Abstract
Objective
Controversy exists over the utility of early post-operative magnetic resonance imaging (MRI) after transsphenoidal pituitary surgery for macroadenomas. We investigate whether valuable information can be derived from current higher resolution scans.
Methods
Volumetric MRI scans were obtained in the early (<10 days) and late (>30 days) post-operative periods in a series of patients undergoing transsphenoidal pituitary surgery. The volume of the residual tumor, resection cavity, and corresponding visual field tests were recorded at each time point. Statistical analyses of changes in tumor volume and cavity size were calculated using the late MRI as the gold standard.
Results
40 patients met the inclusion criteria. Pre-operative tumor volume averaged 8.8 cm3. Early postoperative assessment of average residual tumor volume (1.18 cm3) was quite accurate and did not differ statistically from late post-operative volume (1.23 cm3, p=.64), indicating the utility of early scans to measure residual tumor. Early scans were 100% sensitive and 91% specific for predicting ≥ 98% resection (p<.001, Fisher’s exact test). The average percent decrease in cavity volume from pre-operative MRI (tumor volume) to early post-operative imaging was 45% with decreases in all but 3 patients. There was no correlation between the size of the early cavity and the visual outcome.
Conclusions
Early high resolution volumetric MRI is valuable in determining the presence or absence of residual tumor. Cavity volume almost always decreases after surgery and a lack of decrease should alert the surgeon to possible persistent compression of the optic apparatus that may warrant re-operation.
Keywords: Volumetric MRI, transsphenoidal, pituitary adenoma
Introduction
Magnetic resonance imaging (MRI) is the preferred imaging modality for post-operative follow-up of pituitary tumors (2, 8, 11, 13). Most studies have recommended a delayed (≥ 2 months), rather than early, postoperative follow-up MRI (6, 7, 10, 13). This stems from the difficulty in interpreting early postoperative images due to persistent cavity enlargement, accumulation of hemorrhagic material, fat packing and other post-operative changes (2, 6, 10, 11).
However, many of these studies were performed before high resolution volumetric spoiled gradient recalled echo (SPGR) sequencing was available. The superior soft tissue contrast and thinner sections obtained with SPGR has been shown to have superior sensitivity to the detection of pre-operative tumor in the sella turcica compared with the T1-weighted spin echo technique (9). We hypothesized that the sensitivity and specificity of high resolution early post-operative volumetric imaging would be higher than previously reported and that valuable information could be gained by performing early imaging. Early imaging would provide caregivers with valuable information to direct future care. For example, early hematoma accumulation could be evaluated for possible re-operation, particularly in light of persistent or worsening visual complaints. In addition, certain patients may fail to return for their delayed imaging studies and may be lost to radiographic follow-up in spite of evidence of residual tumor that may require close surveillance.
Methods
This study was approved by the institutional review board at Weill-Cornell Medical College. Data was gathered from a prospective database of all endoscopic endonasal transsphenoidal surgeries at Weill-Cornell Medical College, New York Presbyterian Hospital between January 2010 and March 2013 by the senior authors (THS and VKA). A total of 145 pituitary tumors were operated upon during this period.
Only primary resections of pituitary macroadenomas (> 1cm) in whom pre-operative, early post-operative (< 10 days), and late post-operative (> 30 days) volumetric MRI (1 mm slice thickness) with and without intravenous contrast were performed, were selected for this study. Charts were reviewed for visual complaints and formal visual field testing when available at each time point; pre-operatively, early postoperatively and late post-operatively.
Imaging techniques
Pre-operative MRI was performed the day of surgery for navigation purposes utilizing a whole brain volumetric T1-weighted spoiled gradient recalled echo (SPGR) post-contrast sequence. The median days to early post-operative images were 2 days (range 1–9 days) after operation and 3.6 months, in an outpatient setting, for late post-operative images (range 1–17 months).
Imaging protocols for all exams utilized whole brain sagittal fast spin echo (FSE) T1, axial FSE T2 FLAIR, axial diffusion weighted (DWI), and axial FSE T1-weighted post-contrast images. Dedicated imaging through the sella turcica included volumetric coronal T1-weighted spoiled gradient recalled echo (SPGR) pre and post-contrast using the following parameters: repetition time/echo time: 7.5/2.5 msec, a 20° flip angle, 160 × 256 matrix, 5 excitations, 12-cm field of view (FOV), and 1 mm thick sections without intersection gap. Dedicated imaging through the sella turcica also included coronal FSE T2-weighted fat saturated imaging using the following parameters: repetition time/echo time: 3000/85 msec, 256 × 320 matrix, 5 excitations, an echo train length of 16, 18-cm FOV, and 3 mm thick sections with 0.3 mm spacing. For the purposes of this study, the volumetric post-contrast SPGR T1-weighted images were post-processed on a GE Advantage Workstation version 4.3_05 utilizing the volume viewer.
Image Analysis
Image analysis was performed by an initial (JK) and final (AJT) reviewer on a GE Advantage Workstation version 4.3_05 utilizing the volume viewer software application. These reviewers were not blinded to the other reviewer’s read or the results of the early scans. Careful volumetric analyses were performed on all scans to determine pre-operative, early post-operative and late post-operative tumor volume, early and late post-operative resection cavity volume, the extent of resection at both postoperative time points, and the presence of tumor in the cavernous sinus. The pre- and post-contrast volumetric SPGR sequences were matched and correlation was made to the coronal FSE T2-weighted fat saturated imaging to differentiate post-surgical debris, packing material, graft and hemorrhagic elements from enhancing tumor. Tumor was identified as hypo-enhancing tissue with the same signal characteristics as the pre-operative tumor, as opposed to hyper-enhancing normal pituitary, non-enhancing tissue and T1/T2 bright fat packing material. The pre-operative MR was also helpful in locating residual tumor by analyzing the pre-operative extent of tumor i.e. cavernous extension and scrutinizing those areas. Non-enhancing blood products and fluid were also defined and included in the post-operative cavity measurements as appropriate. Sensitivity and specificity of the early post-operative findings were calculated by comparing the early post-operative to the late post-operative findings, treating the late post-operative findings as the gold standard. Differences in tumor size between the two timepoints were compared by Wilcoxon matched pairs signed-rank tests. P ≤ .05 was considered significant and 95% confidence intervals were used.
Results
Forty patients (20 male and 20 female) were included in the study. The patients’ ages ranged from 24 to 78 years (mean 55.3 years) at the time of surgery. Each patient had a histologically confirmed pituitary macroadenoma. Eight of these patients (20%) had tumor with cavernous sinus infiltration.
Identification of Residual Tumor
The average pre-operative tumor volume was 8.8 cm3 (range 0.53–90.19cm3). On early post-operative MRI, the average residual tumor volume was 1.18(SD ±4.0) cm3. On the late MRI scan, the average tumor volume was 1.23(SD ±5.2) cm3. The difference between these two values was not significant (p=0.64, Wilcoxon matched pairs signed-rank test). On the early post-operative MRI scans, average extent of resection was 95%. On the late MRI scans, the average extent of resection was 96%. There were 30 patients with ≥ 98% resection on the early scan. In all but one of these cases, the late scan did not differ from the early scan by more than 1%. One early scan overestimated extent of resection by 6%, indicating that the early scans were 100% sensitive and 91% specific for predicting ≥ 98% resection (p<.001, Fisher’s exact) (Table 1). For the 10 patients with a subtotal resection (range 53–97% resection), the early scans underestimated the extent of resection in six patients (range 5–27%) and overestimated the extent of resection in 4 patients (range 1–10%).
Table 1.
Residual Tumor
| Greater or equal to 98% resection | Complete Series N = 40 | Cavernous Sinus Infiltration N = 8 | No Cavernous Sinus Infiltration N= 32 |
|---|---|---|---|
| Early MRI | 30 | 2 | 28 |
| Late MRI | 29 | 1 | 28 |
| Specificity (false positives) | 91% (1) | 86% (1) | 100% (0) |
| Sensitivity (false negatives) | 100% (0) | 100% (0) | 100% (0) |
Of the 8 pituitary adenomas with cavernous sinus infiltration, 1/8 had no residual tumor on both the early and late scans and 6/8 had residual tumor on both early and late scans, while one case showed no residual on the early scans but did show residual on the late scan (p=0.25, Fisher’s exact). Although not statistically significant due to low sample size, there were no false negatives and one false positive in these 8 cases for a sensitivity and specificity in detecting absence of residual tumor of 100% and 86% respectively (Table 1).
Change in Cavity Size
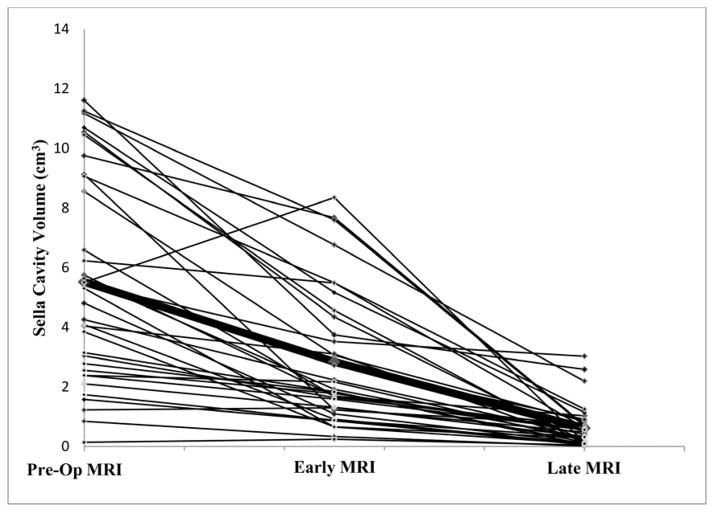
The pre-operative, early post-operative and late post-operative sella cavity volume was measured in 38 patients (Figure 1). Pre-operative cavity volume was the volume of the tumor while the post-operative cavity volume was the volume of residual tumor plus the volume of the resection cavity which includes the expanded diaphragm sella/arachnoid. The average percent decrease in cavity volume from pre-operative imaging (tumor volume) to early post-operative imaging was 45%. The size of the cavity decreased in 35 patients with a minimum of 3% and a maximum of 87%. 3 patients had an increase in cavity size with a minimum increase of 6% and maximum of 75%. The average percent decrease in cavity volume from pre-operative imaging to late post-operative imaging was 86%. On the late post-operative imaging, all patients had a decrease in cavity size from pre-operative scans. There was a significant difference in the average size of the cavity at early compared with late post-operative MRI scans (p<.001, Wilcoxon matched pairs signed-rank test), confirming that tumor cavity shrinks considerably between the two scans. The percent decrease from early to late post-operative imaging was 72%.
Figure 1.
The change sella cavity volume measured in cm3on preoperative (pre-op), early (median 2.0 days), and late MRI (median 3.6 months). Pre-operative cavity volume was the volume of the tumor while the post-operative cavity volume was the volume of residual tumor plus the volume of the resection cavity. Bolded line signifies average. X-axis is not drawn to scale. 3 cases intentionally excluded from the figure due to high tumor volumes.
Cavity volume and Visual Function
The persistence of a large post-operative cavity may appear to persistently cause chiasmal compression after surgery. We correlated post-operative visual change with cavity change on early post-operative MRI scans. Twenty-two patients presented with visual defects pre-operatively. These defects included: bitemporal hemianopsia (63.6%), left homonymous superior quadrantanopia (13.6%), left complete vision loss (9.1%), and right complete vision loss, left nasal hemianopsia and superior temporal quadrantanopia (4.5% each). Shortly after surgery at a timepoint aligned with the early MRI scan (range 1–9 days), subjective vision changes were obtained for 18 of 22 patients with pre-operative visual field deficits. Nine patients (50%) reported improved vision, seven patients (38.8%) reported no change in vision, and two patients (11.1%) reported worsened vision at this time. The two patients with worsening vision had decreases in cavity volume of 45% and 68% and thus no surgical intervention was offered. Subjective vision changes were also obtained for all 22 patients with pre-operative visual field deficits at a time point that aligned with the late MRI scan (range 1–17 months). Seventeen patients (77.3%) reported improved vision from their pre-operative baseline and five patients (22.7%) reported no change in their vision. The two patients with subjectively decreased vision at early MRI both improved by the time of the late MRI. The average change in the volume of the cavity for patients whose vision improved at the time of the early MRI was 47.0%, which was not significantly different from the average decrease in cavity volume for patients with no change or worsening vision, which was 49.0% (p=0.63, Mann-Whitney Test).
Discussion
This paper demonstrates that early post-operative volumetric imaging is 100% sensitive and 91% specific for detecting the presence of residual tumor, as defined by >2% remaining volume. In all but one patient with completely resected tumors (≥ 98% resection), the later scans only differed by 1%, which is within the error for reinterpretation of scans. Although the presence of residual tumor was confirmed on early scans, the actual amount of tumor varied slightly between scans, suggesting that early scans could prove the presence of residual tumor but not reliably determine its exact quantity. Our results support a recent study on comparing immediate and late (3 months) MR imaging in 64 patients with transsphenoidal surgery for pituitary adenoma, which showed similarly high sensitivity and specificity of early MR imaging for detecting residual tumor (14). Although residual tumor was overestimated or underestimated in all 10 patients with residual tumor, we believe the clinical relevance for early MRI detection of residual tumor is not the ability to accurately measure the amount of residual tumor, but the ability to detect the presence or absence of residual tumor. In our series, 10 out of 11 (91%) cases with residual tumors were correctly identified on early MRI scans. The maximum underestimation (27%) or overestimation (10%) of these residuals would not change the decision to reoperate for residual tumor. We also show that careful volumetric measurements should demonstrate a decrease in cavity volume in almost all patients. Even those patients with an early worsening in vision had a decrease in their post-resection cavity volume (presumably from trauma and not residual compression; hence, no re-operation indicated). Each of these patients had an improvement in vision by the later time point. This raises the possibility that if a patient complains of persistent visual complaints and early post-operative imaging shows no change in cavity volume or even an increase (an event which did not occur in our series), they likely would benefit from re-operation. However, if vision worsens and cavity volume decreases, it appears from our series that visual improvement is likely in the long run.
The literature is conflicted on the value of early post-operative MR imaging. Dina et al. found that the height of the resection cavity was increased or unchanged in 5/10 patient at early imaging and only minimally decreased in the remaining 5 (2). Likewise, Rajaraman et al. reported little change in post-operative cavity height in a series of 14 patients and Kremer et al. showed in a series of 50 patients that 83% of patients had a persistent mass in early post-operative MRI scans that was difficult to interpret (6, 10). On the other hand, Kilic et al. and Yoon et al. have described the utility of early post-operative imaging, although some false positives and negative were reported (4, 16). None of these studies used volumetric MRI scans with volumetric measurements. Hence, our study establishes the utility of early post-operative imaging in the modern imaging era and updates earlier studies with more definitive data on the subject.
In addition to determining the presence or absence of residual tumor, early scans can also evaluate the volume of the resection cavity and the presence and quantity of post-operative hematoma in a way that avoids high costs of an intraoperative MRI. Following transsphenoidal surgery for pituitary macroadenomas, patients with pre-operative visual complaints are not always improved after surgery, and sometimes they are worsened. In these situations, the surgeon must decide if a new hematoma has maintained or increased compression on the optic apparatus. Our volumetric measurements demonstrate that a decrease in cavity volume almost always occurs after successful transsphenoidal surgery. This finding is quite different from prior reports that only measured diameter and found little noticeable change in the majority of patients (2, 10). Hence, if an early MRI scan shows an increase in cavity volume and vision is worse or not improved compared with before surgery, early re-operation may be warranted to further decompress the chiasm. On the other hand, unchanged vision or even slight worsening in the face of a decreased cavity volume may warrant careful observation since long-term improvement may occur.
Another use for early post-operative MRI scans would be to detect the presence of significant residual tumor that might warrant early reoperation. It is easier to do a re-operation immediately after surgery, when the scar has not formed around the nasoseptal flap and fat graft. Such a philosophy motivates the justification of intraoperative MRI scanning (1, 12). However, such a situation did not occur in our series since all patients had adequate tumor removal and decompression of the optic apparatus. We attribute this result to our use of extended endoscopic techniques (3, 5, 12, 15). With regards to the issue of cost, one could argue that the cost of an additional early scan does not justify its use in the absence of visual worsening since the re-operation rate is low if vision remains stable. This is a reasonable argument. As such, we only recommend early scans in the following situations; 1) If vision is worse, the scan is useful since a larger or stable cavity size and persistent chiasmal compression should be explored to decompress the chiasm. If the cavity is smaller, re-exploration will not likely improve vision. 2) If vision is not improved, a scan that shows a larger cavity size and persistent chiasmal compression should have exploration to decompress the chiasm. If the cavity is smaller, re-exploration will not likely improve vision. 3) If there is a suspicion of residual tumor and re-operation would be considered, then early high resolution imaging can be very sensitive to residual tumor which, if found, can guide additional early re-operation.
Conclusion
Early high-resolution volumetric MRI scanning is highly sensitive and specific for determining the presence of residual tumor after transsphenoidal surgery. Such information is valuable with regards to patient counseling and determining the interval of follow-up scans. While persistent cavity enlargement may warrant re-operation in the face of visual deterioration, cavity shrinkage predicts visual improvement even in the face of early deterioration, which may also spare patients additional unnecessary surgery. This paper attempts to demonstrate the accuracy of early high resolution volumetric MRI scanning. While cost may prohibit its use routinely, it may be indicated in certain situations.
Abbreviations
- MRI
Magnetic resonance imaging
- SPGR
spoiled gradient recalled echo
- FSE
fast spin echo
- DWI
diffusion weighted imaging
- FLAIR
fluid attenuated inversion recovery
- FOV
field of view
- SD
standard deviation
- MR
magnetic resonance
Footnotes
Conflict of Interest: None
Disclosure of Funding: This investigation was supported by grant UL1RR024996 of the Clinical and Translational Science Center at Weill-Cornell Medical College. This grant is funded through the National Institutes of Health and therefore its publication must comply with the NIH Public Access Policy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bohinski RJ, Warnick RE, Gaskill-Shipley MF, Zucarello M, van Loveren HR, Kormos DW, Tew JM. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transphenoidal surgery. Neurosurg. 2001;49:1133–1144. doi: 10.1097/00006123-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Dina TS, Feaster SH, Laws ER, Jr, Davis DO. MR of the pituitary gland postsurgery: serial MR studies following transsphenoidal resection. AJNR Am J Neuroradiol. 1993;14(3):763–769. [PMC free article] [PubMed] [Google Scholar]
- 3.Hofstetter CP, Nanaszko MJ, Mubita LL, Tsiouris J, Anand VK, Schwartz TH. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary. 2012;15(3):450–463. doi: 10.1007/s11102-011-0350-z. [DOI] [PubMed] [Google Scholar]
- 4.Kiliç T, Ekinci G, Seker A, Elmaci I, Erzen C, Pamir MN. Determining optimal MRI follow-up after transsphenoidal surgery for pituitary adenoma: scan at 24 hours postsurgery provides reliable information. Acta Neurochir (Wien) 2001;143(11):1103–1126. doi: 10.1007/s007010100002. [DOI] [PubMed] [Google Scholar]
- 5.Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of giant pituitary adenomas. Pituitary. 2012;15(2):150–159. doi: 10.1007/s11102-011-0359-3. [DOI] [PubMed] [Google Scholar]
- 6.Kremer P, Forsting M, Ranaei G, Wuster C, Hamer J, Sartor K, Kunze S. Magnetic resonance imaging after transsphenoidal surgery of clinically non-functional pituitary macroadenomas and its impact on detecting residual adenoma. Acta Neurochir (Wien) 2002;144(5):433–443. doi: 10.1007/s007010200064. [DOI] [PubMed] [Google Scholar]
- 7.Mansouri A, Symons S, Schwartz M, Chen J, Pirouzmand F. Quantitative volumetric analysis post transsphenoidal pituitary adenoma surgery. Can J Neurol Sci. 2012;39(5):600–604. doi: 10.1017/s0317167100015328. [DOI] [PubMed] [Google Scholar]
- 8.Mikhael MA, Ciric IS. MR imaging of pituitary tumors before and after surgical and/or medical treatment. J Comput Assist Tomogr. 1988;12(3):441–445. doi: 10.1097/00004728-198805010-00017. [DOI] [PubMed] [Google Scholar]
- 9.Patronas N, Bulakbasi N, Stratakis CA, Lafferty A, Oldfield EH, Doppman J, Nieman LK. Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab. 2003;88(4):1565–1569. doi: 10.1210/jc.2002-021438. [DOI] [PubMed] [Google Scholar]
- 10.Rajaraman V, Schulder M. Postoperative MRI appearance after transsphenoidal pituitary tumor resection. Surg Neurol. 1999;52:592–598. doi: 10.1016/s0090-3019(99)00157-3. discussion 598–599. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez O, Mateos B, de la Pedraja R, Villoria R, hernando JI, Pastor A, Pomposo I, Aurrecoechea J. Postoperative follow-up of pituitary adenomas after trans-sphenoidal resection: MRI and clinical correlation. Neuroradiology. 1996;38(8):747–754. doi: 10.1007/s002340050341. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz TH, Stieg PE, Anand VK. Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery. 2006;58(1 Suppl):ONS44–51. doi: 10.1227/01.neu.0000193927.49862.b6. discussion ONS44–51. [DOI] [PubMed] [Google Scholar]
- 13.Steiner E, Knosp E, Herold CJ, Kramer J, Stiglbauer R, Staniszewski K, Imhof H. Pituitary adenomas: findings of postoperative MR imaging. Radiology. 1992;185(2):521–527. doi: 10.1148/radiology.185.2.1410366. [DOI] [PubMed] [Google Scholar]
- 14.Stofko DL, Nickles T, Sun H, Dehdashti AR. The value of immediate postoperative MR imaging following endoscopic endonasal pituitary surgery. Acta Neurochir (Wien) 2013 doi: 10.1007/s00701-013-1834-6. [DOI] [PubMed] [Google Scholar]
- 15.Theodosopoulos PV, Leach J, GKR, Zimmer LA, Denny AM, Guthikonda B, Froehlich S, Tew JM. Maximizing the extent of tumor resection during transsphenoidal surgery for pituitary macroadenomas: can endoscopy replace imtraoperative imaging? J Neurosurg. 2010;112:736–743. doi: 10.3171/2009.6.JNS08916. [DOI] [PubMed] [Google Scholar]
- 16.Yoon PH, Kim DI, Jeon P, Lee SI, Lee SK, Kim SH. Pituitary adenomas: early postoperative MR imaging after transsphenoidal resection. AJNR Am J Neuroradiol. 2001;22(6):1097–1104. [PMC free article] [PubMed] [Google Scholar]