Abstract
Previous reports indicate that among healthy individuals low Aerobic Fitness (AF) and high Body-Mass Index (BMI) predict poor neurocognition and daily-functioning. It is unknown whether these associations extend to disorders characterized by poor neurocognition, such as schizophrenia. Therefore, we compared AF and BMI in individuals with schizophrenia and non-clinical controls, and then within the schizophrenia group we examined the links between AF, BMI, neurocognition and daily-functioning. Thirty-two individuals with schizophrenia and 64 gender- and age-matched controls completed assessments of AF (indexed by VO2max) and BMI. The former also completed measures of neurocognition, daily-functioning and physical activity. The schizophrenia group displayed significantly lower AF and higher BMI. In the schizophrenia group, AF was significantly correlated with overall neurocognition (r=0.57), along with executive functioning, working memory, social cognition, and processing speed. A hierarchical regression analysis indicated that AF accounted for 22% of the neurocognition variance. Furthermore, AF was significantly correlated with overall daily-functioning (r=0.46). In contrast, BMI displayed significant inverse correlations with neurocognition, but no associations to daily-functioning. AF was significantly correlated physical activity. The authors discuss the potential use of AF-enhancing interventions to improve neurocognitive and daily-functioning in schizophrenia, along with putative neurobiological mechanisms underlying these links, including Brain-Derived Neurotrophic Factor.
Keywords: schizophrenia, aerobic fitness, body mass index, neurocognition, daily functioning
1. Introduction
Individuals with schizophrenia display a broad range of neurocognitive deficits across multiple domains including working memory, executive function, attention, verbal and visual memory, processing speed, and social cognition (Gold, 2004; Nuechterlein et al., 2004). These deficits have been identified as major determinants of poor functioning and disability (Barch and Keefe, 2010), representing a serious public health concern (Green, 2007). At present, available pharmacological and cognitive-remediation treatment approaches offer only minimal to limited benefits to ameliorate these deficits (Buchanan et al., 2011; Dixon et al., 2010; Javitt et al., 2012; McGurk et al., 2007; Vinogradov et al., 2013; Wykes et al., 2011). Thus, there remains an urgent need to identify novel approaches to improve neurocognitive functioning in people with schizophrenia.
1.1. Aerobic fitness, body-mass index, and neurocognition: insights from basic science
The ability to execute goal-directed cognitive operations and function effectively is contingent, in part, on the capacity to mobilize resources in order to initiate activities and sustain them over time. Supporting this view, converging lines of animal and human research link Aerobic Fitness (AF) to neurocognitive functioning (Hillman et al., 2008; van Praag, 2008). Specifically, among non-clinical populations, increases in AF have been found to predict improvements in neurocognitive functioning - in a meta-analysis of 29 randomized clinical trials with 2049 adults, increases in AF have been found to predict improvements in executive functioning, processing speed, attention, and declarative memory, with marginal support for working memory (Smith et al., 2010). Additionally, improvements in AF have been found to counteract age-related memory decline (Colcombe and Kramer, 2003; Kramer et al., 1999), delay onset of neurodegenerative diseases (Adlard et al., 2005; Kaspar et al., 2005), as well as enhance recovery from brain injury (Gobbo and O‧Mara, 2005; Grealy et al., 1999). Altogether, these findings provide strong support for the link between AF and neurocognition. Along with AF, another physical indicator that attracted considerable interest for its link to neurocognition is Body Mass Index (BMI), with the number of publications listed in PubMed that focus on this association growing 8-fold over the past decade (2002:18 → 2012:145). Consistent with the AF-neurocognition findings, a recent meta-analysis found significant associations between higher BMI and poorer performance on measures of memory, attention and executive functioning among non-clinical individuals (Siervo et al., 2011).
1.2. Aerobic fitness, body-mass index, and neurocognition: empirical findings in individuals with schizophrenia
Individuals with schizophrenia display a highly sedentary lifestyle that is associated with both low AF and high BMI (Daumit et al., 2005; Vancampfort et al., 2013; Vancampfort et al., 2011). Consistent with reports among non-clinical populations, preliminary findings among individuals with schizophrenia suggest associations between low AF, high BMI, and neurocognitive difficulties - Pajonk and colleagues (Pajonk et al., 2010) reported that improvements in AF among 16 males with schizophrenia were associated with enhancements in short-term memory, along with increases in hippocampal volume. Similarly, Friedman et al. (Friedman et al., 2010) found that higher BMI and hypertension in individuals with schizophrenia were associated with poor immediate, delayed and recognition memory. In another recent report, the number of metabolic syndrome symptoms, a correlate of AF and BMI, were associated with neurocognitive impairments (Boyer et al., 2013).
While these findings are suggestive of links between low AF, high BMI, and poor neurocognitive functioning in people with schizophrenia, these associations have not been examined systematically to date despite their clinical relevance. Specifically, a number of important gaps in the literature remain unaddressed. First, previous studies in schizophrenia have focused primarily on memory indices. Thus, the links between AF, BMI and other domains of neurocognitive functioning remain undetermined. Second, the contribution of AF and BMI to neurocognitive deficits in people with schizophrenia is undetermined. Finally, it is unknown whether the putative associations between AF, BMI and neurocognition extend to “real-world” daily functioning.
1.3. The present study
The primary goals of the present study were: 1) To confirm previously reported differences in AF and BMI between individuals with schizophrenia and healthy controls using “state-of-the-art” methodology; 2) To examine the links between AF, BMI, and neurocognition in the schizophrenia group and assess whether AF and BMI would predict neurocognitive functioning; and 3) To examine whether the putative associations between AF, BMI, and neurocognition would extend to “real world” daily functioning. We hypothesize that within the schizophrenia group, individuals with lower AF and higher BMI will display poorer neurocognitive functioning across multiple domains. Finally, we hypothesize that these associations will extend to daily functioning, with individuals with lower AF and higher BMI displaying poorer daily functioning.
2. Method
2.1. Participants
Data were obtained from two unrelated studies of aerobic fitness conducted at the New York State Psychiatric Institute (NYSPI) at the Columbia University Medical Center (CUMC). The studies were approved by the NYSPI’s Institutional Review Board and all participants provided written informed consent. Participants were recruited from outpatient mental health clinics affiliated with the NYSPI/CUMC (schizophrenia), as well as advertising (non-clinical controls) and were medically cleared for participation in the fitness assessment using the American College of Sports Medicine’s (2009) standards for exercise.
For the schizophrenia group, the inclusion criteria were a DSM-IV diagnosis of schizophrenia or related disorders; age 18–50 years; English-speaking, taking antipsychotic medication for at least 8 weeks and on current doses for 4 weeks and/or injectable depot antipsychotics with no change in last 3 months; and medically cleared by a physician to take part in a AF assessment. The exclusion criteria were a DSM-IV diagnosis of alcohol/substance abuse within the past month or alcohol/substance dependence within the past 6 months; a history of seizures or head trauma with loss of consciousness (>1 hour) resulting in cognitive sequelae and/or rehabilitation; significant clinical abnormalities in physical examination, electrocardiogram, or lab assessments; neurological/medical conditions that could interfere with study participation; untreated hyper- or hypothyroidism; extreme obesity (BMI≥40); recent use of street drugs (confirmed by urine toxicology tests); being pregnant/nursing; having serious suicidal/homicidal risk; presence of moderate or more severe symptoms of disorganization (SAPS positive formal thought disorder global rating >3); more than a mild level of depressive symptoms (BDI>18); and participation in a study involving neurocognitive assessment in the previous 3 months. For the non-clinical group, the inclusion criteria were age 20–45 years and English-speaking. The exclusion criteria were taking antipsychotic medications; having current or past major depressive disorder; history of heart disease, hypertension, diabetes mellitus, asthma or neurological/medical conditions that could interfere with study participation; and being pregnant or nursing. A total of 32 individuals with a DSM-IV diagnosis of schizophrenia or related disorders (25 schizophrenia, 7 schizoaffective disorder) were included in the study along with 64 gender- and age-matched non-clinical controls. Their demographic and clinical data are presented in Table 1.
Table 1.
Demographic and Clinical Information
| Schizophrenia (n=32) |
Non-Clinical Controls (n=64) |
t / Z | p | |
|---|---|---|---|---|
| Age (Mean) | 37.29 (9.41) | 35.17 (5.51) | 1.39 | N.S. |
| Sex (% female) | 37% | 37% | 0 | N.S. |
| Race (% Caucasian) | 31% | 39% | 0.75 | N.S. |
| Ethnicity (% Hispanic) | 41% | 34% | 0.60 | N.S. |
| Schizophrenia | Mean | SD | ||
| Positive Symptoms (SAPS Global Ratings) | ||||
| Hallucinations | 1.56 | 1.97 | ||
| Delusions | 1.87 | 1.58 | ||
| Bizarre Behavior | 0.30 | 0.82 | ||
| Positive Formal Thought Disorder | 0.57 | 0.99 | ||
| Negative Symptoms (SANS Global Ratings) | ||||
| Affective Flattening | 1.96 | 1.33 | ||
| Alogia | 1.09 | 1.24 | ||
| Avolition-Apathy | 2.27 | 1.16 | ||
| Anhedonia-Asociality | 2.48 | 1.16 | ||
| Attention | 2.04 | 1.29 | ||
| Mood Symptoms (Total Scores) | ||||
| Depression (BDI) | 7.59 | 7.78 | ||
| Anxiety (BAI) | 5.25 | 5.03 | ||
| Antipsychotic medication (Chlorpromazine Equivalence) | 354.38 | 291.77 | ||
| Antidepressant medication (% Prescribed) | 25% | -- | ||
Note: N.S. – Not significant; SAPS–Scale for Assessment of Positive Symptoms; SANS–Scale for Assessment of Negative; Symptoms; BDI–Beck Depression Inventory; BAI–Beck Anxiety Inventory.
2.2. Measures
Diagnostic and Clinical Assessments were established using The Structured Clinical Interview for DSM-IV (SCID-I). Clinical symptoms were assessed using the Scales for Assessment of Positive and Negative Symptoms (SAPS/SANS), the Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI). All diagnostic and clinical interviews were administered by raters and trained personnel with a Master’s level or above education.
Aerobic fitness and body mass index were determined by a cardiopulmonary exercise test to establish VO2max, considered a “gold standard” index of AF. VO2max is an index of the maximum capacity of an individual‧s body to transport and use oxygen during incremental aerobic exercise, reflecting the individual’s AF level. Assessments in both studies were supervised by one of the co-authors (MNB), a physician with extensive experience conducting cardiopulmonary assessments. The assessments in both studies were conducted in the same laboratory (the Human Performance Laboratory at Columbia University Medical Center), with identical lab technicians (one of the co-authors, HFA), equipment and assessment protocols. All tests were completed on weekdays, typically at ~10am. The tests were performed on an electronically-braked cycle ergometer (Ergometrics 800, SensorMedics Inc., Yorba Linda, CA) with a Viasys Encore metabolic cart (Viasys Corporation, Loma Linda, CA). The equipment was calibrated prior to every test. Continuous 12-lead telemetry was monitored via CardioSoft electro-cardiogram software (GE/CardioSoft, Houston, TX). Participants completed measurements of a 5-minute resting baseline, 3-minute of no-resistance warm-up, ramping exercise protocol of 10–15 watts with maximum exercise norms reached in ~12 minutes, followed by an active recovery of 3 minutes. During the test, the workload was increased 10–15 Watts every 1-minute until one of these criteria was reached: VO2 plateau, 85% of maximal heart rate (220-age), respiratory quotient≥1.1, or self-reported exhaustion as index by the Borg Scale (Borg, 1998). We used VO2maxpeak (mL/kg/min) scores in all analyses. Body Mass Index (BMI) was determined using an online calculator available at the web site of the U.S. Department of Health and Human Services’ National Heart Lung and Blood Institute (http://nhlbisupport.com/bmi/bminojs.htm) based on measurements of height and weight collected on the morning of the VO2max assessment using a SECA model 220 scale.
Neurocognition among the individuals with schizophrenia was assessed using the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein and Green, 2006). The MCCB assesses functioning in several neurocognitive domains including processing speed, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. The MCCB have been used widely in trials of neurocognition in schizophrenia (Buchanan et al., 2011; Javitt et al., 2012). We used MCCB T-scores adjusted for age, gender, and demographics in all analyses. The primary variable of interest for neurocognition was the MCCB composite score.
Daily functioning among the individuals with schizophrenia was measured using The Specific Levels of Functioning scale (SLOF; Schneider and Struening, 1983), a behavioral survey administered to an informant familiar with the study participant. The SLOF assess functioning and behavior across six domains including: physical functioning; personal care skills; interpersonal relationships; social acceptability; activities of community living; and work skills. The alpha coefficients for the various domains among 173 mental-health patients residing in the community were .57, .92, .92, .68, .95, and .93, respectively (Schneider and Struening, 1983). The NIMH-sponsored Validation of Everyday Real-World Outcomes (VALERO) study found the SLOF to be most robustly related to performance-based measures of neurocognition and everyday living skills among individuals with schizophrenia (Harvey, 2011). The primary variable of interest for daily functioning was the SLOF total score.
Degree of physical Activity was assessed among the individuals with schizophrenia using “real world” behavioral and self-report measures. Physical activity during “Real World” functioning was assessed using actigraph devices (ActiGraph wGT3X+ monitors). Such devices offer a non-invasive method of monitoring human rest and physical activity behaviors and have been used successfully in studies of individuals with schizophrenia (Tahmasian et al., 2013). Participants wore the actigraph devices around their waist over a continuous 36-hour period (10am Day 1 → 10pm Day 2), including during sleep time. All actigraph assessments were conducted during weekdays. Self-reports of physical activity were assessed using The International Physical Activity Questionnaire (IPAQ; Craig et al., 2003). Responders were asked to report their physical activities during the previous week. The IPAQ was developed as an instrument for cross-national monitoring of physical activity and inactivity among 18-to 65-year-old adults in diverse settings. The IPAQ has been used successfully with individuals with schizophrenia and demonstrated measurement properties that are comparable to those reported in the general population (Faulkner et al., 2006).
2.3. Statistical analyses
For group comparisons, we evaluated variables using two-tailed t-tests. Shapiro–Wilk tests indicated that all primary variables of interest were normally distributed (BMI (p=.291), Aerobic fitness (p=.052), neurocognition (p=.918), and daily functioning (p=.389)). Thus, associations between variables within the schizophrenia group were examined first using Pearson correlations coefficients, along with partial correlations controlling for depression and antipsychotic medication (as indexed by chlorpromazine equivalence; Woods, 2003). We used the Holm’s sequential Bonferroni procedure to reduce risk for type I error for correlational analyses. As the primary variables of interest were AF and BMI and their links to the summary scores of neurocognition and daily functioning, the initial significance level was set at p<.0125 (=0.05/4) across the 4 correlations of the primary variables of interest. Significance levels for exploratory tests (sub-domains of neurocognition and daily functioning) were set at p<.05 for all tests. Finally, assessment of whether AF or BMI would predict neurocognition after controlling demographic and clinical variables was tested using hierarchical regression analyses. The MCCB overall composite score was entered as dependent variable, demographic and clinical variables were entered in block 1, and AF or BMI were entered in block 2. The statistical package IBM SPSS version 21.0 was employed for all data analyses.
2.4. Study procedures
After satisfying the inclusion and exclusion criteria, participants completed the diagnostic, clinical, AF, and BMI assessments. The individuals with schizophrenia also completed assessments of neurocognition and daily functioning along with behavioral and self-report measures of physical activity. All assessments were conducted during weekdays and were typically completed within 3 weeks of study entry. The raters conducting the neurocognitive and clinical assessments were blind to the participants’ AF and BMI data, which were typically obtained last. Likewise, the physician and technician administering the VO2max tests were blind to the participants’ neurocognitive and daily functioning data.
3. Results
3.1. Aerobic fitness and body mass index: comparison of individuals with schizophrenia and non-clinical controls
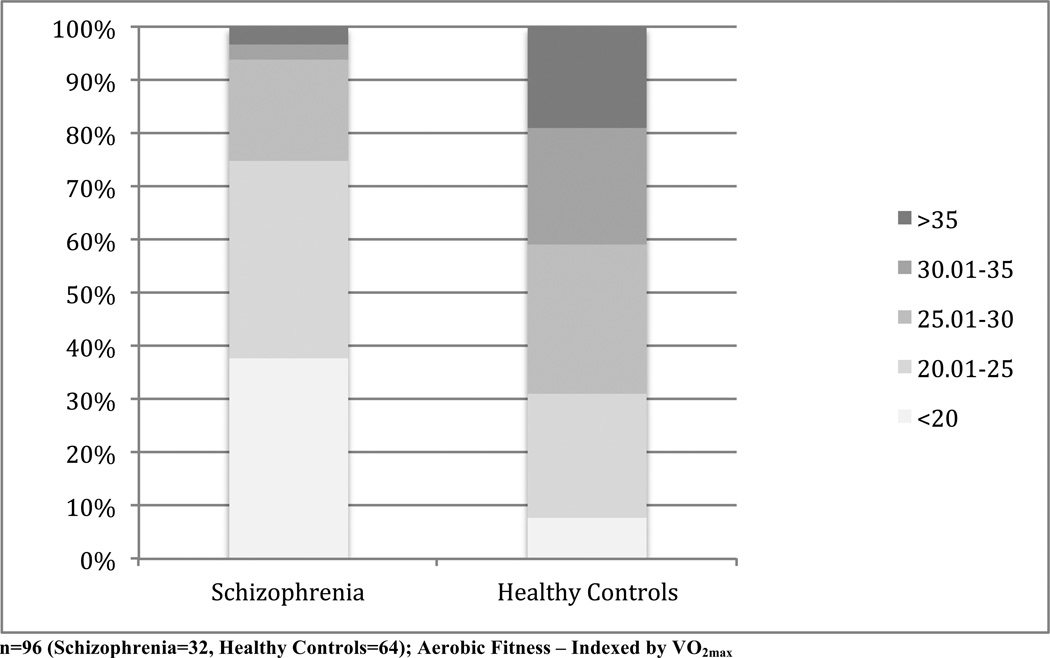
Comparison of the participants’ mean AF, as indexed by VO2max (mL/kg/min), indicated significant group differences (t=4.78, p<.001; df=94), with the schizophrenia group displaying lower AF than the non-clinical control group (mean=21.51, SD=6.49 vs. mean=28.92, SD=6.99, respectively). The distribution of AF among the individuals with schizophrenia and non-clinical controls is presented in Figure 1.
Figure 1.
Distribution of Aerobic Fitness Among Individuals with Schizophrenia and Healthy Controls
Similarly, the schizophrenia group had significantly higher BMI compared to the non-clinical group (t=5.93, p<.001; df=94; mean=32.10, SD=6.07 vs. mean=26.05, SD=3.75, respectively). Among the schizophrenia group, 69% of participants were obese (BMI≥30) and 28% were extremely obese (BMI≥35). As expected, there was a significant inverse association between AF and BMI among both groups (r=−.46, p<.001; n=95).
Among the individuals with schizophrenia, assessment of daily physical activity as indexed by the actigraph devices indicated that in the course of the 36-hour assessment (2160 min), participants spent on average 81.4% of the assessment time engaged in sedentary behavior (1759.53 min, SD=228.96), defined as sitting or lying down. On average, only 5.7% of the time was spent engaged in moderate or more intense physical activity (123.48 min, SD=81.02; defined as walking or more intense physical activity). Both AF and BMI displayed significant correlations with the percentage of time individuals were engaged in such activities during daily functioning (r=0.41, p=0.05 and r=−0.42, p=0.04, respectively). Likewise, self-reports of physical activity among the individuals with schizophrenia indicated that AF was significantly correlated with the number of minutes subject reported to engage in walking (r=0.56, p=0.007), doing moderately intense physical activity (r=0.65, p=0.001), and doing vigorously intense physical activity (r=0.60, p=0.003) during the previous week. In contrast, BMI was not associated with self-reported physical activities. Altogether, these findings suggest that the AF, as an indexed of by VO2max, corresponds with degree of physical activity among individuals with schizophrenia.
Previous reports indicated that obesity-related cardio-metabolic comorbidities (e.g., diabetes, hypertension, cardiovascular diseases) have been linked to cognitive decline in individuals with schizophrenia (Boyer et al., 2013). To address the potential impact of such cardio-metabolic comorbidities, we recorded the presence of cardio-metabolic comorbidities for each participant with schizophrenia. Employing a strategy used by Boyer et al. (2013), we created a continuous variable indexing the number of cardio-metabolic comorbidities criteria present. Accordingly, the number of cardio-metabolic comorbidities displayed a trend association with neurocognition (r=−0.28, p=0.08) and no significant relationship with AF or daily functioning. Thus, we did not include cardio-metabolic comorbidities in any further analyses.
3.2. Association of aerobic fitness and body mass index with neurocognitive functioning
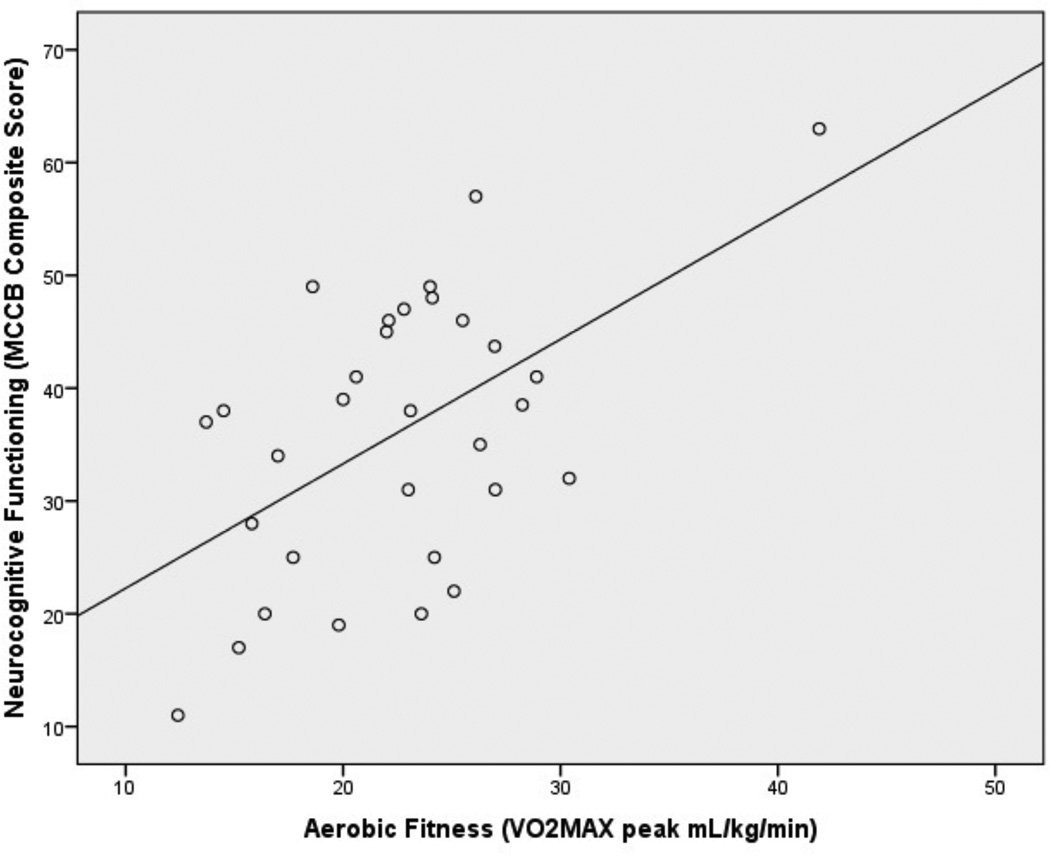
The mean neurocognitive functioning scores of participants with schizophrenia and their correlations with AF and BMI are presented in Table 2. Neurocognitive functioning was not associated with age or gender. Overall, AF was significantly correlated with overall MCCB composite score (controlling for depression and antipsychotic medication indexed by Chlorpromazine equivalence; See Figure 2). The results remain significant after correction using Holm’s sequential Bonferroni procedure. Exploratory analyses indicated significant correlations with a number of MCCB neurocognitive domains including reasoning and problem solving, speed of processing, working memory, and social cognition, with a trend association with visual learning (controlling for depression and antipsychotic medication indexed by Chlorpromazine equivalence). Notably, the association between AF and MCCB social cognition was driven primarily by significant correlation between AF and the Social Management Task of the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; r=0.48, p=0.03).
Table 2.
Neurocognitive and Daily Functioning: Mean, Standard Deviation, and Association with Aerobic Fitness and Body Mass Index
| Domain of Functioning | Mean (SD) | Partial Correlations# |
|
|---|---|---|---|
| Aerobic Fitness (VO2max) |
Body Mass Index (BMI) |
||
| Neurocognitive Functioning | |||
| MCCB (T-Scores) | |||
| Speed of Processing | 36.53 (11.96) | **0.46 | **−0.40 |
| Attention / Vigilance | 43.97 (12.26) | 0.17 | −0.08 |
| Working Memory | 39.57 (11.57) | **0.45 | ***−0.53 |
| Verbal Learning | 37.83 ( 7.31) | 0.19 | −0.02 |
| Visual Learning | 40.40 (14.51) | *0.37 | −0.10 |
| Reasoning & Problem Solving | 41.13 ( 9.46) | ***0.55 | −0.31 |
| Social Cognition | 42.33 (16.32) | **0.44 | **−0.46 |
| MCCB Composite Score | 33.70 (13.56) | ***0.57 | **−0.44 |
| Daily Functioning | |||
| SLOF (Informant reported) | |||
| Physical Functioning | 24.60 ( .74) | **0.43 | −0.22 |
| Personal Care Skills | 33.89 ( 2.64) | 0.13 | 0.15 |
| Interpersonal Relationships | 26.00 ( 5.98) | **0.42 | 0.07 |
| Social Acceptability | 34.07 ( 1.82) | 0.16 | 0.01 |
| Daily Activities | 51.46 ( 5.95) | **0.43 | 0.01 |
| Work Skills | 23.93 ( 5.99) | ***0.57 | −0.12 |
| SLOF Total Score | 193.93 (18.89) | **0.46 | −0.01 |
Note: n=32;
<.01;
< .05,
< .10;
partial correlations controlling for depression and antipsychotic medication;
VO2max - maximal oxygen consumption test; MCCB - MATRICS Consensus Cognitive Battery; SLOF – Specific Level of Functioning scale; Higher VO2max, MCCB and SLOF scores indicate better performance.
Figure 2.
Association of Aerobic Fitness with Neurocognitive Functioning in Individuals with Schizophrenia
Likewise, BMI displayed significant inverse correlations with the overall MCCB composite score (controlling for depression and antipsychotic medication). The results remain significant after correction using Holm’s sequential Bonferroni procedure. Exploratory analyses indicated significant correlations between BMI and the MCCB domains of working memory, social cognition, and speed of processing (controlling for depression and antipsychotic medication).
To estimate the relative contribution of AF to neurocognitive functioning, an exploratory hierarchical step-wise multiple regression analysis was conducted with MCCB composite score entered as the dependent variable, demographic and clinical variables including age, gender, depression, avolition, delusions, hallucinations and antipsychotic medication entered in block 1, and AF entered in block 2. The overall model accounted for 46% of the MCCB composite score. After controlling for potential covariates, AF accounted for 22% of the MCCB composite score variance (F1,31=8.54, p=0.006), contributing uniquely to the model‧s validity (β=0.46, t=2.92, p=0.006). A similar regression analysis with BMI entered in block 2 indicated the overall model accounted for 37% of the MCCB composite score. After controlling for potential covariates, AF accounted for 14% of the MCCB composite score variance (F1,31=5.03, p=0.032), contributing uniquely to the model‧s validity (β=−.37, t=-2.24, p=0.032). Ascertainment of the combined contributions of AF and BMI to neurocognitive functioning by adding the BMI to AF in block 2 did not contribute significantly to the model.
3.3. Association of aerobic fitness and body mass index with daily functioning
AF displayed an association with overall daily functioning as indexed by SLOF total score (see Table 2). This association remained significant after correction using Holm’s sequential Bonferroni procedure. The association was driven primarily by AF’s significant correlations with a number of daily functioning domains including work skills, physical functioning, daily activities, and interpersonal relationships (controlling for depression and anti-psychotic medication). Specifically, the association between AF and the SLOF work skills domain was propelled by relationships with SLOF work skills domain items as reported by informant including “Has Employable Skills” (r=0.63, p<.01), “Works with Minimal Supervision” (r=0.49, p=0.02), and “Completes Assigned Tasks” (r=0.48, p=0.03), controlling for depression and antipsychotic medication. In contrast, BMI was not associated with the SLOF total score or any of the SLOF sub-domains.
4. Discussion
To the best of our knowledge, the present study is the first to systematically characterize the associations between AF, BMI, neurocognition and daily functioning in individuals with schizophrenia.
4.1. Aerobic fitness and body mass index in individuals with schizophrenia
Consistent with published reports (Ozbulut et al., 2013; Daumit et al., 2005; Vancampfort et al., 2011), the participants with schizophrenia displayed significantly lower AF along with significantly higher BMI compared to the non-clinical controls. Specifically, over two-thirds of the schizophrenia participants were obese (BMI>30), and a nearly a third were extremely obese (BMI>35). These findings are in agreement with previous reports, which indicate that poor AF and sedentary behavior are highly prevalent among individuals with schizophrenia (Roick et al., 2007; Vancampfort et al., 2010).
Likewise, our actigraph and self-report physical activity data indicated a highly sedentary lifestyle - participants in our sample engaged in sedentary behavior on average ~29 hours and 20 min out of the 36 hours assessment time (81%). Likewise, self-reports confirm that the vast majority of participants did not engaged in more than light physical activity during the previous week. Only a small minority (7%) engaged in exercise activity (>2 hours/week) approaching the guidelines recommended by the American College of Sports Medicine (ACSM) and the U.S. federal government (Physical Activity Guidelines for Americans, 2008). These findings are consistent with previous reports - Strassnig and colleagues (Strassnig et al., 2011) reported that 98% of subjects with schizophrenia in their study displayed age-adjusted low AF. In other studies, 25–30% of individuals with schizophrenia reported no physical activity and only 30% were classified as being regularly active vs. 62% of healthy controls (Faulkner et al., 2006; Lindamer et al., 2008). Similarly, the energy expenditure of individuals with schizophrenia has been found to be 20% lower than the ACSM’s minimal recommendations (Sharpe et al., 2006).
4.2. Impact of aerobic fitness and body mass index on neurocognition
The participants with schizophrenia displayed substantial deficits in neurocognitive functioning, obtaining MCCB scores one to one-and-half standard deviations below published means for healthy individuals (Nuechterlein and Green, 2006). Supporting our hypothesis, AF was significantly correlated with overall neurocognitive functioning, as well as performance on multiple sub-domains including reasoning and problem solving, social cognition, speed of processing, and working memory. Moreover, AF significantly contributes to overall neurocognitive functioning, accounting for 22% of the MCCB composite score variance. These results are consistent with previously published reports among individuals with schizophrenia (Pajonk et al., 2010), as well as healthy individuals (Smith et al., 2010). Likewise, our group has previously found significant associations between cardiac vagal control, an index of parasympathetic contribution to cardiac regulation that is highly associated with AF, and enhanced executive functioning in a large and diverse sample of non-clinical individuals (Kimhy et al., 2013),
Results for BMI displayed similar pattern, with lower BMI being associated with better performance on working memory and social cognition neurocognitive domains. Similarly, BMI predicted 14% of the overall neurocognition variance. These results are consistent with previous reports among individuals with schizophrenia (Abdul Rashid et al., 2013; Friedman et al., 2010; Guo et al., 2013) and non-clinical populations (Stanek et al., 2013), suggesting a small, but significant impact of BMI on neurocognition.
4.3. Impact of aerobic fitness and body mass index on daily functioning
The most intriguing finding of the present study was the association between AF and daily functioning. Specifically, better AF was significantly correlated with overall daily functioning (as indexed by informants’ reports), as well as multiple sub-domains including work skills, interpersonal relationships, and physical functioning. These findings are consistent with previous reports, suggesting enhanced AF is associated with improved functioning among individuals with schizophrenia (Vancampfort et al., 2012b). However, in contrast to AF there were no significant associations between BMI and overall daily functioning or between BMI and any of the daily functioning sub-domains. The modest correlation found between AF and BMI is consistent with these results, suggesting AF is uniquely associated with daily functioning. Thus, despite the associations to a number of neurocognitive domains, BMI may not serve as useful target for interventions aiming to improve daily functioning in individuals with schizophrenia. These findings also add to a growing literature questioning the overall usefulness of using BMI as an index of physical health (Shah and Braverman, 2012).
4.4. Aerobic fitness, neurocognition and daily functioning in schizophrenia: implications for development of interventions
Our findings have potentially important clinical implications, as they point to the possibility of utilizing AF-enhancing interventions as a novel strategy to enhance neurocognitive deficits in people with schizophrenia. The need for new strategies is particularly urgent given that available pharmacological and cognitive-remediation treatment approaches offer only minimal to limited benefits to ameliorate such deficits. To date, first- and second-generation antipsychotics have demonstrated minimal efficacy in improving neurocognition (Goff et al., 2011; Keefe et al., 2007). Similarly, results from NIMH’s Treatment Units for Research of Neurocognition in Schizophrenia (TURNS) initiative to test novel pharmacological compounds targeting neurocognitive dysfunction have been disappointing (Buchanan et al., 2011; Javitt et al., 2012). Cognitive remediation studies provided more promising results – a recent meta-analysis found a small to moderate effect size for improvement in neurocognition (Wykes et al., 2011). However, concerns remain about previous trials’ “real-world” efficacy and methodological rigor (Dixon et al., 2010; Vinogradov et al., 2013; Wykes et al., 2011).
Because a sedentary person may experience as much as a 25% increase in AF after 8 weeks of aerobic exercise training (Wilmore et al., 2001), it is reasonable to hypothesize that aerobic exercise can improve neurocognitive and daily functioning in individuals with schizophrenia. Previous studies have concluded that aerobic exercise training is feasible and acceptable to individuals with schizophrenia and that aerobic exercise training can increase AF in this population (Gorczynski and Faulkner, 2010; Vancampfort et al., 2012a). Preliminary reports suggest that aerobic exercise training leads to increases in hippocampal volume and memory improvements in people with schizophrenia (Pajonk et al., 2010). Additionally, AE have been found to reduced need of care along with symptoms of schizophrenia (PANSS Total Score) and depression, compared with occupational therapy (Scheewe et al., 2013). Thus, low AF may potentially represent a unique modifiable risk factor for neurocognitive and functional difficulties in this population. Therefore, in addition to its well-documented cardiovascular, weight-management and other physical health benefits, aerobic exercise training may offer the potential to improve neurocognitive and daily functioning deficits in individuals with schizophrenia via a non-stigmatizing, side-effects-free, and cost-effective intervention. Future studies should aim to assess the efficacy and effectiveness of this treatment strategy.
4.5. Aerobic fitness and neurocognition in schizophrenia: putative neurobiological links
Our results invite speculation about the neurobiological mechanisms underlying the links between AF and neurocognitive dysfunction in schizophrenia. Improvement in neurocognitive functioning is related to the brain’s neuroplasticity, which is dependent on the support of neurotrophins that signal neurons to survive, differentiate and grow (Hennigan et al., 2007; Vaynman and Gomez-Pinilla, 2005). Among neurotrophins, Brain-Derived Neurotrophic Factor (BDNF) has been found to be particularly relevant to such processes as BDNF is the most abundant in the growth factor family (Binder and Scharfman, 2004), has a wide repertoire of neurotrophic and neuroprotective properties (Castren et al., 1998; Knaepen et al., 2010), and an extensive literature indicates that low serum-BDNF has negative pleiotropic effects on neurocognition (Casey et al., 2009). Germane to the links between AF and neurocognition, aerobic exercise training is known to induce a cascade of molecular and cellular processes that mediate brain plasticity (Hennigan et al., 2007; Johnston, 2009; van Praag, 2009). Specifically, among neurotrophins, BDNF has been found to be the most susceptible to regulation by physical activity (Johnston, 2009), with synthesis and release into the blood circulation increasing in a dose-response manner (Rasmussen et al., 2009; Seifert et al., 2010). Consistent with this view, a meta-analysis of 16 studies found lower serum BDNF levels in individuals with schizophrenia compared to healthy controls (Green et al., 2011), including among drug-naïve patients (Jindal et al., 2010; Rizos et al., 2008). Likewise, lower serum BDNF have been linked to poor short-term memory (Zhang et al., 2012) and smaller hippocampal volume (Rizos et al., 2011) in this population. Altogether, these converging lines of research provide support for a putative model in which aerobic exercise-related increases in AF leads to BDNF up-regulation, resulting in enhanced neurocognitive functioning. Future studies should aim to experimentally test and confirm this model in individuals with schizophrenia.
4.6. Limitations
Our study has a number of potential limitations. First, given the cross-sectional design and the relatively small sample size, our findings should be considered preliminary until replicated by other research groups. While a larger sample size would have been clearly preferable, the highly significant results provide support for our conclusions and represent the first systematic characterization of the links between AF, BMI, neurocognition, and daily functioning in people with schizophrenia. Thus, our findings represent an important, though admittedly preliminary, examination of these links.
Second, given the relatively small sample size and the differences in BMI between the individuals with schizophrenia and healthy controls, we did not match the samples for BMI. Thus, it cannot be excluded that differences in aerobic fitness between the groups are due to differences in BMI. Future studies comparing AF should control for potential BMI group differences.
Third, our daily functioning data were based on informants’ reports, rather than objective measures of functioning. Future studies should aim to replicate these findings with laboratory measures of functioning. Additionally, the lack of association between AF and the SLOF domains of personal care skills and social acceptability was potentially a result of a “ceiling” effect, as most participants in our sample received scores within the very upper limit range of these domains. This response pattern may be due to unique sample characteristics – outpatient individuals with schizophrenia who elect to participate in research and therefore less likely to have deficits in these functional domains. Alternatively, the SLOF may potentially have lower sensitivity to accurately discern functioning in these domains.
Finally, previous reports indicate that measurements of VO2max among individuals with extreme obesity (BMI>40) are less precise due to distinct hemodynamic parameters in this population (Fornitano and Godoy, 2010). Thus, we elected to exclude from our study individuals with such extreme obesity levels. As a result, until supported by further studies, our findings should not be generalized to individuals with schizophrenia with extreme obesity (BMI≥40).
Highlights.
Aerobic fitness predicts neurocognitive functioning in people with schizophrenia.
Higher aerobic fitness is associated with better daily functioning.
BMI is associated with neurocognition, but not with daily functioning.
Acknowledgment
This work was supported by grants 1R21MH096132 from The National Institute of Mental Health, Bethesda, MD (Dr. Kimhy) and 1R01HL094423 from The National Heart, Lung, and Blood Institute, Bethesda, MD (Dr. Sloan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Dr. Ballon has received investigator-initiated research funding from Novartis relating to another project. None of the other authors had any conflict of interest relating to this project.
References
- Abdul Rashid N, Lim J, Chong S, Keefe R, Lee J. Unraveling the relationship between obesity, schizoprhenia, and cognition. Schizophrenia Research. 2013;151:107–112. doi: 10.1016/j.schres.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. The Journal of Neuroscience. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Keefe RS. Anticipating DSM-V: opportunities and challenges for cognition and psychosis. Schizophrenia Bulletin. 2010;36:43–47. doi: 10.1093/schbul/sbp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Borg’s Perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- Boyer L, Richieri R, Dassa D, Boucekine M, Fernandez J, Vaillant F, Padovani R, Auquier P, Lancon C. Association of metabolic syndrome and inflammation with neurocognition in patients with schizophrenia. Psychiatry Research. 2013;210:381–386. doi: 10.1016/j.psychres.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Keefe RS, Lieberman JA, Barch DM, Csernansky JG, Goff DC, Gold JM, Green MF, Jarskog LF, Javitt DC, Kimhy D, Kraus MS, McEvoy JP, Mesholam-Gately RI, Seidman LJ, Ball MP, McMahon RP, Kern RS, Robinson J, Marder SR. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biological Psychiatry. 2011;69:442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, Altemus M, Pattwell S, Jones R, Levita L, McEwen B, Magarinos AM, Gunnar M, Thomas KM, Mezey J, Clark AG, Hempstead BL, Lee FS. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164:108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E, Berninger B, Leingartner A, Lindholm D. Regulation of brain-derived neurotrophic factor mRNA levels in hippocampus by neuronal activity. Progress in Brain Research. 1998;117:57–64. doi: 10.1016/s0079-6123(08)64007-8. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, Kreyenbuhl J, Wohlheiter K, Dixon LB. Physical activity patterns in adults with severe mental illness. Journal of Nervous and Mental Disease. 2005;193:641–646. doi: 10.1097/01.nmd.0000180737.85895.60. [DOI] [PubMed] [Google Scholar]
- Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, Lehman A, Tenhula WN, Calmes C, Pasillas RM, Peer J, Kreyenbuhl J, Schizophrenia Patient Outcomes Research T. The 2009 schizophrenia PORT psychosocial treatment recommendations and summary statements. Schizophrenia Bulletin. 2010;36:48–70. doi: 10.1093/schbul/sbp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner G, Cohn T, Remington G. Validation of a physical activity assessment tool for individuals with schizophrenia. Schizophrenia Research. 2006;82:225–231. doi: 10.1016/j.schres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Fornitano LD, Godoy MF. Exercise testing in individuals with morbid obesity. Obesity Surgery. 2010;20:583–588. doi: 10.1007/s11695-008-9692-7. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Wallenstein S, Moshier E, Parrella M, White L, Bowler S, Gottlieb S, Harvey PD, McGinn TG, Flanagan L, Davis KL. The effects of hypertension and body mass index on cognition in schizophrenia. The American Journal of Psychiatry. 2010;167:1232–1239. doi: 10.1176/appi.ajp.2010.09091328. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O’Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behavioural Brain Research. 2005;159:21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Goff DC, Hill M, Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacology, Biochemistry, and Behavior. 2011;99:245–253. doi: 10.1016/j.pbb.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophrenia Research. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gorczynski P, Faulkner G. Exercise therapy for schizophrenia. Cochrane Database of Systematic Reviews. 2010:CD004412. doi: 10.1002/14651858.CD004412.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Archives of Physical Medicine and Rehabilitation. 1999;80:661–667. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- Green MF. Stimulating the development of drug treatments to improve cognition in schizophrenia. Annual Review of Clinical Psychology. 2007;3:159–180. doi: 10.1146/annurev.clinpsy.3.022806.091529. [DOI] [PubMed] [Google Scholar]
- Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Molecular Psychiatry. 2011;16:960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- Guo X, Zhang Z, Wei Q, Lv H, Wu R, Zhao J. The relationship between obesity and neurocognitive function in Chinese patients with schizophrenia. BMC Psychiatry. 2013;13:109. doi: 10.1186/1471-244X-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD. Assessment of everyday functioning in schizophrenia. Innovations in Clinical Neuroscience. 2011;8:21–24. [PMC free article] [PubMed] [Google Scholar]
- Hennigan A, O’Callaghan RM, Kelly AM. Neurotrophins and their receptors: roles in plasticity, neurodegeneration and neuroprotection. Biochemical Society Transactions. 2007;35:424–427. doi: 10.1042/BST0350424. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Review Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, Lieberman J, Goff DC, Csernansky JG, McEvoy JP, Jarskog F, Seidman LJ, Gold JM, Kimhy D, Nolan KS, Barch DS, Ball MP, Robinson J, Marder SR. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophrenia Research. 2012;136:25–31. doi: 10.1016/j.schres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Jindal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naive first episode schizophrenia. Schizophrenia Research. 2010;119:47–51. doi: 10.1016/j.schres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Developmental Disabilities Research Reviews. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Annals of Neurology. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Archives of General Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Crowley OV, McKinley PS, Burg MM, Lachman ME, Tun PA, Ryff CD, Seeman TE, Sloan RP. The association of cardiac vagal control and executive functioning - Findings from the MIDUS study. Journal of Psychiatric Research. 2013;47:628–635. doi: 10.1016/j.jpsychires.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Medicine. 2010;40:765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lindamer LA, McKibbin C, Norman GJ, Jordan L, Harrison K, Abeyesinhe S, Patrick K. Assessment of physical activity in middle-aged and older adults with schizophrenia. Schizophrenia Research. 2008;104:294–301. doi: 10.1016/j.schres.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. The American Journal of Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine, A.C.o.S. Clinical Exercise Testing. In: Thompson WR, editor. ACSM’s Guidelines for Exercise Testing and Supervision. 8th Edition. Woulters Kluwer: Lippincott, Williams and Wilkins, Philadeplhia, PA; 2009. pp. 105–135. [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Tesearch. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS Consensus Battery Manual. Los Angeles: MATRICS Assessment Inc; 2006. [Google Scholar]
- Pajonk F-G, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Muller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry. 2010;67:133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Experimental Physiology. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Rizos EN, Papathanasiou M, Michalopoulou PG, Mazioti A, Douzenis A, Kastania A, Nikolaidou P, Laskos E, Vasilopoulou K, Lykouras L. Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophrenia Research. 2011;129:201–204. doi: 10.1016/j.schres.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Rizos EN, Rontos I, Laskos E, Arsenis G, Michalopoulou PG, Vasilopoulos D, Gournellis R, Lykouras L. Investigation of serum BDNF levels in drug-naive patients with schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:1308–1311. doi: 10.1016/j.pnpbp.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Roick C, Fritz-Wieacker A, Matschinger H, Heider D, Schindler J, Riedel-Heller S, Angermeyer MC. Health habits of patients with schizophrenia. Social Psychiatry and Psychiatric Epidemiology. 2007;42:268–276. doi: 10.1007/s00127-007-0164-5. [DOI] [PubMed] [Google Scholar]
- Scheewe TW, Backx FJ, Takken T, Jorg F, van Strater AC, Kroes AG, Kahn RS, Cahn W. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatrica Scandinavica. 2013;127:464–473. doi: 10.1111/acps.12029. [DOI] [PubMed] [Google Scholar]
- Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Social Work Research and Abstracts. 1983;19:9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Adser H, Jakobsen AH, Pilegaard H, Nielsen HB, Secher NH. Endurance training enhances BDNF release from the human brain. Am Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PloS One. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe JK, Stedman TJ, Byrne NM, Wishart C, Hills AP. Energy expenditure and physical activity in clozapine use: implications for weight management. Australian and New Zealand Journal of Psychiatry. 2006;40:810–814. doi: 10.1080/j.1440-1614.2006.01888.x. [DOI] [PubMed] [Google Scholar]
- Siervo M, Arnold R, Wells JC, Tagliabue A, Colantuoni A, Albanese E, Brayne C, Stephan BC. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obesity Reviews. 2011;12:968–983. doi: 10.1111/j.1467-789X.2011.00903.x. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek KM, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Mitchell JE, Gunstad J. Body mass index and neurocognitive functioning across the adult lifespan. Neuropsychology. 2013;27:141–151. doi: 10.1037/a0031988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig M, Brar JS, Ganguli R. Low cardiorespiratory fitness and physical functional capacity in obese patients with schizophrenia. Schizophrenia Research. 2011;126:103–109. doi: 10.1016/j.schres.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Medicine. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends in Neurosciences. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, De Hert M, De Herdt A, Vanden Bosch K, Soundy A, Bernard PP, De Wachter D, Probst M. Associations between physical activity and the built environment in patients with schizophrenia: a multi-centre study. General Hospital Psychiatry. 2013;35:653–658. doi: 10.1016/j.genhosppsych.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Knapen J, Probst M, van Winkel R, Deckx S, Maurissen K, Peuskens J, De Hert M. Considering a frame of reference for physical activity research related to the cardiometabolic risk profile in schizophrenia. Psychiatry Research. 2010;177:271–279. doi: 10.1016/j.psychres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, Helvik Skjaerven L, Catalan-Matamoros D, Lundvik-Gyllensten A, Gomez-Conesa A, Ijntema R, De Hert M. Systematic review of the benefits of physical therapy within a multidisciplinary care approach for people with schizophrenia. Physical Therapy. 2012a;92:11–23. doi: 10.2522/ptj.20110218. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, Scheewe T, Knapen J, De Herdt A, De Hert M. The functional exercise capacity is correlated with global functioning in patients with schizophrenia. Acta Psychiatrica Scandinavica. 2012b;125:382–387. doi: 10.1111/j.1600-0447.2011.01825.x. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, Sweers K, Maurissen K, Knapen J, De Hert M. Relationships between obesity, functional exercise capacity, physical activity participation and physical self-perception in people with schizophrenia. Acta Psychiatrica Scandinavica. 2011;123:423–430. doi: 10.1111/j.1600-0447.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabilitation and neural repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Nagarajan S. Cognitive training in schizophrenia: golden age or wild west? Biological psychiatry. 2013;73:935–937. doi: 10.1016/j.biopsych.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Wilmore JH, Green JS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C. Relationship of changes in maximal and submaximal aerobic fitness to changes in cardiovascular disease and non-insulin-dependent diabetes mellitus risk factors with endurance training: the HERITAGE Family Study. Metabolism: Clinical and Experimental. 2001;50:1255–1263. doi: 10.1053/meta.2001.27214. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liang J, Chen D, Xiu M, De Yang F, Kosten T, Kosten T. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology. 2012:1–8. doi: 10.1007/s00213-012-2643-y. [DOI] [PubMed] [Google Scholar]