Abstract
Pancreatic ductal adenocarcinoma (PDA) has a poor prognosis due to late detection and resistance to conventional therapies. Published studies show that the PDA tumor microenvironment (TME) is predominantly infiltrated with immune suppressive cells and signals that if altered, would allow effective immunotherapy. However, single-agent checkpoint inhibitors including agents that alter immune suppressive signals in other human cancers such as cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death 1 (PD-1) and its ligand PD-L1, have failed to demonstrate objective responses when given as single agents to PDA patients. We recently reported that inhibition of the CTLA-4 pathway when given together with a T cell inducing vaccine gives objective responses in metastatic PDA patients. In this study, we evaluated blockade of the PD-1/PD-L1 pathway. We found that PD-L1 is weakly expressed at a low frequency in untreated human and murine PDAs but treatment with a GM-CSF secreting PDA vaccine (GVAX) significantly upregulates PD-L1 membranous expression after treatment of tumor bearing mice. In addition, combination therapy with vaccine and PD-1 antibody blockade improved murine survival compared to PD-1 antibody monotherapy or GVAX therapy alone. Furthermore, PD-1 blockade increased effector CD8+ T lymphocytes and tumor-specific interferon-γ production of CD8+ T cells in the TME. Immunosuppressive pathways, including regulatory T cells (Tregs) and CTLA-4 expression on T cells were overcome by the addition of vaccine and low dose cyclophosphamide to PD-1 blockade. Collectively, our study supports combining PD-1 or PD-L1 antibody therapy with a T cell inducing agent for PDA treatment.
Keywords: PD-1, PD-L1, pancreatic cancer, immune checkpoint, immunotherapy
Introduction
Pancreatic ductal adenocarcinoma (PDA) is the fourth leading cause of cancer related deaths in the United States.1 Over 80% of those diagnosed with PDA are ineligible for curative resection with five-year survival less than 5%.2, 3 Current treatment modalities, including surgical resection, chemotherapy, and radiation have failed to significantly improve PDA survival in the last 30 years, necessitating new, novel treatment modalities.3
Immunotherapy has shown promise against solid tumors such as melanoma, renal cell carcinoma (RCC), non-small lung cancer (NSLC) and prostate cancer.4-6 PDA is classically considered a non-immunogenic tumor because very few effector T cells infiltrate these tumors.7, 8 Although a better survival was seen in surgically resected PDA patients with higher levels of CD4 and CD8 tumor infiltrating lymphocytes (TIL) within the tumor microenvironment (TME)9, the majority of PDAs contain a strong immunosuppressive network which limits the immune system's ability to actively eradicate the disease.10 The development of PDA is associated with alterations in its TME from pro-inflammatory to tolerogenic, characterized by infiltration of immunosuppressive cells such as regulatory T cells (Tregs) and myeloid derived suppressive cells (MDSCs) along with pro-cancerous inflammatory signals.7, 11, 12
Program death receptor-1 (PD-1) and one of its major ligands, program death ligand 1 (PDL1), constitute a major tolerance mechanism.13 PD-L1 (or B7-H1) is expressed by tumor cells, antigen presenting cells, B cells, and parenchymal cells. It binds to PD-1 which is mainly expressed on activated T cells14, 15 and results in T cell anergy or death thereby blunting anti-tumor immune responses and promoting tumor growth.14, 16 The expression of PD-1/PD-L1 has been characterized in PDAs.17-22 Despite some reports correlating PD-L1 expression with a poorer prognosis, the overall knowledge on the role of this pathway in PDA is still limited.18 In contrast to clinical trials of anti-PD-1 (αPD-1) and αPD-L1 antibody treatment in NSCLC, melanoma and RCC where durable tumor regression and prolonged stabilization of disease was achieved, when αPD-L1 antibodies were tested in clinical trials in a limited number of metastatic PDA patients, no objective responses were seen.23-26
A possible explanation for the therapeutic failure of PD-1 or PD-L1 blockade therapy in PDA is the lack of a natural infiltration of effector immune cells in the majority of PDAs. A potential strategy to activate effector T cell trafficking into the TME is vaccine-based immunotherapy. We previously developed a human whole cell granulocyte macrophage colony-stimulating factor (GM-CSF) secreting pancreatic cancer vaccine (GVAX) composed of allogeneic PDA tumor cell lines engineered to secrete GM-CSF.27 Phase I and II clinical trials of this vaccine demonstrated its safety and its ability to enhance tumor antigen (mesothelin)-specific interferon-γ (IFNγ) producing T cells in peripheral lymphocytes, which correlated with prolonged survival.27-32 More recently, through a clinical trial of GVAX as a neoadjuvant therapy for resectable PDAs, we observed vaccine-induced tertiary lymphoid aggregates in PDAs surgically resected from the majority of patients who received the vaccine therapy two weeks prior to the surgery and also observed the infiltration of PD-L1+ cells within these lymphoid aggregates.33
Similar to αPD-1 and αPD-L1, ipilimumab therapy, which is a checkpoint blockade antibody against cytotoxic T lymphocyte antigen-4 (CTLA-4) approved by the United States Food and Drug Administration (FDA) for the treatment of unresectable melanoma,34, 35 failed to demonstrate durable and effective anti-tumor activity in metastatic PDA patients as a single agent. However, we recently reported that the combination of ipilimumab with PDA GVAX demonstrated objective clinical responses that were associated with prolonged survival when compared to single agent ipilimumab.31 Notably, immune related adverse effects are common and severe with ipilimumab therapy in contrast to those seen with PD-1/PD-L1 blockade which are less frequent and more manageable.23, 26, 36 Therefore, we explored the potential combination of GVAX with PD-1/PD-L1 blockade therapies in a PDA mouse model.
Mechanistically, PD-L1/PD-1 and CTLA-4 function differently in T cell regulation.37 Although PD-L1 expression can be induced by oncogenic signals, it is mainly activated by adaptive immune responses.38 Spranger et al showed that the up-regulation of PD-L1 in the TME was dependent on CD8+ T cells and IFN-γ.39 Taube et al demonstrated that 98% of PD-L1 expressing melanomas were associated with tumor-infiltrating lymphocytes (TILs) as opposed to PD-L1 negative tumors where only 28% of these were associated with TIL presence.40 Moreover, PD-L1 expression on the tumor was preferentially seen at the tumor-TIL interface correlating with the presence of IFNγ.40 These findings suggest that upregulation of PD-L1 expression within the TME in response to an endogenous anti-tumor immune response subsequently generates an immune checkpoint signal, a process termed adaptive resistance.26, 37, 38, 40 Therefore, it is also intriguing to explore whether similar mechanisms underlie tolerance to vaccine-based cancer immunotherapy and whether PD-1 or PD-L1 blockade therapy can overcome this tolerance mechanism.
In this study, we investigated whether PD-L1 mediated adaptive resistance occurs in PDAs following vaccine-based immunotherapy in mouse PDAs, and whether αPD-1 or αPD-L1 antibody treatment in combination with GVAX can induce a greater anti-tumor immune response than either immunotherapy alone.
Materials and Methods
Study subjects and tissue specimens
Tumor tissues for immunohistochemistry staining (IHC) were obtained from specimens collected after vaccine exposure and unvaccinated patients who underwent surgery concurrently at our institution under IRB approved protocol NA_0007422133, 41. Formalin-fixed paraffin-embedded tissue blocks were obtained from our pathology archive.
Cell lines and medium
Panc02 is a highly tumorigenic cell line derived from methylcholanthrene treated C57Bl6 mice.42, 43 Panc02 cells were maintained in DMEM media (Life Technologies, Frederick, MD, USA), 10% Fetalclone II (ThermoScientific, Rockville, MD, USA), 1% L-glutamine (Life Technologies, Frederick, MD, USA), and 0.5% Penicillin/Streptomycin (Life Technologies, Frederick, MD, USA) at 37°C in 10% CO2. B78H1 cells are an MHC class I-negative variant of B16 melanoma cell line engineered to secrete GM-CSF.43, 44 B78H1 cells were maintained in RPMI media (Life Technologies, Frederick, MD, USA), 10% Fetalclone II, 0.5% L-glutamine, and 1% Penicillin/Streptomycin at 37°C in 5% CO2. Immune analysis was performed using CTL medium which consisted of RPMI media, 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO, USA), 1% L-glutamine, 0.5% Penicillin/Streptomycin, and 0.1% 2-mercaptoethanol (Life Technologies, Frederick, MD, USA).
Human PDA PD-L1 immunohistochemistry
Human PD-L1 IHC staining of paraffin embedded pancreatic tumor specimens was performed using the Dako Catalyzed Signal Amplification system as previously described.45 A PDA was considered to be positive for PD-L1 expression if membranous staining is present in more than 5% of the neoplastic cells in the PDA, as previously described.25, 40
PD-L1 murine immunofluorescence staining
After hemispleen injection, murine liver necropsies were performed. Liver tissue (frozen in OCT at −80°C) was cryo-cut for slides. At the time of staining, slides were thawed, fixed in 4% PFA for 5 min and subsequently washed in TBS with 0.1% tween (TBST) for 5 minutes three separate times. Slides were then blocked with 10% goat serum in PBS for 30 minutes followed by a repeat wash as above. Primary rat anti-mouse B7-H1 antibody (MIH5, eBioscience) and rat anti-mouse IgG 2ak (R35-95, BD Pharmingen) at 1:50 dilution was added for 60 minutes. Slides were washed as above and secondary goat anti-rat IgG-FITC antibody (Southern Biotech) at 1:200 dilution was added for 30 minutes. Slides were washed with TBST for 10 minutes three times and mounted with Vectashield Dapi containing kit.
Mice and in vivo experiments
Six to eight week old C57Bl6 female mice were purchased from Harlan Laboratories (Frederick, MD, USA) and maintained in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines. Tumor inoculation was performed via hemispleen technique on day 0 as previously described.46-48 Briefly, the spleen is eviscerated from the anesthetized mouse, clipped and divided in half. One half of the spleen is injected with 2x106 Panc02 tumor cells. The injected hemispleen is subsequently removed. On day 3, a single dose of cyclophosphamide (Cy) (100 mg/kg) was administered intraperitoneally (IP). Hamster anti-mouse PD-1 G4 antibodies (100 ug IP), hamster anti-mouse PD-L1 10B5 (100 ug IP), and hamster IgG control (100 ug IP) (Rockland Immunochemicals Inc, Boyertown, PA, USA) was administered on day 3 and twice weekly until death. Murine GVAX vaccine was formulated as previously described.43 Vaccine cells were washed in PBS, irradiated at 50 Gy and administered subcutaneously in three limbs (0.1 mL) on days 4, 7, 14, and 21. Mice were monitored three times per week for survival analysis and euthanized by CO2 inhalation following IACUC approved criteria or at study endpoint of 90 days.
Analysis of Spleen and Liver Infiltrating Lymphocytes
On day 13 following hemispleen injection, murine livers and spleens were collected. Each liver was mashed through 100-μm and 40-μm nylon filter and brought to a volume of 25 mL CTL medium. Each spleen was mashed through 100-μm nylon filter and brought to a volume of 15 mL CTL medium. All suspensions were centrifuged at 1500 rpm for 5 minutes. Liver cell pellets were suspended in 4 mL of ACK lysis (Quality Biological, Gaithersburg, MD, USA) and spleen cell pellets were suspended in 2 mL ACK lysis for 2 minutes and all were subsequently spun at 1500 rpm for 5 minutes. Liver cell pellets were then suspended in 5 mL 80% Percoll (GE Healthcare Life Sciences, USA), overlaid with 5 mL 40% Percoll and centrifuged at room temperature for 25 min at 3200 rpm, without brake. The lymphocyte layer was removed and suspended in 10 mL CTL media.
Cell staining and flow cytometry
Following the isolation of spleen and liver infiltrating lymphocytes from murine livers and hemispleens, cells were stained with Live Dead Near-IR Dead Cell kit (Invitrogen), CD3-APC (Biolegend), CD3-APC-Cy7 (Biolegend), CD8-PeCy7 (Biolegend), CD4-V500 (BD Horizon), CD25-BV421 (Biolegend), CTLA4-BV421 (Biolegend) and CD69-FITC (BD Pharmingen) for 30 minutes and assayed on an LSR II flow cytometer (BD Biosciences).
Intracellular staining for Foxp3 and flow cytometry
After staining for CD4 and CD25 following the above protocol, isolated liver infiltrating lymphocytes and splenocytes were suspended in cold Fix/Perm buffer (eBioscience) and incubated for 30 minutes at 4°C. The cells were then washed with Perm Buffer (eBioscience) and blocked with mouse Fc antibody (BD Pharmingen) for 15 minutes. Anti-mouse forkhead box P3 (FoxP3)-AF488 (MF23; BD Pharmingen) antibody was added and incubated at 4°C for 30 min. Cells were washed and assayed on an LSR II flow cytometer.
Intracellular cell staining for IFNγ and flow cytometry
Isolated liver infiltrating lymphocytes and splenocytes were enriched for CD8 cells using CD8 negative isolation kits (Life Technologies, Frederick, MD, USA) according to manufacturer's protocol. CD3:CD28 stimulation beads (Life Technologies, Frederick, MD, USA) were added to isolated CD8+ T cells and incubated for 12 hours at 37°C in 5% CO2 according manufacturer's protocol. Golgistop (1:1000; BD Biosciences) was added and incubated for 5 hours at 37°C in 5% CO2. After removing the beads according to manufacturer's protocol and washing the cells twice with flow buffer, cells were stained with CD8, CD3 and live dead Near-IR stain according to the above protocol. The cells were then washed twice, suspended in cytofix/cytoperm buffer (BD Biosciences), incubated at 4°C for 30 minutes and then washed with Permwash (BD Biosciences). IFNγ-BV421 (Biolegend) antibody was added in Permwash and incubated at 4°C for 20 minutes. Flow cytometry assays were completed on an LSR II flow cytometer.
Mouse IFNγ enzyme-linked immunosorbent assay
Isolated liver infiltrating lymphocytes and splenocytes were enriched for CD8 cells using CD8 negative isolation kits (Life Technologies, Frederick, MD, USA) according to manufacturer's protocol. Irradiated Panc02 tumor cells were added to isolated CD8+ T cells at a ratio of 5:1 (2x105 CD8+ T cells with 4x104 Panc02 tumor cells) and incubated for 18 hours at 37°C. Mouse IFNγ ELISA Ready-SET-Go assay was conducted per manufacturer protocol (eBioscience).
Statistical analysis
Statistical analyses for survival were conducted using Kaplan-Meier curves and log-rank test for survival. For comparison of cure rates the values were evaluated using chi-square test. For comparison of cell number, percentage, and cytokine expression between two groups, the mean values were evaluated using unpaired student's t-test. P < 0.05 was considered statistically significant.
Results
PD-L1 expression is upregulated following GVAX administration when compared to untreated human and mouse PDA tumors
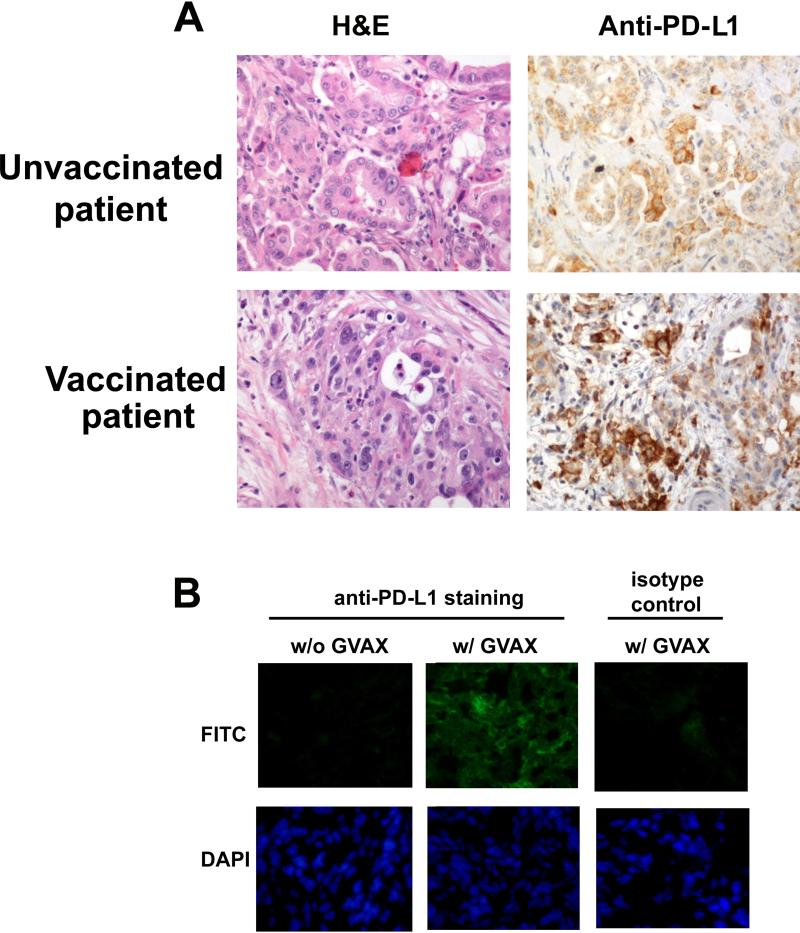
To study the role of PD-L1/PD-1 signaling in regulating anti-tumor immune responses in PDA, we first examined PD-L1 expression in the neoplastic cells of surgically resected PDA. We performed an updated analysis and examined PDAs resected from 25 patients who underwent pancreaticoduodenectomies at our institution. Similar to how the PD-L1 expression was characterized in melanoma25, 40, a PDA was considered to be positive for PD-L1 expression if membranous staining was present in more than 5% of the neoplastic cells in the PDA. IHC analysis revealed that approximately 12.5% (3 out of 25 analyzed) of resected PDAs from unvaccinated patients were positive for PD-L1 expression based on this previously published criteria and, that the intensity of the membranous staining of PD-L1 in these PDAs was also weak (Figure 1A). We then examined the PD-L1 membranous expression in PDAs from patients who received the GVAX vaccine 2 weeks prior to surgical resection in the aforementioned clinical trial.33 We found an increased intensity of PD-L1 membranous staining on the epithelial tumor cells of PDAs from these vaccinated patients when compared to those from unvaccinated patients. The frequency of PDAs considered positive for PD-L1 membranous expression was moderately increased to 25% (10 out of 40 analyzed) in vaccinated patients and strong PD-L1 positive signals were observed in all the vaccine-induced intratumoral tertiary lymphoid aggregates found in the majority (>80%) of PDAs from vaccinated patients. 33
Figure 1. Pancreatic cancer Cy/GVAX therapy upregulates pancreatic tumor expression of PD-L1 in human & murine pancreatic ductal adenocarcinoma (PDA).
(A) Representative H&E staining and IHC with αPD-L1 antibody on resected PDA from unvaccinated patients and patients who received the Cy/GVAX vaccine therapy two weeks prior to surgical resection. (B) Immunofluorescence staining with αPD-L1 antibody and FITC conjugated secondary antibody in liver tumors after Panc02 hemispleen injection comparing mice treated with Cy/GVAX on day 4 and 7 after tumor inoculation (w/GVAX) against mice not receiving GVAX therapy (w/o GVAX) (upper panel). Livers were harvested two weeks after tumor cell inoculation. DAPI staining of nuclei is shown in lower panel.
To better understand the significance of PD-1/PD-L1 regulation of immune responses within the PDA TME, we next tested whether GVAX therapy can also induce the upregulation of PD-L1 expression in a preclinical model of metastatic PDA. We used a previously reported experimental model of liver metastases in which Panc02 tumor cells are injected directly into the spleen. A hemisplenectomy is performed to remove residual tumor cells and to allow the establishment of liver metastases where all untreated mice die from the development of diffuse liver metastases within 6 weeks (Figure S1).48, 49 Metastasis-bearing mice were treated with GVAX four and seven days after hemispleen injection and harvested the liver two weeks after tumor inoculation to perform immunofluorescence staining for PD-L1 expression. Similar to our findings in human PDAs, livers from untreated mice receiving no treatment had no evidence of PD-L1 expression whereas livers from GVAX-treated mice had significant induction of PDL1 membranous expression (Figure 1B). The addition of αPD-1 antibody to GVAX therapy did not alter PD-L1 expression in murine liver metastases when compared to GVAX monotherapy. Thus, these data demonstrate that similar to human PDA following GVAX treatment (Figure 1A), GVAX is also able to induce PD-L1 expression in murine PDAs.
Combination therapy with GVAX and PD-1 or PD-L1 blockade improves survival in a PDA mouse model
Next, we examined whether blocking PD-L1, or its receptor PD-1, can augment the anti-tumor activity of GVAX in the PDA hemisplenectomy model. GVAX was administered on days 4, 7, 14 and 21 (Figure 2A). A single low dose of Cy was given on day 3 for Treg depletion as reported for other GVAX preclinical models.50-52 and hamster anti-mouse PD-1, PD-L1 monoclonal antibodies (mAbs) or IgG control were administered on day 3 as either monotherapy or in combination with Cy/GVAX.
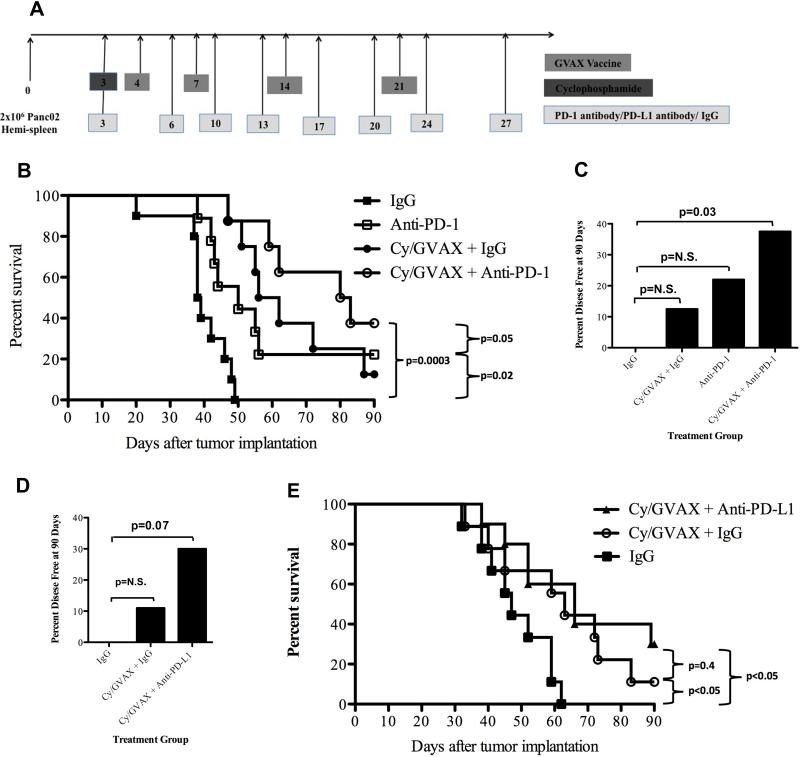
Figure 2. Combination therapy with Cy/GVAX and PD-1 or PD-L1 blockade improves clinical outcomes in a PDA mouse model.
(A) Schema of tumor implantation by the hemispleen procedure and treatment with Cy, GVAX and αPD-1/αPD-L1 blockade as indicated. C57Bl/6 mice were challenged on day 0 with 2 x 106 Panc02 tumor cells followed by administration of 100 mg/kg of Cy on day 3 and irradiated whole-cell vaccine on day 4, 7, 14 and 21. Anti-PD-1, anti-PD-L1 or IgG (5 mg/kg IP) were administered IP twice weekly until death starting on day 3. (B) Kaplan-Meier survival curves of mice that were implanted with PDA cells and were treated with different combinations of Cy, GVAX and the αPD-1 antibody. The percentages of mice that remained disease free at day 90 following tumor implantation and therapy with (C) Cy, GVAX and/or αPD-1 or (D) Cy, GVAX and αPD-L1 are shown. All the p values were yielded by comparing GVAX and/or αPD-1/αPD-L1 treatment groups with IgG treated group. (E) Kaplan-Meier survival curves of mice that were implanted with Panc02 cells via hemispleen technique and treated with different combinations of Cy, GVAX and αPD-L1 antibody. Data are represented as results obtained from experiments with 8-10 mice per group that were repeated at least twice. N.S. not significant.
Although both αPD-1 monotherapy (median OS: 50 days) and Cy/GVAX therapy alone (OS: 59 days) improved the survival of mice compared to IgG control treatment (OS: 38.5 days, p<0.05), Cy/GVAX + αPD-1 combination therapy significantly increased median survival compared to αPD-1 monotherapy (OS: 81.5 days vs. 50 days, p=0.05) (Figure 2B). A trend toward improved survival was seen with Cy/GVAX + αPD-1 combination therapy compared to Cy/GVAX therapy alone (OS: 81.5 days vs. 59 days, p=0.22). Moreover, the combination therapy cured a larger percentage of mice (38%) (Figure 2C) when compared to Cy/GVAX (12.5%) therapy or αPD-1 monotherapy (22%).
Similar experiments were performed to investigate the Cy/GVAX + αPD-L1 combination therapy. This combination cured 30% of mice (Figure 2D and 2E), compared to an 11% cure rate with Cy/GVAX therapy alone. These data suggest that PD-1 or PD-L1 blockade therapy enhances the antitumor activity of Cy/GVAX.
GVAX combined with PD-1 blockade increases CD8+ T lymphocytes in PDAs
To define the immune mechanisms by which PD-1 or PD-L1 blockade enhances the antitumor activity of Cy/GVAX, we first evaluated the effect of each single immunotherapy and combined treatment on the composition of T lymphocytes infiltrating the metastatic PDA TME. Tumor-bearing mice were treated with either αPD-1 or IgG control. Cy was administrated on day 3 and GVAX was administered twice on days 4 and 7 (Figure 3A). On day 13, livers and spleen were harvested for fluorescence-activated cell sorting (FACS) analysis of splenocytes and liver infiltrating lymphocytes (TIL).
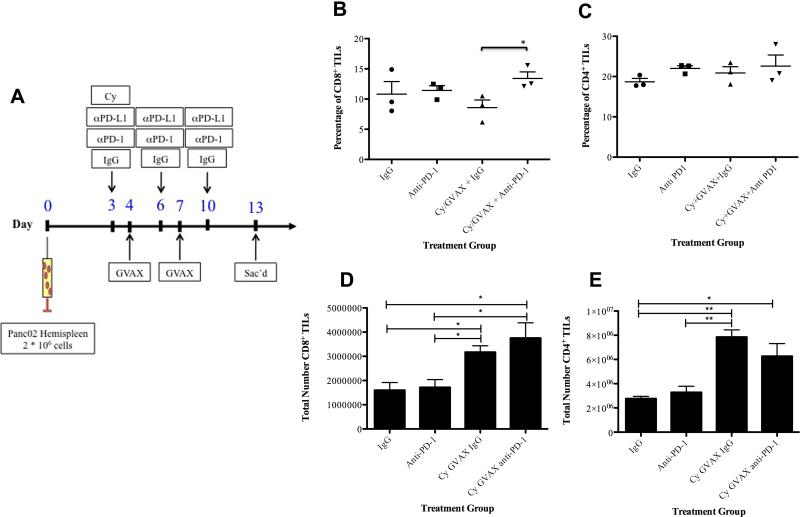
Figure 3. Cy/GVAX combined with PD-1 blockade increases CD8+ T cells in PDAs.
(A) Schema of immune analysis following tumor implantation by the hemispleen procedure and treatment with Cy (100 mg/kg) on day 3, GVAX on day 4, 7 and IgG/αPD-1/αPD-L1 (5 mg/kg IP) on day 3, 6, 10. Each experimental group consisted of five mice, pooled and analyzed individually in triplicates. Percentage of (B) CD8+ and (C) CD4+ tumor infiltrating lymphocytes among total lymphocytes in murine livers and total numbers of (D) CD8+ and (E) CD4+ tumor infiltrating lymphocytes after Panc02 hemispleen and indicated therapy. Data represent mean ± SEM from one representative experiment that was repeated at least twice. * p=0.04, TIL tumor-infiltrating lymphocytes.
TIL numbers were increased in the livers of mice treated with Cy/GVAX + αPD-1 combination therapy where a statistically significant and approximately 60% increase in the percentage of CD8+ T cells among lymphocytes infiltrating the TME was seen in mice treated by the combination when compared with Cy/GVAX alone (13.4% vs. 8.57%, p=0.04) (Figure 3B). By contrast, there was no significant change in CD4+ T cells in the TILs of mice treated with combination therapy compared to Cy/GVAX alone (22.6% vs. 20.9%) (Figure 3C). Interestingly, Cy/GVAX alone significantly increased the absolute numbers of CD8+ and CD4+ TILs, but not the percentage of CD8+ and CD4+ T cells among TILs, compared to no treatment controls (Figure 3D,E). This result suggests that other lymphocyte subtypes, which remain to be explored, were also increased in the TILs following the Cy/GVAX treatment. It should be noted that the Cy/GVAX + αPD-1 combination significantly increases the number of CD8+ TILs per mouse compared to αPD-1 alone but not to Cy/GVAX alone, suggesting that addition of αPD-1 to Cy/GVAX mainly changes the T cell composition in the TILs. Systemically, mice treated with combination therapy had less of an increase in CD8+ T cell composition in their splenocytes compared to those treated with the Cy/GVAX alone (36.8% vs. 32.5%, p<0.01) (Figure S2). These data suggest that αPD-1 therapy may enhance the antitumor activity of Cy/GVAX by selectively increasing the composition of CD8+ T cells in the TME.
Cy/GVAX combined with PD-1 blockade enhances the activation of tumor specific IFNγ production in CD8+ T cells within the metastatic PDA TME
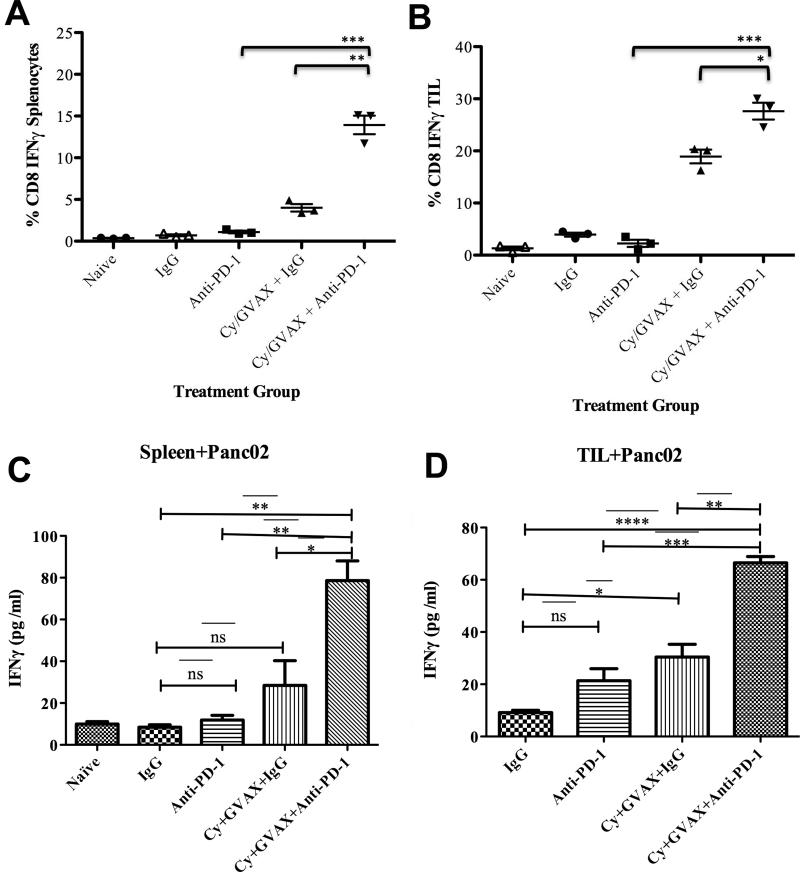
To determine whether PD-1 blockade enhances T cell activation in the TME, we examined IFNγ production by CD8+ T cells in splenocytes and TIL. There were significantly greater numbers of IFNγ producing CD8+ T cells in the spleens of mice treated with Cy/GVAX + αPD-1 combination versus Cy/GVAX alone (13.9% vs. 4%, p<0.01) or αPD-1 monotherapy (13.9% vs. 1.1%, p<0.001) (Figure 4A). Additionally, the Cy/GVAX + αPD-1 combination resulted in a significant increase in the percentage of IFNγ producing CD8+ T cells within TIL when compared with αPD-1 monotherapy (27.6% vs. 2.3%, p<0.001) or Cy/GVAX alone (27.6% vs. 18.9%, p<0.05) (Figure 4B). The total number of IFNγ producing CD8+ T cells in splenocytes (Figure S3A) and in TILs (Figure S3B) were also significantly increased with the combinational therapy compared to Cy/GVAX or αPD-1 alone. Similar results were observed when αPD-L1 blockade was used instead of αPD-1 blockade (data not shown). Although we observed the increase of CD69+CD8+ cells in the TIL from mice treated with Cy/GVAX + αPD-1 combination compared to either monotherapy, we did not observe an increase in the percentage of CD69+ activated cells among CD8+ TIL (Figure S4). These results suggest that the addition of αPD-1 to Cy/GVAX does not further activate CD8+ TIL, but may have increased its IFNγ-mediated cytotoxic activity.
Figure 4. Combinatorial treatment increases the percentage of IFNγ secreting CD8+ T cells and tumor specific CD8+ T cells in the tumor microenvironment.
CD8+ T cells were isolated and purified from spleen and livers on day 13 after hemispleen implantation of Panc02 tumor cells. Tumor-bearing mice were treated with Cy, GVAX or αPD-1/αPD-L1 therapy as indicated. The percentage of IFNγ+ producing CD8+ T cells amongst all CD8+ T cells in (A) splenocytes and (B) tumor infiltrating lymphocytes is shown. ELISA assays were performed using autologous irradiated Panc02 tumor cells as antigenic targets for CD8+ T cells isolated from (C) spleen and (D) tumor infiltrating lymphocytes. Each experimental group consisted of five mice, pooled and analyzed individually in triplicates. Data represent mean ± SEM from one representative experiment that was repeated at least twice. *p<0.05, ** p<0,01, *** p< 0.001, TIL tumor-infiltrating lymphocytes
Next, we assessed for tumor specific CD8+ T cell activity both systemically and within the TME with the murine IFNγ ELISA analysis by using irradiated autologous tumor cells as a target. Compared to IgG controls, neither Cy/GVAX nor αPD-1 alone significantly enhanced the tumor-specific IFNγ secretion by CD8+ T cells in splenocytes. In contrast, Cy/GVAX alone enhanced the tumor-specific IFNγ secretion by CD8+ T cells in the TME when compared with αPD-1 alone. Importantly, mice treated by Cy/GVAX + αPD-1 combination therapy demonstrated significantly greater IFNγ secretion by CD8+ T cells compared to either Cy/GVAX or αPD-1 alone in both splenocytes (Figure 4C) and TIL (Figure 4D) in response to Panc02 tumor cells.
Cy/GVAX and αPD-1 combination overcomes Treg, CTLA-4, and PD-1/PD-L1 immunosuppressive pathways
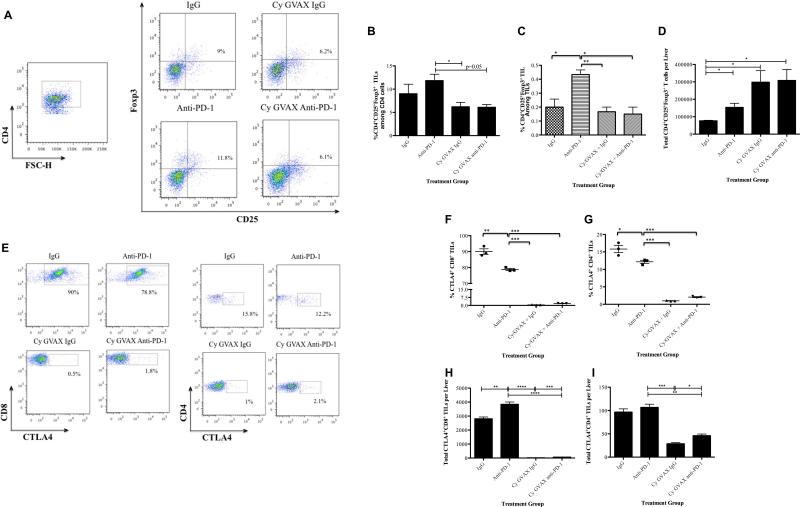
The effect of PD-1/PD-L1 blockade on immune activation is likely the result of blocking the PD-L1/PD-1 immune checkpoint pathway. However, other checkpoint pathways may also be involved. Thus, we sought to determine the effect of αPD-1 blockade therapy on Treg population and CTLA-4 expression levels in splenocytes and TIL. Anti-PD-1 monotherapy significantly increased the percentage of CD4+CD25+Foxp3+ Tregs among TILs (Figure 5A,B,C) and modestly increased that in splenocytes (Figure S5A) when compared to Cy/GVAX or IgG alone . The addition of Cy/GVAX to αPD-1 blockade resulted in a decrease in the percentage of Treg among CD4+ TILs in comparison to αPD-1 monotherapy. Interestingly, increasing total numbers of Tregs within the TME were seen in mice treated by αPD-1 alone, Cy/GVAX alone, or Cy/GVAX + αPD-1 combination therapy comparing to mice treated by IgG alone (Figure 5D). Although it cannot be excluded that FOXP3 is upregulated by PD-1 blockade in effector T cells without acquiring suppressive activity, our results suggest another possibility that Cy/GVAX abrogates the effect of PD-1 blockade on the percentage of CD4+CD25+Foxp3+ among TILs.
Figure 5. Cy/GVAX therapy with αPD-1 blockade overcomes immunosuppressive pathways.
Following hemispleen implantation of Panc02 cells, tumor-bearing mice were treated with Cy (100 mg/kg) on day 3, GVAX on day 4, 7 and IgG/αPD-1/αPD-L1 (5 mg/kg IP) on day 3, 6, 10. Mice were sacrificed on day 13. (A) Fluorescence-activated cell sorting (FACS) density plot of CD4+CD25+Foxp3+ Tregs in TILs. (B) Histogram showing the percentage of CD4+CD25+Foxp3+ Tregs in CD4+ TILs. (C) Histogram showing the percentage of CD4+CD25+Foxp3+ Tregs in all TILs. (D) Histogram showing the total number of CD4+CD25+Foxp3+ Tregs infiltrating the tumors. (E) Representative FACS analysis of CTLA-4+CD8+ T cells in TILs. (F) Histogram showing the percentage of CTLA-4+CD8+ T cells within CD8+ T cells. (G) Histogram showing the percentage of CTLA-4+CD4+ T cells within CD4+ T cells. (H) Histogram showing the total number of CTLA-4+CD8+ TILs. (I) Histogram showing the total number of CTLA-4+CD4+ TILs. Each experimental group consisted of five mice, pooled and analyzed individually in triplicates. Data represent mean ± SEM from one representative experiment that was repeated at least twice. *p<0.05, **p<0.01, ***p<0.001. IgG, hamster IgG; TIL, tumor infiltrating lymphocytes
Anti-PD-1 monotherapy significantly decreased CTLA-4+CD4+ and CTLA-4+CD8+ T cells in the TME compared with IgG controls. However, Cy/GVAX alone had a greater effect on decreasing CTLA4 expression and the addition of Cy/GVAX to αPD-1 blockade therapy significantly decreases both CTLA-4+CD4+ and CTLA-4+CD8+ T cells compared to αPD-1 monotherapy (Figure 5E-I). Notably, the addition of Cy/GVAX to αPD-1 therapy did not significantly decrease CTLA-4+ T cells in the spleen (Figure S5B and S5C). Taken together, these data suggest that Cy/GVAX and αPD-1 antibodies cooperate to overcome multiple immunosuppressive pathways including Tregs, CTLA-4 and PD-L1/PD-1 signaling.
Discussion
In this study, we showed that PD-L1 expression is upregulated in both human and murine PDAs when IFNγ-producing CD8+ T cells infiltrate the TME after Cy/GVAX therapy, supporting for the first time that the process of adaptive resistance can occur in PDA. We further demonstrated that PD-1 blockade therapy may overcome this vaccine-induced adaptive resistance and enhance vaccine-induced effector T cell response in mouse PDA.
A few published studies reported delayed tumor growth in mouse models of PDA with αPD-L1 monotherapy; however αPD-L1 single agent therapy did not eradicate tumors that were either implanted subcutaneously or orthotopically in the pancreas.18, 53 Our study showed some effect of αPD-1 monotherapy in prolonging survival and curing a small percentage of metastasis bearing mice. This modest effect of αPD-1 monotherapy in our model may be exerted on the PD-L1/PD-1 pathway mediated by PD-L1-expressing monocytes or antigen presenting cells that have not yet been examined in our study.54 However, overall survival and cure rates were significantly improved when Cy/GVAX was combined with αPD-1/PD-L1 blockade. We therefore propose that a T cell augmenting agent that increases IFNγ-expressing T cells in the PDA TME should be given with αPD-1/αPD-L1 blockade to achieve a significant clinical response in patients with PDA.
In accordance with our report, previous studies have documented the additive benefit of combining vaccine therapy with PD-1 or PD-L1 blockade in other tumor models.55-58 However, in both CT26 colorectal tumor and ID8 ovarian tumor subcutaneous models, αPD-1/αPD-L1 or αCTLA-4 monotherapy appeared to result in significantly more enhanced effector T cell immune responses and antitumor activity than αPD-1/αPD-L1 did in the liver metastasis model of PDA in our study.56 In addition, we observed that αPD-1/αPD-L1 monotherapy increased the percentage of Tregs in the lymphocytes infiltrating the tumors formed by Panc02 cells whereas others have shown that αPD-1/αPD-L1 monotherapy reduces Tregs in tumors formed by CT26 or ID8 cells.56 The differences in treatment response are likely attributed to the difference in the tumor types. Both αPD-1/αPD-L1 monotherapies were associated with objective responses in colorectal and ovarian cancer patients, but were not effective in metastatic PDA patients in clinical trials.23, 26 Additionally, the liver metastasis model is a more physiologically relevant model than subcutaneous models since the liver is the most common site of metastasis for PDA and the majority of PDA patients have metastatic disease at the time of diagnosis.
Our data show that αPD-1 monotherapy increased the percentage of Tregs in lymphocytes infiltrating the TME. We have also observed a similar increase of Tregs in the TME when GVAX is given without Cy (data not shown). Moreover, the addition of Cy to GVAX therapy has been previously shown to induce the recruitment of high avidity CD8+ T cells which was attributed to Treg depletion.59 Previous reports have studied the effectiveness of GVAX in the treatment of murine colorectal cancer hepatic metastases and demonstrated that Cy in conjunction with CT26 GVAX resulted in transient depletion of Tregs as well as expansion of tumor antigen specific T cells.46, 52 Our current study also shows that Cy/GVAX had a more significant effect in suppressing CTLA-4+ T cells than αPD-1 blockade. The addition of Cy/GVAX to αPD-1 therapy abrogates the αPD-1 induced upregulation of Tregs and significantly downregulates CTLA-4 expression in CD4+ and CD8+ T cells. We did not attempt to distinguish the role of Cy with or without GVAX and the role of GVAX with or without Cy, since this was not within the scope of this study. However, we have not observed an enhancement in anti-tumor activity with PD-1/PD-L1 blockade and a single intraperitoneal dose of Cy alone, or with GVAX alone (data not shown).
In conclusion, this study is the first to provide evidence of therapy induced adaptive resistance with induction of PD-L1 expression in a murine PDA. It supports the addition of αPD-1 or αPD-L1 blockade to Cy/GVAX-based immunotherapy to achieve durable tumor responses. Adding Cy/GVAX therapy to αPD-1 or αPD-L1 therapy may also overcome additional immune checkpoint mechanisms. These findings warrant a direct test of the Cy/GVAX and PD-1 or PDL1 blockade combination therapy in pancreatic cancer patients.
Supplementary Material
Acknowledgments
We wish to thank Dr. Drew Pardoll and Dr. Todd Armstrong for helpful discussion and Ada Tam and Lee Blosser for technical help. K.C.S. is an AHPBA Research Fellow under AHPBA mentor R.D.S. This work was supported in part by the AHPBA Fellowship (K.C.S.), NIH K23 CA148964-01 (L.Z.), Johns Hopkins School of Medicine Clinical Scientist Award (L.Z.), Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (D.L., E.M.J., L.Z.), Lefkofsky Family Foundation (L.Z.), the NCI SPORE in Gastrointestinal Cancers P50 CA062924 (E.M.J., L.Z.), Lustgarten Foundation (L.Z.), the Sol Goldman Pancreatic Cancer Center grants (K.C.S., B.H.E., L.Z.), and NIH NIDDK T32 DK 7713-18 (K.C.S.).
Funding Support:
This work was supported in part by the AHPBA Fellowship (K.C.S.), NIH K23 CA148964-01 (L.Z.), Johns Hopkins School of Medicine Clinical Scientist Award (L.Z.), Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (D.L., E.M.J., L.Z.), Lefkofsky Family Foundation (L.Z.), the NCI SPORE in Gastrointestinal Cancers P50 CA062924 (E.M.J., L.Z.), Lustgarten Foundation (L.Z.), the Sol Goldman Pancreatic Cancer Center grants (K.C.S., B.H.E., L.Z.) and NIH NIDDK T32 DK 7713-18 (K.C.S.).
Footnotes
Conflict of Interests:
Under a licensing agreement between Aduro BioTech, Inc. and the Johns Hopkins University and Dr. Elizabeth Jaffee, the University is entitled to milestone payments and royalty on sales of the vaccine product described in this manuscript. We do not have any other relevant conflicts of interest to be disclosed. RAA is supported by a commercial research grant from Bristol-Meyers Squibb. The other authors have no conflict to disclose.
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin. 2014 [Google Scholar]
- 2.Moon HJ, An JY, Heo JS, et al. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37–43. doi: 10.1097/01.mpa.0000194609.24606.4b. [DOI] [PubMed] [Google Scholar]
- 3.Ma J, Siegel R, Jemal A. Pancreatic Cancer Death Rates by Race Among US Men and Women, 1970-2009. J Natl Cancer Inst. 2013;105:1694–700. doi: 10.1093/jnci/djt292. [DOI] [PubMed] [Google Scholar]
- 4.Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20:241–6. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma PW, Wolchok K, Allison JD. J.P. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2012;11:805–12. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Xue J, Jaffee EM, et al. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230–40. doi: 10.1053/j.gastro.2012.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200–5. doi: 10.1016/j.coi.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Ene-Obong A, Clear AJ, Watt J, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121–32. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 13.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–60. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 14.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Loos M, Giese NA, Kleeff J, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 18.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 19.Batra SK, Metzgar RS, Hollingsworth MA. Isolation and characterization of a complementary DNA (PD-1) differentially expressed by human pancreatic ductal cell tumors. Cell Growth Differ. 1991;2:385–90. [PubMed] [Google Scholar]
- 20.Basso D, Fogar P, Falconi M, et al. Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One. 2013;8:e54824. doi: 10.1371/journal.pone.0054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Ma Q, Chen X, et al. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059–65. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 22.Geng L, Huang D, Liu J, et al. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134:1021–7. doi: 10.1007/s00432-008-0364-8. [DOI] [PubMed] [Google Scholar]
- 23.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 28.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schueneman AJ, Sugar EA, Uram J, et al. Low Total Lymphocyte Count Is Associated with Poor Survival in Patients with Resected Pancreatic Adenocarcinoma Receiving a GM-CSF Secreting Pancreatic Tumor Vaccine. Ann Surg Oncol. 2013;20(Suppl 3):725–30. doi: 10.1245/s10434-013-3262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–31. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 36.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014 doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 37.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–12. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J, Ahuja N, Makary MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford) 2014;16:83–90. doi: 10.1111/hpb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corbett TH, Roberts BJ, Leopold WR, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–26. [PubMed] [Google Scholar]
- 43.Leao IC, Ganesan P, Armstrong TD, et al. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008;1:228–39. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levitsky HI, Lazenby A, Hayashi RJ, et al. In vivo priming of two distinct antitumor effector populations: the role of MHC class I expression. J Exp Med. 1994;179:1215–24. doi: 10.1084/jem.179.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigelow E, Bever KM, Xu H, et al. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J Vis Exp. 2013 doi: 10.3791/4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain A, Slansky JE, Matey LC, et al. Synergistic effect of a granulocyte-macrophage colony-stimulating factor-transduced tumor vaccine and systemic interleukin-2 in the treatment of murine colorectal cancer hepatic metastases. Ann Surg Oncol. 2003;10:810–20. doi: 10.1245/aso.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Olino K, Wada S, Edil BH, et al. Tumor-associated antigen expressing Listeria monocytogenes induces effective primary and memory T-cell responses against hepatic colorectal cancer metastases. Ann Surg Oncol. 2012;19(Suppl 3):S597–607. doi: 10.1245/s10434-011-2037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soares KC, Foley K, Olino K, et al. A Preclinical Murine Model of Hepatic Metastases. J Vis Exp. 2014 doi: 10.3791/51677. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng L, Jaffee EM. Annexin A2 is a new antigenic target for pancreatic cancer immunotherapy. Oncoimmunology. 2012;1:112–4. doi: 10.4161/onci.1.1.18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machiels JP, Reilly RT, Emens LA, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 51.Wada S, Yoshimura K, Hipkiss EL, et al. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–18. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radojcic V, Bezak KB, Skarica M, et al. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2010;59:137–48. doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okudaira K, Hokari R, Tsuzuki Y, et al. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–9. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 54.Selenko-Gebauer N, Majdic O, Szekeres A, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–44. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 55.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–12. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duraiswamy J, Kaluza KM, Freeman GJ, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li B, VanRoey M, Wang C, et al. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–34. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 58.Mkrtichyan M, Najjar YG, Raulfs EC, et al. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur J Immunol. 2011;41:2977–86. doi: 10.1002/eji.201141639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ercolini AM, Ladle BH, Manning EA, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.