Abstract
In an effort to better understand and treat mental disorders, the Wnt pathway and adult hippocampal neurogenesis have received increased attention in recent years. One is a signaling pathway regulating key aspects of embryonic patterning, cell specification, and adult tissue homeostasis. The other is the generation of newborn neurons in adulthood that integrate into the neural circuit and function in learning and memory, and mood behavior. In this review, we discuss the growing relationship between Wnt signaling-mediated regulation of adult hippocampal neurogenesis as it applies to neuropsychiatric disorders. Evidence suggests dysfunctional Wnt signaling may aberrantly regulate new neuron development and cognitive function. Indeed, altered expression of key Wnt pathway components are observed in the hippocampus of patients suffering from neuropsychiatric disorders. Clinically-utilized mood stabilizers also proceed through modulation of Wnt signaling in the hippocampus, while Wnt pathway antagonists can regulate the antidepressant response. Here, we review the role of Wnt signaling in disease etiology and pathogenesis, regulation of adult neurogenesis and behavior, and the therapeutic targeting of disease symptoms.
Keywords: Wnt signaling, adult neurogenesis, hippocampus, psychiatric disorders
1. Introduction
The high prevalence of neuropsychiatric disorders continues to be a public health concern for patients and the medical community worldwide. According to the Center for Disease Control and Prevention, mental illness accounted for a $300 billion economic cost in 2004 with a reported 25% of American adults in 2002 with mental illness (Reeves et al., 2011). According to the US Department of Health and Human Services, over a ten year period from 1996 to 2006 alone the total expenditure on mental health services increased by 63.4% while the number of Americans that sought these services increased by 87.6% (Soni, 2009). Amidst such statistics, the scientific community has embraced the need to better understand the causative mechanism and treatment of such disorders. Adult neurogenesis is one biological process that has received increased attention in recent years because of its potential role in neurological and psychiatric disorders.
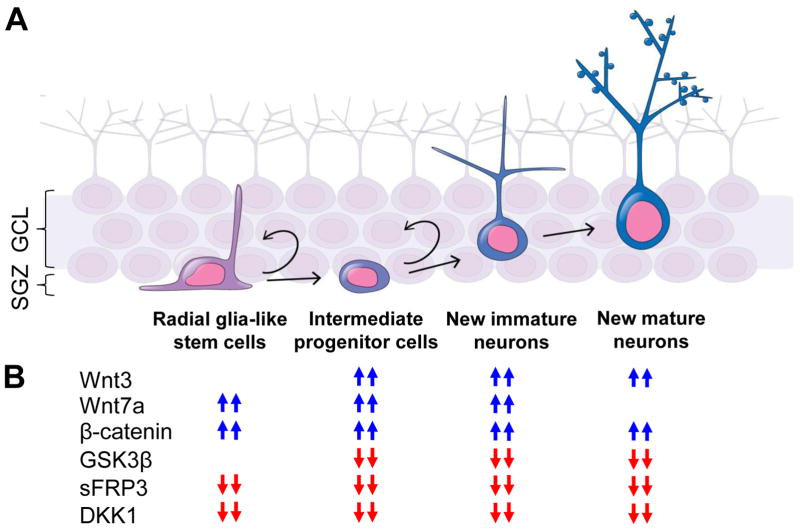
Maintained well into adulthood in mammals, adult neurogenesis continuously generates new neurons from neural stem cells in two specialized neurogenic regions or niches of the adult brain, the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (Ming and Song, 2011). Adult neurogenesis in the hippocampus, the central focus of this review article, has well evidenced roles allowing for striking cellular plasticity (Zhao et al., 2008) and has been tied to specific brain functions in learning and memory (Dupret et al., 2007; Shors et al., 2001), pattern separation (Clelland et al., 2009; Sahay et al., 2011) and emotional behavior (Santarelli et al., 2003; Snyder et al., 2011). It is a sequential process wherein a radial glia-like (RGL) or Type-1 quiescent neural stem cell within the SGZ generates proliferative precursors known as Type-2 intermediate progenitor cells (IPCs) that become committed to the neuronal fate as Type-3 neuroblasts and consequently differentiate into mature granule neurons to integrate into the hippocampal circuit (Ming and Song, 2011) (Fig. 1A). These newborn neurons show distinct morphological and excitability properties that allow them to take on unique roles in memory encoding and learning behavior (Ge et al., 2008; Saxe et al., 2006; Schmidt-Hieber et al., 2004; Snyder et al., 2005). The surrounding microenvironment provides a rich niche of regulatory signals and growth factors that directs newborn neuron development and function during both the normal and diseased condition (Faigle and Song, 2013; Ming and Song, 2011; Zhao et al., 2008). It is an example of an elaborate system where the finer changes in the cellular phenotype feed into the larger behavioral response.
Figure 1.
Summary of the role of Wnt signaling in adult hippocampal neurogenesis. (A) A schematic diagram of adult hippocampal neurogenesis is depicted across the subgranular zone (SGZ) and granular cell layer (GCL) of the dentate gyrus. An activated precursor cell passes through distinct phases as it differentiates and integrates with an elaborate morphology into the circuit; (B) The effect of different components of the canonical Wnt signaling pathway are shown across different stages of neurogenesis.
Wnt signaling has emerged as a crucial pathway in the regulation of adult hippocampal neurogenesis. Its role is well documented in stem cell maintenance, neuronal maturation, axon remodeling, dendritic morphogenesis and adult tissue homeostasis (Ciani and Salinas, 2005; Clevers and Nusse, 2012; Logan and Nusse, 2004; Rosso et al., 2005). Recent post-mortem and pharmacological studies have tied together dysfunction in key Wnt pathway components to disorders such as depression, schizophrenia, bipolar disorder, autism and Alzheimer’s disease (Clevers and Nusse, 2012; De Ferrari et al., 2007). Animal studies pursuing members of the canonical Wnt pathway, such as Wnt3, beta (β)-catenin, glycogen synthase kinase-3β (GSK3β), secreted frizzled related protein 3 (sFRP3) and Dickkopf 1 (DKK1) have shown that targeted manipulation of their activity can induce specific changes in the developmental trajectory of newborn neurons in the dentate gyrus (Jang et al., 2013; Lie et al., 2005; Llorens-Martin et al., 2013; Qu et al., 2010), and alter behavior including anxiety, working and spatial memory, hyperactivity and depression (Jang et al., 2012; Kaidanovich-Beilin et al., 2004; Seib et al., 2013). Indeed, treatments involving electroconvulsive seizures and lithium are observed to proceed through an enhancement of Wnt signaling in the hippocampus (Contestabile et al., 2013; Madsen et al., 2003) and recently a significant association between the Wnt pathway component, sFRP3 and early antidepressant response in patients was observed (Jang et al., 2012). Wnt signaling sits comfortably at a crossroad as both its own regulation and influence extends beyond and into other intersecting pathways including the dopaminergic and serotonergic systems (Li and Jope, 2010; Li et al., 2004b).
Investigation using animal models has provided a better mechanistic understanding of how Wnt signaling may regulate adult hippocampal neurogenesis at the molecular, cellular and behavioral levels. This comes as no surprise as each step of adult hippocampal neurogenesis is a finely tuned sequential process, impacted by physiological and pathological stimuli that regulate neural progenitor cells as they proliferate, differentiate, mature and integrate into the circuit (Ming and Song, 2011; Zhao et al., 2008). In this review article, we discuss Wnt signaling-mediated regulation of adult hippocampal neurogenesis and behavior in relation to neuropsychiatric disorders.
2. Adult hippocampal neurogenesis: understanding preclinical models and human studies
Historically, the hippocampus has been recognized as a crucial component of the limbic system with roles primarily in learning and memory. This was typified by the famous patient H.M. whose temporal lobe was removed in treatment of his epilepsy resulting in unexpected and severe anterograde amnesia (Scoville and Milner, 2000). Modern neuroscience has since expanded the biological significance of the hippocampus to include new roles in cognition, spatial memory and mood regulation. With a better understanding of the hippocampus, the crucial discovery of SGZ neurogenesis in the hippocampus was made (Altman and Das, 1967; Eriksson et al., 1998). For much of the 20th century, it was believed the mammalian brain did not generate new neurons in adulthood. Evidence of neurogenesis occurring in the adult mammalian brain spurred new lines of research and discovery that has since expanded our understanding of adult hippocampal neurogenesis (Ming and Song, 2011). Some of the strongest evidence connecting adult hippocampal neurogenesis to neurological disorders comes from studies of various antidepressants, antipsychotics, mood stabilizers and behavioral treatments that virtually all proceed through an enhancement of SGZ neurogenesis (Jun et al., 2012).
Several lines of evidence from human studies and animal models have linked a dysfunctional hippocampal circuitry and adult hippocampal neurogenesis to neuropsychiatric disorders. For instance, post-mortem studies and imaging techniques such as MRI have shown an associated hippocampal atrophy or loss in hippocampal volume in depressed patients (MacQueen et al., 2008; Maller et al., 2007; Malykhin et al., 2010; Neumeister et al., 2005; Sawyer et al., 2012). In one human study of depression, use of selective serotonin reuptake inhibitors (SSRIs) and tricyclic and tetracyclic antidepressants (TCAs) was correlated with an increase in overall dentate gyrus volume and also increased neural progenitor cells in the anterior hippocampus (Boldrini et al., 2009). Other studies in schizophrenic patients have found cytoarchitectural abnormalities in neuron size, shape and orientation in the hippocampus and its surrounding subfields (Arnold et al., 1995; Zaidel et al., 1997). Animal studies of disrupted-in-schizophrenia 1 (DISC1), a susceptibility gene strongly implicated in schizophrenia, have shed light on its role in regulating SGZ neurogenesis (Duan et al., 2007; Mao et al., 2009). Similarly, other structural and functional changes in the hippocampus including atrophy or volume loss have been reported in epilepsy, addiction, schizophrenia, and depression and other mood disorders (Geuze et al., 2005; Keller and Roberts, 2008; Sapolsky, 2000; Velakoulis et al., 2001).
Outside of such direct observation of hippocampal changes associated with neuropsychiatric disorders, the majority of our knowledge on adult hippocampal neurogenesis and its roles come from animal models. Technical limitations have hindered our ability to apply rodent approaches to humans and quantify SGZ neurogenesis, especially in vivo. A crucial question that thus remains in the field is to what extent does neurogenesis in the human brain contribute to mental function and behavior? One recent study has provided key details on active hippocampal neurogenesis in adult humans (Spalding et al., 2013). The group developed a strategy to retrospectively birthdate cells and study cell turnover dynamics by taking advantage of atmospheric 14C levels from nuclear bomb testing that occurred during the Cold War between 1955–1963 (Spalding et al., 2005). The study results show that there is substantial hippocampal neurogenesis that occurs throughout life in the human hippocampus. Compared to hippocampal neurogenesis in rodents, there is a much slower decline in human neurogenesis with aging with approximately 700 new neurons being added per day and an annual turnover rate of 1.75% within the renewing fraction. Further, approximately 35% of hippocampal neurons are subject to change compared to 10% in rodents. The rate of neurogenesis in a human would make it comparable to that of a 9-month old mouse, possibly conferring it with similar hippocampal function in cognition and memory (Spalding et al., 2013).
Yet, some of the strongest evidence connecting adult hippocampal neurogenesis to neuropsychiatric disorders comes from animal models of study and direct observation of antidepressants and other treatment protocols for mood disorders that proceed through an increase in SGZ neurogenesis. Notwithstanding the structural changes observed in the hippocampus of patients with neuropsychiatric disorders, sustained and significant presence of hippocampal neurogenesis in humans opens the possibility of it functioning as a critical mediator of disease pathogenesis and in the efficacy of targeted treatment regimens. Within this rich neurogenic niche of the hippocampus, research in the past decade has provided us powerful insight on the mechanism of action of clinically relevant treatments and their behavioral outcomes. We have targeted our review at this critical juncture where the Wnt signaling pathway has emerged a key player. In the following sections, we present the role of the Wnt pathway in mediating adult hippocampal neurogenesis and modulating behavioral outcomes through our current understanding of clinical and preclinical models.
3. Wnt/β-catenin signaling during neurogenesis: an overview
3.1. Canonical Wnt signaling
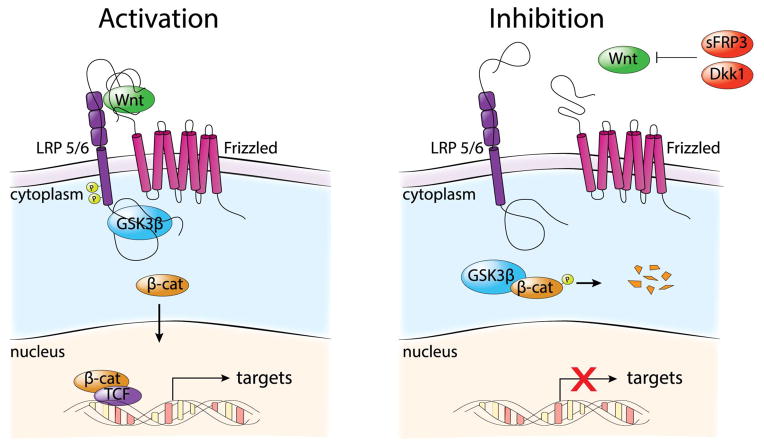
The canonical Wnt pathway that controls gene expression through β-catenin is the primary focus of this review article (Fig. 2). The Frizzled class of receptors (Fzs) are seven transmembrane receptor proteins that form the primary class of receptors while the LRP family of single transmembrane proteins act as co-receptors (Logan and Nusse, 2004). Fz and LRP5/6 receptors work together in binding the ligand and effecting downstream changes in the pathway through Dishevelled (Dvl), GSK3β, adenomatous polyposis coli (APC), Axin, CK1 and β-catenin (MacDonald et al., 2009). In the absence of the Wnt ligand, β-catenin is phosphorylated, ubiquitinylated and targeted by proteasomes for degradation. This phosphorylation is mediated through GSK3β, which is a key biomarker in neuropsychiatric disorders, and is considered a prime therapeutic target for mood stabilizers such as lithium (Contestabile et al., 2013; Wexler et al., 2008). In the presence of a Wnt ligand, β-catenin is subsequently stabilized as Dvl is phosphorylated after interaction with Fz and GSK3β is recruited away from β-catenin. The stabilized β-catenin can now translocate into the nucleus and bind Tcf/Lef to promote transcription of Wnt target genes. The sFRP and DKK family of proteins comprise major antagonists. sFRP3 proteins mediate inhibition through binding to both Wnt or the Fz receptor while the DKK family targets the LRP5/6 receptors (MacDonald et al., 2009). sFRP3 and DKK1 are crucial factors regulating adult neurogenesis, anxiety behavior and cognition (Jang et al., 2012; Seib et al., 2013) while DKK3 is found downregulated in brains of schizophrenia patients (Ftouh et al., 2005).
Figure 2.
Schematic illustration of canonical Wnt/β-catenin signaling. Left panel shows the downstream Wnt pathway following stimulation by a Wnt ligand and subsequent translocation of β-catenin (β-cat) into the nucleus. Right panel shows the downstream pathway following inhibition by Wnt antagonists and subsequent proteasomal degradation of β-catenin.
3.2. Wnt-mediated regulation of adult hippocampal neurogenesis and behavior
The earliest studies demonstrated hippocampal astrocytes mediated proliferation of adult neural stem cells and promoted a neuronal fate commitment (Song et al., 2002). Subsequently, it was shown this function of mature astrocytes was mediated by Wnt3, a stimulator of the Wnt/β-catenin signaling pathway that is released by astrocytes (Lie et al., 2005). The study showed that Wnt signaling components are expressed in the adult dentate gyrus and the regulation of Wnt expression is accompanied with an apparent change in neurogenesis, suggesting that Wnt signaling is a principal regulator of adult hippocampal neurogenesis (Lie et al., 2005). Evidence over the past decade has elucidated the Wnt pathway’s role in mediating the distinct steps of adult hippocampal neurogenesis: self-renewal, proliferation, differentiation, maturation and functional integration. For instance, Wnt signaling is now understood to maintain self-renewal and stimulate proliferation of neural stem cells through the nuclear receptor tailless (TLX) and Wnt7a (Qu et al., 2010). Induced deletion of Tlx was previously shown to cause a marked reduction in neural stem cell proliferation and neurogenesis in the dentate gyrus, and selectively impair spatial learning with no effect on contextual fear conditioning and locomotion (Zhang et al., 2008). Wnt3-mediated activation of the Wnt signaling pathway enhances neuronal fate commitment and promotes adult hippocampal progenitor proliferation specific to neuroblasts (Lie et al., 2005).
The transition between self-renewal and neuronal lineage differentiation may also be coordinated by Wnt signaling through its activation of NeuroD1, a transcription factor essential for generation of granule cells in the hippocampus and cerebellum (Kuwabara et al., 2009; Liu et al., 2000). While early evidence suggested astrocyte-mediated Wnt3 activation of the pathway did not specifically improve adult hippocampal progenitor survival (Lie et al., 2005), it now appears that NeuroD1 expression mediated by Wnt signaling may improve survival and maturation of adult-born neurons (Gao et al., 2009; Kuwabara et al., 2009). These studies have utilized β-catenin and Neurod1 conditional knockout (cKO) mice to show the role of NeuroD1 in survival and maturation of newborn granule cells (Gao et al., 2009; Kuwabara et al., 2009). Additionally they have elucidated the presence of a Sox2 and TCF/LEF regulatory element on its promoter region that allows NeuroD1 to function across these stages (Kuwabara et al., 2009; Liu et al., 2000). GSK3β too has been shown to be involved in mediating survival and apoptosis of immature precursors (Fuster-Matanzo et al., 2013; Sirerol-Piquer et al., 2011). Finally, another target of the canonical Wnt pathway is the prospero-related homeobox 1 gene (Prox1) that plays a stage-specific role during adult hippocampal neurogenesis. It is highly expressed in the mouse dentate gyrus and strongly associated with TCF/LEF signaling (Karalay et al., 2011). Prox1 overexpression enhances differentiation of the newborn neurons while its shRNA-mediated knockdown does not affect the proliferative ability of RGLs coexpressing Sox2 and GFAP, or the survival of mature granule neurons (Karalay et al., 2011).
During adult hippocampal neurogenesis, the immature neuron migrates deeper into the granular cell layer, and extends its dendrites to the molecular layer (Esposito et al., 2005; Ge et al., 2006) and axons into the CA3 subfield (Faulkner et al., 2008; Toni et al., 2008) to incorporate into the existing neural circuit. Both β-catenin and GSK3β play an important role here in dendritic development of postnatal-born neurons in vivo (Gao et al., 2007; Sirerol-Piquer et al., 2011). For instance, newly born neurons of the dentate gyrus that lack β-catenin show reduced dendrite number and branching, and cannot become fully mature granule neurons and survive less than a month from their birth (Gao et al., 2007; Sirerol-Piquer et al., 2011). When deleted, HIF1a, which stimulates neural stem cells proliferation through Wnt signaling under hypoxia can cause reduced dendritic outgrowth of adult-born neurons due to reduced Wnt signaling (Mazumdar et al., 2010). Similarly, Wnt antagonists such as sFRP3 and DKK1 mediate dendritic development through the Wnt signaling pathway (Jang et al., 2013; Seib et al., 2013). Several clinically-utilized treatments have also been shown to modulate both adult hippocampal neurogenesis and Wnt signaling (Table 1). The Wnt pathway has also been the target of several studies that have looked at associated behavioral modulation with the targeting of Wnt pathway components (Table 2). Several of these will be elucidated in this review. Because of its involvement across each step of adult hippocampal neurogenesis (Fig. 1B), the therapeutic targeting of the Wnt pathway has allowed us to better understand causes associated with neuropsychiatric and age-related disorders. It has opened up new avenues for potential targeting of disease symptoms.
Table 1.
Summary of key studies reviewed in paper that demonstrates relationships between major clinical treatments, adult hippocampal neurogenesis and Wnt signaling. SSRIs; Selective serotonin reuptake inhibitors, TCAs; Tricyclic and tetracyclic antidepressants. N.T.; not tested or reported. FST; Forced swimming test, NSF; Novelty suppressed feeding, TST; Tail suspension test, EPM; Elevated plus maze, OF; Open field, MWM; Morris water maze. MDD; Major depressive disorder.
| Treatment | Experimental model & dose | Cellular or molecular effect on hippocampus | Behavioral effect | Reference |
|---|---|---|---|---|
| Anti-depressants (SSRIs, TCAs) | C57BL/6 mice; chronic fluoxetine | Reduces sFRP3 expression. | Reduces immobility time in FST and TST. | Jang et al., 2012 |
| MDD patients; Caucasian | N.T. | FRZB (human SFRP3 gene) polymorphisms associated with antidepressant response. | ||
| Male Sprague Dawley rats; chronic fluoxetine | Increases cell proliferation. Upregulates Wnt3a, pCREB. |
N.T. | Pinnock et al., 2010 | |
| SvEv129 mice; chronic fluoxetine | Increases cell proliferation, improves dendritic maturation and complexity of newborn neurons, enhances maturation of newborn neurons. | Reduces latency to feed in NSF test. | Wang et al., 2008 | |
| 129/Sv mice; chronic fluoxetine | Increases cell proliferation. | Reduces latency to feed in NSF test. Improved coat score and decreased grooming latency. | Santarelli et al., 2003 | |
| X-ray irradiation of hippocampus decreases proliferation. | X-ray irradiation suppresses behavioral response to chronic fluoxetine treatment in NSF test. X-ray irradiation abolishes the effects of chronic fluoxetine treatment. | |||
| Non human primate (female bonnets); chronic fluoxetine | Repeated separation stress reduces adult hippocampal neurogenesis | Repeated separation stress resulted in depression like-behavior measured by anhedonia and subordinance scores. | Perera et al., 2011 | |
| Chronic fluoxetine treatment stimulates neurogenesis in repeated separation stress animals. | Fluoxetine treatment prevented emergence of depression like-behavior in stressed animals. This effect abolished with X-ray irradiation mediated reduction of adult hippocampal neurogenesis. | |||
| C57BL/6Ntac, β-arrestin 2 +/ mice; chronic glucocorticoids | Chronic fluoxetine treatment rescued neurogenesis suppressed under chronic corticosterone treatment. | Anxiety and depressive behavior initiated under chronic corticosterone treatment was reversed by fluoxetine treatment as measured on TST and EPM. | David et al., 2009 | |
| Human brain specimens | Decreased Ki-67+ population of cells in the dentate gyrus of patients suffering from schizophrenia, but not depression. No significant effect of antidepressants on neural stem cell proliferation. | Brain specimens were from patients with depression, bipolar disorder or schizophrenia. | Reif et al., 2006 | |
| Human brain specimens; SSRIs, TCAs | Antidepressant treatment increased overall dentate gyrus volume, neural progenitors in the anterior dentate gyrus, and angiogenesis. | Brain specimens from patients with major depressive disorder. | Boldrini et al, 2009; Boldrini et al, 2012 | |
| Human brain specimens; SSRIs, TCAs | SSRI treatment caused region- specific increase in granule cell number, granular cell layer volume, and dentate gyrus volume. | Brain specimens from patients with major depressive disorder. | Boldrini et al., 2013 | |
| MDD patients, Chinese; 4 or 8- week SSRIs (fluoxetine or citalopram) | N.T. | GSK3β polymorphic genetic variants associated with 4-week SSRI treatment. | Tsai et al., 2008 | |
| Electroconvulsive stimulation (ECS) | Male Wistar rats; single or chronic | Dose-dependent increase in neurogenesis. | N.T. | Madsen et al., 2000 |
| Male Sprague Dawley rats; chronic | Upregulates β-catenin, Wnt2. | N.T. | Madsen et al., 2003 | |
| C57BL/6 mice; single | Increases proliferation and accelerates dendritic maturation of newborn cells. Reduces sFRP3 expression. | N.T. | Jang et al., 2013 | |
| Sprague- Dawley rats Single or chronic | Dose-dependent increase in cell proliferation, but differentiation of newborn cells not affected by ECS. | No negative behavioral change with chronic ECS. Locomotor activity measured with OF test, and spontaneous alternation with Y-maze test. |
Nakamura et al., 2013 | |
| Non-human primates; chronic | Increases proliferation and neurogenesis. | N.T. | Perera et al., 2007 | |
| Lithium | Ts65Dn mice; chronic | Promotes proliferation and restores neurogenesis. Upregulates β-catenin. | Improves hippocampal dependent memory as measured by contextual fear conditioning, object location and novel object recognition tests. No effect on working memory or spontaneous alternation in T- maze test. | Contestabile et al., 2013 |
| In vitro, adult hippocampal progenitors from female Fisher 344 rats. | Dose-dependent increase in cell proliferation. Inhibits GSK3β and upregulates β-catenin. | N.T. | Wexler et al., 2008 | |
| Valproate | Pregnant female Wistar rats; single injection; pre- natal valproate exposure | Upregulates β-catenin expression in hippocampus, overall upregulation of wnt1 and wnt2 expression observed in brain. | N.T. | Wang et al., 2010 |
| Wistar rats; valproic acid (VPA) and/or sunlidac single injection; prenatal exposure | Reduced GSK3β and increased β-catenin mRNA in the prefrontal cortex and hippocampus with VPA treatment. | VPA reduces pain sensitivity, increased stereotypic/repetitive behavior and reduced spatial learning and memory ability as measured by OF and MWM. Sunlidac (VPA inhibitor) reverses and ameliorates such changes. | Zhang et al., 2012b | |
| Loxapine | In vitro, schizophrenia patient fibroblasts | Improves neuronal connectivity. Rescues WNT7A and TCF4 levels, but not DISC1. | N.T. | Brennand et al., 2011 |
| Clozapine/ Haloperiodol | Sprague- Dawley rats; chronic | Upregulates β-catenin, GSK3β, Dvl- 3 expression in hippocampus. | N.T. | Alimohamad et al., 2005 |
Table 2.
Summary of key studies reviewed in paper that demonstrates relationships between Wnt pathway components and behavioral modulation. FST; Forced swimming test, OF; Open field, MWM; Morris water maze, LH; Learned helplessness, SPT; Sucrose preference test, TST; Tail suspension test, EPM; Elevated plus maze.
| Component | Animal Type | Experiment | Effect on target | Effect on behavior | Reference |
|---|---|---|---|---|---|
| GSK3β or β-catenin | C57BL/6J mice | L803-mts GSK3B inhibitor |
Reduced β-catenin expression. | Reduced immobility time on FST. | Kaidonavich-Beilin et al., 2004 |
| C57BL6; Gsk- 3β knock-out mice | Gsk-3β+/− vs. lithium in WT | Increased β-catenin expression in hippocampus. | Gsk-3β+/− and lithium-treated mice showed overall similar effects on behavior; including reduced immobility time on FST, reduced frequency in hole pokes. | O’Brien et al., 2004 | |
| FVB/NTac; Heterozygous GSK mice | No treatment | GSK3β overexpression. | Increased total locomotor activity on OF, reduced immobility time on FST, increased acoustic startle response. | Prickaerts et al., 2006 | |
| Ts65Dn mice | Lithium administration | GSK3β inhibition. | Improved spatial memory as measured through object location, novel object recognition and fear conditioning test. | Contestabile et al., 2013 | |
| TgCRND8 mice | Lithium administration | GSK3β inhibition. | Improved performance on inhibitory avoidance and MWM. | Fiorentini et al., 2010 | |
| Wnt2 | Male Sprague- Dawley rats | AAV-Wnt2 into dentate gyrus | Increased Wnt2 expression. | Reduced escape failures on LH and increased sucrose uptake on SPT. | Okamoto et al., 2010 |
| sFRP3 | C57BL/6 mice | Fluoxetine administration | Reduced sFRP3 expression. | Reduced immobility time in FST and TST. | Jang et al., 2012 |
| Dkk1 | C57BL/6 mice; Dkk1–/f Nestin-Cre, Dkk1f/f Nestin- CreERT2 | Aged mutants with Dkk1 deficiency | Reduced DKK1. | Increased preference on SPT, reduced immobility time on TST, increased spontaneous alternation on T-maze, increased 24-hr retention in place avoidance. | Seib et al., 2013 |
| DISC1 | Mutant hDISC1 mice | Inducible expression of human DISC1 in transgenic mice | Increased hDISC1 expression. | Male mice exhibited spontaneous hyperactivity in OF and decreased social non-aggressive activity in in social interaction, and female mice showed deficient spatial memory. No effects of hDISC1 overexpression found for novelty-induced locomotion in OF and anxiety levels in EPM. | Pletnikov et al., 2008 |
| Dvl | Female Wistar rats | Sulindac (Dvl inhibitor) and valproic acid (VPA) co- administration | Direct effect on Dvl expression not studied. Increased GSK3β and reduced β-catenin. | Improved spatial learning and memory ability on MWM, reduced stereotypic/repetitive behavior compared to VPA treatment alone. | Zhang et al., 2012b |
3.3. Wnt-mediated regulation of adult subventricular zone (SVZ) neurogenesis
The central focus of this review article is adult hippocampal neurogenesis. However, it is worth briefly appreciating the role of Wnt signaling in regulating SVZ neurogenesis and directing the readers’ attention to significant studies and reviews on the topic. During the course of this review, correlations to SVZ neurogenesis will be occasionally mentioned where helpful. Similar to the SGZ, the SVZ of the lateral ventricle retains the capacity to produce new neurons in adulthood (Alvarez-Buylla and Garcia-Verdugo, 2002). After activation of resident RGL cells in the lateral ventricles, new neurons are generated that migrate across the rostral migratory stream as neuroblasts and consequently reach the olfactory bulb to mature and integrate into the surrounding circuit as interneurons (Ming and Song, 2011). The B cells comprise RGLs, the C cells comprise transient-amplifying cells and the A cells are the neuroblasts. In vitro, both Wnt3a and Wnt5a can increase the proliferation and differentiation of isolated neuronal progenitors from the postnatal and adult mice SVZ (Yu et al., 2006). The expression and role of non-canonical Wnt pathway components in the SGZ has also been shown (Pino et al., 2011; Shimogori et al., 2004). In vivo, β-catenin signaling is active in the B and C cells, and can promote proliferation of C cells possibly through DKK1 and GSK3β (Adachi et al., 2007). Similar to SGZ neurogenesis (Zhang et al., 2008) the TLX nuclear receptor plays a significant role in SVZ neurogenesis (Liu et al., 2008) with the potential involvement of Wnt/β-catenin signaling (Qu et al., 2010). In both Wnt7a−/− and TLX−/− mice, there are significant reductions in BrdU+ cells in the SVZ, and in wide-type mice the lentiviral transduction of active β-catenin can increase the number of GFAP+ B cells in the SVZ (Qu et al., 2010).
A number of studies have attempted to determine the significance of the SVZ in cognitive function and neuropsychiatric conditions. At least one study has shown that the SVZ may be sensitive to environmental triggers of stress (Hitoshi et al., 2007). In their study, Hitoshi et al utilized a forced-swim model of stress to show that chronic stress decreased SVZ neurogenesis, an effect that was sustained over weeks after cessation of the stress. In this study, fluoxetine and imipramine were successful in reversing the effects of stress, while glucocorticoids and serotonin had unique and opposing effects in regulating neurogenesis (Hitoshi et al., 2007). Similarly, another study showed administration of paroxetine stimulated a 34% increase in SVZ neurogenesis while an 18% decrease was observed with corticosterone administration, an effect that could be attenuated with paroxetine (Lau et al., 2007). Other contradictory reports have shown adult SVZ neurogenesis in fact decreases in mice chronically treated with fluoxetine for more than 6 weeks (Ohira and Miyakawa, 2011). Aside from stress, physiological aging is also a critical regulator and is implicated in spatio-temporal changes and decreased SVZ neurogenesis with age (Luo et al., 2006; Shook et al., 2012). Antipsychotics such as paliperidone may also play a stimulatory role in increasing neurogenesis in the olfactory epithelium or the posterior SVZ (Nasrallah et al., 2010). In a study of Down syndrome, the mood stabilizer lithium induced proliferation in both euploid and Ts65Dn mice (Bianchi et al., 2010a). The latter group is the most widely used mouse model for Down syndrome where lithium increased proliferation up to euploid levels. Finally, a recent study has shown that electroconvulsive treatment (ECT), one of the more widely used treatments for major depression induces neurogenesis in frontal rat brain areas adjacent to the SVZ and increases small-size calretenin-positive interneurons (Inta et al., 2013). Despite such studies, a significant role of SVZ neurogenesis in neuropsychiatric disorders remains debatable due to the lack of evidence from clinical cohorts, post-mortem specimens, or preclinical models. At this point, it may be more pertinent to conclude that between the two regions undergoing constant neurogenesis in the adult brain, SGZ neurogenesis holds a significant relationship with Wnt signaling in the etiology, pathogenesis and treatment of neuropsychiatric disorders.
4. Wnt signaling in psychiatric disorders
4.1. Depression
Major depression is among the most prevalent psychiatric disorders worldwide and is associated with high rates of morbidity, death by suicide, and significant functional disability. In early onset depression and major depressive disorder (MDD) patients, structural changes including significant volume loss and functional deficits are observed in the hippocampus (MacQueen et al., 2008; Maller et al., 2007; Malykhin et al., 2010; Neumeister et al., 2005; Sawyer et al., 2012). Antidepressants have been shown to increase adult hippocampal neurogenesis in rodents (Santarelli et al., 2003), monkeys (Perera et al., 2007) and even human MDD brains (Boldrini et al., 2009).
In animal models, techniques used to model depression include stress, glucocorticoids, or selective impairment of neurogenesis through irradiation or transgenic means to observe behavioral outcomes. Stress has been shown previously to play a causal relationship with onset of major depression (Kendler et al., 1999; Kendler et al., 1995). Similarly, depressed patients may show impaired glucocorticoid signaling (Anacker et al., 2011) and hyperactivity of the hypothalamo-pituitary-adrenal (HPA) axis (Pariante, 2009). Indeed, treatment-resistant depressed patients may have cortisol levels twice that of healthy controls (Anacker et al., 2011). In rodent models, depression produced via stress models can inhibit one or more steps of adult hippocampal neurogenesis and mediate neural plasticity (Pham et al., 2003; Yun et al., 2010; Zunszain et al., 2011). Learned helplessness and forced swimming tests are two widely used tests in producing stress models for modeling depression and antidepressant effects (Cryan et al., 2002). In the learned helplessness model of depression, administering inescapable footshock to animals can down regulate neurogenesis while fluoxetine can counteract such an effect (Malberg and Duman, 2003). A number of studies have shown that glucocorticoid administration can reduce neurogenesis, hippocampal volume and induce anxiety-related or depressive-like behavior that can be reversed with antidepressant treatment such fluoxetine or imipramine (David et al., 2009; Murray et al., 2008).
More direct evidence for a role of adult hippocampal neurogenesis in depression comes from a series of experiments that indicate a role for neurogenesis in mediating the normal HPA axis and stress response (Snyder et al., 2011). Neurogenesis-deficient mice showed slower recovery of glucocorticoid levels and reduced suppression by dexamethasone following a moderate stress event. The neurogenesis-deficient mice also showed depressive-behavior with increased food avoidance on novelty suppressed feeding (NSF) and increased total immobility on the forced swimming test (FST). Finally, these mice showed anhedonia in the form of reduced preference on the sucrose test (Snyder et al., 2011). Whether impaired adult neurogenesis is a risk factor in the development of depressive behavior remains controversial (van der Worp et al., 2010). Animal studies show that specific impairment of adult hippocampal neurogenesis can lead to depressive behavior. For instance, selectively impairing adult hippocampal neurogenesis through the inducible over-expression of the apoptotic protein Bax in neuronal precursors can increase anxiety-related behavior on elevated plus maze (EPM) and light-dark tests with avoidance of potentially threatening stimuli, but spare depressive-behavior on NSF or FST (Revest et al., 2009; Snyder et al., 2011). At least one animal model study returned contradictory results showing no change in depressive-like behavior with irradiation (Santarelli et al., 2003) and another clinical study has shown no change in hippocampal cell proliferation in MDD patients (Reif et al., 2006). Other studies have shown SSRI or TCA treatment in MDD patients are associated with increased dentate gyrus volume, granule cells, and neural progenitors, as well as increased hippocampal angiogenesis (Boldrini et al., 2012; Boldrini et al., 2013; Boldrini et al., 2009). At this juncture, it may be more pertinent to conclude that newborn neurons may be a major contributor in normalizing or ameliorating against disease states rather than being causally involved in the etiology of depression.
Independent lines of research including post-mortem, pharmacogenetic and preclinical studies have established a significant role for aberrant Wnt signaling in depression. GSK3β is one important downstream effector of Wnt signaling that has been associated with depression and anti-depressant effects. Increased GSK3β mRNA levels are found in the hippocampus of major depression patients, altered GSK3β activity is found in ventral prefrontal cortex of depressed suicide victims and in patients with recurrent MDD, and GSK3β polymorphisms are found associated with structural variation in the hippocampus (Inkster et al., 2009; Karege et al., 2007; Oh et al., 2010). Similarly, GSK3β genetic variants were found to be associated with antidepressant therapeutic response in Chinese MDD patients (Tsai et al., 2008). In the hippocampus, lithium treatment which is used for treating depression and bipolar disorder is strongly tied to GSK3β inhibition and β-catenin upregulation in promoting adult hippocampal progenitor development (Wexler et al., 2008). Remarkably, the proliferative effects of lithium are abolished with inhibition of β-catenin, suggesting a correlation between the Wnt pathway and the therapeutic effect of lithium (Wexler et al., 2008).
Much evidence has accumulated since the pioneering work that first showed that chronic administration of antidepressants promoted proliferation of adult hippocampal progenitors in the rat hippocampus and increased the number of newborn neurons that matured and integrated into the hippocampal circuit (Malberg et al., 2000). Further study provided evidence that in some animal models and non-human primates, the antidepressant-induced behavioral responses require adult hippocampal neurogenesis (Santarelli et al., 2003). One such antidepressant is fluoxetine, which accelerates maturation of immature neurons and enhances neurogenesis-dependent long-term potentiation in the dentate gyrus (Wang et al., 2008), an effect that is interestingly opposite to what is observed in the SVZ (Ohira and Miyakawa, 2011). In non-human primates, fluoxetine treatment can stimulate adult hippocampal neurogenesis and treat anhedonia and subordinance depression-like behavior arising from repeated separation stress (Perera et al., 2011). Such behavioral amelioration was abolished with X-ray irradiation of the hippocampus, again showing the requirement of neurogenesis for antidepressant effect (Perera et al., 2011). Consequently, it was demonstrated electroconvulsive stimulation (ECS) can increase hippocampal neurogenesis in a dose-dependent manner in rats (Madsen et al., 2000; Nakamura et al., 2013) and proceeds by upregulating β-catenin expression in the dentate gyrus of the rat hippocampus (Madsen et al., 2003).
A molecular signaling mechanism linking this process has been identified. Upstream signaling molecules in the Wnt/β-catenin pathway may play a role in such chronic antidepressant treatment (Okamoto et al., 2010). Using microarray analysis, Okamota et al. investigated the effects of citalopram, fluoxetine, venlafaxine, atomoxetine and ECS on hippocampal gene expression of Wnt pathway components. Microarray analysis for gene expression following chronic treatment of antidepressants showed increased expression of subtypes of Wnt, Fz, TCF, β-catenin as well as NeuroD1 expression in the hippocampus (Okamoto et al., 2010). Wnt2, an agonist of the canonical pathway in the hippocampus, was upregulated by all classes of antidepressants. To validate a potential mechanism of action for these anti-depressant effects, the group proceeded to increase Wnt2 expression selectively in the dentate gyrus via an adeno-associated viral (AAV)-mediated approach. This produced an antidepressant effect as measured by the decreased number of escape failures on the learned helplessness paradigm, and reduced anhedonic behavior as measured by the increased sucrose consumption relative to AAV controls (Okamoto et al., 2010). Similarly, administrating a GSK3β inhibitor in mice can increase β-catenin mRNA levels in the hippocampus, and reduce immobility time on the FST (Kaidanovich-Beilin et al., 2004). In lieu of recent and previous evidence, a potential role of Wnt signaling components in mediating the antidepressant effect is not unexpected (Lie et al., 2005; Pinnock et al., 2010). One of the earliest studies by Lie et al. (Lie et al., 2005) demonstrated Wnt3a expression promotes adult hippocampal neurogenesis. Interestingly, the administration of fluoxetine has been shown to mediate an antidepressant effect through an increase in Wnt3a expression in the dentate gyrus (Pinnock et al., 2010). It is also worth noting a potential involvement of SVZ neurogenesis in depression which while sparse indicates an opposite effect to what is observed in the SGZ. For instance, chronic fluoxetine and corticosterone treatment decreases SVZ neurogenesis where the effect of the latter may be prevented with paroxetine administration (Lau et al., 2007; Ohira and Miyakawa, 2011). A more direct relationship between Wnt signaling and such effects in the SVZ is however lacking.
While the evidence presented thus far suggests antidepressant effects are strongly mediated by changes to downstream effectors of canonical Wnt signaling pathway or Wnt ligands in the hippocampus, a potentially versatile role of Wnt pathway antagonists such as sFRP3 and DKK1 in these mechanisms is now being explored. The SFRP family of proteins are important regulators of the Wnt pathway whose expression is changed in disease states (Jones and Jomary, 2002). Recently, we showed that chronic fluoxetine treatment significantly reduced sFRP3 protein expression in the adult dentate gyrus and improved mood behavior in mice as measured by the forced swimming and the tail suspension test (Jang et al., 2012). While contradictory to results from the Okamato et al. (Okamoto et al., 2010) study presented above, such an opposite effect may be explained due to the different animal models used. Consequently, we also showed single-nucleotide polymorphisms (SNPs) in the FRZB (the human sfrp3 ortholog) were significantly associated with antidepressant response in clinically depressed patients (Jang et al., 2012). Further, it is a potent regulator of activity-dependent adult hippocampal neurogenesis and deletion of the sfrp3 gene promotes activation of the quiescent neural stem cell population, promotes newborn neuron maturation and improves dendritic growth and complexity, ultimately assisting the immature neuron in functionally integrating into the hippocampal circuit (Jang et al., 2013). Thus, sFRP3 may potentially be the bridge between stem cell activation and activity-dependent regulation of neurogenesis and depressive-like behavior. However, whether sFRP3 may directly mediate depressive-like behavior through its effects on adult hippocampal neurogenesis remains to be addressed.
The Wnt pathway antagonist DKK1 has also emerged as an important player in mediating depressive-behavior especially during aging. DKK1 belongs to the Dickkopf family of Wnt modulators (Niehrs, 2006) that acts by binding to the LRP5/6 co-receptor preventing any Fz/LRP6 complex formation (Semenov et al., 2008). Recent work on DKK1 provides us one of the first clues to the molecular basis of declining neurogenesis and cognitive function with age (Seib et al., 2013). DKK1 expression in mice increases with age and its loss during old age can reduce depressive symptoms, and restore working memory and memory consolidation (Seib et al., 2013). In their experiments, Seib et al noted DKK1 mutants showed reduced anhedonia as measured by the increased preference for sucrose on the sucrose preference test. These DKK1-deficient mice also showed reduced immobility time on TST, an effect similar to those produced by anti-depressant drugs. In experiments measuring spatial memory and memory consolidation, 18-month-old DKK1 mutants showed increased spontaneous alternation on the T-maze paradigm with a success rate similar to that of 3-month-old wild-type mice. Along with this increase in hippocampal dependent spatial memory, other 10-month DKK1 mutants showed increased 24-hour retention in the place avoidance paradigm, and with success comparable to that of 3-month-old wild-type mice. The loss of DKK1 specifically enhances the self-renewal and survival of quiescent neural progenitors, increases the number of neural progenitors and committed neuroblasts and improves dendritic complexity (Seib et al., 2013). Evidence such as this bolsters the role of Wnt activity in declining neurogenesis with associated changes in affective behavior and anhedonia. It also provides DKK1 as a potential therapeutic target in improving depressive and memory-related behavior and in protecting against the age-related decline in neurogenesis.
Pre-clinical and clinical evidence thus far suggests the Wnt signaling components could be potential targets for effective drug treatment in treating depression. The molecular and cellular mechanisms underlying the efficacy of antidepressant treatment still remain largely unclear and along with the role of Wnt signaling in depression etiology require careful future study.
4.2. Schizophrenia
Schizophrenia is a debilitating neurodevelopmental disorder characterized by aberrant thought processes, social behavior and emotional responses. A complex disorder influenced by both genetic and environmental factors, the role of genetics or heritability in liability to schizophrenia is 81% (Sullivan et al., 2003). Post-mortem studies investigating the histopathology associated with schizophrenia have found smaller neuron size in the hippocampal subfields including the subiculum, entorhinal cortex and CA1 regions and altered neuron shape in the CA1-CA4 regions (Arnold et al., 1995; Zaidel et al., 1997). Structural changes and a reduced hippocampal volume may also be associated with schizophrenia (Antonova et al., 2004; Velakoulis et al., 2001). Reduced amounts of newly formed cells are also found in the human hippocampus in schizophrenia suggesting reduced neural progenitors may contribute to the pathogenesis (Reif et al., 2006). Our understanding of the relationship between Wnt signaling and adult hippocampal neurogenesis in mediating schizophrenia is in its nascent stages. Studies have shown β-catenin expression is reduced in the CA3 and CA4, and gamma-catenin in the CA3 and CA4 hippocampal subregions (Cotter et al., 1998). Wnt1 expression is increased in the CA3 and CA4 regions (Miyaoka et al., 1999), and the Wnt pathway inhibitor DKK3 downregulated in schizophrenic brains (Ftouh et al., 2005). At this point as per our knowledge, the role of DISC1 and its physical interaction with GSK3β could play a key role in Schizophrenia.
DISC1 is implicated as a risk gene for schizophrenia, bipolar disorder and depression. First identified in a Scottish family with high-risk of these disorders, studies have since elucidated the role of DISC1 in regulating the integration of newborn neurons into the hippocampal circuit (Duan et al., 2007; Mao et al., 2009). For instance, down regulating DISC1 can lead to aberrant morphogenesis and mispositioning of newborn neurons in the circuit while DISC1 knockdown in these newborn neurons can enhance excitability and dendritic development (Duan et al., 2007). It was confirmed that DISC1 could mediate such changes during neurogenesis through its direct physical interaction and inhibition of GSK3β activity allowing DISC1 to regulate β-catenin stability (Mao et al., 2009). Indeed, administration of GSK3β inhibitors in adult hippocampal progenitors with DISC1 knockdown is enough to rescue proliferation deficits. This study is the first to show a direct interaction between a major schizophrenia risk gene and a Wnt pathway component in the context of new neuron development in the hippocampus (Mao et al., 2009). Mice lacking a functional form of DISC1 show a wide range of behavioral abnormalities. These include selective impairment in working memory (Kvajo et al., 2008; Li et al., 2007), spontaneous hyperactivity and deficient spatial memory (Pletnikov et al., 2008), depressive-like behavior (Clapcote et al., 2007; Hikida et al., 2007), and deficits in prepulse and latent inhibition (Clapcote et al., 2007). DISC1 may also exert different effects on behavior depending on developmental age (Ayhan et al., 2011). It has been shown the DIX domain containing-1 (Dixdc1) gene regulates neural progenitor proliferation during embryonic cortical development by complexing with DISC1 and co-modulating the Wnt/β-catenin pathway (Singh et al., 2010). Interestingly, it was recently shown Dixdc1−/− mice at 3–4 months display spontaneous locomotor activity, increased anxiety and startle reactivity (Kivimae et al., 2011). More work is needed to confirm the differential role played by Wnt signaling during embryonic, post-natal and adult stages in contributing to schizophrenia etiology and any contribution it could make as a factor in the neurodevelopmental hypothesis of schizophrenia.
One comprehensive study has looked at the neuronal phenotype and gene expression profiles associated with neurons that were generated after reprogramming fibroblasts from schizophrenia patients into human induced pluripotent stem cells (hiPSCs) (Brennand et al., 2011). Decreased neuronal connectivity, neurite outgrowth and reduced synaptic protein levels and density of PSD95 was observed while normal electrophysiological and spontaneous calcium transient activity was maintained in the schizophrenia hiPSC neurons (Brennand et al., 2011). The gene expression profile revealed this neuronal phenotype could be regulated by altered cyclic AMP and Wnt signaling. Indeed, GO analysis of microarray data revealed Wnt components including WNT2B, WNT3, WNT7B, AXIN, LEF1, TCF4 were significantly upregulated while WNT7A and LRP5 were significantly downregulated (Brennand et al., 2011). Previous studies have shown that administration of antipsychotic medication for schizophrenia such as clozapine or haloperidol can significantly enhance expression of β-catenin, GSK3β and Dvl-3 in the rat hippocampus (Alimohamad et al., 2005). In the in vitro study of Brennand et al. (Brennand et al., 2011), it was found that administration of loxapine, a drug for treating schizophrenia, was able to improve neuronal connectivity while clozapine, risperidone and olanzapine were not. Interestingly, while loxapine was able to rescue WNT7A and TCF4 levels to control expression, it had no effect on DISC1 expression.
Studies presented thus far provide two separate models that could explain the role of Wnt/β-catenin signaling in schizophrenia via regulation of adult hippocampal neurogenesis. One is a watershed model that posits that schizophrenia is the result of multiple genetic causes across different pathways including the Wnt signaling pathway (Brennand et al., 2011; Cannon and Keller, 2006). In the other, dysfunctional interaction between DISC1 and GSK3β can effect downstream changes in the Wnt pathway and cause aberrant morphology and integration of newborn neurons in the dentate gyrus (Duan et al., 2007; Mao et al., 2009). Keeping in mind the involvement of adult hippocampal neurogenesis in learning and mood regulation, it could be that across-the-board changes in risk factors for schizophrenia could push Wnt signaling to aberrantly regulate newborn neuron development. Further evidence will be required to understand how Wnt/β-catenin signaling components interact with other pathways in their regulation of newborn neuron development.
4.3. Other neurological and psychiatric disorders
Autism
A strong case for Wnt signaling involvement in autism has been put forth through family and twin studies, animal models of autism, studies of co-occurrence between tuberous sclerosis complex and autism, and epidemiological studies using Valproic Acid (VPA), a teratogen known to increase the risk of autism in offspring (Contestabile and Sintoni, 2013; De Ferrari and Moon, 2006). Hippocampus and amygdala volumes are enlarged in autistic individuals with the persistence of an enlarged hippocampus at all life stages (Barnea-Goraly et al., 2014; Groen et al., 2010; Schumann et al., 2004). Transgenic mice over-expressing β-catenin can develop enlarged, distorted hippocampi (Chenn and Walsh, 2003). In the anterior hippocampus, these distortions may include a less defined and thicker layer of neurons comprising the dentate gyrus and changes in the smooth laminar structure of the dentate gyrus (Chenn and Walsh, 2003). These changes may be even more pronounced in the posterior hippocampus (Chenn and Walsh, 2003). Recently, VPA has assisted in uncovering potential links between Wnt signaling-mediated regulation of hippocampal neurogenesis in autism. Using a VPA-treated autism model of male rats, one study has looked at the effect of VPA on Wnt signaling and oxidative homeostasis (Zhang et al., 2012b). Prenatal VPA administration activated the Wnt pathway as measured through mRNA and protein levels in the prefrontal cortex and the hippocampus (Zhang et al., 2012b). An increase in expression of 4-HNE, an oxidative stress marker, was observed in both regions with VPA administration (Zhang et al., 2012b). Interestingly, prenatal co-administration of VPA and sunlidac, a small molecular inhibitor of the Wnt pathway reduced the expression of 4-HNE and inhibited the Wnt pathway by increasing GSK3β and reducing β-catenin expression (Zhang et al., 2012b). These changes corresponded with enhanced pain sensitivity, amelioration of stereotypic/repetitive behavior, and improved spatial learning and memory ability measured using the Morris water maze (MWM) test (Zhang et al., 2012b). This study provides an interesting correlation between prenatal VPA administration and oxidative homeostatis playing a role in susceptibility to autism where certain autism-like phenotypes may be ameliorated through regulation of the Wnt pathway (Zhang et al., 2012b).
Other studies have considered more direct effects of VPA on dentate gyrus neurogenesis. For instance, in rats VPA administration (possibly through upregulation of NeuroD) can promote neuronal differentiation and inhibit astrocyte and oligodendrocyte differentiation in the dentate gyrus (Hsieh et al., 2004). VPA also demethylates Wnt1 and Wnt2, upregulates their mRNA and protein expression, and increases β-catenin levels in the rat hippocampus (Wang et al., 2010). While there is evidence supporting Wnt2 as an autism-susceptibility gene (Wassink et al., 2001) there is also contradictory evidence showing no association between Wnt2 and autism (Li et al., 2004a; McCoy et al., 2002). Thus, while an association exists between Wnt signaling and the pharmacological effect, more work is required to discern its relationship, if any, in the etiology.
Additional information on neurogenesis and Wnt signaling involvement comes from studies in Ts65Dn mice that comprise a widely used animal model used in studying behavioral phenotypes found in Down syndrome. These mice contain extra genetic information at distal chromosome 16 that correspond to the genes present at human chromosome 21q21–22.1 resulting in a behavioral phenotype similar to one seen in Down syndrome (Reeves et al., 1995). Behavioral impairments in these mice include deficits in tasks requiring visual and spatial information integration on tests such as novel object recognition, spontaneous alternative, radial arm maze and MWM (Cramer and Galdzicki, 2012; Gotti et al., 2011; Reeves et al., 1995). These mice express deficient neurogenesis and functional connectivity within the hippocampus that may be improved with fluoxetine treatment (Bianchi et al., 2010b; Clark et al., 2006; Stagni et al., 2013). The effects of the mood stabilizer lithium are noteworthy in this mouse model. Lithium can increase rescue synaptic plasticity and memory deficits by activating the Wnt/β-catenin pathway and restoring adult hippocampal neurogenesis to physiological levels (Contestabile et al., 2013). This included restoring the number of proliferating cells, increasing the number of amplifying progenitors, restoring neuroblasts numbers and improving spatial memory as measured through object location, novel object recognition and fear conditioning (Contestabile et al., 2013). Finally, while the mechanistic or behavioral implications are lacking, lithium may also rescue deficient SVZ neurogenesis in these mice (Bianchi et al., 2010a).
Alzheimer’s Disease
Due to the inherent plasticity associated with adult hippocampal neurogenesis and its role in learning and memory, adult hippocampal neurogenesis has long been considered to play a key role in Alzheimer’s disease (AD). There is an age-related decline in adult hippocampal neurogenesis owing to the continuous depletion of resident neural stem cells (Encinas et al., 2011; Olariu et al., 2007). Reduced hippocampal neurogenesis is tied to loss in spatial memory performance in aged rats (Clelland et al., 2009; Drapeau et al., 2003). In mouse models, classic AD pathology components such as amyloid β-peptide (Aβ) reduce the proliferation and survival of neuronal progenitors during adult hippocampal neurogenesis (Haughey et al., 2002). On the contrary, some studies have found increased number of neural progenitors in AD condition (Gan et al., 2008; Jin et al., 2004). Still others have demonstrated using an AD mouse model that there is greater dendritic development in early stages but functional impairment during later maturation (Sun et al., 2009), suggesting impaired neurogenesis may contribute to AD-related cognitive deficits. Wnt pathway involvement has been increasingly recognized in AD pathophysiology (De Ferrari and Moon, 2006). In particular, the Wnt antagonist DKK1 that regulates hippocampal neurogenesis, memory and affective behavior during aging (Seib et al., 2013) is also involved in regulating classic AD pathology in the hippocampus. In hippocampal brain slices, Aβ exposure rapidly increased DKK1 levels (Caricasole et al., 2004) while in mature hippocampal neurons DKK1 exposure caused synapse loss (Purro et al., 2012). Interestingly, the Aβ associated neurotoxicity in the hippocampal neurons could be overcome with administration of Wnt3a (Alvarez et al., 2004). In other established AD mice models including APP mice, administration of lithium inhibits GSK3β and stimulates adult hippocampal neurogenesis (Fiorentini et al., 2010). Improved performance was observed on the inhibitory avoidance task and on the MWM. Interestingly, these effects on neurogenesis and cognitive function were age-dependent and declined as brain Aβ deposition and pathology increases (Fiorentini et al., 2010). Evidence thus far suggests that Wnt-mediated regulation of AD pathology and hippocampal-dependent aging are closely related and may work in tandem in mediating associated cognitive decline.
Bipolar Disorder
In Bipolar Disorder, extensive post-mortem studies tying Wnt signaling to structural changes in the brain are lacking, but there is growing pharmacological and gene expression data, and evidence from monozygotic twin pairs (Sani et al., 2012). Lithium is a drug often prescribed for bipolar disorder and as discussed, its therapeutic action may proceed through its inhibition of GSK3β activity and stimulation of newborn neuron production in the hippocampus (Contestabile et al., 2013; Gould et al., 2004; Klein and Melton, 1996; Wexler et al., 2008). A recent study has also associated a larger hippocampal volume in lithium treated bipolar I disorder (BP1) patients compared to a non-lithium treated BP1 group (van Erp et al., 2012). Other studies have delved further into lithium’s association with GSK3β and changes in behavior. For instance, chronic oral lithium administration in mice at clinically relevant doses increases Wnt dependent transcription in vivo in the dentate gyrus and the amygdala, and produces a behavioral phenotype similar to mice carrying only one functional copy of GSK3β (O'Brien et al., 2004). These include reduced immobility time on the FST and reduced exploratory behavior on the open field test (O'Brien et al., 2004). Lithium treatment also increased Wnt-Tcf signaling in the dentate gyrus, amygdala and hypothalamus as measured through increased β-galactosidase activity in X-gal incubated coronal sections from BAT-gal mice. Further, both lithium administration and loss of a functional GSK3β copy resulted in increased β-catenin expression.
Similarly, utilization of a GSK3β inhibitor that has been intracerebroventricularly injected into mice increases the levels of β-catenin in the hippocampus and induces an antidepressant effect as measured using the FST (Kaidanovich-Beilin et al., 2004). Other behavioral aberrations include hyperactivity, mania and increased BDNF expression in the hippocampus in mice that overexpress GSK3β (Prickaerts et al., 2006). In some human studies, peripheral blood mononuclear cells (PBMCs) from BD patients have been used to understand effects of lithium, VPA and other atypical antipsychotics to study molecular or behavioral changes (Li et al., 2010; Polter et al., 2010). However, due to the lack of relevant BD animal models, it remains unknown whether dysregulation of Wnt signaling is involved in the pathogenesis of BD. Currently, there also exists contradictory evidence that GSK3β and β-catenin levels are in fact not modified in the BD post-mortem brain (Kozlovsky et al., 2000; Lesort et al., 1999). While such studies are not hippocampus-specific, they present the possibility that Wnt signaling could possibly be involved in mediating the pharmacological effect mediated through adult hippocampal neurogenesis.
5. Crosstalk and Wnt/β-catenin signaling beyond the hippocampus
The Wnt signaling pathway is a dynamic pathway where both the canonical and non-canonical forms are involved across various stages of embryonic, post-natal and adult tissue development (Ciani and Salinas, 2005; Clevers and Nusse, 2012; Logan and Nusse, 2004; Rosso et al., 2005). In the context of neuropsychiatric disorders, it should be recognized and appreciated that its crosstalk with other cellular pathways and factors can add to the diversity of function presented in this review and found across the wide spectrum of the field. For instance, neural Insulin Growth Factor (IGF) signaling which is crucial for the development of the hippocampal formation and dentate gyrus (Liu et al., 2009) interacts with the canonical Wnt/β-catenin pathway to promote neural proliferation in the post-natal brain through type 1 IGF receptor (IGFR1) (Hu et al., 2012). During aging, crosstalk between astrocytes and Wnt-signaling is modified and may decrease adult hippocampal neurogenesis via Wnt-mediated survivin signaling (Miranda et al., 2012).
In MDD, impaired serotonergic activity may also interact with Wnt signaling. Stimulating serotonin (5HT) release using d-fenfluramine can inhibit levels of GSK3β in vivo in the prefrontal cortex, hippocampus and the striatum; an effect that is mimicked by fluoxetine and imipramine (Li et al., 2004b). Similarly, GSK3β may also mediate the effect of lithium on dopamine-dependent locomotor behaviors (Beaulieu et al., 2004). Indeed, there is a wealth of evidence supporting the role of GSK3β as key player in mood regulation with its ability to interact with multiple pathways and get regulated by serotonin, dopamine, the Akt signaling pathway, BDNF and a number of psychotropic drugs (Li and Jope, 2010). Moving beyond neuropsychiatric disorders, canonical Wnt signaling plays crucial roles across the CNS in the adult. These include maintaining bone homeostasis and its role in osteosarcoma (Baron and Kneissel, 2013; Li et al., 2013). Recent work has elucidated a role for Wnt signaling in gliomas (Zhang et al., 2012a) and in oncogenesis including interaction with microbial pathogens (Al-Harthi, 2012) such as the Hepatitis C virus (Liu et al., 2011). Similarly, Wnt5a, a component of the non-canonical Wnt pathways may interact with other Wnts from the canonical pathway in regulating midbrain dopaminergic neuron development (Andersson et al., 2008; Andersson et al., 2013). The canonical Wnt pathway has also been demonstrated to function in mesodiencephalic dopaminergic systems which has defined roles in depression, schizophrenia, addiction and Parkinson’s disease (Alves dos Santos and Smidt, 2011).
6. Perspectives
A quarter century of research in Wnt signaling has significantly enhanced our understanding of the pathway. Canonical Wnt/β-catenin signaling has emerged a key regulatory pathway in development and human disease. Technical advances in classic genetics, molecular and whole genome proteomics have allowed us to cross the first prerequisite hurdle of characterizing the different components of this canonical pathway. Several components including GSK3β and β-catenin have been identified as biomarkers of neuropsychiatric health in patients. Alongside such strides, the scientific field devoted itself to the study of adult neurogenesis and its role in learning, cognition and behavior. This growth spurt allowed appreciation of the striking neuroplasticity afforded through newborn neuron development. A closer examination of its regulation has revealed a role for Wnt signaling in mediating each step, and key activators and inhibitors have been identified that allow modulation of this pathway. Targeting the molecules of this canonical pathway through animal models has underscored the therapeutic efficacy afforded through fine-tuning of the Wnt pathway. However, many questions remain unanswered. First, a definitive role of Wnt signaling in the causative mechanism of the disorders presented is still lacking. There is favorable evidence that could suggest a more definite role in schizophrenia and depression (Jang et al., 2013; Mao et al., 2009; Okamoto et al., 2010), while evidence in AD suggests aberrant Wnt signaling could be a result or the by-product of disease pathogenesis, and in BD and autism further work is required to establish a relationship. Second, the interaction of canonical Wnt/β catenin signaling with other pathways needs to be further investigated to obtain a clearer picture of how Wnt may regulate disease symptoms. Third, while therapeutic efficacy has been observed through targeting of individual Wnt components, the cellular and molecular mechanisms that link Wnt signaling and adult neurogenesis to the final effect on cognition are still unclear. Utilization of new technologies in animal genetics that allow spatial and temporal precision in targeting neuronal activity can provide us a greater mechanistic understanding of the Wnt pathway in neuropsychiatric disorders and how we may modulate it toward therapeutic benefit in clinic.
Highlights.
We review Wnt/β-catenin signaling pathway in neuropsychiatric disorders.
We evaluate its regulation of adult hippocampal neurogenesis and behavior.
We focus on disease etiology, pathogenesis and promising therapeutic targets.
We discuss the results and evaluate current clinical and preclinical models.
Discussion includes depression, schizophrenia, Alzheimer’s disease, bipolar disorder and autism.
Acknowledgments
We thank Dr. Mark A. Frye for his insightful comments and Kiley Schmidt for editorial comments and assistance with the illustrations. The research in Dr. Jang laboratory was supported by NIMH (R00MH090115), NARSAD (Young investigator award), Whitehall Foundation, Accelerated Regenerative Medicine Award and Career Development Award from Center for Regenerative Medicine at Mayo, Fraternal Order of Eagles Funds from Mayo Clinic Cancer Center, and start-up funds from Mayo foundation.
Footnotes
Financial Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Al-Harthi L. Wnt/beta-catenin and its diverse physiological cell signaling pathways in neurodegenerative and neuropsychiatric disorders. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7:725–730. doi: 10.1007/s11481-012-9412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimohamad H, Sutton L, Mouyal J, Rajakumar N, Rushlow WJ. The effects of antipsychotics on beta-catenin, glycogen synthase kinase-3 and dishevelled in the ventral midbrain of rats. Journal of neurochemistry. 2005;95:513–525. doi: 10.1111/j.1471-4159.2005.03388.x. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214:1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Experimental cell research. 2004;297:186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Alves dos Santos MT, Smidt MP. En1 and Wnt signaling in midbrain dopaminergic neuronal development. Neural development. 2011;6:23. doi: 10.1186/1749-8104-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson ER, Prakash N, Cajanek L, Minina E, Bryja V, Bryjova L, Yamaguchi TP, Hall AC, Wurst W, Arenas E. Wnt5a regulates ventral midbrain morphogenesis and the development of A9-A10 dopaminergic cells in vivo. PloS one. 2008;3:e3517. doi: 10.1371/journal.pone.0003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson ER, Salto C, Villaescusa JC, Cajanek L, Yang S, Bryjova L, Nagy II, Vainio SJ, Ramirez C, Bryja V, Arenas E. Wnt5a cooperates with canonical Wnts to generate midbrain dopaminergic neurons in vivo and in stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E602–610. doi: 10.1073/pnas.1208524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophrenia research. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Gur RC, Gur RE, Shapiro RM, Moberg PJ, Trojanowski JQ. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. The American journal of psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Molecular psychiatry. 2011;16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Frazier TW, Piacenza L, Minshew NJ, Keshavan MS, Reiss AL, Hardan AY. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Progress in neuro-psychopharmacology & biological psychiatry. 2014;48:124–128. doi: 10.1016/j.pnpbp.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature medicine. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Ciani E, Contestabile A, Guidi S, Bartesaghi R. Lithium restores neurogenesis in the subventricular zone of the Ts65Dn mouse, a model for Down syndrome. Brain pathology. 2010a;20:106–118. doi: 10.1111/j.1750-3639.2008.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Ciani E, Guidi S, Trazzi S, Felice D, Grossi G, Fernandez M, Giuliani A, Calza L, Bartesaghi R. Early pharmacotherapy restores neurogenesis and cognitive performance in the Ts65Dn mouse model for Down syndrome. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010b;30:8769–8779. doi: 10.1523/JNEUROSCI.0534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biological psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annual review of clinical psychology. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cerebral cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nature reviews Neuroscience. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, Mackie S, Christie S, Ogawa F, Lerch JP, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay MD, Henkelman RM, Sled JG, Gondo Y, Porteous DJ, Roder JC. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Clark S, Schwalbe J, Stasko MR, Yarowsky PJ, Costa AC. Fluoxetine rescues deficient neurogenesis in hippocampus of the Ts65Dn mouse model for Down syndrome. Experimental neurology. 2006;200:256–261. doi: 10.1016/j.expneurol.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Contestabile A, Greco B, Ghezzi D, Tucci V, Benfenati F, Gasparini L. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Invest. 2013;123:348–361. doi: 10.1172/JCI64650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A, Sintoni S. Histone acetylation in neurodevelopment. Current pharmaceutical design. 2013;19:5043–5050. doi: 10.2174/1381612811319280003. [DOI] [PubMed] [Google Scholar]
- Cotter D, Kerwin R, al-Sarraji S, Brion JP, Chadwich A, Lovestone S, Anderton B, Everall I. Abnormalities of Wnt signalling in schizophrenia--evidence for neurodevelopmental abnormality. Neuroreport. 1998;9:1379–1383. doi: 10.1097/00001756-199805110-00024. [DOI] [PubMed] [Google Scholar]
- Cramer N, Galdzicki Z. From abnormal hippocampal synaptic plasticity in down syndrome mouse models to cognitive disability in down syndrome. Neural plasticity. 2012;2012:101542. doi: 10.1155/2012/101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in pharmacological sciences. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, Wollmer MA, Nitsch RM, Hock C, Morris CM, Hardy J, Moon RT. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS biology. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochimica et biophysica acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner RL, Jang MH, Liu XB, Duan X, Sailor KA, Kim JY, Ge S, Jones EG, Ming GL, Song H, Cheng HJ. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:14157–14162. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PloS one. 2010;5:e14382. doi: 10.1371/journal.pone.0014382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ftouh S, Akbar MT, Hirsch SR, de Belleroche JS. Down-regulation of Dickkopf 3, a regulator of the Wnt signalling pathway, in elderly schizophrenic subjects. Journal of neurochemistry. 2005;94:520–530. doi: 10.1111/j.1471-4159.2005.03239.x. [DOI] [PubMed] [Google Scholar]
- Fuster-Matanzo A, Llorens-Martin M, Sirerol-Piquer MS, Garcia-Verdugo JM, Avila J, Hernandez F. Dual effects of increased glycogen synthase kinase-3beta activity on adult neurogenesis. Human molecular genetics. 2013;22:1300–1315. doi: 10.1093/hmg/dds533. [DOI] [PubMed] [Google Scholar]
- Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, Yan Liu Q, Liu R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer's disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiology of disease. 2008;29:71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Arlotta P, Macklis JD, Chen J. Conditional knock-out of beta-catenin in postnatal-born dentate gyrus granule neurons results in dendritic malformation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:14317–14325. doi: 10.1523/JNEUROSCI.3206-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nature neuroscience. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. The Journal of physiology. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Molecular psychiatry. 2005;10:160–184. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- Gotti S, Caricati E, Panzica G. Alterations of brain circuits in Down syndrome murine models. Journal of chemical neuroanatomy. 2011;42:317–326. doi: 10.1016/j.jchemneu.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Groen W, Teluij M, Buitelaar J, Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:552–560. doi: 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. Journal of neurochemistry. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Maruta N, Higashi M, Kumar A, Kato N, Ikenaka K. Antidepressant drugs reverse the loss of adult neural stem cells following chronic stress. Journal of neuroscience research. 2007;85:3574–3585. doi: 10.1002/jnr.21455. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Lee SY, O'Kusky JR, Ye P. Signaling Through the Type 1 Insulin-Like Growth Factor Receptor (IGF1R) Interacts with Canonical Wnt Signaling to Promote Neural Proliferation in Developing Brain. ASN neuro. 2012 doi: 10.1042/AN20120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, Matthews PM. Association of GSK3beta polymorphisms with brain structural changes in major depressive disorder. Archives of general psychiatry. 2009;66:721–728. doi: 10.1001/archgenpsychiatry.2009.70. [DOI] [PubMed] [Google Scholar]
- Inta D, Lima-Ojeda JM, Lau T, Tang W, Dormann C, Sprengel R, Schloss P, Sartorius A, Meyer-Lindenberg A, Gass P. Electroconvulsive therapy induces neurogenesis in frontal rat brain areas. PloS one. 2013;8:e69869. doi: 10.1371/journal.pone.0069869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Bonaguidi MA, Kitabatake Y, Sun J, Song J, Kang E, Jun H, Zhong C, Su Y, Guo JU, Wang MX, Sailor KA, Kim JY, Gao Y, Christian KM, Ming GL, Song H. Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell stem cell. 2013;12:215–223. doi: 10.1016/j.stem.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MH, Kitabatake Y, Kang E, Jun H, Pletnikov MV, Christian KM, Hen R, Lucae S, Binder EB, Song H, Ming GI. Secreted frizzled-related protein 3 (sFRP3) regulates antidepressant responses in mice and humans. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.158. published online 4 December 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. BioEssays : news and reviews in molecular, cellular and developmental biology. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- Jun H, Mohammed Qasim Hussaini S, Rigby MJ, Jang MH. Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural plasticity. 2012;2012:854285. doi: 10.1155/2012/854285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biological psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, Tarabykin V, Lie DC, Jessberger S. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, Malafosse A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biological psychiatry. 2007;61:240–245. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. The American journal of psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Walters EE, MacLean C, Neale MC, Heath AC, Eaves LJ. Stressful life events, genetic liability, and onset of an episode of major depression in women. The American journal of psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kivimae S, Martin PM, Kapfhamer D, Ruan Y, Heberlein U, Rubenstein JL, Cheyette BN. Abnormal behavior in mice mutant for the Disc1 binding partner, Dixdc1. Translational psychiatry. 2011;1:e43. doi: 10.1038/tp.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3beta immunoreactivity in postmortem frontal cortex of schizophrenic patients. The American journal of psychiatry. 2000;157:831–833. doi: 10.1176/appi.ajp.157.5.831. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nature neuroscience. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvajo M, McKellar H, Arguello PA, Drew LJ, Moore H, MacDermott AB, Karayiorgou M, Gogos JA. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WM, Qiu G, Helmeste DM, Lee TM, Tang SW, So KF, Tang SW. Corticosteroid decreases subventricular zone cell proliferation, which could be reversed by paroxetine. Restorative neurology and neuroscience. 2007;25:17–23. [PubMed] [Google Scholar]
- Lesort M, Greendorfer A, Stockmeier C, Johnson GV, Jope RS. Glycogen synthase kinase-3beta, beta-catenin, and tau in postmortem bipolar brain. Journal of neural transmission. 1999;106:1217–1222. doi: 10.1007/s007020050235. [DOI] [PubMed] [Google Scholar]
- Li C, Shi X, Zhou G, Liu X, Wu S, Zhao J. The canonical Wnt-beta-catenin pathway in development and chemotherapy of osteosarcoma. Frontiers in bioscience. 2013;18:1384–1391. doi: 10.2741/4187. [DOI] [PubMed] [Google Scholar]
- Li J, Nguyen L, Gleason C, Lotspeich L, Spiker D, Risch N, Myers RM. Lack of evidence for an association between WNT2 and RELN polymorphisms and autism. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2004a;126B:51–57. doi: 10.1002/ajmg.b.20122. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, Hennah W, Peltonen L, Lonnqvist J, Huttunen MO, Kaprio J, Trachtenberg JT, Silva AJ, Cannon TD. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu M, Cai Z, Wang G. Regulation of glycogen synthase kinase-3 during bipolar mania treatment. Bipolar disorders. 2010;12:741–752. doi: 10.1111/j.1399-5618.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004b;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schutz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes & development. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F, Shan X, Cai X, Chen Q, Ling N, Zhang B, Bi Y, Chen K, Ren H, Huang A, He TC, Tang N. Enhancement of canonical Wnt/beta-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PloS one. 2011;6:e27496. doi: 10.1371/journal.pone.0027496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes & development. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ye P, O'Kusky JR, D'Ercole AJ. Type 1 insulin-like growth factor receptor signaling is essential for the development of the hippocampal formation and dentate gyrus. Journal of neuroscience research. 2009;87:2821–2832. doi: 10.1002/jnr.22129. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M, Fuster-Matanzo A, Teixeira CM, Jurado-Arjona J, Ulloa F, Defelipe J, Rabano A, Hernandez F, Soriano E, Avila J. GSK-3beta overexpression causes reversible alterations on postsynaptic densities and dendritic morphology of hippocampal granule neurons in vivo. Molecular psychiatry. 2013;18:451–460. doi: 10.1038/mp.2013.4. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Developmental cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biological psychiatry. 2008;64:880–883. doi: 10.1016/j.biopsych.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Newton SS, Eaton ME, Russell DS, Duman RS. Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biological psychiatry. 2003;54:1006–1014. doi: 10.1016/s0006-3223(03)00700-5. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biological psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17:1023–1027. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. Journal of psychiatry & neuroscience : JPN. 2010;35:337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, Biechele T, Petryshen TL, Moon RT, Haggarty SJ, Tsai LH. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, O'Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC. O2 regulates stem cells through Wnt/beta-catenin signalling. Nature cell biology. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy PA, Shao Y, Wolpert CM, Donnelly SL, Ashley-Koch A, Abel HL, Ravan SA, Abramson RK, Wright HH, DeLong GR, Cuccaro ML, Gilbert JR, Pericak-Vance MA. No association between the WNT2 gene and autistic disorder. American journal of medical genetics. 2002;114:106–109. doi: 10.1002/ajmg.10182. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, Wang H, Rao M, Altura RA, Kaspar BK. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging cell. 2012;11:542–552. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophrenia research. 1999;38:1–6. doi: 10.1016/s0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. European journal of pharmacology. 2008;583:115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ito M, Liu Y, Seki T, Suzuki T, Arai H. Effects of single and repeated electroconvulsive stimulation on hippocampal cell proliferation and spontaneous behaviors in the rat. Brain research. 2013;1491:88–97. doi: 10.1016/j.brainres.2012.10.052. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain research. 2010;1354:23–29. doi: 10.1016/j.brainres.2010.07.075. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, Bain EE, Charney DS, Drevets WC. Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biological psychiatry. 2005;57:935–937. doi: 10.1016/j.biopsych.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DH, Park YC, Kim SH. Increased glycogen synthase kinase-3beta mRNA level in the hippocampus of patients with major depression: a study using the stanley neuropathology consortium integrative database. Psychiatry investigation. 2010;7:202–207. doi: 10.4306/pi.2010.7.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Miyakawa T. Chronic treatment with fluoxetine for more than 6 weeks decreases neurogenesis in the subventricular zone of adult mice. Molecular brain. 2011;4:10. doi: 10.1186/1756-6606-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, Dileone RJ, Newton SS, Duman RS. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry. 2010;68:521–527. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. The Journal of comparative neurology. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Annals of the New York Academy of Sciences. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. The European journal of neuroscience. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pinnock SB, Blake AM, Platt NJ, Herbert J. The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PloS one. 2010;5:e13652. doi: 10.1371/journal.pone.0013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino D, Choe Y, Pleasure SJ. Wnt5a controls neurite development in olfactory bulb interneurons. ASN neuro. 2011;3:e00059. doi: 10.1042/AN20100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Molecular psychiatry. 2008;13:173–186. 115. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, Jope RS. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid beta-mediated synaptic loss. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q, Sun G, Li W, Yang S, Ye P, Zhao C, Yu RT, Gage FH, Evans RM, Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nature cell biology. 2010;12:31–40. doi: 10.1038/ncb2001. sup pp 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nature genetics. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Reeves WC, Strine TW, Pratt LA, Thompson W, Ahluwalia I, Dhingra SS, McKnight-Eily LR, Harrison L, D'Angelo DV, Williams L, Morrow B, Gould D, Safran MA. Mental illness surveillance among adults in the United States. MMWR Surveill Summ. 2011;60(Suppl 3):1–29. [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Molecular psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Molecular psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature neuroscience. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, Caloro M, Telesforo CL, Caltagirone SS, Panaccione I, Simonetti A, Demontis F, Girardi P. The role of memantine in the treatment of psychiatric disorders other than the dementias: a review of current preclinical and clinical evidence. CNS Drugs. 2012;26:663–690. doi: 10.2165/11634390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of general psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging & mental health. 2012;16:753–762. doi: 10.1080/13607863.2012.678478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. The Journal of neuropsychiatry and clinical neurosciences. 2000;12:103–113. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A. Loss of dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Zhang X, He X. DKK1 antagonizes Wnt signaling without promotion of LRP6 internalization and degradation. The Journal of biological chemistry. 2008;283:21427–21432. doi: 10.1074/jbc.M800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. The Journal of comparative neurology. 2004;473:496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Shook BA, Manz DH, Peters JJ, Kang S, Conover JC. Spatiotemporal changes to the subventricular zone stem cell pool through aging. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6947–6956. doi: 10.1523/JNEUROSCI.5987-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48. doi: 10.1016/j.neuron.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirerol-Piquer M, Gomez-Ramos P, Hernandez F, Perez M, Moran MA, Fuster-Matanzo A, Lucas JJ, Avila J, Garcia-Verdugo JM. GSK3beta overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus. 2011;21:910–922. doi: 10.1002/hipo.20805. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Soni A. Statistical Brief #248. Agency for Healthcare Research and Quality; Rockville, MD: 2009. Jul, The Five Most Costly Conditions, 1996 and 2006: Estimates for the U.S. Civilian Noninstitutionalized Population. [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Stagni F, Magistretti J, Guidi S, Ciani E, Mangano C, Calza L, Bartesaghi R. Pharmacotherapy with fluoxetine restores functional connectivity from the dentate gyrus to field CA3 in the Ts65Dn mouse model of down syndrome. PloS one. 2013;8:e61689. doi: 10.1371/journal.pone.0061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Archives of general psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Sun B, Halabisky B, Zhou Y, Palop JJ, Yu G, Mucke L, Gan L. Imbalance between GABAergic and Glutamatergic Transmission Impairs Adult Neurogenesis in an Animal Model of Alzheimer's Disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nature neuroscience. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ, Liou YJ, Hong CJ, Yu YW, Chen TJ. Glycogen synthase kinase-3beta gene is associated with antidepressant treatment response in Chinese major depressive disorder. The pharmacogenomics journal. 2008;8:384–390. doi: 10.1038/sj.tpj.6500486. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS medicine. 2010;7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Thompson PM, Kieseppa T, Bearden CE, Marino AC, Hoftman GD, Haukka J, Partonen T, Huttunen M, Kaprio J, Lonnqvist J, Poutanen VP, Toga AW, Cannon TD. Hippocampal morphology in lithium and non-lithium-treated bipolar I disorder patients, non-bipolar co-twins, and control twins. Human brain mapping. 2012;33:501–510. doi: 10.1002/hbm.21239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis D, Stuart GW, Wood SJ, Smith DJ, Brewer WJ, Desmond P, Singh B, Copolov D, Pantelis C. Selective bilateral hippocampal volume loss in chronic schizophrenia. Biological psychiatry. 2001;50:531–539. doi: 10.1016/s0006-3223(01)01121-0. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xu L, Zhu X, Cui W, Sun Y, Nishijo H, Peng Y, Li R. Demethylation of specific Wnt/beta-catenin pathway genes and its upregulation in rat brain induced by prenatal valproate exposure. Anatomical record. 2010;293:1947–1953. doi: 10.1002/ar.21232. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Huang J, Swiderski RE, Pietila J, Braun T, Beck G, Folstein SE, Haines JL, Sheffield VC. Evidence supporting WNT2 as an autism susceptibility gene. American journal of medical genetics. 2001;105:406–413. doi: 10.1002/ajmg.1401. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Molecular psychiatry. 2008;13:285–292. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- Yu JM, Kim JH, Song GS, Jung JS. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Molecular and cellular biochemistry. 2006;288:17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- Yun J, Koike H, Ibi D, Toth E, Mizoguchi H, Nitta A, Yoneyama M, Ogita K, Yoneda Y, Nabeshima T, Nagai T, Yamada K. Chronic restraint stress impairs neurogenesis and hippocampus-dependent fear memory in mice: possible involvement of a brain-specific transcription factor Npas4. Journal of neurochemistry. 2010;114:1840–1851. doi: 10.1111/j.1471-4159.2010.06893.x. [DOI] [PubMed] [Google Scholar]
- Zaidel DW, Esiri MM, Harrison PJ. Size, shape, and orientation of neurons in the left and right hippocampus: investigation of normal asymmetries and alterations in schizophrenia. The American journal of psychiatry. 1997;154:812–818. doi: 10.1176/ajp.154.6.812. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhang J, Han L, Pu P, Kang C. Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharmacol. 2012a;7:740–749. doi: 10.1007/s11481-012-9359-y. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sun Y, Wang F, Wang Z, Peng Y, Li R. Downregulating the canonical Wnt/beta-catenin signaling pathway attenuates the susceptibility to autism-like phenotypes by decreasing oxidative stress. Neurochemical research. 2012b;37:1409–1419. doi: 10.1007/s11064-012-0724-2. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]