounds in patients with mild cognitive impairment and alzheimer's disease. Neuro-Degenerative Diseases. 2010;7(4):251–259. doi: 10.1159/000287953. [DOI] [PubMed] [Google Scholar]
- Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. Journal of the American College of Cardiology. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64(7):1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim KW, Yu BP, Chung HY. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free Radical Biology & Medicine. 2000;28(5):683–692. doi: 10.1016/s0891-5849(99)00274-9. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurology. 2006;5(9):735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Langstrom B. Imaging brain amyloid in alzheimer's disease with pittsburgh compound-B. Annals of Neurology. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knecht S, Wersching H, Lohmann H, Berger K, Ringelstein EB. How much does hypertension affect cognition?: Explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. Journal of the Neurological Sciences. 2009;283(1–2):149–152. doi: 10.1016/j.jns.2009.02.362. [DOI] [PubMed] [Google Scholar]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of alzheimer's disease. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2002;22(5):1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with alzheimer's disease. Nature Genetics. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Experimental Gerontology. 2000;35(5):521–532. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- Leoni V. The effect of apolipoprotein E (ApoE) genotype on biomarkers of amyloidogenesis, tau pathology and neurodegeneration in alzheimer's disease. Clinical Chemistry and Laboratory Medicine : CCLM / FESCC. 2011;49(3):375–383. doi: 10.1515/CCLM.2011.088. [DOI] [PubMed] [Google Scholar]
- Lescai F, Chiamenti AM, Codemo A, Pirazzini C, D’Agostino G, Ruaro C, Ghidoni L. An APOE haplotype associated with decreased ε4 expression increases the risk of late onset alzheimer’s disease. Journal of Alzheimer’s disease. 2011;24:235–245. doi: 10.3233/JAD-2011-101764. [DOI] [PubMed] [Google Scholar]
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin in patients with alzheimer's disease: Peripheral inflammation or signals from the brain? Journal of Neuroimmunology. 2000;103(1):97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging. 1994;9(3):339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lotz M, Ebert S, Esselmann H, Iliev AI, Prinz M, Wiazewicz N, Nau R. Amyloid beta peptide 1–40 enhances the action of toll-like receptor-2 and -4 agonists but antagonizes toll-like receptor-9-induced inflammation in primary mouse microglial cell cultures. Journal of Neurochemistry. 2005;94(2):289–298. doi: 10.1111/j.1471-4159.2005.03188.x. [DOI] [PubMed] [Google Scholar]
- Ludwig FC, Smoke ME. The measurement of biological age. Experimental Aging Research. 1980;6(6):497–522. doi: 10.1080/03610738008258384. [DOI] [PubMed] [Google Scholar]
- MacDonald SW, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: Towards improving characterizations of developmental time. Journal of Gerontology: Psychological Sciences. 2011;66(Suppl 1):59–70. doi: 10.1093/geronb/gbr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: Findings from the victoria longitudinal study. Gerontology. 2004;50(2):64–81. doi: 10.1159/000075557. [DOI] [PubMed] [Google Scholar]
- Marchesi VT. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: Implications for early detection and therapy. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2011;25(1):5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. Journal of the American Geriatrics Society. 2009;57(2):225–230. doi: 10.1111/j.1532-5415.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Annals of Neurology. 2005;58(5):730–735. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for alzheimer's disease and cardiovascular disease. Molecular Psychiatry. 2006;11(8):721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Innate immunity in alzheimer's disease: A model for local inflammatory reactions. Molecular Interventions. 2001;1(1):22–29. [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neuroscience Letters. 1987;79(1–2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- Mendes de Leon CF. Aging and the elapse of time: A comment on the analysis of change. The Journals of Gerontology.Series B, Psychological Sciences and Social Sciences. 2007;62(3):S198–S202. doi: 10.1093/geronb/62.3.s198. [DOI] [PubMed] [Google Scholar]
- Mondadori CR, de Quervain DJ, Buchmann A, Mustovic H, Wollmer MA, Schmidt CF, Henke K. Better memory and neural efficiency in young apolipoprotein E epsilon4 carriers. Cerebral Cortex (New York, N.Y.: 1991) 2007;17(8):1934–1947. doi: 10.1093/cercor/bhl103. 1991. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau alzheimer pathology in cognitively normal aging. Annals of Neurology. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaart E, Boerrigter ME, Ravid R, Swaab DF, Vijg J. Increased levels of DNA breaks in cerebral cortex of alzheimer's disease patients. Neurobiology of Aging. 1990;11(3):169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- Murray IV, Sindoni ME, Axelsen PH. Promotion of oxidative lipid membrane damage by amyloid beta proteins. Biochemistry. 2005;44(37):12606–12613. doi: 10.1021/bi050926p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset alzheimer's disease. Nature Genetics. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2007;62(10):1096–1105. doi: 10.1093/gerona/62.10.1096. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Miyao K. Further evaluation of the basic nature of the human biological aging process based on a factor analysis of age-related physiological variables. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences. 2003;58(3):196–204. doi: 10.1093/gerona/58.3.b196. [DOI] [PubMed] [Google Scholar]
- Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. cardiovascular heart study (CHS) collaborative research group. Circulation. 1993;88(3):837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- Nordberg A. Amyloid imaging in early detection of alzheimer's disease. Neuro-Degenerative Diseases. 2010;7(1–3):136–138. doi: 10.1159/000289223. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in alzheimer's disease. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1999;19(6):1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Research.Molecular Brain Research. 1991;11(2):97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- Pratico D, MY Lee V, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in alzheimer's disease: Evidence for enhanced lipid peroxidation in vivo. The FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 1998;12(15):1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- Raffaitin C, Feart C, Le Goff M, Amieva H, Helmer C, Akbaraly TN, Barberger-Gateau P. Metabolic syndrome and cognitive decline in french elders: The three-city study. Neurology. 2011;76(6):518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- Randall CN, Strasburger D, Prozonic J, Morris SN, Winkie AD, Parker GR, Poduslo SE. Cluster analysis of risk factor genetic polymorphisms in alzheimer's disease. Neurochemical Research. 2009;34(1):23–28. doi: 10.1007/s11064-008-9626-8. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Wyss-Coray T. Classification and prediction of clinical alzheimer's diagnosis based on plasma signaling proteins. Nature Medicine. 2007;13(11):1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in alzheimer's disease. Neurobiology of Aging. 2003;24(7):915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertension Research : Official Journal of the Japanese Society of Hypertension. 2011;34(4):431–440. doi: 10.1038/hr.2010.264. [DOI] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in alzheimer's disease: Amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Progress in Neurobiology. 2009;87(3):181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. From the present to the future. commentary on Luszcz and Bryan. Gerontology. 1999;45(6):345–347. doi: 10.1159/000022118. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Alberts MJ. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic alzheimer's disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia aging study. Annals of Neurology. 2002;52(2):168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Fazekas F, Kapeller P, Roob G, Lechner A, Hartung HP. MRI cerebral white matter lesions and paraoxonase PON1 polymorphisms : Three-year follow-up of the austrian stroke prevention study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(7):1811–1816. doi: 10.1161/01.atv.20.7.1811. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, de Craen AJ, Witteman JC, Frolich M, Hofman A, Westendorp RG. Systemic markers of inflammation and cognitive decline in old age. Journal of the American Geriatrics Society. 2007;55(5):708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Schroots JJF, Birren JE. Concepts of time and aging in science. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging 3. San Diego, CA: Academic Press; 1990. pp. 45–64. [Google Scholar]
- Seeman TE, Huang MH, Bretsky P, Crimmins E, Launer L, Guralnik JM. Education and APOE-e4 in longitudinal cognitive decline: MacArthur studies of successful aging. The Journals of Gerontology.Series B, Psychological Sciences and Social Sciences. 2005;60(2):P74–P83. doi: 10.1093/geronb/60.2.p74. [DOI] [PubMed] [Google Scholar]
- Seo JS, Lee KW, Kim TK, Baek IS, Im JY, Han PL. Behavioral stress causes mitochondrial dysfunction via ABAD up-regulation and aggravates plaque pathology in the brain of a mouse model of alzheimer disease. Free Radical Biology and Medicine. 2011;50(11):1526–1535. doi: 10.1016/j.freeradbiomed.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, Wolf PA. Plasma homocysteine as a risk factor for dementia and alzheimer's disease. The New England Journal of Medicine. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- Singh VK, Guthikonda P. Circulating cytokines in alzheimer's disease. Journal of Psychiatric Research. 1997;31(6):657–660. doi: 10.1016/s0022-3956(97)00023-x. [DOI] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L. Apolipoprotein E and cognitive performance: A meta-analysis. Psychology and Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Small GW, Siddarth P, Kepe V, Ercoli LM, Burggren AC, Bookheimer SY, Barrio JR. Prediction of cognitive decline by positron emission tomography of brain amyloid and tau. Archives of Neurology. 2012;69(2):215–222. doi: 10.1001/archneurol.2011.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sng J, Meaney MJ. Environmental regulation of the neural epigenome. Epigenomics. 2009;1(1):131–151. doi: 10.2217/epi.09.21. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of alzheimer disease. the nun study. JAMA : The Journal of the American Medical Association. 1997;277(10):813–817. [PubMed] [Google Scholar]
- Soares HD, Chen Y, Sabbagh M, Roher A, Schrijvers E, Breteler M. Identifying early markers of alzheimer's disease using quantitative multiplex proteomic immunoassay panels. Annals of the New York Academy of Sciences. 2009;1180:56–67. doi: 10.1111/j.1749-6632.2009.05066.x. [DOI] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict alzheimer disease and dementia. Neurology. 2011;77(3):227–234. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. Journal of the American Geriatrics Society. 2010;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- Spiro A, Brady CB., III . Integrating health into cognitive aging research and theory: Quo vadis? In: Hofer SM, Alwin DF, editors. The handbook of cognitive aging: Interdisciplinary perspectives. Thousdand Oaks, CA: Sage; 2008. pp. 260–282. [Google Scholar]
- Spulber G, Niskanen E, MacDonald S, Smilovici O, Chen K, Reiman EM, Soininen H. Whole brain atrophy rate predicts progression from MCI to alzheimer's disease. Neurobiology of Aging. 2010;31(9):1601–1605. doi: 10.1016/j.neurobiolaging.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Su JH, Deng G, Cotman CW. Neuronal DNA damage precedes tangle formation and is associated with up-regulation of nitrotyrosine in alzheimer's disease brain. Brain Research. 1997;774(1–2):193–199. doi: 10.1016/s0006-8993(97)81703-9. [DOI] [PubMed] [Google Scholar]
- Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Takahashi W, Tsukamoto Y, Takizawa S, Kawada S, Takagi S. Relationship between chronic kidney disease and white matter hyperintensities on magnetic resonance imaging. Journal of Stroke and Cerebrovascular Diseases : The Official Journal of National Stroke Association. 2010 doi: 10.1016/j.jstrokecerebrovasdis.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Takata Y, Ansai T, Soh I, Kimura Y, Yoshitake Y, Sonoki K, Takehara T. Physical fitness and cognitive function in an 85-year-old community-dwelling population. Gerontology. 2008;54(6):354–360. doi: 10.1159/000129757. [DOI] [PubMed] [Google Scholar]
- Tan L, Schedl P, Song HJ, Garza D, Konsolaki M. The toll-NFkappaB signaling pathway mediates the neuropathological effects of the human alzheimer's Abeta42 polypeptide in drosophila. PloS One. 2008;3(12):e3966. doi: 10.1371/journal.pone.0003966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay T, Wang JJ, Kifley A, Lindley R, Newall P, Mitchell P. Sensory and cognitive association in older persons: Findings from an older australian population. Gerontology. 2006;52(6):386–394. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- Thomas T, Nadackal TG, Thomas K. Aspirin and non-steroidal anti-inflammatory drugs inhibit amyloid-beta aggregation. Neuroreport. 2001;12(15):3263–3267. doi: 10.1097/00001756-200110290-00024. [DOI] [PubMed] [Google Scholar]
- Thorvaldsson V, Macdonald SW, Fratiglioni L, Winblad B, Kivipelto M, Laukka EJ, Backman L. Onset and rate of cognitive change before dementia diagnosis: Findings from two swedish population-based longitudinal studies. Journal of the International Neuropsychological Society : JINS. 2011;17(1):154–162. doi: 10.1017/S1355617710001372. [DOI] [PubMed] [Google Scholar]
- Torres LL, Quaglio NB, de Souza GT, Garcia RT, Dati LM, Moreira WL, Marcourakis T. Peripheral oxidative stress biomarkers in mild cognitive impairment and alzheimer's disease. Journal of Alzheimer's Disease : JAD. 2011;26(1):59–68. doi: 10.3233/JAD-2011-110284. [DOI] [PubMed] [Google Scholar]
- Vanguilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: Past progress and future directions. Frontiers in Aging Neuroscience. 2011;3:8. doi: 10.3389/fnagi.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Salvioli S, Franceschi C. Oxidative stress and the ageing endocrine system. Nature Reviews. Endocrinology. 2013;9(4):228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the lifespan. Journal of Applied Physiology (Bethesda, Md.: 1985) doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vural P, Degirmencioglu S, Parildar-Karpuzoglu H, Dogru-Abbasoglu S, Hanagasi HA, Karadag B, Uysal M. The combinations of TNFalpha-308 and IL-6 -174 or IL-10 -1082 genes polymorphisms suggest an association with susceptibility to sporadic late-onset alzheimer's disease. Acta Neurologica Scandinavica. 2009;120(6):396–401. doi: 10.1111/j.1600-0404.2009.01230.x. [DOI] [PubMed] [Google Scholar]
- Wahlin A, MacDonald SW, deFrias CM, Nilsson LG, Dixon RA. How do health and biological age influence chronological age and sex differences in cognitive aging: Moderating, mediating, or both? Psychology and Aging. 2006;21(2):318–332. doi: 10.1037/0882-7974.21.2.318. [DOI] [PubMed] [Google Scholar]
- Wahlin A. Health, disease, and cognitive aging. In: Dixon RA, Bäckman L, Nilsson LG, editors. New frontiers in cognitive aging. Oxford, UK: Oxford University Press; 2004. pp. 279–302. [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in alzheimer's disease. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology. 2007;20(6):947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Weindruch R. Interventions based on the possibility that oxidative stress contributes to sarcopenia. The Journals of Gerontology.Series A, Biological Sciences and Medical Sciences, 50 Spec No. 1995:157–161. doi: 10.1093/gerona/50a.special_issue.157. [DOI] [PubMed] [Google Scholar]
- Wohlwill JF. The study of behavioral development. New York: Academic Press; 1973. [Google Scholar]
- Wollmer MA, Sleegers K, Ingelsson M, Zekanowski C, Brouwers N, Maruszak A, Papassotiropoulos A. Association study of cholesterol-related genes in alzheimer's disease. Neurogenetics. 2007;8(3):179–188. doi: 10.1007/s10048-007-0087-z. [DOI] [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Zawia NH. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (pb): Evidence for a developmental origin and environmental link for AD. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28(1):3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB Health ABC Study. Predictors of maintaining cognitive function in older adults: The health ABC study. Neurology. 2009;72(23):2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Harris T. Inflammatory markers and cognition in well-functioning african-american and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Yagi S, Akaike M, Aihara K, Iwase T, Yoshida S, Sumitomo-Ueda Y, Sata M. High plasma aldosterone concentration is a novel risk factor of cognitive impairment in patients with hypertension. Hypertension Research : Official Journal of the Japanese Society of Hypertension. 2011;34(1):74–78. doi: 10.1038/hr.2010.179. [DOI] [PubMed] [Google Scholar]
- Zotova E, Nicoll JAR, Kalaria R, Holmes C, Boche D. Inflammation in Alzheimer’s disease: relevance to pathogenesis and therapy. Alzheimer’s Research and Therapy. 2010;2:1. doi: 10.1186/alzrt24. [DOI] [PMC free article] [PubMed] [Google Scholar]