Abstract
The goal of this study was to understand the roles of maternal history of childhood sexual abuse (CSA) and current family functioning on the cortisol awakening response (CAR) in pregnancy. Participants were 185 pregnant women (ages 18–40) who completed items from the Adverse Childhood Experiences scale to measure child maltreatment history and the Family Assessment Device to measure current family functioning. Participants provided saliva samples at wake-up and 30 minutes after wake-up at 25, 29, and 35 weeks gestation to measure CAR. A moderation effect was found such that participants with more severe CSA histories and poorer perceived family functioning had increasing CAR in pregnancy compared to participants with less severe CSA histories and better family functioning. These findings highlight the importance of considering stress in both childhood and current environments in predicting maternal cortisol in pregnancy.
Keywords: Childhood sexual abuse, family functioning, cortisol awakening response, pregnancy
Introduction
Maternal cortisol production during pregnancy is essential to healthy fetal development. Cortisol levels that are unusually elevated or attenuated may lead to poor neonatal outcomes (Rich-Edwards & Grizzard, 2005; P. D. Wadhwa, Entringer, Buss, & Lu, 2011). Over pregnancy baseline cortisol output increases reaching 2–3 times levels observed at the start of pregnancy. Cortisol awakening response and cortisol stress response becomes blunted as pregnancy progresses and circadian rhythms are maintained (Sandman, Davis, Buss, & Glynn, 2011). Diminished neuroendocrine reactivity in late pregnancy is believed to protect the fetus from over-exposure to maternal glucocorticoids (de Kloet, Rosenfeld, van Eekelen, Suntanto, & Levine, 1988). Higher absolute cortisol levels, more pronounced cortisol increases over gestation, and a lack of dampening of cortisol awakening response over pregnancy are associated with adverse neonatal outcomes (Buss et al., 2009; Entringer, Buss, Andersen, Chicz-DeMet, & Wadhwa, 2011; Giurgescu, 2009; P. Wadhwa et al., 2004). Close to term a sharp increase in maternal salivary cortisol and CRH and a decrease in CRH-binding protein (which regulates availability of biologically active CRH) and 11β-HSD2 is observed (which changes cortisol into inactive cortisone) (McLean et al., 1995; Sandman, et al., 2011). These changes in HPA activity play an essential role in favorable pregnancy outcomes. Therefore, it is important to understand factors that influence maternal cortisol regulation in pregnancy because these factors may be targets for interventions aimed at reducing risk for adverse birth outcomes. In this study, we investigated the moderating influence of current family functioning in pregnancy on the association between maternal history of childhood sexual abuse and cortisol in pregnancy. We investigated both total cortisol production and changes in maternal cortisol over late second and third trimesters because both indices of maternal HPA regulation have been associated with poor neonatal outcomes in past research (Buss, et al., 2009; Giurgescu, 2009).
Our group recently reported that pregnant women with childhood sexual abuse (CSA) histories displayed increasing cortisol awakening responses over the course of gestation compared to women with non-sexual child abuse or no abuse histories (Bublitz & Stroud, 2012). The current interpersonal environment has also been shown to influence HPA functioning and may be particularly salient to maternal cortisol levels during pregnancy given increased reliance on family relationships as women prepare for motherhood (Goldenberg & Culhane, 2005). Some evidence shows that, in both non-pregnant (Adam & Gunnar, 2001; Raikkonen et al., 2011) and pregnant samples (A. Nierop, Wirtz, Bratsikas, Zimmerman, & Ehlert, 2008; P. D. Wadhwa, Dunkel Schetter, Chicz-DeMet, Porto, & Sandman, 1996), positive interpersonal relationships are associated with typical diurnal cortisol patterns (i.e., lower overall cortisol levels, diurnal rhythms displaying a steep morning rise followed by a steady decline across the day).
Experiences of CSA are associated with greater interpersonal challenges in adulthood (Lalor & McElvaney, 2010; Parks, Kim, Day, Garza, & Larkby, 2011) including deficits in interpersonal functioning and insecure attachments in adult relationships (Main & Goldwyn, 1994). In childhood, experiences of CSA are associated with poorer emotion regulation, less pro-social behavior, greater relational aggression, greater emotional withdrawal, and greater peer rejection (Alink, Cicchetti, Kim, & Rogosch, 2012; Kim & Cicchetti, 2010). There is also evidence demonstrating that positive interpersonal relationships foster resilience from negative social and emotional outcomes among victims of child maltreatment; victims of CSA who experience greater interpersonal support are less likely to develop post-traumatic stress disorder (PTSD) symptoms (Hyman, Gold, & Cott, 2003) and are less likely to experience high psychological distress as adults (Hill, Kaplan, French, & Johnson, 2010). Victims may also be buffered from elevated cortisol if their interpersonal environment is supportive; Alink et al (Alink, et al., 2012) found that greater pro-social behaviors and lower aggression in maltreated children (assessed using information from peers and summer camp counselors) were associated with lower morning cortisol levels one year later. Thus, the interaction between CSA and the current family environment may yield important insight into maternal cortisol regulation in pregnancy. However, to our knowledge this has not been studied.
In the current study, we investigated the moderating influence of current family functioning on the association between maternal history of CSA and cortisol awakening response (CAR) in pregnancy. We focused specifically on the CAR because previous evidence shows that that a lack of dampening in CAR over pregnancy is associated with adverse neonatal outcomes (Buss, et al., 2009; Entringer et al., 2010). We examine family functioning (conceptualized as the ability of the family system to meet the needs of its members through developmental transitions (Johnson & Maas, 1998)) due to its increased salience during an important transitional period: pregnancy. We hypothesized that participants with a CSA history and poorer perceived family functioning during pregnancy would display greater CAR and increasing CAR trajectories, whereas participants with CSA histories who perceived their family environments as more supportive would be buffered from elevated CAR. Finally, we examined associations between CAR and neonatal outcomes including gestational age at birth, birth weight, and APGAR score at 5 minutes after birth.
Methods
Participants
Maternal and infant characteristics are presented in Table 1. Participants were 185 women with singleton pregnancies who were part of a larger study of the effects of maternal mood on fetal and infant development (Behavior and Mood in Mothers, Behavior in Infants (BAMBI)). Women in the current study were 26.46 years old (SD=5.49), from diverse racial/ethnic backgrounds (43% Non-Hispanic White, 16% Black, 29% Hispanic, 4% Asian, 4% ‘>1 race’, 4% ‘other’), 36% were married, and 36% of pregnancies were planned. Women were excluded from participating for medical conditions in pregnancy (e.g., gestational diabetes, preeclampsia, hyper/hypothyroidism, hypertension) or if they were taking medications that could influence endocrine functioning (e.g., insulin, psychotropic medications, steroids). Finally, women were excluded from participating if they were < 18 or > 40 years old or reported an average of >1 alcoholic drink per week of pregnancy. Seventy-three percent of participants in this study were included in our previous study on the main effect of maternal history of CSA on cortisol awakening responses during late pregnancy (Bublitz & Stroud, 2012). This study was approved by the Women & Infants Hospital and Lifespan Hospital Institutional Review Boards.
Table 1.
Maternal and infant characteristics
| Mean/ % | SD | ||
|---|---|---|---|
| Maternal Characteristics | |||
| Maternal age (years) | 26.46 | 5.52 | |
| Race (% Non-Hispanic White) | 44% | ||
| Marital Status (% married) | 36% | ||
| Yearly Income (% < $30K) | 47% | ||
| Pre-Pregnancy BMI | 26 | 6 | |
| Gravida | 2 | 2 | |
| Planned pregnancy (% yes) | 36% | ||
| Cigarette Smoking (% yes) | 8% | ||
| Depressive symptoms (IDS) | 12 | 9 | |
| Anxiety symptoms (HAM-A) | 6 | 5 | |
| Child Maltreatment Scores | % Any Abuse | ||
| Childhood Sexual Abuse Score | 5 | 3 | 24% |
| Childhood Physical Abuse Score | 7 | 4 | 47% |
| Childhood Neglect Score | 5 | 3 | 27% |
| Witnessed domestic violence Score | 5 | 4 | 30% |
| Adverse Childhood Events Score | 3 | 2 | |
| Infant Characteristics | |||
| Gestational Age at Birth (weeks) | 39.63 | 1.21 | |
| Birth weight (grams) | 3389 | 451 | |
| APGAR score at 5 minutes | 9 | 1 | |
Procedure
Pregnant women completed 1–3 study sessions during second and third trimesters of pregnancy (Session 1: 25 weeks GA (SD=4); Session 2: 29 weeks GA (SD=1); Session 3: 35 weeks GA (SD=1). 100% of participants completed at least 1 study session, 81% completed at least 2 study sessions, and 72% completed all three study sessions. At baseline, participants provided information on history of childhood maltreatment, current family functioning, health behaviors, depression and anxiety symptoms, as well as basic demographic and pregnancy health information. For three days following each session, participants provided saliva samples (passive drool) at wake-up, 30 minutes after wake-up, and bedtime. After participants completed the 3 days of saliva collection, study staff retrieved samples from participants’ homes and provided payment. Samples were stored at room temperature until collection by study staff. Time between saliva collection and retrieval was typically 1–3 days. Participants were instructed to store samples in refrigerators if retrieval was delayed > 3 days.
Maternal medical history was assessed by self-report and confirmed by chart review following delivery. Neonatal outcomes, including gestational age at birth, birth weight, and APGAR scores, were collected from medical chart review following delivery.
Materials
Childhood Maltreatment
Participants completed a self-report measure of childhood abuse history that included 15 items from the Adverse Childhood Experiences (ACE) scale (Dube et al., 2003). In particular, participants were asked how often, before the age of 18, they experienced childhood sexual abuse (e.g. “How often did an adult person at least 5 years older than you touch or fondle your body in a sexual way?”), physical abuse (e.g., “How often did a parent, stepparent or adult living in your home push, grab, slap, or throw something at you?”), neglect (e.g., “How often did you not have enough to eat?”), or witnessed domestic violence (e.g., “How often did your father, stepfather, or mother’s boyfriend push, grab, slap, or throw something at your mother?”). Response options ranged from 1 (never) to 5 (very often). Continuous scores of childhood sexual abuse, physical abuse, neglect, and witnessing domestic violence were computed by summing participants’ responses on these items. We also calculated at total adverse events score by tallying the number of abuse experiences women reported in childhood. To examine the effects of maternal CSA history on cortisol relative to other types of childhood maltreatment experiences, continuous scores on all maltreatment scales, and the total adverse events score, were entered simultaneously into models. Scores ranged from 1–20 for CSA, 3–20 for physical abuse, 1–20 for witnessing domestic violence, 1–12 for neglect in childhood, and 0–7 for number of adverse childhood experiences.
Family Functioning
To measure perceptions of family functioning in pregnancy, participants completed the Family Assessment Device (FAD) at the baseline session (~25 weeks GA) (Epstein, Baldwin, & Bishop, 1983). The FAD is a self-report measure of the family’s ability to solve problems, communicate effectively, fulfill appropriate roles, and regulate emotions and behaviors during the past two months. The measure consists of 53 items; 20 items are reverse-scored. Examples of items include, “You can’t tell how a person is feeling from what they are saying,” “If someone is in trouble, the others become too involved,” “Making decisions is a problem in our family,” and, “We confront problems involving feelings [reversed-scored].” Response options ranged from 1 (Strongly Agree) to 4 (Strongly Disagree), with higher scores indicating more problematic family functioning. The FAD includes a General Functioning Scale as well as six subscales (problem solving, communication, roles, affective responsiveness, affective involvement, and behavior control). The General Functioning Subscale is a sum of 12 items from subscales and assesses the overall health/pathology of the family. Items include, “In times of crisis we can turn to each other for support [reversed-scored],” and, “We don’t get along well together.” Scores for general functioning ranged from 12–41, 6–21 for problem solving, 7–32 for communication, 11–38 for roles, 6–21 for affective responsiveness, 6–23 for affective involvement, and 7–27 for behavior control. Correlations among subscales ranged from r =.43–.70. Both the general functioning scale and individual subscales were analyzed in this study.
Salivary cortisol
Maternal saliva samples were returned to the lab by study staff, aliquoted, and stored at −80°C until analysis. Samples were shipped to the laboratory of Clemens Kirschbaum, Ph.D. (Dresden University). Cortisol concentrations were analyzed with an immunoassay with time-resolved fluorescence detection. The intra and inter-assay coefficients of variation were < 8%. A subset of the sample (17%) were given Medication Event Monitoring System (MEMS) caps (AARDEX, Zurich, Switzerland) that time stamp every opening of bottles containing tubes used to collect maternal saliva. Caps were distributed to participants as they became available. For participants with both self-reported and MEMS-recorded saliva sampling times there was an average time discrepancy of 5 minutes (SD=5) for cortisol samples at awakening and 5 minutes (SD=4) for cortisol samples collected 30 minutes after awakening. These data suggest that, in a subset of participants, participants were adherent to the sampling protocol.
Maternal Characteristics
As part of the larger study, participants provided information on age, race, relationship status, and socioeconomic characteristics. They also reported pre-pregnancy height and weight to compute body mass index (BMI). At each study session participants provided information on medical conditions or medications that could affect endocrine functioning, including gestational diabetes, preeclampsia, hyper/ hypothyroidism, insulin, steroid, and antidepressant use. This information was confirmed by medical chart review following delivery. Participants were interviewed about their current symptoms of depression (assessed using the Inventory for Depressive Symptomatology (Rush et al., 1986)) and anxiety (assessed using the Hamilton Anxiety Rating Scale (Hamilton, 1959)) at each study session. Finally, at each study session, participants were asked to report cigarette or alcohol use over the course of pregnancy using the Timeline Follow-Back Interview (Sobell & Sobell, 1995). These maternal characteristics were considered as covariates in analyses.
Data Analysis
Descriptive statistics were conducted using SPSS v.20 software. We examined associations among maternal childhood abuse scores, general family functioning scores, and average CAR in pregnancy using Pearson correlations. We also examined maternal childhood abuse and family functioning as predictors of change in CAR from 25–35 weeks gestation, using hierarchical linear modeling (HLM) (Raudenbush & Bryk, 2002). Bonferroni corrections were applied to correct for multiple comparisons in bivariate associations. Salivary cortisol values were log-transformed to adjust for skewed distributions (skewness ranged from 0.48–12.88 prior to log-transforming and values ranged from 0.15–1.59 after log-transforming). Morning saliva samples that were < 20 or > 40 minutes apart were omitted from analyses in order to accurately capture the morning awakening response. We calculated the cortisol awakening response (CAR) on each day of saliva collection by taking the difference between log transformed values and averaging CAR at each gestational time period and averaging across the three sampling days. This resulted in a total of 499 CAR measures out of a possible 555 CAR measures (i.e., 3 CAR values per participant, 185 participants). Ten CAR values were missing or excluded due to noncompliance at session 1 (5%), 32 values at session 2 (17%), and 14 values at session 3 (8%).
Hierarchical models were specified as follows; at level-1 we modeled within-person change in CAR from 25 to 35 weeks gestation. Gestational age at each session and average time of awakening over the three days were considered time-varying covariates in level-1 models. At level-2 we modeled the interaction of continuous child maltreatment scores and current family functioning scores (calculated by multiplying child maltreatment scores by family functioning scores). The following equations summarize the model:
Level-1: cortisol awakening responseij = b0j + b1i(wake-up time) + b2 i(gestational age) + rij.
Level-2:b0j = b00 + b01 (childhood sexual abuse score × Family Functioning) + b02 (childhood physical abuse score × Family Functioning) + b03 (childhood neglect score × Family Functioning) + b04 (childhood domestic violence score × Family Functioning) + (number of adverse childhood experiences × Family Functioning) + µ0i b2j = b20 + b21 (childhood sexual abuse score × Family Functioning) + b22 (childhood physical abuse score × Family Functioning) + b23 (childhood neglect score × Family Functioning) + b24 (childhood domestic violence score × Family Functioning) + b25 + b25 (number of adverse childhood experiences × Family Functioning) + µ2i
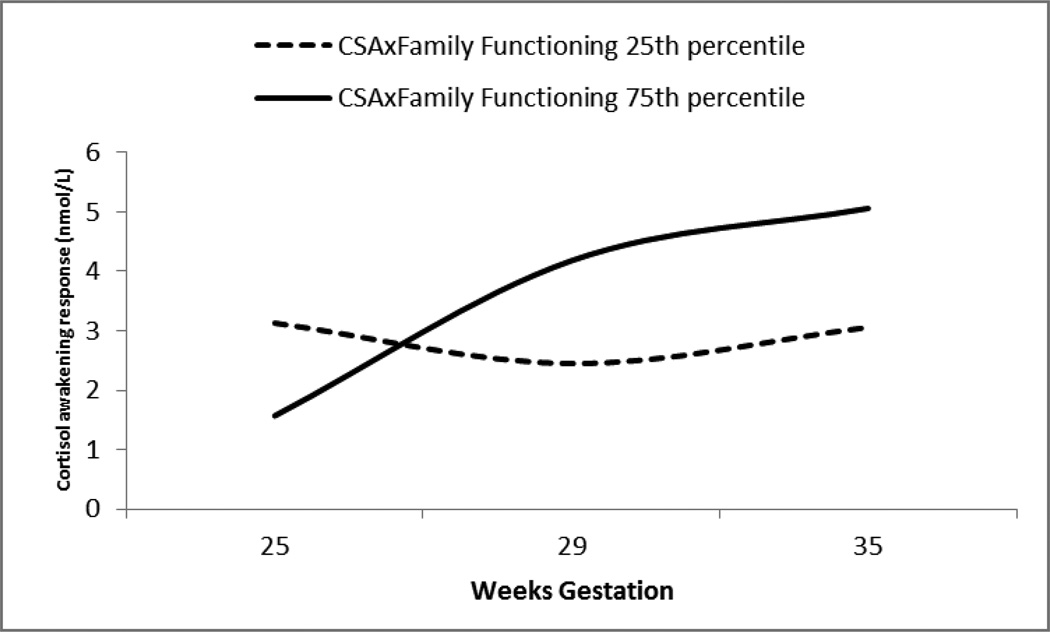
Equations indicate that cortisol awakening response (cortisol awakening responseij) is a function of participants’ intercept (b0j), wake-up time (b1i), the gestational age at which the awakening response was collected (b2 i) and error (rij). At level 2, b2jrepresents the person’s intercept (i.e. the expected value of cortisol awakening response over gestation) as a function of intercepts across all participants (b20), the participants’ scores on child maltreatment X family functioning interaction terms, and error (µ 2i). For illustrative purposes, significant results were graphed at two arbitrary points at the 25thand 75th percentile of continuous CSAxFAD score (Figure 1). CAR was converted back to raw nmol/L in the figure.
Figure 1.
Family functioning moderates the association between maternal childhood sexual abuse and change in cortisol awakening response from 25–35 weeks gestation.
Note. CSA= Childhood sexual abuse. FAD = Family Assessment Device. For illustrative purposes, significant results were graphed at two arbitrary points at the 25th and 75th percentile of continuous CSAxFAD interaction terms.
When models indicated that change in CAR varied by the interaction between maternal childhood maltreatment history and family functioning, we performed post-hoc analyses to examine whether associations were significant at 25, 29 or 35 weeks gestation separately using Pearson correlations. Bonferroni corrections were applied to correct for multiple comparisons when appropriate. Results from post-hoc analyses allowed us to determine whether associations were particularly salient at a specific gestation age.
Results
Preliminary Analyses
To test for significant covariates we examined whether maternal characteristics significantly predicted change in CAR from 25–35 weeks gestation. Results revealed that no maternal characteristics were significant predictors of CAR trajectories from 25– 35 weeks gestation: maternal age (b=.005, SE=.01, p=.65), race (b=−.04, SE=.05, p=.47), income (b=.04, SE=.03, p=.26), marital status (b=−.02, SE=.04, p=.62), pre-pregnancy BMI (b=.002, SE=.01, p=.85), gravida (b=−.001, SE=.07, p=.93), cigarette smoking (b=.004, SE=.009, p=.68), planned nature of the pregnancy (b=.08, SE=.16, p=.63), depressive symptoms (b=−.006, SE=.007, p=.40), or anxiety symptoms (b=−.001, SE=.01, p=.93). Thus, no maternal characteristics were included as covariates in subsequent analyses.
Associations among Childhood Sexual Abuse and Family Functioning Scores
Bivariate correlations between CSA score and family functioning scores were examined. We found that greater experiences of CSA were associated with greater difficulties in a number of domains of family functioning, including family problem solving (r=.17, p=.02), family roles (r=.16, p=.03), and problems with behavioral control (r=.19, p=.01). Greater experiences of CSA was also marginally associated with greater difficulties in overall family functioning (r=.13, p=.08), poorer communication (r=.13, p=.09), and difficulties with affective involvement (r=.14, p=.07). However, associations dropped below significance after correcting for multiple comparisons. CSA was not significantly associated with family affective responsiveness (r=.10, p=.17).
The moderating influence of family functioning on the association between childhood maltreatment and Cortisol Awakening Response
Associations among average CAR in pregnancy and interaction terms were not significant (r values <.14, p values >.13). However, as illustrated in Model 1 in Table 2, current general family functioning significantly moderated the effect of CSA severity on change in CAR across gestation (b=.001, SE=.001, p=.001), such that, among participants with a more severe CSA history, those who reported poorer perceived family functioning displayed increasing CAR compared to participants with less severe CSA histories and better perceived family functioning. Results are illustrated in Figure 1. Family functioning did not significantly moderate the effects of other child maltreatment experiences on CAR (p’s > .10). Post-hoc analyses revealed a significant association among interaction terms and CAR at 35 weeks (r=.22, p<.05) but not at 25 or 29 weeks gestation (r’s<.07, p’s>.10). CSAxFAD score accounted for 3% of the variance in change in CAR change from 25–35 weeks gestation.
Table 2.
|
Model 1: Child Maltreatment × Family Functioning |
Model 2: Childhood Sexual Abuse × Family Functioning Subscales |
|
|---|---|---|
| Fixed Effects | Coeff (SE) | Coeff (SE) |
| Intercept | 13.08 (9.53) | 14.1(21.08)** |
| Childhood sexual abuse × Family Functioning | .002 (.001)** | |
| Child Physical Abuse × Family Functioning | .002 (.001) | |
| Child Domestic Violence × Family Functioning | −.001 (.001) | |
| Child Neglect × Family Functioning | −.001 (.001) | |
| Adverse Events Score × Family Functioning | .03 (.03) | |
| CSA × Problem Solving | −.01(.01) | |
| CSA × Communication | .01(.01) | |
| CSA × Roles | .00(.01) | |
| CSA × Affective Responsiveness | .02(.02) | |
| CSA × Affective Involvement | −.01(.01) | |
| CSA × Behavioral Control | −.004(.03) | |
p <.001
We then examined the associations among the interaction between maternal CSA scores and specific domains of family functioning and average maternal CAR in pregnancy. The associations among average CAR in pregnancy and CSAxProblem Solving (r=.17, p=.05) and CSAxBehavior Control (r=.17, p=.05) were significant, however after correcting for multiple comparisons associations dropped below significance. All other correlations were non-significant (r’s<.16, p’s>.08). As illustrated in Model 2 in Table 2, there were no significant interactions among CSA history and domains of family functioning (p’s > .12).
Associations among maternal cortisol awakening response and neonatal outcomes
We examined bivariate associations between CAR at each gestational age and neonatal outcomes using Pearson correlations. None of the associations were significant (r values <.14, p values > .10). We also examined associations among neonatal outcomes and change in CAR over pregnancy using HLM. Change in maternal CAR was not associated with gestational age at birth (B=−.004, SE=.04, p=.91), birth weight (B<.001, SE<.001, p=.93), or APGAR score (B=.02, SE=.15, p=.90).
Discussion
Childhood maltreatment may have long-term effects on HPA axis regulation, and these effects appear to extend into pregnancy. While stressful experiences in childhood may program HPA regulation, demands of the current environment may influence the level of cortisol output in adulthood. The goal of this study was to examine the moderating influence of current family functioning in pregnancy on the association between maternal CSA history and cortisol awakening responses in late pregnancy.
Results indicate that family functioning moderated the association between CSA and change in CAR over pregnancy, such that participants with more severe CSA histories and who reported poorer perceived family functioning in pregnancy displayed increasing CAR from 25 to 35 weeks gestation. Family functioning did not moderate the association between other forms of childhood maltreatment, or the number of child maltreatment experiences, and CAR suggesting that CSA history may be uniquely dysregulating to HPA activity in pregnancy. This finding complements findings from other studies which have also found that victims of childhood maltreatment who report better interpersonal support in adulthood are buffered from the negative social and emotional outcomes often associated with childhood maltreatment (Hill, et al., 2010; Hyman, et al., 2003). Furthermore, past studies have found that victims of childhood maltreatment demonstrate increased cortisol output specifically within the context of current life challenges, including current psychopathology and deficits in interpersonal competence (Alink, et al., 2012; Cook, Chaplin, Sinha, Tebes, & Mayes, 2012; Heim et al., 2002; Kim & Cicchetti, 2010; Newport, Heim, Bonsall, Miller, & Nemeroff, 2004).
These findings are also consistent with previous evidence showing that hostile or negative behaviors during marital conflict predicted greater increases in endocrine parameters (Kiecolt-Glaser et al., 1997; Malarkey, Kiecolt-Glaser, Pearl, & Glaser, 1994). Difficulties with problem solving may be particularly stressful to pregnant women, and in turn particularly dysregulating to HPA axis activity, as the salience of the need to resolve problems effectively increases as families prepare to integrate a new baby into the family (Cowan & Cowan, 2000). Indeed, lower perceived partner support in pregnancy predicts greater maternal (and infant) emotional distress (Tieman, van der Ende, & Verhulst, 2005), and declines in romantic relationship quality in the prenatal and postpartum period are associated with increases in women’s anxiety symptoms (Smith, Draper, Manktelow, Dorling, & Field, 2007). Given that the majority of pregnancies in this sample were unplanned, increasing cortisol over pregnancy in women with poorer perceived family functioning may be reflective of increasing instability in the relationship with the father of the baby. Consistent with this possibility, an unplanned pregnancy is associated with sharper declines in husbands’ marital satisfaction across the transition to parenthood (McDonald, Han, Mulla, Beyene, & Group, 2010). Future research is needed that measures family functioning at several stages of pregnancy in order to determine if cortisol changes parallel changes in perceived family functioning.
Results of this study indicate that family functioning in pregnancy moderates the association between CSA and change in cortisol over pregnancy specifically, but not absolute CAR output. Maternal HPA activity is dynamic over pregnancy; placental CRH increases as pregnancy progresses, leading to increases in maternal cortisol production over gestation. It is possible that increases in circulating CRH results in increased sensitivity of the HPA axis to maternal distress (i.e., poor family functioning) as pregnancy progresses. Future research is needed that examines change in placental CRH over pregnancy among victims of CSA.
Patterns of cortisol observed in women with more severe CSA histories and poorer family functioning have important implications for understanding adverse maternal and neonatal health outcomes. Specifically, past research has found that a lack of dampening in the CAR over pregnancy is associated with shorter gestational length (Buss, et al., 2009). There is an inverse association between gestational length and offspring morbidity; shorter gestational length places offspring at increased risk for life-long health problems including respiratory and cardiovascular disorders (Saigal & Doyle, 2008). Women at risk for pregnancy complications and adverse neonatal outcomes were excluded from the current sample at the time of recruitment, thus it was not surprising that we did not observe an association between CAR and birth outcomes in this low-risk sample. Among a more representative sample, including high-risk pregnancies, women with CSA histories and poor family environments may be particularly susceptible to adverse neonatal outcomes, potentially due to a lack of dampening in their CAR over gestation.
Results from this study also have implications for maternal health in pregnancy and postpartum. Past studies suggest that elevated cortisol in pregnancy may place women at increased risk for health problems during pregnancy including pre-eclampsia, gestational diabetes, and increased weight gain (Damjanovic et al., 2009; Keller-Wood et al., 2014; Redman & Sacks, 1999). Women may also be at elevated risk for disorders in the postpartum period such as thyroiditis and rheumatoid arthritis (Elenkov et al., 2001; Mastorakos & Ilias, 2000) and postpartum mood disorders (Mastorakos & Ilias, 2000; A Nierop, Bratsikas, Zimmerman, & Ehlert, 2006). Thus interventions aimed at improving family functioning in order to lower cortisol may have beneficial effects on both maternal and offspring health.
This study was limited by the small number of samples used to estimate the CAR (Clow, Hucklebridge, & Thorn, 2010) and the one-time administration of the family assessment device (FAD). In addition, the FAD is a measure of perceived family functioning. It is possible that participants with CSA histories have poorer perceptions of interpersonal relationships that may not accurately reflect the availability and support of their social networks. However, subjective interpersonal experiences may be as important for cortisol regulation as objective measures of support (Endrighi, Hamer, & Steptoe, 2011). In addition, we did not find significant associations among CSA scores and family functioning scores, indicating that women with more severe CSA histories did not have poorer perceptions of their current family environment than women with less severe CSA histories. Another limitation of this study is that women were asked to retrospectively recall childhood experiences of maltreatment. We were not able to verify the accuracy of these reports; women may have under- or over-reported their experiences of child maltreatment. Finally, women included in this study were drawn from a highly selected sample that was at low-risk for adverse maternal and neonatal complications. The null associations among maternal cortisol and neonatal outcomes may have been due to a lack of variability in birth outcomes among women in this sample. Women were also excluded from participating for a variety of medical conditions and medications that could impact maternal cortisol output. Therefore, results from this study may not generalize to high-risk pregnancies and may underestimate the effects of CSA and family functioning on maternal cortisol during pregnancy, given that women with chronic health conditions in pregnancy or medication use may exhibit altered cortisol profiles during gestation (Damjanovic, et al., 2009; McKenna, Wittber, Nagaraja, & Samuels, 2000; Salustiano, De Pinho, Provost, Ruano, & Zugaib, 2013; Shea et al., 2007).
Future studies are needed that examine the moderating influence of family functioning on the association between CSA history and maternal cortisol in a sample of pregnant women at high-risk for adverse neonatal outcomes. Studies are also needed that prospectively assess cortisol regulation in child maltreatment victims across the lifespan and throughout pregnancy in order to better understand the effects of maltreatment on cortisol regulation as the HPA axis undergoes changes associated with gestation. Such prospective studies would allow for the examination of effects of maltreatment timing and duration on maternal cortisol regulation over pregnancy. Previous research suggests that early life stress that occurs in early childhood may have the most detrimental impact on development (Doom & Gunnar, 2013).
Taken together, findings from this study suggest that maternal history of CSA has long-term consequences for HPA axis regulation in pregnancy, and elevated maternal cortisol levels associated with CSA are particularly pronounced when experiencing challenges in the prenatal family environment. A highly functioning family context may buffer victims of CSA from elevated cortisol production and may serve as a protective factor regarding adverse neonatal outcomes that are elevated in CSA victims. These findings highlight the importance of considering stress in both childhood and current environments in predicting maternal cortisol in pregnancy. While women with a history of CSA may be at heightened risk for elevated cortisol levels in pregnancy, this risk may be potentiated or mitigated by the current family environment. Thus, interventions targeted at improving family functioning may be particularly helpful for pregnant women with CSA histories and may have a positive impact on both maternal and neonatal health.
Highlights.
Pregnant women with history of child sexual abuse and poor family functioning had increasing cortisol awakening response.
Women with a history of sexual abuse may be protected from elevated cortisol in a supportive current family environment.
Considering stress in both childhood and current environments is important in predicting cortisol in pregnancy.
Acknowledgements
Preparation of this manuscript was supported by the National Institutes of Health (R01 MH079153) to L.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflict of interest to declare.
References
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26(2):189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Alink LR, Cicchetti D, Kim J, Rogosch FA. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Developmental Psychology. 2012;48(1):224–236. doi: 10.1037/a0024892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublitz MH, Stroud LR. Childhood sexual abuse predicts diurnal cortisol in pregnancy: preliminary findings. Psychoneuroendocrinology. 2012;37(9):1425–1430. doi: 10.1016/j.psyneuen.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Reyes JF, Chicz-DeMet A, Sandman CA, Waffarn F, Wadhwa PD. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009;201(4):398, e391–e398. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Thorn L. The cortisol awakening response in context. International Review of Neurobiology. 2010;93:153–175. doi: 10.1016/S0074-7742(10)93007-9. [DOI] [PubMed] [Google Scholar]
- Cook EC, Chaplin TM, Sinha R, Tebes JK, Mayes L. The stress response and adolescents' adjustment: The impact of child maltreatment. Journal of Youth and Adolescence. 2012;41:1067–1077. doi: 10.1007/s10964-012-9746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CP, Cowan PA. When partners become parents: The big life change for couples. Mahwah, NJ: Lawrence Erlbaum Associations; 2000. [Google Scholar]
- Damjanovic SS, Stojic RV, Lalic NM, Jotic AZ, Macut DP, Ognjanovic SI, Popovic BM. Relationship between basal metabolic rate and cortisol secretion throughout pregnancy. Endocrine. 2009;35(2):262–268. doi: 10.1007/s12020-008-9137-z. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Rosenfeld P, van Eekelen JA, Suntanto W, Levine S. Stress, glucocorticoids, and development. Prog Brain Res. 1988;73:101–120. doi: 10.1016/S0079-6123(08)60500-2. [DOI] [PubMed] [Google Scholar]
- Doom J, Gunnar MR. Stress physiology and developmental psychopathyology: past, present, and future. Development and Psychopathology. 2013;25(4 Pt 2):1359–1373. doi: 10.1017/S0954579413000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Elenkov I, Wilder R, Bakalow V, Link A, Dimitrov M, Fisher S, Chrousos G. IL-12, TNF-a, and hormonal changes during late pregnancy and early postpartum: Implications for autoimmune disease activity during these times. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4933–4938. doi: 10.1210/jcem.86.10.7905. [DOI] [PubMed] [Google Scholar]
- Endrighi R, Hamer M, Steptoe A. Associations of trait optimism with diurnal neuroendocrine activity, cortisol responses to metnal stress, and subjective stress measures in healthy men and women. Psychosomatic Medicine. 2011;73(8):672–278. doi: 10.1097/PSY.0b013e31822f9cd7. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa PD. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med. 2011;73:469–474. doi: 10.1097/PSY.0b013e31821fbf9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, Wadhwa PD. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13(3):258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein NB, Baldwin LM, Bishop DS. The McMaster Family Assessment Device. Journal of Marital and Family Therapy. 1983;9:171–180. [Google Scholar]
- Giurgescu C. Are maternal cortisol levels related to preterm birth? J Obstet Gynecol Neonatal Nurs. 2009;38(4):377–390. doi: 10.1111/j.1552-6909.2009.01034.x. doi: JOGN1034 [pii] 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF. Prepregnancy health status and the risk of preterm delivery. Arch Pediatr Adolesc Med. 2005;159:89–90. doi: 10.1001/archpedi.159.1.89. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: A multiple regression analysis. Depression and Anxiety. 2002;15(3):117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Hill TD, Kaplan LM, French MT, Johnson RJ. Victimization in early life and mental health in adulthood. Journal of Health and Social Behavior. 2010;51(1):48–63. doi: 10.1177/0022146509361194. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Gold SN, Cott MA. Forms of social support that moderate PTSD in childhood sexual abuse victims. Journal of Family Violence. 2003;18(5):295–300. [Google Scholar]
- Johnson M, Maas M. The Nursing Outcomes Classification. Journal of Nursing Care Quality. 1998;12(5):9–20. doi: 10.1097/00001786-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M, Feng X, Wood C, Richards E, Anthony R, Dahl G, Tao S. Elevated maternal coritsol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Americal Journal of Physiology. 2014;11 doi: 10.1152/ajpregu.00530.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Cacioppo JT, MacCallum RC, Snydersmither M, Kim C, Malarkey WB. Marital conflict in older adults: Endocrinological and immunological correlates. Psychosomatic Medicine. 1997;59(4):339–349. doi: 10.1097/00006842-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry. 2010;51(6):706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor K, McElvaney R. Child sexual abuse, links to later sexual exploitation/high-risk sexual behavior, and prevention/treatment programs. Trauma Violence Abuse. 2010;11(4):159–177. doi: 10.1177/1524838010378299. [DOI] [PubMed] [Google Scholar]
- Main M, Goldwyn R. Adult attachment rating and classification systems, version 6. 0. University of California Berkeley; 1994. [Google Scholar]
- Malarkey WB, Kiecolt-Glaser JK, Pearl D, Glaser R. Hostile behavior during marital conflict alters pituitary and adrenal hormones. Psychosomatic Medicine. 1994;56(1):41–51. doi: 10.1097/00006842-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal hypothalamic-pituitary-adrenal axis in pregnancy and the postpartum period: postpartum-related disorders. Annals of the New York Academy of Sciences. 2000;900:95–106. doi: 10.1111/j.1749-6632.2000.tb06220.x. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Han Z, Mulla S, Beyene J, Group KS. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analysis. British Medical Journal. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna DS, Wittber GM, Nagaraja HN, Samuels P. The effects of repeated doses of antenatal corticosteroids on maternal adrenal function. American Journal of Obstetrics and Gynecology. 2000;183(3):669–673. doi: 10.1067/mob.2000.106755. [DOI] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith RA. A placental clock controlling the length of human pregnancy. Nature Medicine. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55(1):10–20. doi: 10.1016/s0006-3223(03)00692-9. doi: S0006322303006929 [pii] [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Zimmerman R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosomatic Medicine. 2006;68(6):931–937. doi: 10.1097/01.psy.0000244385.93141.3b. [DOI] [PubMed] [Google Scholar]
- Nierop A, Wirtz PH, Bratsikas A, Zimmerman R, Ehlert U. Stress-buffering effects of psychosocial resources on physiological and psychological stress response in pregnant women. Biological Psychology. 2008;78:261–268. doi: 10.1016/j.biopsycho.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Parks SE, Kim KH, Day NL, Garza MA, Larkby CA. Lifetime self-reported victimization among low-income, urban women: the relationship between childhood maltreatment and adult violent victimization. Journal of Interpersonal Violence. 2011;26(6):1111–1128. doi: 10.1177/0886260510368158. [DOI] [PubMed] [Google Scholar]
- Raikkonen K, Lahti M, Heinonen K, Pesonen AK, Wahlbeck K, Kajantie E, Eriksson JG. Risk of severe mental disorders in adults separated temporarily from their parents in childhood: the Helsinki Birth Cohort Study. Journal of Psychiatry Research. 2011;45:332–338. doi: 10.1016/j.jpsychires.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, editors. Hierarchical linear models: applications and data analysis methods. 2 ed. Vol. 1. SAGE; 2002. [Google Scholar]
- Redman C, Sacks GSIL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. American Journal of Obstetrics and Gynecology. 1999;180(2):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Grizzard TA. Psychosocial stress and neuroendocrine mechanisms in preterm delivery. American Journal of Obstetrics and Gynecology. 2005;192(5 Suppl):S30–S35. doi: 10.1016/j.ajog.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18(1):65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Saigal S, Doyle L. An overview of mortality and sequelae of preterm birth from infancy to adulthood. The Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Salustiano EM, De Pinho JC, Provost K, Ruano R, Zugaib M. Maternal serum hormonal factors in the pathogenesis of preeclampsia. Obstetrical and Gynecological Survey. 2013;68(2):141–150. doi: 10.1097/OGX.0b013e31827f2500. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn L. Prenatal Programming of Human Neurological Function. International Journal of Peptides. 2011;2011:1–9. doi: 10.1155/2011/837596. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety, and early life trauma on the cortisol awakening response during pregnancy: Preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Smith LK, Draper ES, Manktelow BN, Dorling JS, Field DJ. Socioeconomic inequalities in very preterm birth rates. Arch Dis Child Fetal Neonatal Ed. 2007;92(1):F11–F14. doi: 10.1136/adc.2005.090308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback users' manual. Toronto: Addiction Research Foundation; 1995. [Google Scholar]
- Tieman W, van der Ende J, Verhulst FC. Psychiatric disorders in young adult intercountry adoptees: an epidemiological study. American Journal of Psychiatry. 2005;162(3):592–598. doi: 10.1176/appi.ajp.162.3.592. [DOI] [PubMed] [Google Scholar]
- Wadhwa P, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. American Journal of Obstetrics and Gynecology. 2004;191(4):1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Dunkel Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosomatic Medicine. 1996;58:432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Entringer S, Buss C, Lu M. The contribution of maternal stress to preterm birth: Issues and considerations. Clin Perinatol. 2011;38:351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]