Abstract
Background
We aimed to assess whether chronic obstructive pulmonary disease (COPD) is associated with expansion of the myocardial extracellular volume (ECV) using T1 measurements.
Methods
Adult COPD patients (GOLD stage 2 or higher) and free of known cardiovascular disease were recruited. All study patients underwent measures of pulmonary function, 6-minute walk test, serum measures of inflammation, overnight polysomnography, and a contrast CMR study.
Results
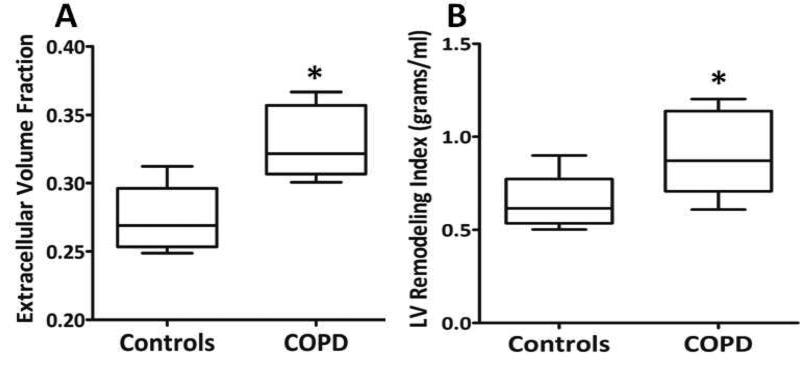
Eight patients with COPD were compared to 8 healthy control subjects. The mean predicted FEV1% of COPD subjects was 68%. Compared to controls, patients had normal left ventricular (LV) and right ventricular size, mass, and function. However, as compared to controls, the LV remodeling index (median 0.87 IQR 0.43 vs. median 0.62 IQR 0.17, p=0.03) and active left atrial emptying fraction was increased (median 46 IQR 8 vs. median 38 IQR 10, p=0.005), and passive left atrial emptying fraction was reduced (median 24 IQR 10 vs. median 44 IQR 20, p=0.007). The ECV was increased in patients with COPD (median 0.32 IQR 0.05 vs. median 0.27 IQR 0.05, p=0.001). The ECV showed a strong positive association with LV remodeling (r = 0.72, p = 0.04) and an inverse association with the 6-minute walk duration (r = −0.79, p = 0.02) and passive left atrial emptying fraction (r = −0.68, p = 0.003).
Conclusions
Expansion of the ECV, suggestive of diffuse myocardial fibrosis, is present in COPD and is associated with LV remodeling, reduced left atrial function and exercise capacity.
Introduction
Cardiovascular morbidity and mortality among patients with chronic obstructive pulmonary disease (COPD) is high, 1-3 but the mechanisms are unknown. Myocardial fibrosis is a marker of increased risk among broad groups of patients with cardiovascular disease 4, 5 and is confirmed pathologically in patients with advanced lung disease.6 Cardiac magnetic resonance (CMR) with the additive contrast technique of late gadolinium enhancement (LGE) is the gold-standard technique for the detection of replacement myocardial fibrosis.7 However, among patients with COPD, LGE is not detected,8 likely because LGE-based CMR measures for detection of myocardial fibrosis underestimate fibrosis when the entire myocardium is diffusely involved.9 Determination of the myocardial extracellular volume (ECV) by T1 measurements is a robust method for quantifying diffuse myocardial fibrosis.10-12 The ECV by CMR has been validated against histological measures of fibrosis,10, 11 is associated with measures of myocardial function,13 and is an independent predictor of mortality among broad populations with cardiovascular disease.14 We hypothesized that an expanded ECV would provide this marker of increased cardiovascular risk in patients with COPD. To design a definitive study, we first performed a pilot study where we measured the ECV in a small well-characterized cohort of patients with COPD.
Methods
Study population
The study was approved by the local institutional review board and all participants gave their written informed consent. This study was completed over a period of 6 months from January to June, 2011, at Brigham and Women's Hospital. Adult patients (≥18 years of age) with known COPD as diagnosed by a pulmonologist (defined as Global Initiative for Chronic Obstructive Lung Disease, GOLD stage 2 or higher and ≥10 pack-years of smoking history) were screened. Patients with standard contraindications to a CMR (e.g. claustrophobia, ferromagnetic devices etc), receiving positive airway pressure therapy, uncontrolled COPD, known cardiovascular disease, a clinical history of heart failure or reduced ejection fraction, hypertension, diabetes, or pregnant women were excluded. After a telephone screening, patients presented for a history and physical examination, pulmonary function tests, blood tests, overnight polysomnography, 6 minute walk test, and a CMR study the next morning. Pulmonary function tests, including lung volumes and diffusing capacity, were performed according to the guidelines of the American Thoracic Society.15 Lung function measures of specific interest included forced expiratory volume at 1 second (FEV1), the degree of hyperinflation (as measured by residual volume divided by the total lung capacity, RV/TLC%), and the forced expired flow from 25-75% (FEF25-75%). Blood samples were collected for measurement of markers of inflammation (hs-CRP and IL-6) as well as a hematocrit and creatinine. All study subjects with COPD underwent a supervised in-laboratory polysomnography to exclude the co-existence of previously undiagnosed obstructive sleep apnea. Sleep studies were scored by an experienced, blinded sleep technologist according to standard criteria 16 and patients with an apnea-hypopnea index (AHI) ≥10/hour were a priori excluded. These patients were included in a prior published study detailing cardiovascular structure among patients with the overlap syndrome.17 All COPD study participants also completed the Epworth Sleepiness Scale to assess daytime sleepiness 18 and the Modified Medical Research Council (MMRC) score to assess dyspnea before the polysomnography.18We recruited healthy volunteer controls by open enrolment using an IRB approved research website (http://clinicaltrials.partners.org/).11 We specifically excluded volunteers with chest pain on exertion, any active or prior history of heart disease, stroke, diabetes, malignancy, sleep apnea, hypertension, an irregular heart rhythm or atrial fibrillation, or any form of kidney disease. Healthy volunteers underwent testing with a comprehensive questionnaire detailing medical and medication history, peak flow measurement, standard anthropometric data, measurement of blood pressure, pulse, serum creatinine and hematocrit, followed by a full CMR study with contrast. Healthy volunteers underwent a history and a physical examination by a board-certified pulmonologist. Healthy volunteers did not undergo a sleep study, Epworth Sleepiness Scale, MMRC score, 6 minute walk test, full lung function testing or polysomnography.
Cardiac Magnetic Resonance
All images were acquired with EKG gating, breath-holding, and with the patient in a supine position as previously described.4 Subjects were imaged on 3.0-T CMR system (Siemens, Erlangen, Germany). The CMR protocol consisted of cine steady-state free precession imaging for left ventricular (LV), right ventricular (RV) volumes, function and mass. All parameters were indexed by dividing the value by body surface area.19 All patients underwent an LGE imaging protocol for replacement myocardial fibrosis. A segmented inversion-recovery pulse sequence for LGE was used starting 10-15 minutes after cumulative 0.15-mmol/kg dose of gadolinium DTPA (Magnevist, Bayer HealthCare Pharmaceuticals Inc, Wayne, New Jersey). The LV and RV remodeling index, as indices of concentric remodeling,20 were calculated by dividing the LV and RV mass by their respective end-diastolic volume.
Evaluation of Pulmonary Arterial Pressures
Pulmonary hypertension has been reported in patients with COPD. To assess indirectly whether pulmonary hypertension was present in this cohort we measured RV mass index 21 and, among patients with COPD, peak and average PA blood velocity,22, 23 and pulmonary arterial (PA) size.23 The RV mass index has been shown to correlate with invasive measured PA pressures, while peak and mean PA velocities are reduced in patients with pulmonary hypertension.23 Flow imaging was performed perpendicular to the main PA with a velocity-encoded gradient echo sequence and a typical upper velocity limit of 150 cm/s (with further increases if any signal aliasing). The following typical imaging parameters were applied: repetition time/echo time: 5.9–7.5/2.3–3.1 ms; number of averages: 3; slice thickness: 5 mm; in-plane resolution: 1.0–2.0 mm; reconstructed cardiac phases: 30; temporal resolution: 30-60 ms. Values for pulmonary flow velocities were compared to published reports.23
Left Atrial Function
Left atrial (LA) volumes were calculated from using the area-length method from the two and the four-chamber views as previously described.24 LA volumes were measured at the end of ventricular systole (defined as the frame immediately prior to opening of the mitral leaflets, LA VOLmax), at the end of passive LV filling (defined as the frame immediately prior to LA contraction, LA VOLbac) and at the end of ventricular diastole, (LA VOLmin). Left atrial volumes were indexed to body surface area. From the LA volumes the following parameters were calculated: LA passive function (LAPEF) = (LA VOLmax – LA VOLbac) × 100%/LA VOLmax and 2) LA active emptying fraction (LACEF) = (LA VOLbac – LA VOLmin) × 100%/LA VOLbac.
Extracellular Volume Fraction
T1 measurements were performed with a Look-Locker sequence with a non-slice-selective adiabatic inversion pulse, followed by a gradient-echo acquisition (slice thickness 8 mm, TR > 3 RR intervals pre-contrast and 2 RR intervals post-contrast).11 The T1 sequence was performed in a single slice in the mid-ventricle and was repeated in the same mid LV short-axis slice once before and 3 additional times after the injection of gadolinium spanning a 20-30 minute period. For each Look-Locker sequence the endo- and epicardial borders of the LV were traced and divided into 6 standard segments) and segments were numbered 1 through 6 starting from the anterior RV insertion point and proceeding in a clockwise direction (MASS Research, Leiden University Medical Centre, The Netherlands). The slope of the linear relationship (the partition coefficient for gadolinium, λGd) was calculated and an ECV for all 6 myocardial segments was quantified as reported previously. 11, 25 A global ECV for each patient or healthy volunteer was then calculated by averaging the 6 myocardial segmental values.
Statistical methods
Descriptive statistics are presented as medians with interquartile ranges for continuous variables and as numbers and percentages for categorical variables. Data were assessed for normality of distribution and homogeneity of variance. Due to the small sample size, continuous variables were analyzed using Mann–Whitney tests, and categorical variables were analyzed using Fisher's exact test. The relationships between two different parameters were evaluated by simple correlation analysis. Two sided p-values of ≤0.05 were considered significant. All analyses were performed using GraphPad Prism 5 (GraphPad Software Inc; Chicago, IL) and SigmaPlot Version 11.0 (Systat Software Inc; San Jose, CA).
Results
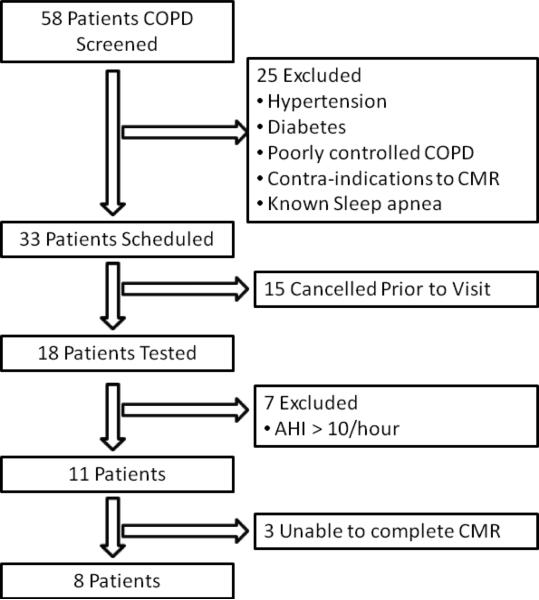
From a consecutive series of 58 patients with known COPD, 25 subjects were excluded (exclusion criteria included hypertension, diabetes, poorly controlled COPD, known sleep apnea, and routine contra-indications to the performance of a CMR, Figure 1). From this group, 33 patients were scheduled for study visits, of whom, 15 cancelled prior to the visit. The cohort of 18 patients all underwent testing; 7 of these had previously undiagnosed sleep apnea and were excluded leaving the final study population of 11 patients. Of these 11 patients all underwent a CMR study but 3 were unable to complete the CMR study, 2 due to claustrophobia and 1 due to an inability to breath-hold. This process left a final study population of 8 patients. Healthy volunteer subjects for comparison were selected from a larger cohort of 32 healthy volunteers to age- and gender-match the 8 subjects with COPD recruited as part of this study. There was no significant difference in age, gender, body mass index (BMI), body surface area (BSA), heart rate, blood pressure or hematocrit between COPD patients and controls (Table 1). All patients, both from the cohort of healthy volunteers used in this study and the study subjects self identified as being Caucasian. The peak flow of the 8 healthy volunteers averaged 398 litres/min for the female volunteers and 560 litres/min for the male volunteers.
Figure 1.
Study flow diagram: AHI: apnea-hypopnea index; COPD: chronic obstructive pulmonary disease
Table 1.
Study subject characteristics
| Variable | Healthy Controls (n=8) | COPD (n=8) | p value |
|---|---|---|---|
| Age, years | 63 (56, 68) | 60 (57, 68) | 0.96 |
| Female gender, n (%) | 6 (86%) | 6 (86%) | 1.00 |
| Body Mass Index (kg/m2) | 25.9 (23.1, 35.1) | 26.6 (22.0, 29.1) | 0.72 |
| Body Surface Area (m2) | 1.8 (1.7, 2.0) | 1.8 (1.7, 1.9) | 0.80 |
| Heart rate (beats/minute) | 70 (59, 81) | 67 (55, 68) | 0.33 |
| Systolic blood pressure (mmHg) | 125 (121, 129) | 128 (117, 129) | 0.88 |
| Diastolic blood pressure (mmHg) | 74 (65, 79) | 69 (68, 70) | 0.38 |
| Hematocrit, % | 45 (39, 46) | 44 (42, 46) | 0.96 |
Results are expressed as median (lower quartile, upper quartile), unless otherwise specified.
COPD severity
Patients with COPD had a median duration of diagnosis of 5.5 years (interquartile range 3.75 to 6.5). The median duration of smoking in pack years was 40 (interquartile range 19.4 to 75). All patients were on an inhaled bronchodilator at the time of the study, three patients (37.5%) were on inhaled steroids, no patient was on oral prednisone and no patient had required in-patient admission for an acute exacerbation of COPD in the 12 month calendar period prior to the study. The average predicted FEV1 was 65±9%, average predicted FVC was 84±12%, FEV1/FVC was 0.6±0.07, DLCO 11.9±1.9mmol CO per min per kPa, FEF25-75 was 37±18%, and RV/TLC was 117±29%. The average apnea-hypopnea index was 4.9±1.5 events per hour, the oxygen desaturation index was 10.4±4.4 events per hour, and the MMRC score was 1.38±0.9. The 6-minute walk distance was 277±27 meters. The mean hs-CRP was 2.4±2.1 mg/l and mean IL-6 was 5.3±1.9 pg/ml.
Cardiac magnetic resonance measures of cardiac size, function and PA pressures
There was no difference in left atrial volume, LV volume, LV mass, or LV ejection fraction between patients with COPD as compared to healthy controls (Table 2). The LV remodeling index was increased in patients with COPD as compared to controls (median 0.87 [IQR 0.71, 1.14] vs. 0.62 [0.60, 0.77], p = 0.03, Figure 2). There was no difference in RV volumes, RV mass or RV function between patients with COPD and healthy controls. The peak and mean PA blood flow velocities were preserved at 95±15 cm/sec and 15±6 cm/sec, respectively.23 In comparison, the peak and mean velocities in a group of healthy controls were 84±22 cm/sec and 16±5 cm/sec, respectively.23 The RV remodeling index was also similar between patients with COPD and controls (median 0.23 [IQR 0.19, 0.29] vs. 0.23 [0.16, 0.25], p=0.72). No patient had replacement myocardial fibrosis detected using LGE imaging.
Table 2.
Comparison of CMR Measures between Healthy Controls and COPD Subjects.
| Variable | Healthy Controls (n=8) | COPD (n=8) | p value |
|---|---|---|---|
| Maximal LA volume index, ml/m2 | 31 (27, 38) | 36 (33, 38) | 0.28 |
| LA volume before atrial contraction LA VOLbac, ml/m2 | 16 (14, 26) | 26 (23, 29) | 0.05 |
| Minimal LA volume index, ml/m2 | 10 (9, 15) | 15 (13, 16) | 0.10 |
| LA passive ejection fraction (LAPEF, %) | 44 (31, 51) | 24 (20, 30) | 0.007 |
| LA contractile ejection fraction (LACEF, %) | 38 (33, 43) | 46 (41, 49) | 0.005 |
| Right atrial area index, cm2/m2 | 5.2 (4.2, 7.9) | 7.2 (6.4, 11.1) | 0.04 |
| Left ventricular end-diastolic volume index, ml/m2 | 66 (55, 81) | 64 (56, 67) | 0.57 |
| Left ventricular end-systolic volume index, ml/m2 | 25 (19, 33) | 24 (21, 28) | 0.96 |
| Left ventricular ejection fraction, percent | 61 (58, 69) | 63 (56, 66) | 0.51 |
| Left ventricular mass index, g/m2 | 46 (39, 56) | 55 (49, 63) | 0.08 |
| Left ventricular remodeling index, g/ml | 0.62 (0.60, 0.77) | 0.87 (0.71, 1.14) | 0.03 |
| Right ventricular end-diastolic volume index, ml/m2 | 65 (56, 70) | 71 (51, 85) | 0.51 |
| Right ventricular end-systolic volume index, ml/m2 | 29 (25, 38) | 32 (24, 43) | 1.00 |
| Right ventricular ejection fraction, percent | 54 (50, 60) | 53 (51, 54) | 0.57 |
| Right ventricular mass index, g/m2 | 13 (11, 16) | 16 (14, 16) | 0.28 |
| Right ventricular remodeling index, g/ml | 0.23 (0.16, 0.25) | 0.23 (0.19, 0.29) | 0.72 |
| Extracellular Volume (ECV) | 0.27 (0.25, 0.30) | 0.32 (0.31, 0.36) | 0.001 |
Results are expressed as median (lower quartile, upper quartile); COPD: chronic obstructive pulmonary disease, LV: left ventricular, RV: right ventricular
Figure 2.
Comparison of the ECV (A) and the LV remodeling index (B) between patients with COPD and healthy controls; * = p < 0.05.
Left atrial volumes and function
There was no difference in the maximum or minimal LA volumes between patients and controls (Table 2); however, LA volumes at the end of passive LV filling (LA VOLbac) was increased in patients as compared to controls (median 26 [IQR 23, 29] vs. 16 [14, 26], p=0.05). Left atrial passive function was reduced (LAPEF, median 24 [IQR 20, 30] vs. 44 [31, 51], p=0.007), while LA active function (LACEF, median 46 [IQR 41, 49] vs. 38 [33, 43], p=0.005) was increased in patients with COPD as compared to controls.
Extracellular volume fraction
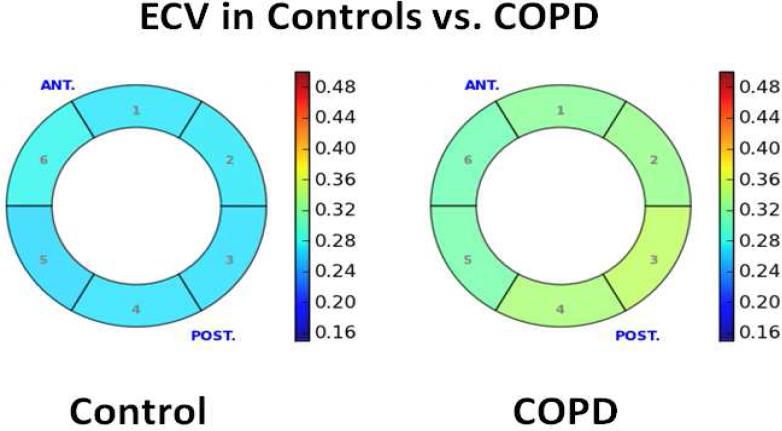
The ECV was increased in patients with COPD as compared to controls (median 0.32 [IQR 0.31, 0.36] vs. median 0.27 [IQR 0.25, 0.30], p=0.001, Figure 2). Among healthy controls there was no difference in the segmental ECV from the anterior segment through to the anteroseptal segment (0.27±0.03, 0.27±0.03, 0.28±0.03, 0.27±0.02, 0.28±0.02, 0.27±0.02, p = 0.87). Similarly, among patients with COPD, there was no difference in the segmental ECV values from the 6 different segments (0.33±0.03, 0.32±0.02, 0.32±0.03, 0.32±0.02, 0.35±0.05, 0.34±0.05, p = 0.73). Representative ECV measurements in a patient with COPD and a healthy control are shown in Figure 3. Among patients with COPD, there was no statistically significant association between the ECV and the duration of COPD (r=0.51, p = 0.20), pack-years history (r = 0.21, p = 0.61), FEV1 (r = 0.04, p = 0.92), FVC (r=0.08, p = 0.88), FEV/FVC (r=−0.14, p = 0.76) or the DLCO (r=0.22, p = 0.62). There was also no association between the ECV and serum measures of inflammation (ECV vs. hs-CRP, r=−0.09, p = 0.83), and measures of RV size or function. ‘Four of the subjects were active smokers and four were prior smokers. We did not find a difference in the ECV between current and active smokers (median 0.33 [IQR 0.31, 0.36] vs. 0.32 [0.30, 0.36], p=1.00). There was, however, a strong inverse association between ECV and the 6-minute walk distance (r=−0.79, p = 0.02) and LA passive emptying fraction (LAPEF, r =− 0.69, p = 0.003), and a positive association between the ECV and the LV remodeling index (r = 0.72, p = 0.04).
Figure 3.
Representative bulls eye comparison of the ECV between a healthy control (average ECV 0.27) and a patient with COPD (average ECV of 0.33). The figure shows a short-axis view of the mid-slice of the left ventricle with the ECV of each segment represented by color using the color chart to the right.
Discussion
In this pilot study, we performed a contrast CMR study in patients with COPD. Our hypothesis was that patients with COPD free of overt cardiovascular disease would have imaging evidence of diffuse myocardial fibrosis. We found that the ECV, a non-invasive measure of diffuse myocardial fibrosis, was elevated in patients with COPD. We found that an elevated ECV was associated with a low 6 minute walk time, reduced LA function, and adverse left ventricular remodeling.
We observed that an increased ECV had a strong association with reduced functional capacity, LA function, and adverse LV remodeling. The LV remodeling index provides an index of wall thickness to cavity size and is the equivalent of the echocardiogram-derived relative wall thickness.20 In patients with COPD, the increase in the LV remodeling index represented a combination of a minimally increased LV wall thickness and mass, with a relative reduction in the volume of the LV cavity. As a result, the ratio of LV mass to end-diastolic volume increased. Concentric LV remodeling is associated with a reduction in systolic and diastolic function,26, 27 and is an independent marker of increased risk in patients with and without cardiovascular disease.28-30 There was also a strong inverse association between the ECV and exercise duration. The 6 minute walk test is a robust measure of outcomes in patients with COPD.31, 32 These finding of an association between the ECV and functional status is novel in adults, and is in agreement with a previous description in a pediatric population.33 Finally, we found an inverse association between the ECV and passive LA function. We did not directly measure LV diastolic function, although it is known that LV diastolic function and LA passive ejection fraction are strongly related.34, 35 The novel finding of a relationship between the ECV as a measure of myocardial fibrosis and LA function passive function, as a surrogate for LV diastolic function, appears reasonable based on published data.36, 37
Ultimately, we aim to test our hypothesis that an expanded ECV will provide a biomarker identifying patients with COPD that are increased cardiovascular risk. The ECV has been validated as a non-invasive surrogate of myocardial fibrosis in both animals and humans,10, 11 in patients with heart failure is associated with measures of diastolic function and atrial enlargement,13 and provides independent prognostic information.14 There are limited data testing the presence of myocardial fibrosis in patients with COPD. Kohama and colleagues measured the extent of myocardial fibrosis in an autopsy study of a heterogeneous group of patients who died of advanced lung disease.6 They found that patients had increased myocardial fibrosis in the LV, specifically, the septum of the LV. There are limited non-invasive studies but Murphy and colleagues tested the prevalence of LGE, or replacement myocardial fibrosis, in a small population of patients with COPD.8 Similar to our study, none of the 25 patients with COPD in the prior study had LGE. However, despite the lack of LGE in our study, we found that patients had evidence of diffuse interstitial fibrosis. While expansion of the ECV can occur due to pathological processes other than fibrosis and collagen, we believe that this explanation represents the most logical possibility in this cohort. We excluded patients with diabetes, making abnormal cross-linking of fibers unlikely, and our population had no clinical or imaging evidence of amyloid or sarcoid.
The causes of an increased ECV and myocardial fibrosis in this population are unclear. We cannot exclude secondary pulmonary hypertension.38 However, we believe that the pulmonary pressures are unlikely to have been markedly elevated for several reasons. Firstly, our patients had relatively preserved FEV1, and important pulmonary hypertension is uncommon in ambulatory COPD patients especially until the FEV1 falls below 50%.39 Secondly, right ventricular mass has been reported to correlate well with catheterization-derived mean pulmonary pressures. We found no significant difference in RVMI. Thirdly, peak and mean PA velocities, which are reduced in patients with pulmonary hypertension,23 were preserved in this population. In our study the FEV1% was 68% of predicted, in comparison to an FEV1% of 41% in the study of Vonk-Noordegraaf and colleagues 40 and an FEV1 of over 40% in the study of Hilde and colleagues.41 We also tested for occult sleep apnea as sleep apnea is a frequently under-diagnosed co-morbidity in patients with COPD,42 and treatment of undiagnosed sleep apnea has been shown to reverse pulmonary hypertension,43 and reduce COPD-exacerbation related hospitalizations and mortality.44 However, none of our patients included in this study had sleep apnea using a threshold of apnea hypopnea index of less than 10 events per hour. We therefore believe that the most likely mechanism for an increase in myocardial fibrosis involves the association between COPD, inspiratory intrathoracic pressures, and LV afterload.45, 46 Patients with COPD can generate markedly negative pleural pressure on inspiration 47 (e.g. during hyperinflation with auto-PEEP), and it is possible that these pressures may increase LV wall stress from an increased afterload and induce myocardial fibrosis.
Our study has some limitations which merit discussion. This was a pilot study with small numbers, which limits some of the statistical interpretations. Our sample size would have limited the ability to detect difference in measures such as LV mass, RV mass, RV remodeling index.46 Further, we were unlikely to detect significant differences in descriptive variables between groups that may have led to bias in interpretation; however, qualitative assessment of the groups suggests that the COPD patients and controls were well-matched. We acknowledge that smoking amongst the COPD patients may have contributed to increased ECV; further studies are needed to clarify this issue. Our study included selected patients from a single medical center, which could affect the generalizability of our findings. The study was approved by the local IRB and all testing and data recording were done prospectively and as part of the pre-specified study protocol. This protocol did not include the performance of an echo and a right heart catheterization. We also did not perform a formal sleep study or full pulmonary function test in our healthy volunteers. However, none of our volunteers were smokers, none had a history of pulmonary disease, were of normal weight, had normal spirometry, and all underwent a thorough history and physical examination by a licensed pulmonologist making the probability for presence of occult pulmonary disease relatively low. A future area of research is to test methods for measurement of the ECV in the RV free wall. However, at present, we are unable to measure the ECV in a non-hypertrophied RV free wall as the spatial resolution is not sufficient. Finally, we did not test LV and RV systolic function beyond measures of EF or test whether an expanded ECV was associated with subtle RV dysfunction.41
Conclusions
In conclusion, patients with COPD without clinical heart failure have an increased extracellular volume. This increase in the ECV in COPD is associated with measures of exercise capacity and adverse LV remodeling. The mechanism for this increase is not clear but is likely due to myocardial fibrosis. Further data through mechanistic research and clinical trials are required to determine if this preliminary finding can be replicated in larger studies, whether the increase in the ECV is associated with the risk for development of subsequent heart failure, and whether intervention based on this measure can reduces the risk for development of adverse clinical outcomes in this high-risk population.
Brief summary.
The mechanistic association between COPD and cardiovascular morbidity and mortality is not completely understood; thus we aimed to assess whether diffuse myocardial fibrosis was evident in patients with COPD compared with controls. Our study indicates that expansion of the myocardial extracellular volume, suggestive of diffuse myocardial fibrosis, is present in COPD and is associated with left ventricular remodeling, reduced left atrial function and exercise capacity.
Acknowledgments
Funding Sources
This work was supported in part by an American Heart Association Fellow to Faculty Grant (12FTF12060588, TGN), and project grants (R01HL090634-01A1, MJH; R01HL091157, RYK). Dr. Malhotra is PI on the following grants: AHA 0840159N, NIH R01 HL090897, NIH K24 HL 093218, NIH 1 P01 HL 095491 (Overall PI: Saper, Brigham PI: Malhotra), NIH R01HL110350, NIH UM1HL108724 (Overall PIs: Talmor/Loring, Brigham PI: Malhotra), NIH R01AG035117, NIH R01 HL085188. The Harvard Catalyst is funded by UL1 RR 025758-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Sidney S, Sorel M, Quesenberry CP, Jr., DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–75. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal SK, Heiss G, Barr RG, et al. Airflow obstruction, lung function, and risk of incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Heart Fail. 2012;14:414–22. doi: 10.1093/eurjhf/hfs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Man SF, Van Eeden S, Sin DD. Vascular risk in chronic obstructive pulmonary disease: role of inflammation and other mediators. Can J Cardiol. 2012;28:653–61. doi: 10.1016/j.cjca.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Neilan TG, Shah RV, Abbasi SA, et al. The Incidence, Pattern, and Prognostic value of Left Ventricular Myocardial Scar by Late Gadolinium Enhancement in Patients with Atrial Fibrillation. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neilan TG, Coelho-Filho OR, Danik SB, et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944–54. doi: 10.1016/j.jcmg.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohama A, Tanouchi J, Hori M, Kitabatake A, Kamada T. Pathologic involvement of the left ventricle in chronic cor pulmonale. Chest. 1990;98:794–800. doi: 10.1378/chest.98.4.794. [DOI] [PubMed] [Google Scholar]
- 7.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 8.Murphy CA, Blyth KG, Chaudhuri R, et al. Assessment of the presence of occult myocardial infarction in chronic obstructive pulmonary disease using contrast-enhanced cardiac magnetic resonance imaging. Respiration. 2009;78:263–9. doi: 10.1159/000203354. [DOI] [PubMed] [Google Scholar]
- 9.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flett AS, Hayward MP, Ashworth MT, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 11.Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6:672–83. doi: 10.1016/j.jcmg.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachour A, Virkkala JT, Maasilta PK. AutoCPAP initiation at home: optimal trial duration and cost-effectiveness. Sleep Medicine. 2007;8:704–10. doi: 10.1016/j.sleep.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. 2013;111:717–22. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TC, Piehler K, Meier CG, et al. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, Miyazaki C, Bruce CJ, Ommen S, Miller FA, Oh JK. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr. 2008;21:1129–37. doi: 10.1016/j.echo.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma B, Neilan TG, Kwong RY, et al. Evaluation of right ventricular remodeling using cardiac magnetic resonance imaging in co-existent chronic obstructive pulmonary disease and obstructive sleep apnea. Copd. 2012;10:4–10. doi: 10.3109/15412555.2012.719050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson PA, Jaffer FA, Neilan TG, Shepard JA, Stone JR. Case records of the Massachusetts General Hospital. Case 34-2006. A 72-year-old woman with nausea followed by hypotension and respiratory failure. N Engl J Med. 2006;355:2022–31. doi: 10.1056/NEJMcpc069025. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 20.Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19:1550–8. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 21.Roeleveld RJ, Marcus JT, Boonstra A, et al. A comparison of noninvasive MRI-based methods of estimating pulmonary artery pressure in pulmonary hypertension. J Magn Reson Imaging. 2005;22:67–72. doi: 10.1002/jmri.20338. [DOI] [PubMed] [Google Scholar]
- 22.Ley S, Mereles D, Puderbach M, et al. Value of MR phase-contrast flow measurements for functional assessment of pulmonary arterial hypertension. Eur Radiol. 2007;17:1892–7. doi: 10.1007/s00330-006-0559-9. [DOI] [PubMed] [Google Scholar]
- 23.Sanz J, Kuschnir P, Rius T, et al. Pulmonary arterial hypertension: noninvasive detection with phase-contrast MR imaging. Radiology. 2007;243:70–9. doi: 10.1148/radiol.2431060477. [DOI] [PubMed] [Google Scholar]
- 24.Farzaneh-Far A, Ariyarajah V, Shenoy C, et al. Left atrial passive emptying function during dobutamine stress MR imaging is a predictor of cardiac events in patients with suspected myocardial ischemia. JACC Cardiovasc Imaging. 2011;4:378–88. doi: 10.1016/j.jcmg.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–34. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen BD, Edvardsen T, Lai S, et al. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation. 2005;112:984–91. doi: 10.1161/CIRCULATIONAHA104.500488. [DOI] [PubMed] [Google Scholar]
- 27.Kuznetsova T, Herbots L, Richart T, et al. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29:2014–23. doi: 10.1093/eurheartj/ehn280. [DOI] [PubMed] [Google Scholar]
- 28.Verdecchia P, Schillaci G, Borgioni C, et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995;25:871–8. doi: 10.1016/0735-1097(94)00424-O. [DOI] [PubMed] [Google Scholar]
- 29.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundstrom J, Lind L, Nystrom N, et al. Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation. 2000;101:2595–600. doi: 10.1161/01.cir.101.22.2595. [DOI] [PubMed] [Google Scholar]
- 31.Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132:1778–85. doi: 10.1378/chest.07-2050. [DOI] [PubMed] [Google Scholar]
- 32.Spruit MA, Polkey MI, Celli B, et al. Predicting outcomes from 6-minute walk distance in chronic obstructive pulmonary disease. J Am Med Dir Assoc. 13:291–7. doi: 10.1016/j.jamda.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teo SG, Yang H, Chai P, Yeo TC. Impact of left ventricular diastolic dysfunction on left atrial volume and function: a volumetric analysis. Eur J Echocardiogr. 2010;11:38–43. doi: 10.1093/ejechocard/jep153. [DOI] [PubMed] [Google Scholar]
- 35.Nikitin NP, Witte KK, Thackray SD, Goodge LJ, Clark AL, Cleland JG. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr. 2003;4:36–42. doi: 10.1053/euje.2002.0611. [DOI] [PubMed] [Google Scholar]
- 36.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–93. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 37.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–7. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 38.Bogaard HJ, Natarajan R, Henderson SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–60. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 39.Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest. 137:39S–51S. doi: 10.1378/chest.10-0087. [DOI] [PubMed] [Google Scholar]
- 40.Vonk-Noordegraaf A, Marcus JT, Holverda S, Roseboom B, Postmus PE. Early changes of cardiac structure and function in COPD patients with mild hypoxemia. Chest. 2005;127:1898–903. doi: 10.1378/chest.127.6.1898. [DOI] [PubMed] [Google Scholar]
- 41.Hilde JM, Skjorten I, Grotta OJ, et al. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol. 2013;62:1103–11. doi: 10.1016/j.jacc.2013.04.091. [DOI] [PubMed] [Google Scholar]
- 42.Bakker JP, Sharma B, Malhotra A. Obstructive sleep apnea: the elephant in the cardiovascular room. Chest. 2011;141:580–1. doi: 10.1378/chest.11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arias MA, Garcia-Rio F, Alonso-Fernandez A, Mediano O, Martinez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375–83. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 44.Machado MC, Vollmer WM, Togeiro SM, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35:132–7. doi: 10.1183/09031936.00192008. [DOI] [PubMed] [Google Scholar]
- 45.Buda AJ, Pinsky MR, Ingels NB, Jr., Daughters GT, 2nd, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301:453–9. doi: 10.1056/NEJM197908303010901. [DOI] [PubMed] [Google Scholar]
- 46.Smith BM, Kawut SM, Bluemke DA, et al. Pulmonary hyperinflation and left ventricular mass: the Multi-Ethnic Study of Atherosclerosis COPD Study. Circulation. 2013;127:1503–11. 11e1–6. doi: 10.1161/CIRCULATIONAHA.113.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potter WA, Olafsson S, Hyatt RE. Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung disease. J Clin Invest. 1971;50:910–9. doi: 10.1172/JCI106563. [DOI] [PMC free article] [PubMed] [Google Scholar]