Abstract
Dental pulp/dentin regeneration using dental stem cells combined with odontogenic factors may offer great promise to treat and/or prevent premature tooth loss. Here, we investigate if BMP9 and Wnt/β-catenin act synergistically on odontogenic differentiation. Using the immortalized SCAPs (iSCAPs) isolated from mouse apical papilla tissue, we demonstrate that Wnt3A effectively induces early osteogenic marker alkaline phosphatase (ALP) in iSCAPs, which is reduced by β-catenin knockdown. While Wnt3A and BMP9 enhance each other’s ability to induce ALP activity in iSCAPs, silencing β- catenin significantly diminishes BMP9-induced osteo/odontogenic differentiation. Furthermore, silencing β-catenin reduces BMP9-induced expression of osteocalcin and osteopontin and in vitro matrix mineralization of iSCAPs. In vivo stem cell implantation assay reveals that while BMP9-transduced iSCAPs induce robust ectopic bone formation, iSCAPs stimulated with both BMP9 and Wnt3A exhibit more mature and highly mineralized trabecular bone formation. However, knockdown of β-catenin in iSCAPs significantly diminishes BMP9 or BMP9/Wnt3A-induced ectopic bone formation in vivo. Thus, our results strongly suggest that β-catenin may play an important role in BMP9-induced osteo/ondontogenic signaling and that BMP9 and Wnt3A may act synergistically to induce osteo/odontoblastic differentiation of iSCAPs. It’s conceivable that BMP9 and/or Wnt3A may be explored as efficacious biofactors for odontogenic regeneration and tooth engineering.
Keywords: dental stem cells, stem cells of apical papilla, BMP signaling, Wnt/β-catenin signaling, odontoblastic differentiation, dontogenesis, osteogenic differentiation, tooth engineering
1. Introduction
Teeth are highly mineralized organs resulting from sequential and reciprocal interactions between the oral epithelium and the underlying cranial neural crest-derived mesenchyme [1-3]. Premature tooth loss caused by caries, pulpitis, and apical periodontitis presents a formidable challenge in managing the patient’s quality of life, and controlling health care costs and loss of economic productivity. While de novo tooth engineering may provide great promise for improving clinical outcomes of dental diseases, harnessing the natural regenerative potential of dental stem cells in dentin-pulp tissues may offer more practical solutions to enhance wound healing and maintain pulp vitality [4-6]. Any successful tissue engineering would require at least three components, including bio-compatible scaffolding materials, effective biological factors, and progenitors that have differentiation potential of becoming intended tissue types. Most of dental structures are derived from dental ectomesenchyme, a zone of condensed cells derived from oral ectoderm during embryonic tooth development [1, 4, 7]. Dental stem cells are considered a population of mesenchymal stem cell (MSC)-like cells, and at least five types of dental stem/progenitor cells have been identified [1, 7], including dental pulp stem cells (DPSCs), stem cells from human exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSCs), dental follicle progenitor cells (DFPCs), and stem cells from apical papilla (SCAP). Although these postnatal populations have MSC-like characteristics, the dental stem cells are isolated from specialized tissues with potent capacities to differentiate into odontogenic cells.
We have recently established a reversibly immortalized mouse stem cells of apical papilla tissue of mouse lower incisor teeth (or iSCAPs), which not only maintain long-term cell proliferation but also retain the ability to differentiate into multiple lineages, including osteo/odontoblastic lineage upon BMP9 stimulation [8]. We previously demonstrated that BMP9 (aka, growth and differentiation factor 2, or GDF2) is one of the most potent factors that can induce osteogenic and adipogenic, to a lesser extent, chondrogenic differentiation of MSCs [9-13]. However, it remains to be thoroughly investigated how odontogenic differentiation is regulated by other major signaling pathways, such as Wnt/β-catenin.
Wnts are a family of secreted glycoproteins that regulate many developmental processes [14-16]. Wnt signaling plays an important role in skeletal development [15, 17-20]. Wnt proteins bind to their cognate receptor frizzled (Fz) and LRP-5/6 co-receptors, and activate distinct signaling pathways, including the canonical β-catenin pathway. In the absence of Wnt signaling, β-catenin is degraded by the proteasome system after GSK3 dependent phosphorylation. In the presence of Wnt signaling, unphosphorylated β-catenin accumulates in the cytoplasm and translocates into the nucleus where it associates with Tcf/LEF transcription factors to regulate the expression of target genes [16, 21-24]. However, the precise function of Wnt/β-catenin in osteo/odontoblastic differentiation remains to be fully elucidated.
In this study, we investigate the effect of Wnt/β-catenin signaling on osteo/odontogenic differentiation of iSCAP cells, and the role of β-catenin in mediating the synergistic effect between BMP9 and Wnt3A on osteo/odontogenic differentiation. Using a stable iSCAP line in which β-catenin expression was effectively silenced, we found that Wnt3A induces early osteogenic marker alkaline phosphatase (ALP) in iSCAP cells, which can be significantly diminished by β-catenin knockdown. While Wnt3A and BMP9 enhance each other’s ability to induce ALP activity in iSCAPs, silencing β-catenin significantly diminishes the BMP9-induced osteo/odontogenic, but not adipogenic, differentiation. Silencing β-catenin leads to a decrease in BMP9-induced expression of osteocalcin and osteopontin and in vitro matrix mineralization of the iSCAPs. In vivo stem cell implantation assay reveals that, while BMP9- transduced iSCAP cells induce robust ectopic bone formation, the iSCAP cells stimulated with both BMP9 and Wnt3A exhibit more mature and highly mineralized trabecular bone formation. However, knockdown of β-catenin in iSCAP cells significantly inhibits BMP9 or BMP9/Wnt3A-induced ectopic bone formation in vivo. These results strongly suggest that β-catenin signaling may play an important role in BMP9-induced osteo/ondontogenic signaling and that BMP9 and Wnt3A may act synergistically to induce osteo/odontoblastic differentiation of iSCAPs, which requires functional β-catenin signaling activity. Thus, it’s conceivable that BMP9 and/or Wnt3A may be explored as novel and efficacious biofactors for odontogenic regeneration and tooth engineering.
2. Materials and Methods
2.1. Cell culture and chemicals
HEK-293 cell line was purchased from ATCC (Manassas, VA) and maintained in complete Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), 100 units of penicillin and 100μg of streptomycin at 37°C in 5% CO2 [25-30]. The immortalized mouse stem cells of dental apical papilla (iSCAPs) were previously characterized and reported [8]. The recently engineered 293pTP line was used for adenovirus amplification [31]. Both 293pTP and iSCAPs were maintained in complete DMEM. Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA).
2.2. Construction of the piggyBac vector expressing three siRNA sites targeting mouse β-catenin and the generation of stable iSCAP-KD and iSCAP-Ctrl lines
Using the three siRNA targeting sites that were previously proven effective [32, 33], we subcloned three siRNA sites, each driven by the opposing U6 and H1 promoters into our modified piggyBac transposon vector pMPB [26, 28], yielding pMPB-simBC. All cloning fragments were verified by DNA sequencing. A vector containing the scrambled sites was also constructed as a control (e.g., pMPB-Ctrl). Subconfluent iSCAP cells were co- transfected with the above vectors and a piggyBac transposase-expressing vector [26, 28]. At 48h after transfection, the cells were selected against blasticidin S (4μg/ml) for 5-7 days. The resultant stable lines were designated as iSCAP-KD and iSCAP-Ctrl, respectively.
2.3. Generation and amplification of recombinant adenoviruses expressing BMP9, Wnt3A, and GFP
Recombinant adenoviruses were generated using the AdEasy technology as described [9, 10, 12, 34]. The coding regions of human BMP9 and mouse Wnt3A were PCR amplified and cloned into an adenoviral shuttle vector, and subsequently used to generate and amplify recombinant adenoviruses in HEK-293 or 293pTP cells [31]. The resulting adenoviruses were designated as AdBMP9 and AdWnt3A, both of which also express GFP [35-38]. Analogous adenovirus expressing only GFP (AdGFP) was used as controls [33, 39-41]. For all adenoviral infections, polybrene (4-8μg/ml) was added to enhance infection efficiency as previously reported [29].
2.4. Cell transfection and firefly luciferase assay
Subconfluent iSCAP cells were transfected with the Tcf/Lef reporter pTOP-Luc using Lipofectamine Reagent (Invitrogen) by following the manufacturer’s instructions. At the end of transfection procedure, the cells were infected with AdWnt3A or AdGFP. At 48h post transfection/infection, cells were lysed for luciferase assays using Luciferase Assay System (Promega, Madison, WI) by following the manufacturer’s instructions. Easy conditions were done in triplicate.
2.5. RNA isolation, quantitative and semi-quantitative RT-PCR (qPCR & sqPCR)
Total RNA was isolated by using TRIZOL Reagents (Invitrogen) and used to generate cDNA templates by reverse transcription reactions with hexamer and M-MuLV reverse transcriptase (New England Biolabs, Ipswich, MA). The cDNA products were used as PCR templates. The sqPCR were carried out as described [42-46]. PCR primers (Suppl. Table 1) were designed by using the Primer3 program and used to amplify the genes of interest (approximately 150-250bp).
For qPCR analysis, SYBR Green-based qPCR analysis was carried out by using the thermocycler Opticon II DNA Engine (Bio-Rad, CA) with a standard pUC19 plasmid as described elsewhere [27, 47-49]. The qPCR reactions were done in triplicate. The sqPCR was also carried out as described [30-32, 39, 46, 50-53]. Briefly, sqPCR reactions were carried out by using a touchdown protocol: 94°C × 20”, 68°C × 30”, 70°C× 20” for 12 cycles, with 1°C decrease per cycle, followed by 25-30 cycles at 94°C× 20”, 56°C × 30”, 70°C × 20”. PCR products were resolved on 1.5% agarose gels. All samples were normalized by the expression level of GAPDH.
2.6. Immunofluorescence staining
Immunofluorescence staining was performed as described [12, 33, 42, 49, 54, 55]. Briefly, cells were infected with AdWnt3A or AdGFP for 48h, fixed with methanol, permeabilized with 1% NP-40, and blocked with 10% BSA, followed by incubating with β-catenin antibody (Santa Cruz Biotechnology). After being washed, cells were incubated with Texas Red-labeled secondary antibody (Santa Cruz Biotechnology). Stains were examined under a fluorescence microscope. Stains without primary antibodies, or with control IgG, were used as negative controls.
2.7. Qualitative and quantitative assays of alkaline phosphatase (ALP) activity
ALP activity was assessed quantitatively with a modified assay using the Great Escape SEAP Chemiluminescence assay kit (BD Clontech, Mountain View, CA) and qualitatively with histochemical staining assay (using a mixture of 0.1 mg/ml napthol AS-MX phosphate and 0.6 mg/ml Fast Blue BB salt), as described [9, 10, 12, 33, 39, 43, 51, 54]. Each assay condition was performed in triplicate and the results were repeated in at least three independent experiments. ALP activity was normalized by total cellular protein concentrations among the samples.
2.8 Matrix mineralization assay (alizarin red S staining)
iSCAP cells were seeded in 24-well cell culture plates and infected with AdBMP9 or AdGFP. Infected cells were cultured in the presence of ascorbic acid (50 mg/mL) and β-glycerophosphate (10mM). At 10 days after infection, mineralized matrix nodules were stained for calcium precipitation with Alizarin Red S as described [9, 10, 12, 33, 39, 43, 51, 54]. Briefly, cells were fixed with 0.05% (v/v) glutaraldehyde at room temperature for 10min and washed with distilled water, fixed cells were incubated with 0.4% Alizarin Red S (Sigma- Aldrich) for 5 min, followed by extensive washing with distilled water. The staining of calcium mineral deposits was recorded under bright field microscopy.
2.9. Oil red O staining assay
iSCAPs were seeded in 12-well cell culture plates and infected with AdBMP9 or AdGFP for 10 days. Oil Red O staining was performed as described [12, 52]. Briefly, cells were fixed with 10% formalin at room temperature for 20 min and washed with PBS. The fixed cells were stained with freshly prepared Oil Red-O solution (6 parts saturated Oil Red O dye in isopropanol plus 4 parts water) at 37°C for 30-60mi n, followed by washing with 70% ethanol and distilled water.
2.10. iSCAP cell implantation, ectopic bone formation, and micro-computed tomography (μCT) analysis
All animal studies were conducted by following the guidelines approved by the Institutional Animal Care and Use Committee (IACUC). Stem cell-mediated ectopic bone formation was performed as described [10, 12, 26, 46, 51, 53, 56-58]. Briefly, iSCAP cells were infected with AdBMP9 and/or AdWnt3A, or AdGFP for 16h, collected and resuspended in PBS for subcutaneous injection (5x106/injection) into the flanks of athymic nude (nu/nu) mice (5 animals per group, 4–6 wk old, female, Harlan Laboratories, Indianapolis, IN). At 4 weeks after implantation, animals were sacrificed, and the implantation sites were retrieved for μCT imaging, histologic evaluation, and special stains. All retrieved specimens were fixed and imaged using the μCT component of a GE triumph (GE healthcare, Piscataway, NJ) trimodality preclinical imaging system. All image data analysis was performed using Amira 5.3 (Visage Imaging, Inc., San Diego, CA), and 3D volumetric data were obtained as described [8, 26, 43, 46, 52, 53, 56, 57].
2.11. Histological evaluation and Trichrome staining
Retrieved tissues were fixed, decalcified in 10% buffered formalin, and embedded in paraffin. Serial sections of the embedded specimens were stained with hematoxylin and eosin (H & E). Trichrome staining was carried out as previously described [9, 10, 12, 26].
2.12. Statistical analysis
The quantitative assays were performed in triplicate and/or repeated three times. Data were expressed as mean ± SD. Statistical significances were determined by one-way analysis of variance and the student’s t test. A value of p<0.05 was considered statistically significant.
3. Results
3.1. Construction and validation of siRNAs targeting mouse β-catenin in iSCAPs
We have recently demonstrated that piggyBac transposon system has significant advantages over retroviral vectors in terms of generating high expression stable lines [26, 28]. In order to effectively and stably silence mouse β-catenin expression in iSCAP cells, we subcloned three siRNA sites (Fig. 1A), each driven by the opposing U6 and H1 promoters into our modified piggyBac pMPB [26, 28], yielding pMPB-simBC. A vector containing the scrambled sites was also constructed as a control (e.g., pMPB-Ctrl). Subconfluent iSCAP cells were co-transfected with the above vectors and a piggyBac transposase-expressing vector [26, 28]. Stable lines were obtained by selecting them against blasticidin S and designated as iSCAP-KD and iSCAP-Ctrl, respectively.
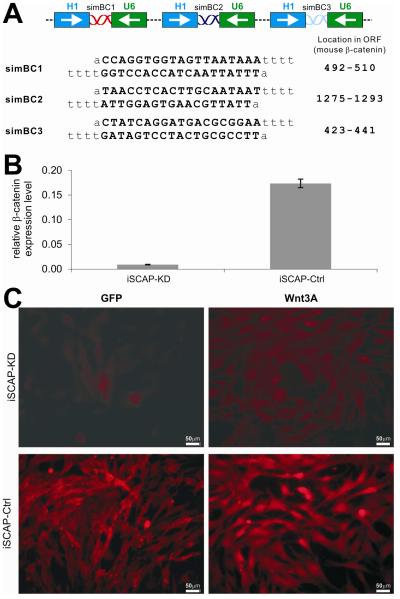
Fig. 1. Construction and validation of siRNAs targeting mouse β-catenin in the iSCAPs.
(A) Schematic representation of the tandem siRNA targeting configuration and the targeting sequences and locations of the three siRNA sites (simBC1, 2, and 3) on mouse β-catenin open reading frame (ORF). (B) Efficient knockdown of endogenous β-catenin expression in iSCAPs. Total RNA was isolated form subconfluent iSCAP cells that were stably transduced with the piggyBac vector expressing three simBC sites (iSCAP-KD) or scrambled controls (iSCAP-Ctrl). The RNA samples were subjected to reverse-transcription and subsequently quantitative real- time PCR (qPCR) using primers specific for mouse β-catenin and GAPDH. The relative β-catenin expression level was calculated by dividing the β-catenin concentration with the respective GAPDH concentration. Each reaction was done in triplicate. (C) simBCs can effectively block Wnt3a-induced β-catenin accumulation. Subconfluent iSCAP-KD and iSCAP- Ctrl cells were infected with AdWnt3A or AdGFP for 30h, fixed and subjected to immunofluorescence staining with an anti-β-catenin antibody.
We first assessed the knockdown efficiency of endogenous β-catenin in iSCAP cells. Using qPCR analysis, we found that the endogenous β-catenin expression in the iSCAP-KD cells was approximately 5% of that in the iSCAP-Ctrl cells (Fig. 1B), indicating that the endogenous β-catenin expression is effectively silenced in the iSCAP-KD cells. Furthermore, when iSCAP-KD cells were transduced with Wnt3A, we found that cytoplasmic/nuclear accumulation of β-catenin was significantly diminished, compared with that in the iSCAP-Ctrl cells (Fig. 1C). Thus, these results strongly demonstrate that β-catenin expression is effectively silenced in the iSCAP-KD cells.
3.2. iSCAP-KD cells are significantly less responsive to Wnt3A-activated canonical signaling pathway
We further analyzed the effect of β-catenin knockdown on the downstream events of Wnt/β-catenin signaling in iSCAP cells. When subconfluent iSCAP-KD and iSCAP-Ctrl cells were transfected with TOP-Luc reporter plasmid and infected with AdWnt3A or AdGFP, we found that the relative reporter activity in Wnt3A-stimulated iSCAP-KD cells decreased to approximately 22% of that of the iSCAP-Ctrl cells (Fig. 2A). Accordingly, when the expression of two well-characterized Wnt/β-catenin downstream target genes, Axin2 [59] and c-Myc [21], was examined, we found that Wnt3A was shown to significantly induce the expression of Axin2 and c-Myc in iSCAP-Ctrl cells, which was effectively inhibited in iSCAP- KD cells (Fig. 2B). These results strongly suggest that knocking down β-catenin expression in ISCAP-KD may effectively blunt the functional activities of canonical Wnt signaling.
Fig. 2.
Fig. 2. The iSCAP-KD cells are significantly less responsive to Wnt3A-activated canonical signaling pathway. (A) iSCAP-KD cells exhibit significantly lower β-catenin/Tcf reporter activity upon Wnt3A stimulation. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were transfected with TOP-Luc reporter plasmid and infected with AdWnt3A or AdGFP. At 48h post transfection/infection, cells were lysed for luciferase assays. Easy conditions were done in triplicate. (B).Wnt3A-induced expression of Wnt/β-catenin target genes was significantly decreased in iSCAP cells. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were infected with AdWnt3A or AdGFP for 36h. Total RNA was isolated and subjected to reverse transcription. The resultant cDNAs were used as templates for qPCR analysis using primers specific for mouse Axin2 and c-Myc transcripts. All samples were normalized by GAPDH levels. Each assay condition was done in triplicate.
3.3. Silencing β-catenin expression significantly diminishes BMP9-induced osteo/odontoblastic differentiation of iSCAP cells
We and others demonstrated that canonical Wnt signaling can effectively induce osteogenic differentiation of mesenchymal stem cells [20, 33, 39, 47]. We sought to determine if Wnt3A can induce early osteo/odontogenic marker alkaline phosphatase (ALP) activity in iSCAPs, and if the induced ALP activity would be reduced when β-catenin is silenced in iSCAP cells. We found that Wnt3A effectively induced ALP activity in iSCAP-Ctrl cells, which was significantly blunted in iSCAP-KD cells (Fig. 3A). Accordingly, quantitative ALP assays indicate that Wnt3A induced ALP activity in a dose-dependent fashion (at least at day 3) in iSCAP-Ctrl cells (Fig. 3B, panel a) while β-catenin knockdown in iSCAP-KD cells significantly decreased ALP activity, compared to that in iSCAP-Ctrl cells (p<0.001) (Fig. 3B, panels a & b), suggesting that Wnt3A-induced oste/odonotoblastic differentiation of iSCAPs requires functional β-catenin.
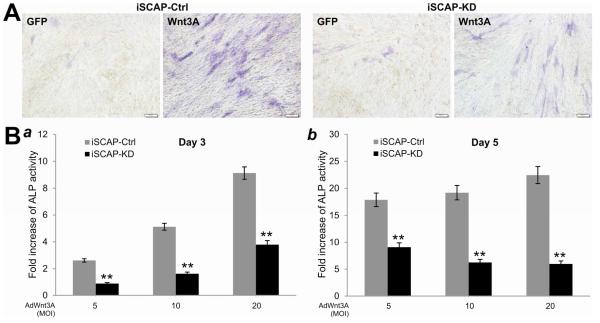
Fig. 3. Silencing β-catenin expression reduces Wnt3A-induced early osteogenic marker alkaline phosphatase (ALP) activity in iSCAP cells.
(A) Wnt3A-induced ALP activity decreases in iSCAP-KD cells. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were infected with AdWnt3A or AdGFP. At the indicated time points post infection, cells were either stained for qualitative ALP activity at day 3. Each assay conditions were done in triplicate. Representative staining results are shown. (B) Wnt3A induces ALP activity in a dose-dependent fashion, which is blunted by silencing β-catenin expression in iSCAP cells. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were infected with the indicated titers (MOIs, multiplicities of infection) of AdWnt3A or AdGFP. At the indicated time points post infection, cells were lysed for quantitative ALP activity assays at days 3 (a) or 5 (b). Each assay conditions were done in triplicate. “**”, p<0.001 (iSCAP-KD vs. iSCAP-Ctrl).
We previously demonstrated that BMP9 is one of the most potent osteogenic BMPs in mesenchymal cell stems and in iSCAPs [8-12]. We sought to test if silencing β-catenin affects BMP9-induced oste/odonotoblastic differentiation of iSCAPs. We found that BMP9 stimulated robust ALP activity qualitatively in iSCAP-Ctrl cells while the BMP9-induced ALP activity was remarkably reduced in iSCAP-KD cells (Fig. 4A). Quantitatively, BMP9 was shown to induce ALP activity in a dose-dependent fashion in both iSCAP-Ctrl and iSCAP-KD cells although the ALP activity was significantly lower in iSCAP-KD cells than that in iSCAP-Ctrl cells at each of the four titers tested (p<0.001) (Fig. 4B). We further tested the late stage mineralization in BMP9-stimulated iSCAP cells and found that Alizarin red staining was markedly decreased in iSCAP-KD cells compared with that in iSCAP-Ctrl cells (Fig. 4C). We previously demonstrated that BMP9 can induce adipogenic differentiation of mesenchymal stem cells [12]. Oil red O staining experiments indicate that BMP9 induced significant adipogenic differentiation in both iSCAP-KD and iSCAP-Ctrl cells, which is seemingly not affected by β-catenin knockdown (Fig. 4D). These results suggest that while Wnt3A itself can induce odontoblastic differentiation of iSCAPs functional β-catenin activity may be essential for BMP9-induced osteo/odontogenic differentiation in iSCAP cells.
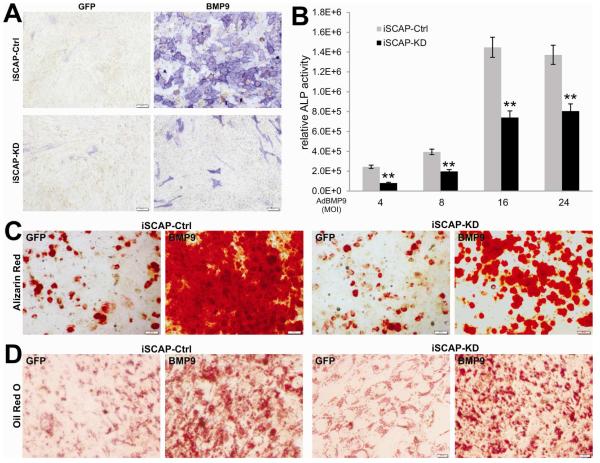
Fig. 4. Silencing β-catenin expression significantly diminishes BMP9-induced osteo/odontoblastic differentiation of iSCAP cells.
(A) and (B) BMP9-induced ALP activity is decreased in the β-catenin silenced iSCAP cells. (A) ALP histochemical staining assay. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were infected with AdBMP9 or AdGFP. At day 5 post infection, cells were fixed for ALP histochemical staining assay. Each assay conditions were done in triplicate. Representative results are shown. (B) Quantitative ALP assay. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were infected with the indicated titers (MOIs) of AdBMP9 or AdGFP. At day 5 post infection, cells were lysed for quantitative ALP activity assays. Each assay conditions were done in triplicate. “**”, p<0.001 (iSCAP-KD vs. iSCAP- Ctrl). (C) Alizarin red S staining. Subconfluent iSCAP-KD or iSCAP-Ctrl cells were infected with AdBMP9 or AdGFP, and maintained in matrix mineralization culture medium for 10 days. Matrix mineralization nodules were stained with Alizarin Red S as described in Methods. Staining results were recorded under a microscope. (D) Oil Red O staining. Subconfluent iSCAP-KD or iSCAP-Ctrl cells were infected with AdBMP9 or AdGFP, and cultured for 10 days. Oil Red O staining was performed as described in Methods. All staining experiments were carried out in duplicate. Representative images are shown.
3.4. Silencing β-catenin diminishes the synergistic osteo/odontogenic activity between BMP9 and Wnt3A in iSCAP cells
We next examined if Wnt3A and BMP9 synergistically regulate odontogenic differentiation of iSCAP cells and if β-catenin plays an important role in this synergistic action. When iSCAP-Ctrl cells were transduced with a fixed titer of AdBMP9 and increasing titers of AdWnt3A, there was an apparent trend of increasing ALP activities at both days 3 and 5 (p<0.05) (Fig. 5A). However, ALP activities were consistently lower in the iSCAP-KD cells under the same conditions (Fig. 5A). Accordingly, when iSCAP-Ctrl cells were transduced with a fixed titer of AdWnt3A and increasing titers of AdBMP9, there was a similar trend of increasing ALP activities at higher Wnt3A titers, especially at day 5 (p<0.05), while β-catenin knockdown significantly diminished the ALP activities induced by BMP9 and Wnt3A (Fig. 5B). Furthermore, both BMP9 and, to a lesser extent, Wnt3A were shown to induce the expression of osteogenic regulator Runx2 in iSCAP-Ctrl cells, which was significantly reduced in iSCAP-KD cells (p<0.001) (Fig. 5C, panel a). The expression of late osteogenic marker osteopontin (OPN) was significantly elevated in Wnt3A and/or Wnt3A stimulated iSCAP-Ctrl cells, which was drastically diminished in iSCAP-KD cells (p<0.001) (Fig. 5C, panel b). These results strongly suggest that BMP9 and Wnt3A may act synergistically to induce osteo/odontoblastic differentiation of iSCAPs, which requires functional β-catenin activity.
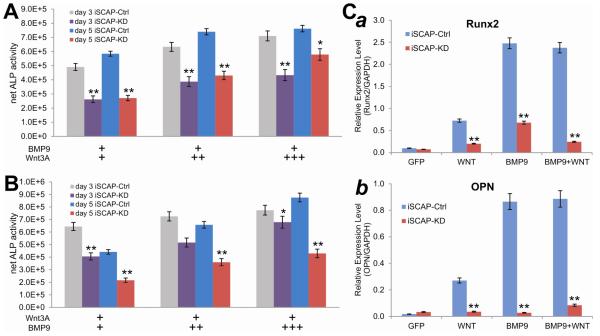
Fig. 5. Silencing β-catenin diminishes the synergistic osteogenic activity between BMP9 and Wnt3A in iSCAP cells.
(A) Increased exogenous Wnt3A expression does not completely recover the BMP9-induced osteogenic activity in iSCAP-KD cells. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were co-infected a fixed titer of AdBMP9 and/or varied titers of AdWnt3A or AdGFP. ALP activity was assayed on days 3 and 5. Each assay conditions were done in triplicate. The net ALP activity was calculated by subtracting ALP readings of BMP9 or Wnt3A only groups from that of respective BMP9+Wnt3A groups. “*”, p<0.05; “**”, p<0.001 (iSCAP-KD vs. iSCAP-Ctrl). (B) Increased exogenous BMP9 expression fails to overcome the decrease of BMP9-induced osteogenic activity in iSCAP-KD cells. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were co-infected a fixed titer of AdWnt3A and/or varied titers of AdBMP9 or AdGFP. ALP activity was assayed on days 3 and 5. Each assay conditions were done in triplicate. The net ALP activity was calculated by subtracting ALP readings of the BMP9 or Wnt3A only groups from that of respective BMP9+Wnt3A groups. “*”, p<0.05; “**”, p<0.001 (iSCAP-KD vs. iSCAP- Ctrl). (C) Wnt3A and/or BMP9-induced expression of bone specific markers is significantly decreased in β-catenin silenced iSCAP cells. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were infected with AdGFP, AdWnt3A, AdBMP9, or AdBMP9+AdWnt3A for 5 days. Total RNA was isolated and subjected to reverse transcription and quantitative real-time PCR using primers specific for mouse Runx2 (a) or osteopontin (OPN) (b), as well as GAPDH. Relative expression level was calculated by dividing Runx expression levels with respective GAPDH expression levels. Each assay condition was done in triplicate. “**”, p<0.001 (iSCAP-KD vs. iSCAP-Ctrl).
3.5. BMP9-induced ectopic bone formation from iSCAP cells can be potentiated by Wnt3A but attenuated by β-catenin knockdown
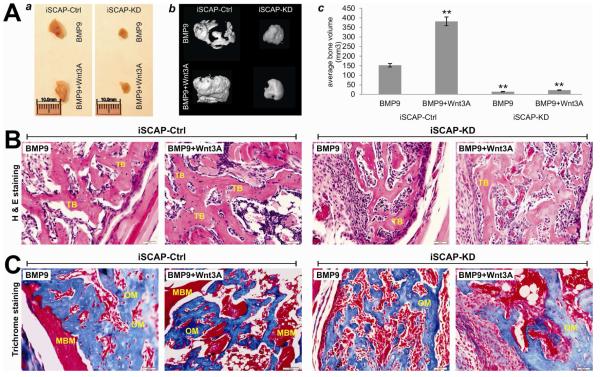
Using our previously established stem cell implantation assay [8, 12, 26, 38, 46, 52, 53, 58], we tested the in vivo effect of BMP9 and Wnt3A signaling crosstalk on ectopic bone formation of iSCAP cells. Subconfluent iSCAP-KD and iSCAP-Ctrl cells were transduced with AdBMP9, AdWnt3A, AdBMP9+AdWnt3A, or AdGFP, and injected subcutaneously into the flanks of athymic nude mice for 4 weeks. No recoverable masses were detected in the GFP or Wnt3A group. Robust bony masses were retrieved from both BMP9 and BMP9+Wnt3A transduced iSCAP-Ctrl groups while much smaller masses were recovered from the iSCAP- KD cells (Fig. 6A, panel a). MicroCT imaging illustrated more distinct size differences among these samples (Fig. 6A, panel b). Quantitative analysis of the total bone volumes indicates that in iSCAP-Ctrl cells co-transduction of BMP9 and Wnt3A induced significantly more robust bone formation than that transduced with BMP9 alone (Fig. 6A, panel c), suggesting that Wnt3A may potentiate BMP9-induced bone formation even though Wnt3A itself failed to induce any ectopic bone formation. However, in iSCAP-KD cells either BMP9 or BMP9+Wnt3A-induced bone formation was significantly diminished when compared with that in iSCAP-Ctrl cells (p<0.001) (Fig. 6A, panel c). Accordingly, H & E histological evaluation revealed that BMP9-transduced iSCAP-Ctrl cells formed evident trabecular bone, which was even more robust in the presence of both BMP9 and Wnt3A (Fig. 6B, two left panels). Silencing β-catenin expression in iSCAP-KD cells, however, significantly reduced trabecular bone formation induced by BMP9 or BMP9+Wnt3A (Fig. 6B). Trichrome staining confirmed that iSCAP-Ctrl cells transduced with BMP9 formed apparently mature and mineralized bone matrices, while a combination of BMP9 and Wnt3A induced more mature and highly mineralized bone matrices (Fig. 6C, two left panels). However, the maturity and mineralization were significantly diminished in iSCAP-KD cells transduced with either BMP9 or BMP9+Wnt3A (Fig. 6C). Taken together, these in vivo results strongly suggest that β- catenin may play an important role in mediating BMP9-induced bone formation, and the BMP9-Wnt3A may crosstalk in inducing osteo/odontoblastic differentiation of iSCAP cells.
Fig. 6. BMP9-induced ectopic bone formation from iSCAP cells is potentiated by Wnt3A but attenuated by β-catenin knockdown.
Subconfluent iSCAP-KD and iSCAP-Ctrl cells were infected with AdBMP9, AdWnt3A, AdBMP9+AdWnt3A, or AdGFP for 16h. Cells were collected for subcutaneous injections (3x106 cells/site in 100μl PBS) into the flanks of athymic nude mice (n=5 each group). (A) Gross images and μCT analysis. At 4 weeks after injection, the animals were sacrificed. Masses formed at the injection sites were retrieved (a), fixed in formalin, and subjected to μCT imaging (b). No masses were detected the animals injected with GFP- transduced iSCAPs. The 3-D reconstruction was performed for all scanned samples (b), and the average total bone volume (c) were determined using the Amira 5.3 software. “**”, p<0.001 when compared with that of the BMP9-transduced iSCAP-Ctrl cells. (B) and (C) Histologic evaluation and Trichrome staining. After μCT imaging was completed, the samples were decalcified and subjected to paraffin-embedded sectioning for histologic evaluation, including H & E staining (B) and Trichrome staining (C). Representative results are shown. TB, trabecular bone; MBM, mineralized bone matrix; OM, osteoid matrix.
4. Discussion
To understand how major signaling pathways regulate osteo/odontogenic differentiation, we investigated if canonical Wnt/β-catenin signaling plays an important role in BMP9-induced osteo/odontogenic signaling in iSCAPs. By leveraging the efficient transduction efficiency of our modified piggyBac transposon system, we established a stable iSCAP line in which β-catenin expression was efficiently silenced. Using this knockdown and control lines we demonstrated that Wnt3A induced early osteogenic marker ALP in iSCAP cells, which was significantly diminished by β-catenin knockdown. While Wnt3A and BMP9 promoted each other’s ability to induce ALP activity, silencing β-catenin significantly diminishes the BMP9-induced osteo/odontogenic, but not adipogenic, differentiation of iSCAP cells. Moreover, silencing β-catenin led to a decrease in BMP9-induced expression of osteocalcin and osteopontin and in vitro matrix mineralization of the iSCAP cells. In vivo studies revealed that while BMP9-transduced iSCAP cells induced robust ectopic bone formation, the iSCAP cells stimulated with both BMP9 and Wnt3A exhibited more mature and highly mineralized trabecular bone formation. Knockdown of β-catenin in iSCAP cells significantly inhibited BMP9 or BMP9/Wnt3A-induced ectopic bone formation in vivo. These results strongly suggest that canonical Wnt/β-catenin signaling may play an important role in BMP9-induced osteo/ondontogenic signaling and that BMP9 and Wnt3A may act synergistically to induce osteo/odontoblastic differentiation of iSCAPs, which requires functional β-catenin signaling activity.
While the detailed molecular mechanisms underlying tooth development remain to be fully elucidated, it’s well established that the interactions between the epithelial and mesenchymal tissue components of developing teeth regulate morphogenesis and cell differentiation, and determine key features of dentitions and individual teeth, such as the number, size, shape, and formation of dental hard tissues [60-62]. It is generally accepted that several conserved signaling pathways, including the BMP, Wnt, FGF, Shh and Notch pathways, play important roles throughout tooth development [60-62]. These signaling pathways may interact or crosstalk through positive and negative feedback loops to regulate not only morphogenesis of individual teeth but also tooth number, shape, and spatial pattern [61, 62]. For example, it has been reported that cessation of epithelial BMP signaling switches the differentiation of crown epithelia to the root lineage in a β-catenin-dependent manner [63]. Krüppel-like family (KLF) transcription factor Epfn was shown to enhance canonical Wnt/β-catenin signaling in the developing dental pulp mesenchyme, a condition that promotes the activity of other downstream signaling pathways, such as BMPs, which are fundamental for cellular induction and ameloblast differentiation [64]. It was reported that Wnt/β-catenin signaling in the dental mesenchyme regulates incisor development by regulating BMP4 [65]. It was reported that Smad4 may play a crucial role in regulating the interplay between TGFβ/BMP and Wnt signaling to ensure the proper cranial neural crest cell fate during tooth morphogenesis [66]. A recent systems biology analysis by integrating the epithelial and mesenchymal gene regulatory networks through the action of diffusible extracellular signaling molecules identified a key epithelial-mesenchymal inter-tissue Wnt-BMP feedback circuit [67].
While Wnt/β-catenin signaling has been shown to play an essential role in activating odontogenic mesenchyme during early tooth development and directing multiple stages of tooth morphogenesis [68, 69], little is known about possible roles of BMP9 in tooth development. We have recently demonstrated that BMP9 can effectively induce iSCAP cells to differentiate into bone, cartilage, and, to lesser extent, adipocytes [8]. BMP9 (aka, GDF2) is one of the least characterized BMPs and yet found by us as one of the most potent oetsogenic BMPs [9-12, 70, 71]. We have recently compared potentially important downstream mediators of Wnt3A and BMP9 induced osteogenic differentiation in MSCs through gene expression profiling, and found that Wnt3A and BMP9 regulated the expression of overlapping but distinct sets of downstream target genes [39, 47], suggesting that there may be an important crosstalk between BMP and Wnt-induced osteogenic signaling pathways. We later demonstrated that canonical Wnt signaling, possibly through β-catenin interaction with Runx2, plays an important role in BMP9-induced osteogenic differentiation of MSCs [33]. In this study, using the immortalized SCAPs isolated from the apical papilla tissue of mouse lower incisor teeth we demonstrated that Wnt3A and BMP9 can potentiate each other’s ability to induce osteo/odontogenic differentiation in vitro and in vivo. Furthermore, β- catenin knockdown significantly diminishes BMP9-induced osteo/odontogenic differentiation of iSCAP cells, indicating that BMP9-induced odontoblastic differentiation requires functional β-catenin signaling. Interestingly, a recent study also found that Wnt3a can enhance BMP9- induced osteogenic differentiation in mesenchymal stem cell-like C3H10T1/2 cells [72]. However, this study did not address if functional canonical Wnt signaling is required for optimal BMP9-induced osteogenic differentiation. Furthermore, our studies have expanded BMP9’s odontogenic activity in dental stem cells. Nonetheless, our work and others’ studies strongly suggest that the synergistic effect between Wnt3a and BMP9 on Osteogenesis may underline a common mechanism in the different types of osteogenic progenitor cells.
Our results also suggest that there may be a synergistic crosstalk between BMP9 and Wnt3A in odontogenic differentiation of iSCAP cells. Although the molecular mechanisms behind this crosstalk are largely undefined, it was reported that β-catenin and Lef1/Tcf can form a complex with Smad4 and/or Smad1 [73-76]. It was also shown that Runx2 formed a complex with Lef1/Tcf4 and regulated downstream target genes [77, 78]. However, the signaling crosstalk may be more complicated, as Lef-1 was found to repress the osteocalcin promoter via interaction with Runx2 [79]; and it has also been reported that canonical Wnt signaling promotes osteogenesis by directly stimulating Runx2 expression [80]. We have recently demonstrated that BMP9 can induce the recruitment of both Runx2 and β-catenin to the osteocalcin promoter in MSCs, suggesting that canonical Wnt signaling, possibly through interactions between β-catenin and Runx2, may play an important role in BMP9-induced osteogenic differentiation of MSCs [33]. Nonetheless, the molecular mechanism underlying the crosstalk between BMP9 and canonical Wnt/β-catenin in iSCAPs needs to be further investigated.
We have demonstrated that the dental stem cells SCAPs retain multipotent potential of differentiating into osteo/odontoblastic, chondrogenic and adipogenic lineages. BMP9 can effectively induce osteo/odontoblastic differentiation, which can be further potentiated by canonical Wnts, such as Wnt3A. The β-catenin may play an essential role in mediating such synergistic crosstalk effect between BMP9 and Wnt3A. Thus, it is conceivable that a combination of SCAPs (as a valuable progenitor source) and BMP9 and Wnt3A (as efficacious biofactors) may allow us to achieve potentially successful dental tissue engineering for odontogenic regeneration.
5. Conclusions
Using the immortalized SCAPs isolated from the apical papilla tissue of mouse lower incisor teeth, we demonstrated that BMP9-induced osteo/odontogenic differentiation can be potentiated by Wnt3A in vitro and in vivo, but significantly diminished by β-catenin knockdown, suggesting that β-catenin may play an important role in BMP9-induced osteo/ondontogenic signaling and that BMP9 and Wnt3A may act synergistically to induce osteo/odontoblastic differentiation of iSCAPs. Thus, it’s conceivable that BMP9 and/or Wnt3A may be explored as novel and efficacious biofactors for odontogenic regeneration and tooth engineering.
Supplementary Material
Acknowledgements
The reported work was supported in part by research grants from the National Institutes of Health (AT004418, AR50142, and AR054381 to TCH, RCH and HHL), the Natural Science Foundation of China (#81271183 to Feng D, and #81301551 to EH), Chongqing Municipal Commissions on Education, and Science & Technology (#cstc2014jcyjA10010 to HZ; and #KJ130303 and #cstc2013jcyjA0093 to Jinhua W), and Chongqing Yubei District Commission on Science & Technology (#2014-#15 HZ). This work was also supported in part by The University of Chicago Core Facility Subsidy grant from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number UL1 TR000430.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- [1].Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of dental research. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bluteau G, Luder HU, De Bari C, Mitsiadis TA. Stem cells for tooth engineering. European cells & materials. 2008;16:1–9. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- [3].Rai S, Kaur M, Kaur S. Applications of stem cells in interdisciplinary dentistry and beyond: an overview. Annals of medical and health sciences research. 2013;3:245–54. doi: 10.4103/2141-9248.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang GT. Dental pulp and dentin tissue engineering and regeneration: advancement and challenge. Frontiers in bioscience (Elite edition) 2011;3:788–800. doi: 10.2741/e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Goldberg M. Pulp healing and regeneration: more questions than answers. Advances in dental research. 2011;23:270–4. doi: 10.1177/0022034511405385. [DOI] [PubMed] [Google Scholar]
- [6].Smith AJ, Smith JG, Shelton RM, Cooper PR. Harnessing the natural regenerative potential of the dental pulp. Dental clinics of North America. 2012;56:589–601. doi: 10.1016/j.cden.2012.05.011. [DOI] [PubMed] [Google Scholar]
- [7].Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. Journal of endodontics. 2010;36:781–9. doi: 10.1016/j.joen.2010.02.006. [DOI] [PubMed] [Google Scholar]
- [8].Wang J, Zhang H, Zhang W, Huang E, Wang N, Wu N, et al. Bone Morphogenetic Protein-9 (BMP9) Effectively Induces Osteo/Odontoblastic Differentiation of the Reversibly Immortalized Stem Cells of Dental Apical Papilla. Stem cells and development. 2014;23:1405–16. doi: 10.1089/scd.2013.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs) J Bone Joint Surg Am. 2003;85-A:1544–52. doi: 10.2106/00004623-200308000-00017. [DOI] [PubMed] [Google Scholar]
- [10].Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene therapy. 2004;11:1312–20. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- [11].Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–77. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- [12].Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem cells and development. 2009;18:545–59. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, et al. BMP-9 Induced Osteogenic Differentiation of Mesenchymal Stem Cells: Molecular Mechanism and Therapeutic Potential. Current gene therapy. 2011;11:229–40. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- [14].Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- [15].Glass DA, 2nd, Karsenty G. In vivo analysis of Wnt signaling in bone. Endocrinology. 2007;148:2630–4. doi: 10.1210/en.2006-1372. [DOI] [PubMed] [Google Scholar]
- [16].Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- [17].Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes & development. 1997;11:3286–305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- [18].Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Developmental cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- [19].Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, et al. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–21. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- [20].Kim JH, Liu X, Wang J, Chen X, Zhang H, Kim SH, et al. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Therapeutic advances in musculoskeletal disease. 2013;5:13–31. doi: 10.1177/1759720X12466608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway [see comments] Science (New York, NY. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- [22].Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- [23].He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–45. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, et al. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- [25].Wang N, Zhang H, Zhang BQ, Liu W, Zhang Z, Qiao M, et al. Adenovirus-mediated efficient gene transfer into cultured three-dimensional organoids. PloS one. 2014;9:e93608. doi: 10.1371/journal.pone.0093608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang N, Zhang W, Cui J, Zhang H, Chen X, Li R, et al. The piggyBac Transposon-Mediated Expression of SV40 T Antigen Efficiently Immortalizes Mouse Embryonic Fibroblasts (MEFs) PloS one. 2014;9:e97316. doi: 10.1371/journal.pone.0097316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lamplot JD, Liu B, Yin L, Zhang W, Wang Z, Luther G, et al. Reversibly Immortalized Mouse Articular Chondrocytes Acquire Long-Term Proliferative Capability while Retaining Chondrogenic Phenotype. Cell transplantation. 2014 doi: 10.3727/096368914X681054. [DOI] [PubMed] [Google Scholar]
- [28].Wen S, Zhang H, Li Y, Wang N, Zhang W, Yang K, et al. Characterization of constitutive promoters for piggyBac transposon-mediated stable transgene expression in mesenchymal stem cells (MSCs) PloS one. 2014;9:e94397. doi: 10.1371/journal.pone.0094397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhao C, Wu N, Deng F, Zhang H, Wang N, Zhang W, et al. Adenovirus-mediated gene transfer in mesenchymal stem cells can be significantly enhanced by the cationic polymer polybrene. PloS one. 2014;9:e92908. doi: 10.1371/journal.pone.0092908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li R, Zhang W, Cui J, Shui W, Yin L, Wang Y, et al. Targeting BMP9-Promoted Human Osteosarcoma Growth by Inactivation of Notch Signaling. Current cancer drug targets. 2014 doi: 10.2174/1568009614666140305105805. [DOI] [PubMed] [Google Scholar]
- [31].Wu N, Zhang H, Deng F, Li R, Zhang W, Chen X, et al. Overexpression of Ad5 precursor terminal protein accelerates recombinant adenovirus packaging and amplification in HEK-293 packaging cells. Gene therapy. 2014;21:629–37. doi: 10.1038/gt.2014.40. [DOI] [PubMed] [Google Scholar]
- [32].Luo Q, Kang Q, Song WX, Luu HH, Luo X, An N, et al. Selection and validation of optimal siRNA target sites for RNAi-mediated gene silencing. Gene. 2007;395:160–9. doi: 10.1016/j.gene.2007.02.030. [DOI] [PubMed] [Google Scholar]
- [33].Tang N, Song WX, Luo J, Luo X, Chen J, Sharff KA, et al. BMP9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signaling. Journal of cellular and molecular medicine. 2009;13:2448–64. doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Luo J, Deng ZL, Luo X, Tang N, Song WX, Chen J, et al. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nature protocols. 2007;2:1236–47. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- [35].Kong Y, Zhang H, Chen X, Zhang W, Zhao C, Wang N, et al. Destabilization of Heterologous Proteins Mediated by the GSK3beta Phosphorylation Domain of the beta-Catenin Protein. Cell Physiol Biochem. 2013;32:1187–99. doi: 10.1159/000354518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu X, Qin J, Luo Q, Bi Y, Zhu G, Jiang W, et al. Cross-talk between EGF and BMP9 signalling pathways regulates the osteogenic differentiation of mesenchymal stem cells. Journal of cellular and molecular medicine. 2013 doi: 10.1111/jcmm.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Y, Hong S, Li M, Zhang J, Bi Y, He Y, et al. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res. 2013;31:1796–803. doi: 10.1002/jor.22427. [DOI] [PubMed] [Google Scholar]
- [38].Gao Y, Huang E, Zhang H, Wang J, Wu N, Chen X, et al. Crosstalk between Wnt/beta-Catenin and Estrogen Receptor Signaling Synergistically Promotes Osteogenic Differentiation of Mesenchymal Progenitor Cells. PloS one. 2013;8:e82436. doi: 10.1371/journal.pone.0082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Luo Q, Kang Q, Si W, Jiang W, Park JK, Peng Y, et al. Connective Tissue Growth Factor (CTGF) Is Regulated by Wnt and Bone Morphogenetic Proteins Signaling in Osteoblast Differentiation of Mesenchymal Stem Cells. The Journal of biological chemistry. 2004;279:55958–68. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- [40].Zhang Y, Chen X, Qiao M, Zhang BQ, Wang N, Zhang Z, et al. Bone morphogenetic protein 2 inhibits the proliferation and growth of human colorectal cancer cells. Oncology reports. 2014 doi: 10.3892/or.2014.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen X, Luther G, Zhang W, Nan G, Wagner ER, Liao Z, et al. The E-F Hand Calcium-Binding Protein S100A4 Regulates the Proliferation, Survival and Differentiation Potential of Human Osteosarcoma Cells. Cell Physiol Biochem. 2013;32:1083–96. doi: 10.1159/000354508. [DOI] [PubMed] [Google Scholar]
- [42].Huang J, Bi Y, Zhu GH, He Y, Su Y, He BC, et al. Retinoic acid signalling induces the differentiation of mouse fetal liver-derived hepatic progenitor cells. Liver Int. 2009;29:1569–81. doi: 10.1111/j.1478-3231.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- [43].Zhang W, Deng ZL, Chen L, Zuo GW, Luo Q, Shi Q, et al. Retinoic acids potentiate BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. PloS one. 2010;5:e11917. doi: 10.1371/journal.pone.0011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rastegar F, Gao JL, Shenaq D, Luo Q, Shi Q, Kim SH, et al. Lysophosphatidic acid acyltransferase beta (LPAATbeta) promotes the tumor growth of human osteosarcoma. PloS one. 2010;5:e14182. doi: 10.1371/journal.pone.0014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Su Y, Wagner ER, Luo Q, Huang J, Chen L, He BC, et al. Insulin-like growth factor binding protein 5 suppresses tumor growth and metastasis of human osteosarcoma. Oncogene. 2011;30:3907–17. doi: 10.1038/onc.2011.97. [DOI] [PubMed] [Google Scholar]
- [46].Huang E, Zhu G, Jiang W, Yang K, Gao Y, Luo Q, et al. Growth hormone synergizes with BMP9 in osteogenic differentiation by activating the JAK/STAT/IGF1 pathway in murine multilineage cells. J Bone Miner Res. 2012;27:1566–75. doi: 10.1002/jbmr.1622. [DOI] [PubMed] [Google Scholar]
- [47].Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, et al. CCN1/Cyr61 Is Regulated by the Canonical Wnt Signal and Plays an Important Role in Wnt3A-Induced Osteoblast Differentiation of Mesenchymal Stem Cells. Molecular and cellular biology. 2006;26:2955–64. doi: 10.1128/MCB.26.8.2955-2964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Peng Y, Kang Q, Cheng H, Li X, Sun MH, Jiang W, et al. Transcriptional characterization of bone morphogenetic proteins (BMPs)-mediated osteogenic signaling. Journal of cellular biochemistry. 2003;90:1149–65. doi: 10.1002/jcb.10744. [DOI] [PubMed] [Google Scholar]
- [49].Zhu GH, Huang J, Bi Y, Su Y, Tang Y, He BC, et al. Activation of RXR and RAR signaling promotes myogenic differentiation of myoblastic C2C12 cells. Differentiation; research in biological diversity. 2009;78:195–204. doi: 10.1016/j.diff.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Luo X, Sharff KA, Chen J, He TC, Luu HH. S100A6 expression and function in human osteosarcoma. Clin Orthop Relat Res. 2008;466:2060–70. doi: 10.1007/s11999-008-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, et al. Hey1 Basic Helix-Loop-Helix Protein Plays an Important Role in Mediating BMP9-induced Osteogenic Differentiation of Mesenchymal Progenitor Cells. The Journal of biological chemistry. 2009;284:649–59. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hu N, Jiang D, Huang E, Liu X, Li R, Liang X, et al. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. Journal of cell science. 2013;126:532–41. doi: 10.1242/jcs.114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Huang E, Bi Y, Jiang W, Luo X, Yang K, Gao JL, et al. Conditionally Immortalized Mouse Embryonic Fibroblasts Retain Proliferative Activity without Compromising Multipotent Differentiation Potential. PloS one. 2012;7:e32428. doi: 10.1371/journal.pone.0032428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Luo X, Chen J, Song WX, Tang N, Luo J, Deng ZL, et al. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest. 2008;88:1264–77. doi: 10.1038/labinvest.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC, et al. Wnt antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. Journal of cellular biochemistry. 2009;108:295–303. doi: 10.1002/jcb.22254. [DOI] [PubMed] [Google Scholar]
- [56].Luo J, Tang M, Huang J, He BC, Gao JL, Chen L, et al. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. The Journal of biological chemistry. 2010;285:29588–98. doi: 10.1074/jbc.M110.130518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25:2447–59. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang J, Weng Y, Liu X, Wang J, Zhang W, Kim SH, et al. Endoplasmic reticulum (ER) stress inducible factor cysteine-rich with EGF-like domains 2 (Creld2) is an important mediator of BMP9-regulated osteogenic differentiation of mesenchymal stem cells. PloS one. 2013;8:e73086. doi: 10.1371/journal.pone.0073086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14973–8. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thesleff I, Jarvinen E, Suomalainen M. Affecting tooth morphology and renewal by fine-tuning the signals mediating cell and tissue interactions. Novartis Foundation symposium. 2007;284:142–53. doi: 10.1002/9780470319390.ch10. discussion 53-63. [DOI] [PubMed] [Google Scholar]
- [61].Fraser GJ, Bloomquist RF, Streelman JT. Common developmental pathways link tooth shape to regeneration. Developmental biology. 2013;377:399–414. doi: 10.1016/j.ydbio.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lan Y, Jia S, Jiang R. Molecular patterning of the mammalian dentition. Seminars in cell & developmental biology. 2014;25-26:61–70. doi: 10.1016/j.semcdb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yang Z, Hai B, Qin L, Ti X, Shangguan L, Zhao Y, et al. Cessation of epithelial Bmp signaling switches the differentiation of crown epithelia to the root lineage in a beta-catenin-dependent manner. Molecular and cellular biology. 2013;33:4732–44. doi: 10.1128/MCB.00456-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ibarretxe G, Aurrekoetxea M, Crende O, Badiola I, Jimenez-Rojo L, Nakamura T, et al. Epiprofin/Sp6 regulates Wnt-BMP signaling and the establishment of cellular junctions during the bell stage of tooth development. Cell and tissue research. 2012;350:95–107. doi: 10.1007/s00441-012-1459-8. [DOI] [PubMed] [Google Scholar]
- [65].Fujimori S, Novak H, Weissenbock M, Jussila M, Goncalves A, Zeller R, et al. Wnt/beta-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Developmental biology. 2010;348:97–106. doi: 10.1016/j.ydbio.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li J, Huang X, Xu X, Mayo J, Bringas P, Jr., Jiang R, et al. SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development (Cambridge, England) 2011;138:1977–89. doi: 10.1242/dev.061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].O'Connell DJ, Ho JW, Mammoto T, Turbe-Doan A, O'Connell JT, Haseley PS, et al. A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Science signaling. 2012;5:ra4. doi: 10.1126/scisignal.2002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Developmental biology. 2008;313:210–24. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chen J, Lan Y, Baek JA, Gao Y, Jiang R. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Developmental biology. 2009;334:174–85. doi: 10.1016/j.ydbio.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lamplot JD, Qin J, Nan G, Wang J, Liu X, Yin L, et al. BMP9 signaling in stem cell differentiation and osteogenesis. American journal of stem cells. 2013;2:1–21. [PMC free article] [PubMed] [Google Scholar]
- [71].Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, et al. Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes & Diseases. 2014;1:87–105. doi: 10.1016/j.gendis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhang X, Lin LB, Xu DJ, Chen RF, Tan JX, Liang X, et al. Wnt3a enhances bone morphogenetic protein 9-induced osteogenic differentiation of C3H10T1/2 cells. Chinese medical journal. 2013;126:4758–63. [PubMed] [Google Scholar]
- [73].Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, et al. Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann's organizer. Nature. 2000;403:781–5. doi: 10.1038/35001602. [DOI] [PubMed] [Google Scholar]
- [74].Hu MC, Rosenblum ND. Smad1, beta-catenin and Tcf4 associate in a molecular complex with the Myc promoter in dysplastic renal tissue and cooperate to control Myc transcription. Development (Cambridge, England) 2005;132:215–25. doi: 10.1242/dev.01573. [DOI] [PubMed] [Google Scholar]
- [75].Hussein SM, Duff EK, Sirard C. Smad4 and beta-catenin co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2. The Journal of biological chemistry. 2003;278:48805–14. doi: 10.1074/jbc.M305472200. [DOI] [PubMed] [Google Scholar]
- [76].Nakashima A, Katagiri T, Tamura M. Cross-talk between Wnt and bone morphogenetic protein 2 (BMP-2) signaling in differentiation pathway of C2C12 myoblasts. The Journal of biological chemistry. 2005;280:37660–8. doi: 10.1074/jbc.M504612200. [DOI] [PubMed] [Google Scholar]
- [77].Xiao G, Jiang D, Ge C, Zhao Z, Lai Y, Boules H, et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. The Journal of biological chemistry. 2005;280:30689–96. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- [78].Reinhold MI, Naski MC. Direct interactions of Runx2 and canonical Wnt signaling induce FGF18. The Journal of biological chemistry. 2007;282:3653–63. doi: 10.1074/jbc.M608995200. [DOI] [PubMed] [Google Scholar]
- [79].Kahler RA, Westendorf JJ. Lymphoid enhancer factor-1 and beta-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. The Journal of biological chemistry. 2003;278:11937–44. doi: 10.1074/jbc.M211443200. [DOI] [PubMed] [Google Scholar]
- [80].Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. The Journal of biological chemistry. 2005;280:33132–40. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.