Abstract
Rationale
Platelets contain abundant thymidine phosphorylase (TYMP), which is highly expressed in diseases with high risk of thrombosis, such as atherosclerosis and type II diabetes.
Objective
Test the hypothesis that TYMP participates in platelet signaling and promotes thrombosis.
Methods and Results
By using a ferric chloride (FeCl3) induced carotid artery injury thrombosis model, we found time to blood flow cessation was significantly prolonged in Tymp−/− and Tymp+/− mice compared to wild type (WT) mice. Bone marrow transplantation and platelet transfusion studies demonstrated that platelet TYMP was responsible for the antithrombotic phenomenon in the TYMP deficient mice. Collagen-, collagen-related peptide (CRP)-, adenosine diphosphate-and/or thrombin-induced platelet aggregation were significantly attenuated in Tymp+/− and Tymp−/− platelets, and in WT or human platelets pretreated with TYMP inhibitor KIN59. Tymp deficiency also significantly decreased agonist-induced P-select in expression. TYMP contains an N-terminal SH3 domain binding proline-rich motif and forms a complex with the tyrosine kinases Lyn, Fyn and Yes in platelets. TYMP-associated Lyn was inactive in resting platelets, and TYMP trapped and diminished active Lyn after collagen stimulation. Tymp/Lyn double haploinsufficiency diminished the antithrombotic phenotype of Tymp+/− mice. TYMP deletion or inhibition of TYMP with KIN59 dramatically increased PECAM-1 tyrosine phosphorylation and diminished CRP or collagen induced AKT phosphorylation. In vivo administration of KIN59 significantly inhibited FeCl3 induced carotid artery thrombosis without affecting hemostasis.
Conclusion
TYMP participates in multiple platelet signaling pathways and regulates platelet activation and thrombosis. Targeting TYMP might be a novel anti-platelet and anti-thrombosis therapy.
Keywords: Thymidine phosphorylase, platelet, arterial thrombosis, anti-platelet therapy, cell signaling, thrombosis
INTRODUCTION
Thrombotic events are a major cause of morbidity and mortality in developed nations and remain an important area for new therapeutic discoveries. Inappropriate or uncontrolled platelet activation at site of vascular injury is an important pathogenic component of thrombosis, which causes myocardial infarction or stroke. Therefore, understanding the complex signaling pathways involved in promoting and inhibiting platelet activity remains high priority.
Human platelets contain more than 4000 proteins1, and for the most part the functions of these proteins are not known with certainty. Thymidine phosphorylase (TYMP) was initially purified from human platelets, and each human platelet contains about 116,000 copies of TYMP1. TYMP reversibly converts thymidine to thymine and 2-deoxy-D-ribose-1-phosphate (2DDRP)2, and the latter sugar is further degraded to 2-deoxy-D-ribose (2DDR). TYMP thus maintains the nuclear pool of these molecules and contributes to the nucleotide salvage pathway. In 1987 a potent angiogenic protein, platelet-derived endothelial cell growth factor, was purified from human platelets3 and was later shown to be identical to human TYMP4. The angiogenic effect of TYMP5 is supported by observations that TYMP expression in tumors is associated with enhanced-angiogenesis6,7. The angiogenic effect of TYMP is believed to be mediated by its mobilizing effect on endothelial cells, but the actual mechanism(s) remain undefined6,8.
We have found that direct injection of a plasmid vector encoding human TYMP into ischemic canine myocardium or rabbit hindlimb promotes angiogenesis in cardiac and skeletal muscle5,9,10 and improves myocardial function and prevents hindlimb necrosis. We also found that TYMP inhibited vascular smooth muscle cell (VSMC) proliferation and migration11–13. The inhibitory role of TYMP on VSMC is partly mediated by regulating the phosphorylation of Lyn12, an important non-receptor tyrosine kinase of the Src family. TYMP is also expressed in monocytes/macrophages and stromal cells, suggesting that it may have additional roles in the circulatory systems. Interestingly, although TYMP was initially isolated from platelets, its role in platelet physiology is largely unexplored.
In this study we examined the hypothesis that TYMP participates in platelet activation and promotes thrombosis. To this end, we used a combination of ex vivo studies of human platelets treated with a pharmacologic TYMP inhibitor or platelets purified from mice with TYMP deficiency along with in vivo models of arterial thrombosis in genetically modified mice or TYMP inhibitor treated mice. These approaches show that TYMP contributes to platelet activation and promotes thrombosis in a platelet-dependent manner. We also defined a signaling pathway in which TYMP interacts with Lyn, Fyn and Yes in platelets to regulate their function.
METHODS
All animal uses have been approved by the IACUC of The Cleveland Clinic. Details please find online Supplemental Material.
RESULTS
TYMP deficiency in mice is anti-thrombotic and platelets account for the prothrombotic role of TYMP
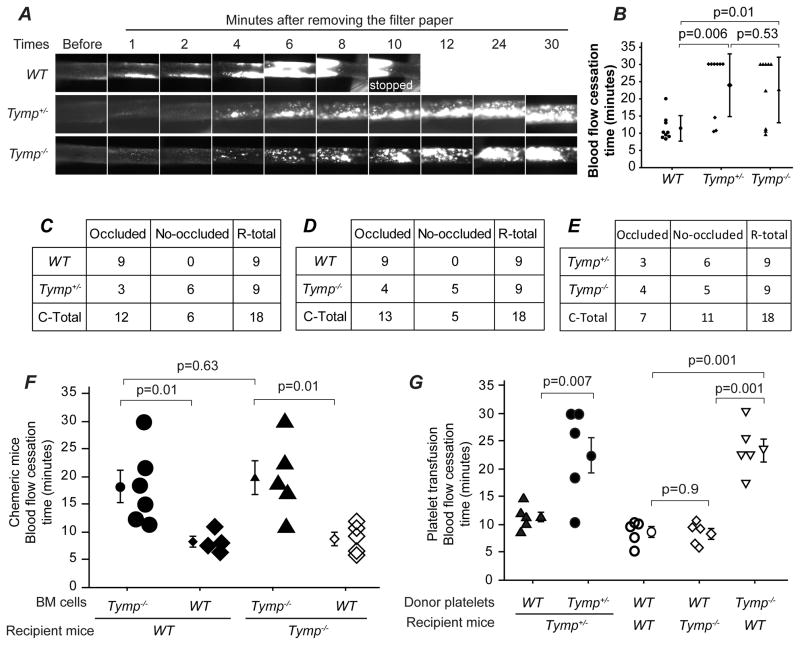
To examine whether TYMP affects thrombosis, 8 to 12 week old male Tymp+/− and Tymp−/− mice were subjected to the FeCl3-induced carotid artery injury thrombosis model14. Male WT mice were used as controls. Figure 1A and Online Video I–III show representative thrombi formation in the three strains over time. Thrombi were confirmed immediately after removing the 7.5% FeCl3 solution saturated filter paper (1 min in Figure 1A), and no gross differences were observed among the three groups. As usual, parts of the initially formed thrombi were washed away by blood flow in all groups (Figure 1A, 2 min). Thrombi started to enlarge 3–4 min after removing the filter paper and these later thrombi were stable and not washed away. The thrombi in WT mice formed faster than those in Tymp+/− or Tymp−/− mice. Cessation of blood flow was seen in all WT mice (n=9) with an average vessel occlusion time of 11.4 min (Figure B, C and D). Only 3 of the 9 Tymp+/− mice and 4 of the 9 Tymp−/− mice showed flow cessation within the 30 min of observation with average occlusion times >20 min (Figure 1B, C, D and E). Occlusion times were significantly different between WT and mice with any defect in Tymp, buy were not different when comparing Tymp−/− to Tymp+/− mice. These data show TYMP contributes to thrombus formation and suggest that even partial insufficiency of TYMP is enough to provide anti-thrombotic benefit.
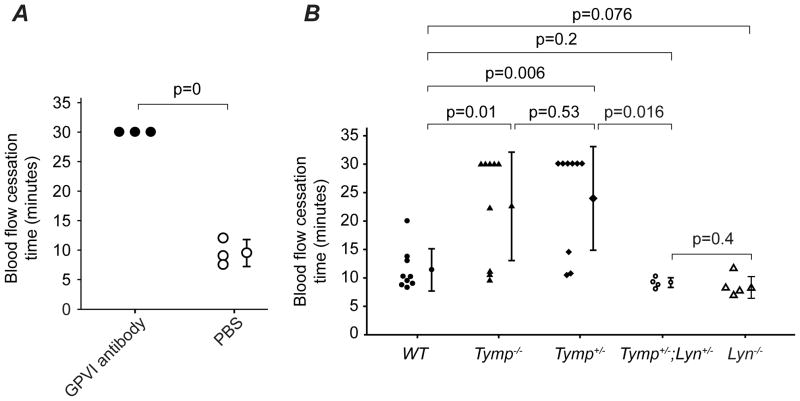
Figure 1. Deletion of thymidine phosphorylase (Tymp) protects against arterial thrombosis in mice.
A. Representative video images of carotid artery thrombi formation after 7.5% FeCl3 treatment. Platelets were labeled by direct jugular vein injection of rhodamine 6G and arteries were imaged in real time by fluorescent intravital microscopy. B. Blood flow cessation time. Data are presented as Mean ± SEM, n=9 for each group. C, D and E are contingency tables showing mice number based on whether occluded thrombus formed or not within the observed 30 min, and Fisher’s exact test was used for statistical analysis. Two side p=0.009 in C, p=0.029 in D and p=1 in E. F. WT and Tymp−/− recipient mice were exposed to 10.5 Gy of external beam irradiation, and then received bone marrow (BM) transplantation as indicated. The successful BM engrafted mice were subjected to 7.5% FeCl3 induced injury on carotid arteries 4 weeks later (n=4~6). G. Tymp+/−, Tymp−/− or WT recipient mice were exposed to 11 Gy of irradiation and allowed to live for 5 days to induce serious thrombocytopenia. Donor platelets isolated from WT, Tymp+/− or Tymp−/− mice were labeled with Rhodamine 6G, and total 109 platelets in 200 μl saline were transfused into the recipient mouse via jugular vein injection. The mice were then subjected to 7.5% FeCl3 induced carotid artery injury. N=5 in each group.
Since TYMP deficiency has been associated with mitochondrial dysfunction in nucleated human cells, we assessed platelet mitochondrial function by MTT assay. We found no differences in platelet mitochondrial function among the three strains [Supplemental Figure (SF) IA], which was identical to the original description in this mouse strain15. TYMP deletion also did not affect circulating platelet counts (SF IB). TYMP and its ultimate product 2DDR induce integrin β1 and β3 activation and/or expression in endothelial cells16,17. We found that TYMP haplodeficiency or completely deletion did not change the expression of integrin β1 and β3 in platelets; von Willebrand factor levels were also comparable among the three strains (SF IC).
Vera et al. reported that activated platelets secrete 2DDRP, which acts as a cooperative agonist and potentiates platelet activation in response to thrombin18. To examine the potential that 2DDRP is low and thus induces antithrombotic phenotype in the TYMP deficient mice, murine plasma obtained by ultracentrifugation of platelet poor plasma was analyzed by liquid chromatography on-line tandem mass spectrometry (LC/MS). As shown in SF ID, plasma 2DDRP was similar among the WT, Tymp+/− and Tymp−/− mice, suggesting that 2DDRP levels are not responsible for the observed phenotype in the Tymp+/− and Tymp−/− mice.
We thus performed bone marrow transplant using Tymp−/− and WT mice to determine the cellular basis of the TYMP-mediated pro-thrombotic effect. Recipient mice were exposed to 10.5Gy of external beam irradiation and then infused with 2×106 donor bone marrow cells by jugular vein injection 4 hours after irradiation. Surviving chimeric mice were subjected to FeCl3-induced carotid artery thrombosis 4 weeks later. The time necessary to form occlusive thrombi were significantly prolonged in WT mice that received Tymp−/− bone marrow when compared to WT mice that received WT marrow (Figure 1F); Tymp−/− mice that received WT bone marrow displayed significantly shorter arterial occlusion times when compared with Tymp−/− mice that received Tymp−/− marrow. These data demonstrated that repleting TYMP in circulating cells reverses the anti-thrombotic effect of systemic TYMP deletion and that TYMP in cells of the vessel wall is not involved in the observed phenotype.
Erythrocytes do not express TYMP so we next distinguished the effect of platelet vs. leukocyte TYMP on thrombosis. To do this, platelet transfusion experiments were performed in mice rendered severely thrombocytopenic by irradiation, which decreased platelet counts to ~5% of normal after 5~6 days19–21. Donor platelets were isolated from Tymp+/−, Tymp−/− or WT mice, labeled with rhodamine 6G, and then transfused into the thrombocytopenic mouse via jugular vein injection 10 min before FeCl3 injury. Each mouse received 109 platelets, which resulted in a final circulating platelet concentration of 6 × 1011/L ± 1.1 × 1011/L in ~25 g mice (n=3). Reconstitution of Tymp+/− platelets in Tymp+/− recipient mice resulted in similar occlusion times as non-irradiated Tymp+/− mice. However, thrombocytopenic Tymp+/− mice receiving WT platelets showed an average flow cessation time of 11 min, which was significantly shorter than the time to occlusion in thrombocytopenic Tymp+/− mice received Tymp+/− platelets. Similarly, WT recipients transfused with Tymp−/− platelets showed significantly prolonged blood flow cessation time when compared to WT or Tymp−/− animals reconstituted with WT platelets (Figure 1G). These studies demonstrate that platelet TYMP is an important determinant of the anti-thrombotic phenotype of the Tymp deficient animals.
TYMP is involved in platelet activation
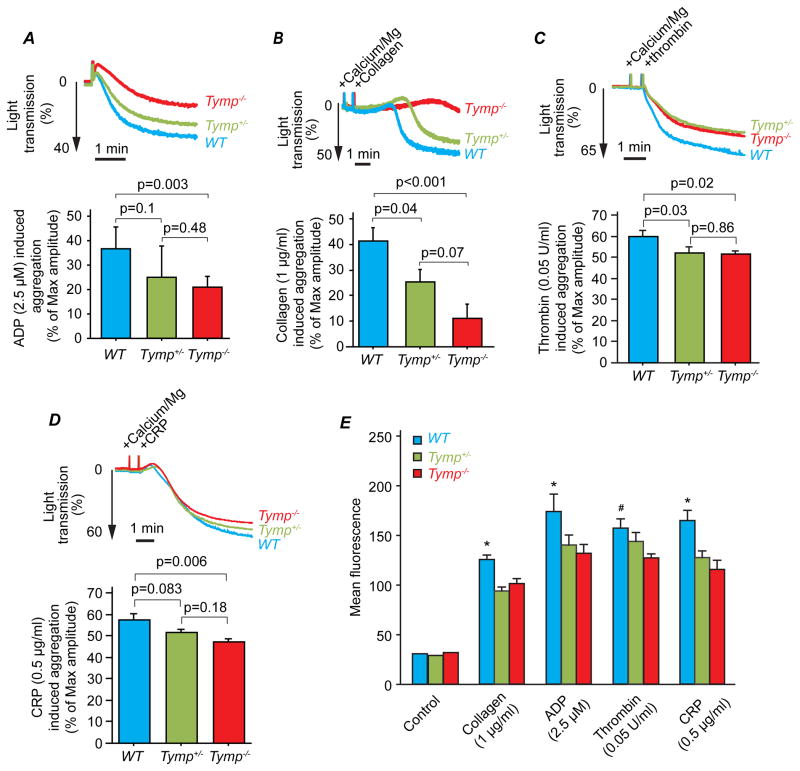
To clarify how TYMP affects thrombosis, we examined agonist induced platelet aggregation and granule secretion. As shown in Figure 2A, Tymp deletion or haploinsufficiency significantly attenuated ADP induced platelet aggregation in platelet rich plasma; the maximum extent of aggregation was reduced ~33% compared to WT platelets. Tymp deletion nearly abolished platelet aggregation in response to 1μg/ml collagen (Figure 2B). Tymp haploinsufficiency also significantly blunted platelet aggregation in response to the low dose collagen, but to a lesser extent than homozygous deletion. We further demonstrated that TYMP deletion significantly decreased thrombin and collagen related peptide (CRP) induced aggregation of washed platelet (Figure 2C&D). All platelet activating receptors ultimately trigger platelet α-granule release, resulting in P-select in translocation to the platelet surface. As shown in Figure 2E, P-select in expression detected by flow cytometry was significantly attenuated in Tymp deficient platelets in response to collagen (1 μg/ml), ADP (2.5 μM), thrombin (0.05 U/ml) or CRP (0.5 μg/ml). Tymp deletion also significantly attenuated high dose agonist-induced platelet activation (SF II). These data suggest that TYMP participates in platelet signaling and activation in response to multiple receptors.
Figure 2. TYMP deficiency attenuates platelet activation in vitro.
A & B. Platelet-rich plasma isolated from Tymp−/−, Tymp+/− or WT mice was used for platelet aggregation assay induced by (A) 2.5 μM ADP, and (B) 1 μg/ml collagen using a standard turbidimetric assay. N=6. C & D. Washed murine platelets were used for aggregation assay induced by (C) 0.05 U/ml thrombin or (D) 0.5 μg/ml collage-related peptide (CRP) (n=4 or 5). E. Platelets in platelet-rich plasma (20 μl) were mixed with Tyrode’s buffer (80 μl), and stimulated with indicated agonists for 5 min at room temperature. Reactions were stopped by adding 2% formaldehyde, 1 mM EDTA in PBS. P-select in expression was stained by FITC-conjugated antibody and examined by flow cytometry. *p<0.05 WT vs. Tymp+/− and Tymp−/−; # P<0.05, WT vs. Tymp−/−, N=3.
TYMP forms a complex with Src family kinases in platelets
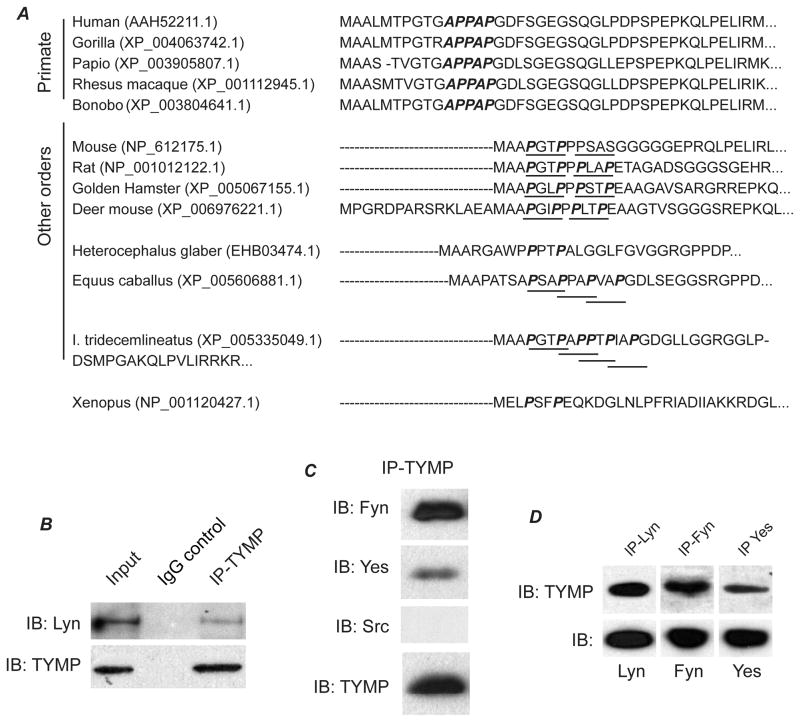
We previously found that TYMP overexpression induced constitutive phosphorylation of Lyn kinase in VSMC12. Lyn is a major SFK in human and mouse platelets, and plays important and complex roles in platelet activation22. All SFKs contain aSH3 domain that serves as a site for protein interactions by binding to proline-rich peptide sequences in its binding partners23. We analyzed the sequence of human TYMP and discovered a consensus SH3-binding sequence APPAP24 at the N-terminus of TYMP (Figure 3A), which is highly conserved in different primate species. Murine TYMP also contains a core binding motif PxxP25, which is also highly preserved in other orders. These findings suggest that TYMP could directly influence platelet signaling via SFKs or other SH3 domain containing signaling proteins. To test this hypothesis, we first examined whether TYMP interacts with SFKs in platelets. Resting human platelet lysates were immunoprecipitated with specific monoclonal antibodies to TYMP and then the precipitates were assessed for immunoblotting the SFKs including Src, Lyn, Fyn and Yes. Normal mouse or rabbit IgG did not pull down any Src, Lyn, Fyn or Yes, nor TYMP (SFIII and not shown). Anti-TYMP precipitates however contained Lyn, Fyn and Yes, but not Src (Figure 3B and C). Conversely, anti-Lyn, Fyn and Yes precipitates also contained TYMP (Figure 3D). These findings were reproduced using platelets from 4 different donors and were also confirmed using mouse platelets (data not shown).
Figure 3. TYMP forms a complex with Src family kinases in platelets.
A. A consensus SH3-binding sequence APPAP was found in different primate TYMPs; and a core binding motif PxxP was also found in murine and other orders. B, C and D. Immunoprecipitation-immunoblotting assays were performed using indicated antibodies to determine the interaction of TYMP and Src family kinases including Lyn, Fyn, Src and Yes in human platelets.
Inhibition of TYMP reduces TYMP/Lyn association and GPVI-mediated Lyn activation
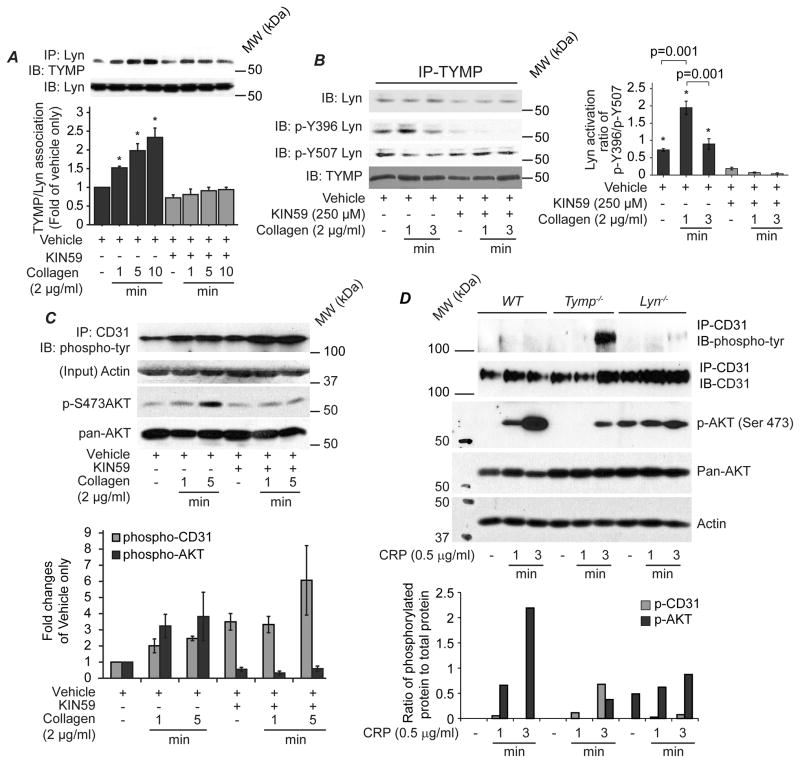
Insights gained from structure studies suggest that substrate (or inhibitor) binding induces a conformational change in TYMP, which is essential for its phosphorylase action26. Conformation changes are also essential for protein-protein interaction. TYMP metabolites including thymidine, 2DDR and 2DDRP did not affect agonist-induced platelet aggregation (SFIV), suggesting that the phosphate and the nucleoside-binding sites of TYMP are not responsible for its effect on platelet function. We thus examined whether another element of the active site of human TYMP, as partic acid-20327, affected its association with SFKs using a potent Asp203-binding TYMP inhibitor, KIN5928,29. As shown in Figure 4A, collagen stimulation significantly increased the amount of TYMP that co-precipitated with Lyn. Inhibition of TYMP with KIN59 significantly decreased the amount of Lyn that co-precipitated with TYMP (Figure 4A and B). Immunoblotting of the TYMP precipitates with antibodies specific for phosphorylated Lyn showed that the TYMP-associated Lyn (Figure 4B) was phosphorylated at Y507, an inactive status in resting platelets. Upon collagen stimulation, TYMP-associated Lyn was rapidly phosphorylated at Y396. However, activity of the TYMP associated Lyn (detected by phosphorylation at Y396) was only higher at the initial phase (within 1 min), but was significantly decreased over time (3 min). This phenomenon was dramatically blocked by KIN59, suggesting that TYMP may act by trapping and diminishing active Lyn.
Figure 4. TYMP interacts with Lyn and discriminately regulates phosphorylation of Lyn, PECAM-1 (CD31) and AKT in platelets.
A. Human platelet lysates were used for immunoprecipitation of Lyn and then immunoblotting was performed for TYMP and Lyn. Blots represent at least 3 independent experiments. *p<0.05 vs. corresponding times of KIN59 treatments. B. Human platelets were pretreated with vehicle or KIN59 and then stimulated with collagen for indicated times. Platelets lysates were analyzed by immunoprecipitation and immunoblotting assays using indicated antibodies. Bar graph shows Lyn activity from 3 independent experiments. *p<0.05 vs. corresponding times of KIN59 treatments. C. Human platelets treated as in panel A were analyzed by Western blot using indicated antibodies. Bar graph represents 3 independent experiments. D. Mouse platelets pooled from 10–12 mice were divided into 3 groups and stimulated with CRP as indicated. Platelet lysates were used for immunoprecipitation and immunoblotting assays using indicated antibodies. Bar graph shows the ratio of phosphorylated AKT and PECAM1 to their total proteins.
Lyn phosphorylates platelet-endothelial cell adhesion molecule 1 (PECAM1, CD31) within its immunoreceptor tyrosine-based inhibitory (ITIM) motif that inhibits platelet activation in response to collagen stimulation30. In the presence of the TYMP inhibitor KIN59 we found that inhibition of TYMP activity significantly increased tyrosine phosphorylated PECAM1 (Figure 4C), potentially from Lyn release. It is known that activated PECAM1 recruits and activates tyrosine phosphatase SHP-2, which subsequently binds to phosphoinositol 3-kinase (PI3K) and diminishes PI3K signaling31. As a marker of PI3K signaling pathway activation, we assessed AKT phosphorylation, and found KIN59 treatment dramatically inhibited collagen induced AKT phosphorylation (Figure 4C). To confirm this finding, we pooled platelets harvested from 10–12 mice, divided them to three groups and then stimulated the platelets with CRP for indicated times. As shown in Figure 4D, in comparison with WT platelets, CRP-induced AKT phosphorylation was dramatically reduced in Tymp−/− and Lyn−/− platelets. Tyrosine phosphorylated PECAM1 was significantly increased in Tymp−/− platelets in response to CRP stimulation, but not in WT and Lyn−/− platelets. These data suggest TYMP and Lyn form an association that regulates Lyn function and subsequently affects glycoprotein (GP) VI signaling mediated platelet activation.
Lyn deficiency reverses the anti-thrombotic phenotype of Tymp deficient mice
Having shown that TYMP interacts with SFKs and regulates SFKs activity in platelets, we hypothesized that deletion of SFK would diminish the anti-thrombotic phenotype of TYMP deficiency. Yes is not expressed in mouse platelets. While Fyn only plays a minor role in mediating GPVI signaling22, which plays critical roles in response to vascular injury32 including the FeCl3 induced arterial thrombosis model33. By platelet transfusion experiments, we found blocking WT platelets with GPVI antibody significantly prolonged thrombosis time in the 7.5% FeCl3 injury induced carotid artery thrombosis model (Figure 5A). Transfusion of Fyn null platelets to the thrombocytopenic WT mice did not affect thrombosis time when compared WT mice received WT platelets (data not shown), supporting that platelet Fyn plays a minor role in this model. We thus focused on the effect of Lyn and crossed Tymp−/− mice with Lyn−/− animals34 to generate Tymp+/−; Lyn+/− mice. Consistent with our hypothesis, Lyn haploinsufficiency dramatically diminished the prolonged thrombosis time displayed by the Tymp+/− and Tymp−/− mice to the levels of WT and Lyn−/− mice (Figure 5B).
Figure 5. Lyn deficiency reverses the anti-thrombotic phenotype of Tymp deficient mice.
A. Platelet GPVI signaling plays an important role in the FeCl3 induced thrombosis model. B. The time to blood flow cessation was assessed in Tymp+/−;Lyn+/− and Lyn−/− mice (N=4 in each group) using the FeCl3 injury induced carotid artery thrombosis model and data were compared with Tymp+/−, Tymp−/− and WT mice.
KIN59 inhibits platelet aggregation in vitro and has an anti-thrombotic effect in vivo without affecting hemostasis
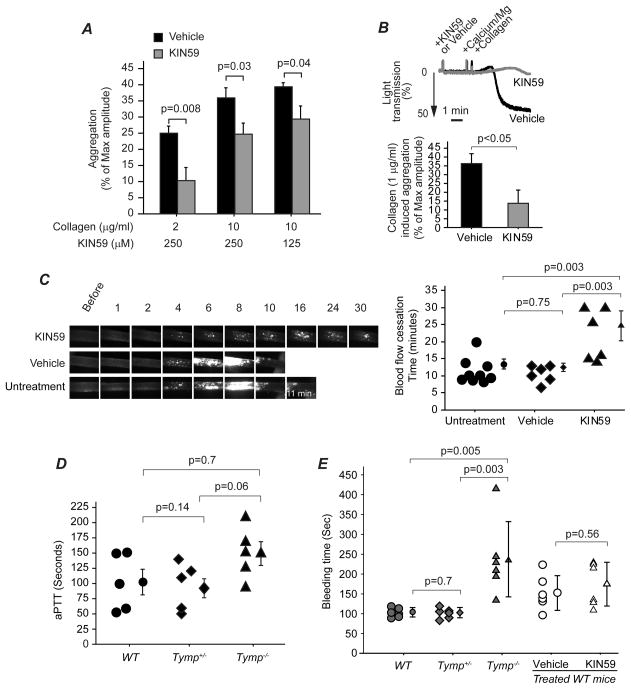
Having shown that inhibition of TYMP with KIN59 affected activity of platelet signaling molecules, we next examined whether inhibition of TYMP with KIN59 affects platelet activation. KIN59 reversibly and in a dose-dependent fashion inhibited collagen-induced platelet aggregation (SF V). Figure 6A shows cumulative data for different concentrations of KIN59 on different doses of collagen-induced platelet aggregation. These studies demonstrate that inhibition of TYMP activity significantly inhibited platelet aggregation in response to collagen stimulation. Treatment of WT platelets in platelet-rich plasma with KIN59 also dramatically inhibited aggregation in response to 1 μg/ml collagen (Figure 6B), replicating the pattern seen with Tymp−/− platelets. KIN59 also significantly inhibited aggregation induced by ADP, thrombin and CRP (not shown).
Figure 6. Pharmacologic inhibition of TYMP activity inhibits platelet aggregation and thrombosis without affecting hemostasis.
A. The effect of different concentrations of KIN59 on different doses of collagen induced human platelet aggregation. N=6~9. B. The effect of KIN59 (250 μM) on murine platelet aggregation in response to 1 μg/ml collagen stimulation, N=6. C. The effect of KIN59 on thrombosis was assessed on eight weeks old WT mice that were treated with KIN59 (30 mg/kg/day) for 3 days. The mice were then subjected to 7.5% FeCl3 induced carotid artery thrombosis model, n=6. D. aPTT assay was performed using platelet-poor-plasma from 5 different mice. E. Tail bleeding time was assessed in anesthetized WT, Tymp+/− and Tymp−/− mice by cutting 1 cm of tail from the tip, or in WT mice received KIN59 (30 mg/kg/day) or vehicle treatment for 3 days. N=6.
Given that TYMP plays an important role in platelet activation in response to stimulation by any of examined agonists, we determined whether pharmacologic inhibition of TYMP activity affects thrombosisin vivo. KIN59, 30 mg/kg/day, has previously been delivered in vivo to mice and shown to inhibit TYMP induced angiogenesis35,36 without producing obvious side effects. We treated WT and Tymp−/− mice with intraperitoneal injection of KIN59 (30 mg/kg/day) for 3 days, and then subjected the mice to FeCl3 induced carotid artery injury. Vehicle treatment did not affect carotid artery occlusion times when compared with the untreated WT mice; KIN59 administration, however significantly prolonged the time to form occlusive thrombus in the WT mice (Figure 6C and Online Video IV and V). KIN59 did not further prolong blood cessation time in the Tymp−/− mice (not shown) suggesting that the effect is TYMP-dependent.
Hemorrhagic complications are associated with all currently available anti-platelet and anticoagulation therapies. We assessed activated partial thromboplastin time (aPTT) and tail bleeding time to determine whether TYMP deficiency affects hemostasis. aPTT was not significantly different among the WT, Tymp+/− and Tymp−/− mice (Figure 6D). Tymp haploinsufficiency also did not influence bleeding time (Figure 6E). Bleeding times were prolonged ~2 fold in Tymp−/− mice; however, this level of increase was not associated with any increase in surgical mortality or in observable bleeding at the surgical site. KIN59 treatment for 3 days also did not affect bleeding time (Figure. 6E) as well as plasma 2DDRP (SFVI) when compared with the mice received vehicle injection. These data suggest that TYMP may play a role in normal hemostasis, but that haploinsufficiency or significant enzymatic inhibition is not sufficient to cause a hemostatic defect.
DISCUSSION
In this study, we demonstrated for the first time that TYMP promotes thrombosis by regulating platelet activation. We found that:(1) TYMP deletion or haploinsufficiency in platelets significantly prolonged thrombotic occlusion times in response to FeCl3 induced carotid artery injury without affecting hemostasis; (2) TYMP deletion or haploinsufficiency significantly attenuated ADP-, collagen-, CRP-and thrombin-induced platelet aggregation and α-granule release;(3) in response to platelet agonists, TYMP interacts with Lyn, Fyn and Yes and modulates activity of Lyn and its downstream signaling molecules such as PECAM1 and AKT; (4) Lyn haploinsufficiency diminished the phenotype found in the Tymp+/− mice; (5) pharmacologic inhibition of TYMP activity reduced agonist-induced human and murine platelet aggregation in vitro and prolonged murine thrombosis times in vivo without affecting hemostasis. This study indicates that targeting TYMP might be a novel anti-platelet or anti-thrombosis therapy.
TYMP has been found in atherosclerotic plaques37,38, serum of type II diabetic patients39 and cancer40. All of these diseases have high risk of thrombosis, suggesting that TYMP may play a role in these diseases-associated thrombophilia. This hypothesis is supported by one study reported a decade ago in which increased expression of TYMP in human hepatocellular carcinomas was correlated with high incidence of portal vein thrombosis41. This hypothesis is also supported by a recent study, in which perfusion of erythrocyte-encapsulated TYMP to mice resulted in thrombi in the lungs42. Interestingly, Aspirin, an anti-platelet drug, inhibits TYMP production in a human monocyte cell line, THP1 cells43, suggesting that Aspirin may also inhibit TYMP production in platelet and thus results in the anti-thrombotic effect. We found that TYMP deficiency did not affect expression of GPIb, GPVI (not shown), von Willebrand Factor or integrin β1 and β3 expression in platelets nor was the number of circulating platelets or their mitochondrial function affected by the loss of TYMP. These observations exclude platelet quantity and quality as the source of defective platelet action in the Tymp haploinsufficient or null mice. Activated platelets secrete 2DDRP which can act as a cooperative agonist and potentiates platelet activation18. We found no difference in plasma 2DDRP between Tymp deficient and WT mice or between KIN59 and vehicle treated mice by LC/MS. Mouse uridine phosphorylase also catalyzes thymidine metabolism15. Since uridine phosphorylase is not expressed in platelets1 and thymidine levels were not significantly higher in the Tymp−/− mice15, we presume 2DDRP in platelets of the Tymp deficient or null mice should not be high. Indeed, pretreatment of platelets with thymidine, 2DDRP and 2DDR did not alter ADP and collagen induced platelet aggregation (SFIV). We thus exclude levels of the TYMP metabolites as the source of the antithrombotic phenotype in the Tymp deficient mice.
By in vivo thrombosis studies in chimeric mice generated by bone marrow transplant or thrombocytopenic mice reconstituted with WT or Tymp haploinsufficient platelets, we demonstrated that platelet TYMP plays a role in platelet activation and thrombosis, suggesting that TYMP participates in platelet signaling pathway to regulate platelet activation. By analyzing the amino acid sequence of TYMP proteins we found the N-terminus of most mammalian TYMPs contain a SH3 domain binding proline-rich motif (APPAP in primate) or a core binding motif (PxxP, in rodents or other species)25. This finding suggests a molecular mechanism enabling TYMP to participate in signaling by directly binding to SH3 domain containing proteins, such as SFKs. We found that TYMP overexpression induced constitutive Lyn tyrosine phosphorylation in VSMC12. Here we demonstrate that TYMP forms a complex with Fyn, Lyn and Yes in human and murine platelets. We found that TYMP associates with Lyn, and that this depends on Asp203 mediated TYMP conformation formation. Furthermore, association of Lyn with TYMP rapidly diminished Lyn phosphorylation at Y396 residue induced by collagen suggesting Lyn activity was inhibited. These data suggest that TYMP acts to sequester active Lyn to decrease Lyn function. Thus, loss of TYMP increases Lyn inhibition of platelet activation.
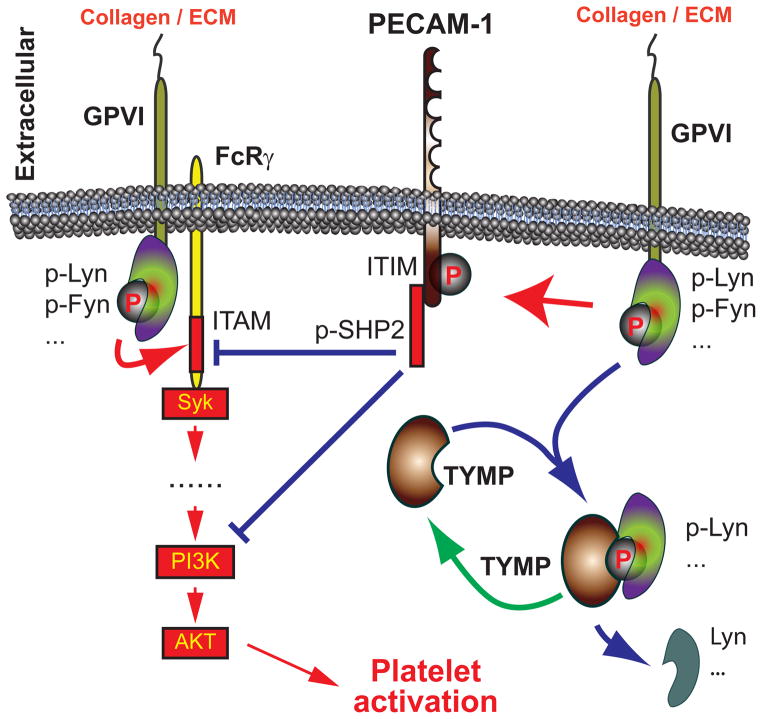
SFKs share individual and overlapping roles in platelet activation22. Fyn mainly acts as a stimulation factor; however, Lyn modulates both activation and inhibition of platelet44. Lyn and Fyn are constitutively associated with the cytoplasmic domain of GPVI45,46, and Lyn and Fyn mediated phosphorylation of the FC receptor γ chain immunoreceptor tyrosine-based activation motif domain promotes GPVI mediated platelet activation. In vitro studies thus showed that Lyn mediates a rapid response to collagen stimulation, especially obvious at low collagen concentrations46,47, and a delayed response of platelets to low doses of collagen induced aggregation was found in Lyn null platelets22. We also showed that Lyn functions downstream of CD36 in activating platelets through danger-associated molecular patterns, such as oxidized LDL20. Lyn, however also inhibits collagen induced platelet activation via phosphorylation of PECAM-1 ITIM domain48,49 and Lyn-deficient platelets are thus hyper-responsive to collagen stimulation49. By examining PECAM-1 phosphorylation we found TYMP deletion or inhibition its activity significantly increased platelet PECAM-1 tyrosine phosphorylation in vitro (Figure 4C&D). In combination with the fact that TYMP diminishes Lyn phosphorylation at Y396 our data suggest that TYMP attenuates Lyn mediated PECAM-1/ITIM phosphorylation in platelets. TYMP deficiency thus increases Lyn/PECAM-1 mediated platelet inhibition and inhibits thrombosis. Our in vivo data suggest this concept as Lyn−/− mice showed a modest acceleration of thrombosis times (Figure 5B, SF VII and Online Video VI), and haploinsufficiency of Lyn significantly diminished the anti-thrombotic phenotype found in Tymp+/− mice. Therefore, our studies indicate that TYMP participates in GPVI signaling, and regulates Lyn, PECAM-1 and PI3K/AKT signaling and platelet function (Figure 7). We also found TYMP influences ADP and thrombin mediated platelet activation, suggesting a potential role of TYMP in G protein-coupled receptor signaling. This phenomenon may also due to the effect of TYMP on Lyn activation as Lyn also mediates thrombin50 and ADP51 induced platelet activation.
Figure 7. TYMP functions on platelet GPVI signaling and promote platelet activation.
Red arrows indicate stimulatory and blue arrows indicate inhibitory effects.
A human rare autosomal recessive syndrome known as MNGIE (Mitochondrial Neuro Gastro Intestinal Encephalomyopathy) has been associated with TYMP loss of function. Patients with this disorder generally die in early adulthood, although at this point no bleeding or thrombotic diathesis has been described. Heterozygous carriers have no discernible phenotype52. Indeed, no clinical symptoms of MNGIE were present in the Tymp−/− mice15. Tymp−/− mice generated by a different group also did not show any symptoms of MNGIE53. ATYMP inhibitor known as Tipiracilis a component of an experimental anti-cancer drug TAS-102 and has been used in phase I, II and III clinical trials. Available data from these studies did not show any bleeding problems or any symptoms similar to the MNGIE. By examination of aPTT and tail bleed assays, we found neither Tymp deficiency nor inhibition of TYMP with KIN59 affected coagulation hemostasis or bleeding. These data suggest that regulation of TYMP expression or activity is safe, and may achieve therapeutic effects.
In summary, our study demonstrated for the first time that TYMP participates in platelet signaling and plays an important role in regulating platelet activation. Genetic deletion of TYMP or pharmacological inhibition of TYMP activity dramatically inhibited platelet activation in vitro and significantly prolonged thrombosis time in vivo. We conclude that pharmacological inhibition of TYMP activity might be a novel approach to develop antiplatelet and anti-thrombotic therapy.
Supplementary Material
Novelty and Significance.
What Is Known?
Thymidine phosphorylase (TYMP) is abundantly expressed in human platelets but its function in platelet physiology is not known.
TYMP has been found in atherosclerotic plaques and in serum from patients with type II diabetes and cancer; diseases with elevated risk of thrombosis.
TYMP overexpression in vascular smooth muscle cell induced Src family kinase Lyn phosphorylation.
What New Information Does This Article Contribute?
Genetic deletion or haploinsufficiency of TYMP in mice significantly attenuated ADP-, collagen-, CRP- and thrombin-induced platelet activation.
Co-immunoprecipitation studies showed that platelet TYMP interacts with Lyn, Fyn and Yes.
TYMP modulates Lyn activity as well as activities of signaling molecules downstream of Lyn, such as PECAM1 and AKT.
Lyn haploinsufficiency diminished the antithrombotic phenotype found in the Tymp+/− mice.
Pharmacologic inhibition of TYMP activity reduced agonist-induced human and murine platelet aggregation in vitro and prolonged murine thrombosis times in vivo without affecting hemostasis.
This study was designed to examine the role of platelet TYMP on platelet function and thrombus formation. By using in vivo murine arterial thrombosis model, we discovered that TYMP deletion or haploinsufficiency achieved significant anti-thrombotic effect in platelet-dependent manner. TYMP plays important role in various agonists induced platelet activation and aggregation, which are critical events in clot formation. TYMP regulates PECAM1 and AKT phosphorylation through regulating Lyn activity. Inhibition of TYMP activity significantly inhibited thrombosis without affecting hemostasis in vivo. This study suggests that targeting TYMP might be a novel antithrombotic strategy.
Acknowledgments
The authors thank Dr. Peter Newman (Blood Center of Wisconsin) for providing the collagen related peptide, Dr. Anthony L. DeFranco (UCSF School of Medicine) for allowing to use the Lyn−/− mice and Dr. Neetu Gupta (Cleveland Clinic) for providing the Lyn−/− mice for this study; thank Dr. Renliang Zhang (Cleveland Clinic) for Mass Spectrometry analysis; thank Dr. Stan Hazen (Cleveland Clinic) for supporting this work and its animal husbandry.
SOURCES OF FUNDING
This study was supported by NIH grants HL81011 and HL092747 (to R.L.S.) and AA017748 (to T.M.) and grants of the Spanish CICYT (SAF2009-13914-C02-01 and SAF2012-39760-C02-01). A. G. has a JAE-predoctoral fellowship financed by the CSIC and the FSE (Fondo Social Europeo).
Nonstandard Abbreviations and Acronyms
- TYMP
Thymidine phosphorylase
- FeCl3
Ferric chloride
- CRP
Collagen related peptide
- ADP
Adenosine diphosphate
- 2DDRP
2-deoxy-D-ribose-1-phosphate
- 2DDR
2-deoxy-D-ribose
- VSMC
Vascular smooth muscle cell
- SFKs
Src family kinases
- WT
Wild type C57BL6/J
- LC/MS
Liquid chromatography on-line tandem mass spectrometry
- GP
Glycoprotein
Footnotes
DISCLOSURES
None.
References
- 1.Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120:e73–82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 2.Friedkin M, Roberts D. The enzymatic synthesis of nucleosides. II. Thymidine and related pyrimidine nucleosides. The Journal of biological chemistry. 1954;207:257–266. [PubMed] [Google Scholar]
- 3.Miyazono K, Okabe T, Urabe A, Takaku F, Heldin CH. Purification and properties of an endothelial cell growth factor from human platelets. The Journal of biological chemistry. 1987;262:4098–4103. [PubMed] [Google Scholar]
- 4.Moghaddam A, Bicknell R. Expression of platelet-derived endothelial cell growth factor in Escherichia coli and confirmation of its thymidine phosphorylase activity. Biochemistry. 1992;31:12141–12146. doi: 10.1021/bi00163a024. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Tanaka K, Ihaya A, Fujibayashi Y, Takamatsu S, Morioka K, Sasaki M, Uesaka T, Kimura T, Yamada N, Tsuda T, Chiba Y. Gene therapy for chronic myocardial ischemia using platelet-derived endothelial cell growth factor in dogs. Am J Physiol Heart Circ Physiol. 2005;288:H408–415. doi: 10.1152/ajpheart.00176.2004. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama S, Furukawa T, Sumizawa T, Takebayashi Y, Nakajima Y, Shimaoka S, Haraguchi M. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 2004;95:851–857. doi: 10.1111/j.1349-7006.2004.tb02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu EJ, Lee Y, Rha SY, Kim TS, Chung HC, Oh BK, Yang WI, Noh SH, Jeung HC. Angiogenic factor thymidine phosphorylase increases cancer cell invasion activity in patients with gastric adenocarcinoma. Mol Cancer Res. 2008;6:1554–1566. doi: 10.1158/1541-7786.MCR-08-0166. [DOI] [PubMed] [Google Scholar]
- 8.Koukourakis MI, Giatromanolaki A, O’Byrne KJ, Comley M, Whitehouse RM, Talbot DC, Gatter KC, Harris AL. Platelet-derived endothelial cell growth factor expression correlates with tumour angiogenesis and prognosis in non-small-cell lung cancer. Br J Cancer. 1997;75:477–481. doi: 10.1038/bjc.1997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada N, Li W, Ihaya A, Kimura T, Morioka K, Uesaka T, Takamori A, Handa M, Tanabe S, Tanaka K. Platelet-derived endothelial cell growth factor gene therapy for limb ischemia. J Vasc Surg. 2006;44:1322–1328. doi: 10.1016/j.jvs.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Tanaka K, Morioka K, Takamori A, Handa M, Yamada N, Ihaya A. Long-term effect of gene therapy for chronic ischemic myocardium using platelet-derived endothelial cell growth factor in dogs. J Gene Med. 2008;10:412–420. doi: 10.1002/jgm.1156. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Tanaka K, Morioka K, Uesaka T, Yamada N, Takamori A, Handa M, Tanabe S, Ihaya A. Thymidine phosphorylase gene transfer inhibits vascular smooth muscle cell proliferation by upregulating heme oxygenase-1 and p27KIP1. Arterioscler Thromb Vasc Biol. 2005;25:1370–1375. doi: 10.1161/01.ATV.0000168914.85107.64. [DOI] [PubMed] [Google Scholar]
- 12.Yue H, Tanaka K, Furukawa T, Karnik SS, Li W. Thymidine phosphorylase inhibits vascular smooth muscle cell proliferation via upregulation of STAT3. Biochimica et biophysica acta. 2012;1823:1316–1323. doi: 10.1016/j.bbamcr.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handa M, Li W, Morioka K, Takamori A, Yamada N, Ihaya A. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48:1566–1574. doi: 10.1016/j.jvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Li W, McIntyre T, Silverstein R. Ferric chloride-induced murine carotid arterial injury: a model of redox pathology. Redox Biology. 2013;1:50–55. doi: 10.1016/j.redox.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez LC, Akman HO, Garcia-Cazorla A, Dorado B, Marti R, Nishino I, Tadesse S, Pizzorno G, Shungu D, Bonilla E, Tanji K, Hirano M. Unbalanced deoxynucleotide pools cause mitochondrial DNA instability in thymidine phosphorylase-deficient mice. Hum Mol Genet. 2009;18:714–722. doi: 10.1093/hmg/ddn401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss KA, Ashton AW, Schwartz EL. Thymidine phosphorylase and 2-deoxyribose stimulate human endothelial cell migration by specific activation of the integrins alpha 5 beta 1 and alpha V beta 3. The Journal of biological chemistry. 2003;278:19272–19279. doi: 10.1074/jbc.M212670200. [DOI] [PubMed] [Google Scholar]
- 17.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, Astroulakis Z, Xiao Q, Hill J, Xu Q, Mayr M. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circulation research. 2009;104:32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 18.Vara DS, Campanella M, Canobbio I, Dunn WB, Pizzorno G, Hirano M, Pula G. Autocrine amplification of integrin alphaIIbbeta3 activation and platelet adhesive responses by deoxyribose-1-phosphate. Thromb Haemost. 2013:109. doi: 10.1160/TH12-10-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature medicine. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K, Febbraio M, Li W, Silverstein RL. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circulation research. 2008;102:1512–1519. doi: 10.1161/CIRCRESAHA.108.172064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsakiris DA, Scudder L, Hodivala-Dilke K, Hynes RO, Coller BS. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost. 1999;81:177–188. [PubMed] [Google Scholar]
- 22.Severin S, Nash CA, Mori J, Zhao Y, Abram C, Lowell CA, Senis YA, Watson SP. Distinct and overlapping functional roles of Src family kinases in mouse platelets. J Thromb Haemost. 2012;10:1631–1645. doi: 10.1111/j.1538-7836.2012.04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingley E. Functions of the Lyn tyrosine kinase in health and disease. Cell Commun Signal. 2012;10:21. doi: 10.1186/1478-811X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witter DJ, Famiglietti SJ, Cambier JC, Castelhano AL. Design and synthesis of SH3 domain binding ligands: modifications of the consensus sequence XPpXP. Bioorganic & medicinal chemistry letters. 1998;8:3137–3142. doi: 10.1016/s0960-894x(98)00577-0. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen JT, Porter M, Amoui M, Miller WT, Zuckermann RN, Lim WA. Improving SH3 domain ligand selectivity using a non-natural scaffold. Chemistry & biology. 2000;7:463–473. doi: 10.1016/s1074-5521(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 26.Pugmire MJ, Cook WJ, Jasanoff A, Walter MR, Ealick SE. Structural and theoretical studies suggest domain movement produces an active conformation of thymidine phosphorylase. Journal of molecular biology. 1998;281:285–299. doi: 10.1006/jmbi.1998.1941. [DOI] [PubMed] [Google Scholar]
- 27.Bronckaers A, Aguado L, Negri A, Camarasa MJ, Balzarini J, Perez-Perez MJ, Gago F, Liekens S. Identification of aspartic acid-203 in human thymidine phosphorylase as an important residue for both catalysis and non-competitive inhibition by the small molecule “crystallization chaperone” 5′-O-tritylinosine (KIN59) Biochemical pharmacology. 2009;78:231–240. doi: 10.1016/j.bcp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Liekens S, Hernandez AI, Ribatti D, De Clercq E, Camarasa MJ, Perez-Perez MJ, Balzarini J. The nucleoside derivative 5′-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action. The Journal of biological chemistry. 2004;279:29598–29605. doi: 10.1074/jbc.M402602200. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Perez MJ, Priego EM, Hernandez AI, Camarasa MJ, Balzarini J, Liekens S. Thymidine phosphorylase inhibitors: recent developments and potential therapeutic applications. Mini Rev Med Chem. 2005;5:1113–1123. doi: 10.2174/138955705774933301. [DOI] [PubMed] [Google Scholar]
- 30.Moraes LA, Vaiyapuri S, Sasikumar P, Ali MS, Kriek N, Sage T, Gibbins JM. Antithrombotic actions of statins involve PECAM-1 signaling. Blood. 2013;122:3188–3196. doi: 10.1182/blood-2013-04-491845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moraes LA, Barrett NE, Jones CI, Holbrook LM, Spyridon M, Sage T, Newman DK, Gibbins JM. Platelet endothelial cell adhesion molecule-1 regulates collagen-stimulated platelet function by modulating the association of phosphatidylinositol 3-kinase with Grb-2-associated binding protein-1 and linker for activation of T cells. J Thromb Haemost. 2010;8:2530–2541. doi: 10.1111/j.1538-7836.2010.04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lockyer S, Okuyama K, Begum S, Le S, Sun B, Watanabe T, Matsumoto Y, Yoshitake M, Kambayashi J, Tandon NN. GPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolism. Thrombosis research. 2006;118:371–380. doi: 10.1016/j.thromres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl (3) -induced thrombosis. J Thromb Haemost. 2011;9:1423–1426. doi: 10.1111/j.1538-7836.2011.04328.x. [DOI] [PubMed] [Google Scholar]
- 34.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. Journal of immunology. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liekens S, Bronckaers A, Hernandez AI, Priego EM, Casanova E, Camarasa MJ, Perez-Perez MJ, Balzarini J. 5′-O-tritylated nucleoside derivatives: inhibition of thymidine phosphorylase and angiogenesis. Molecular pharmacology. 2006;70:501–509. doi: 10.1124/mol.105.021188. [DOI] [PubMed] [Google Scholar]
- 36.Liekens S, Bronckaers A, Belleri M, Bugatti A, Sienaert R, Ribatti D, Nico B, Gigante A, Casanova E, Opdenakker G, Perez-Perez MJ, Balzarini J, Presta M. The thymidine phosphorylase inhibitor 5′-O-tritylinosine (KIN59) is an antiangiogenic multitarget fibroblast growth factor-2 antagonist. Mol Cancer Ther. 2012;11:817–829. doi: 10.1158/1535-7163.MCT-11-0738. [DOI] [PubMed] [Google Scholar]
- 37.Ignatescu MC, Gharehbaghi-Schnell E, Hassan A, Rezaie-Majd S, Korschineck I, Schleef RR, Glogar HD, Lang IM. Expression of the angiogenic protein, platelet-derived endothelial cell growth factor, in coronary atherosclerotic plaques: In vivo correlation of lesional microvessel density and constrictive vascular remodeling. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2340–2347. doi: 10.1161/01.atv.19.10.2340. [DOI] [PubMed] [Google Scholar]
- 38.Boyle JJ, Wilson B, Bicknell R, Harrower S, Weissberg PL, Fan TP. Expression of angiogenic factor thymidine phosphorylase and angiogenesis in human atherosclerosis. J Pathol. 2000;192:234–242. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH699>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Hamed EA, Zakary MM, Abdelal RM, Abdel Moneim EM. Vasculopathy in type 2 diabetes mellitus: role of specific angiogenic modulators. J Physiol Biochem. 2011;67:339–349. doi: 10.1007/s13105-011-0080-8. [DOI] [PubMed] [Google Scholar]
- 40.Pauly JL, Schuller MG, Zelcer AA, Kirss TA, Gore SS, Germain MJ. Identification and comparative analysis of thymidine phosphorylase in the plasma of healthy subjects and cancer patients. J Natl Cancer Inst. 1977;58:1587–1590. doi: 10.1093/jnci/58.6.1587. [DOI] [PubMed] [Google Scholar]
- 41.Guo L, Kuroda N, Toi M, Miyazaki E, Hayashi Y, Enzan H, Jin Y. Increased expression of platelet-derived endothelial cell growth factor in human hepatocellular carcinomas correlated with high Edmondson grades and portal vein tumor thrombosis. Oncol Rep. 2001;8:871–876. doi: 10.3892/or.8.4.871. [DOI] [PubMed] [Google Scholar]
- 42.Levene M, Coleman DG, Kilpatrick HC, Fairbanks LD, Gangadharan B, Gasson C, Bax BE. Preclinical toxicity evaluation of erythrocyte-encapsulated thymidine phosphorylase in BALB/c mice and Beagle dogs: an enzyme replacement therapy for mitochondrial neurogastrointestinal encephalomyopathy. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfs278. [DOI] [PubMed] [Google Scholar]
- 43.Zhu GH, Schwartz EL. Expression of the angiogenic factor thymidine phosphorylase in THP-1 monocytes: induction by autocrine tumor necrosis factor-alpha and inhibition by aspirin. Molecular pharmacology. 2003;64:1251–1258. doi: 10.1124/mol.64.5.1251. [DOI] [PubMed] [Google Scholar]
- 44.Quek LS, Pasquet JM, Hers I, Cornall R, Knight G, Barnes M, Hibbs ML, Dunn AR, Lowell CA, Watson SP. Fyn and Lyn phosphorylate the Fc receptor gamma chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–4253. [PubMed] [Google Scholar]
- 45.Suzuki-Inoue K, Tulasne D, Shen Y, Bori-Sanz T, Inoue O, Jung SM, Moroi M, Andrews RK, Berndt MC, Watson SP. Association of Fyn and Lyn with the proline-rich domain of glycoprotein VI regulates intracellular signaling. The Journal of biological chemistry. 2002;277:21561–21566. doi: 10.1074/jbc.M201012200. [DOI] [PubMed] [Google Scholar]
- 46.Schmaier AA, Zou Z, Kazlauskas A, Emert-Sedlak L, Fong KP, Neeves KB, Maloney SF, Diamond SL, Kunapuli SP, Ware J, Brass LF, Smithgall TE, Saksela K, Kahn ML. Molecular priming of Lyn by GPVI enables an immune receptor to adopt a hemostatic role. Proc Natl Acad Sci U S A. 2009;106:21167–21172. doi: 10.1073/pnas.0906436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Zhang G, Liu J, Stojanovic A, Ruan C, Lowell CA, Du X. An important role of the SRC family kinase Lyn in stimulating platelet granule secretion. J Biol Chem. 2010;285:12559–12570. doi: 10.1074/jbc.M109.098756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cicmil M, Thomas JM, Sage T, Barry FA, Leduc M, Bon C, Gibbins JM. Collagen, convulxin, and thrombin stimulate aggregation-independent tyrosine phosphorylation of CD31 in platelets. Evidence for the involvement of Src family kinases. The Journal of biological chemistry. 2000;275:27339–27347. doi: 10.1074/jbc.M003196200. [DOI] [PubMed] [Google Scholar]
- 49.Ming Z, Hu Y, Xiang J, Polewski P, Newman PJ, Newman DK. Lyn and PECAM-1 function as interdependent inhibitors of platelet aggregation. Blood. 2011;117:3903–3906. doi: 10.1182/blood-2010-09-304816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho MJ, Pestina TI, Steward SA, Lowell CA, Jackson CW, Gartner TK. Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with gamma-thrombin. Blood. 2002;99:2442–2447. doi: 10.1182/blood.v99.7.2442. [DOI] [PubMed] [Google Scholar]
- 51.Shankar H, Kahner BN, Prabhakar J, Lakhani P, Kim S, Kunapuli SP. G-protein-gated inwardly rectifying potassium channels regulate ADP-induced cPLA2 activity in platelets through Src family kinases. Blood. 2006;108:3027–3034. doi: 10.1182/blood-2006-03-010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Douglas GV, Wiszniewska J, Lipson MH, Witt DR, McDowell T, Sifry-Platt M, Hirano M, Craigen WJ, Wong LJ. Detection of uniparental isodisomy in autosomal recessive mitochondrial DNA depletion syndrome by high-density SNP array analysis. J Hum Genet. 2011;56:834–839. doi: 10.1038/jhg.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haraguchi M, Tsujimoto H, Fukushima M, et al. Targeted deletion of both thymidine phosphorylase and uridine phosphorylase and consequent disorders in mice. Molecular and cellular biology. 2002;22:5212–5221. doi: 10.1128/MCB.22.14.5212-5221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.