Abstract
Background
Qualitative research provides insight into the cancer experience through the perspective of the pediatric patient. However, somewhat small sample sizes can hinder full discovery of new knowledge and limit interpretation of data.
Objective
To describe health-related quality of life (HRQOL) reported by children and adolescents in responses to two interview questions during treatment for acute lymphoblastic leukemia (ALL) and compare their responses by age, gender, risk group, and time in treatment through a quantitative content analysis approach.
Interventions/Methods
Children and adolescents (N=150) were asked two validated questions in pediatric patients receiving treatment for ALL: “What makes a good day for you” and “How has being sick been for you” over six treatment time points. Interview data were coded analyzed quantitatively.
Results
Code frequencies differed significantly by age, gender, risk group, and time in treatment. Adolescents had a greater focus on being with friends and females generally reported more codes representing negative experiences. Children and adolescents reported being affected by symptoms resulting from cancer treatment. Some adolescents described that being sick positively changed their lives, and viewed their illness as a new life experience.
Conclusion
The two proposed questions are feasible to use clinically to assess HRQOL in children and adolescents with ALL, and the qualitative codes from their descriptions can be used to identify factors affecting HRQOL of children and adolescents with leukemia
Implications for practice
Nurses can use these two questions to assess the HRQOL of children and adolescents during and following treatment for ALL.
Introduction
Assessing health-related quality of life (HRQOL) during cancer therapy is an important indicator of patient reported outcomes and provides measurement of the physical, emotional, and social role functioning of children and adolescents,1,2 with the intent of developing interventions and thus improving the patients HRQOL during and after treatment.2 Assessing HRQOL over the cancer treatment trajectory provides important evidence of the impact of treatment on the ill child or adolescent. Numerous instruments have been used to assess HRQOL in children and adolescents with cancer, with the more commonly measured cancer- specific domains being pain, anxiety, cancer worry, nausea, and body image.3
A recent review of pediatric HRQOL instruments revealed specific instrument limitations, including the lack of input or feedback from the patient in item development. 3 Without the patient perspective, the conceptual and empirical HRQOL is incomplete and may contribute to inaccurate descriptions.4 Most instruments that measure HRQOL in children with cancer do not have age-sensitive items, but instead use the same item in all age forms or in the single form available. Furthermore, HRQOL instruments include universal items that may not address gender-related differences in perceptions, even though such differences are known to exist.3,5 Together, these limitations of available HRQOL instruments can compromise their content validity in measuring the full spectrum of HRQOL experiences5-7 and raise questions about the clinical validity of HRQOL instruments.4 Self-reported, open-ended questionnaires may provide more accurate information about how individual children and adolescents perceive their HRQOL during cancer therapy3 and yield information specific to the individual child or adolescent that can be used to guide clinical care.
To develop self-reported questionnaires, we conducted two pilot studies using open-ended interview questions to explore the perceptions of children and adolescents regarding their HRQOL during cancer therapy. In these studies, pediatric oncology patients were asked four questions; however, responses from two questions were found to sufficiently assess HRQOL from the child’s or adolescent’s perspective, as responses to the remaining two questions were duplicative of the responses to the two interview questions. Patients reported a sense of well-being based on the ability to participate in their usual activities, interact with others, feel cared for, cope with uncomfortable physical and emotional reactions, and to find meaning in their illness.4 This kind of qualitative approach allows children and adolescents to describe their cancer experiences and provides a premise from which new hypotheses for assessing HRQOL can be generated.
The pilot studies identified 6 domains that comprise HRQOL of children with cancer. The six domains were (1) symptoms (both disease and treatment related), (2) usual activities, (3) social/family interactions, (4) health status, (5) mood, and (6) meaning of illness. The conceptual definition of HRQOL derived from the initial pilots was: “an overall sense of well-being based on being able to participate in usual activities; to interact with others and feel cared about; to cope with uncomfortable physical, emotional, and cognitive reactions; and to find meaning in the illness experience.”4
Study Purpose
The previously completed pilot studies provided guidance for the development of our HRQOL study, labeled the Good Day Study, which was incorporated into a pediatric Phase III clinical trial for ALL. The Good Day Study purpose was to describe HRQOL over the course of treatment for ALL from the perspective of children and adolescents using two validated interview questions. Our aims were to identify the components of their HRQOL and the factors related to any differences in their responses according to age, gender, risk, and time in treatment.
Methods
Study Population
The Good Day Study was a secondary objective of a larger nurse-led quality-of-life objective nested in an institutional therapeutic protocol (Total XV) for the treatment of ALL. The overall objective of the institutional protocol was to improve the treatment response rate in children and adolescents with ALL through risk-directed therapy. Inclusion criteria for the Good Day Study were enrollment in the institutional ALL protocol, ability to understand English, ages 8 to 18 years, and providing informed consent or assent. The study was approved by the institutional review board.
The Good Day study sample included 150 children and adolescents interviewed at six time points. The demographics were consistent with ALL, in that more children than adolescents and more males than females were eligible for the study (Table 1). For logistic considerations of group sizes and responses at each time point, and the limitations of the coding and analysis software, each patient response (i.e., entry) during the interview was treated as a case. Thus, the total number of study cases from the 150 patients was 578. Figure 1 depicts the number of patients (n=150) to the number of first-level codes for Question 1 (n=1207) and Question 2 (n=987).
Table 1.
Patient Demographics by Age, Gender, and Risk Group Stratified by Number of Patients, Cases, and Codes
| Risk
|
Total
|
|||||
|---|---|---|---|---|---|---|
| Low | Standard | High | Patients | Cases | Codes | |
|
| ||||||
| Female | 20 | 35 | 2 | 57 | 210 | 855 |
| Child | 17 | 17 | 1 | 35 | 131 | 461 |
| Adolescent | 3 | 18 | 1 | 22 | 79 | 394 |
| Male | 21 | 69 | 3 | 93 | 368 | 1339 |
| Child | 15 | 32 | 2 | 49 | 196 | 717 |
| Adolescent | 6 | 37 | 1 | 44 | 172 | 622 |
| Total | 41 | 104 | 5 | 150 | 578 | 2194 |
Figure 1. Diagram Depicting Enrollment, Attrition by Data Point, and Frequency of Codes.
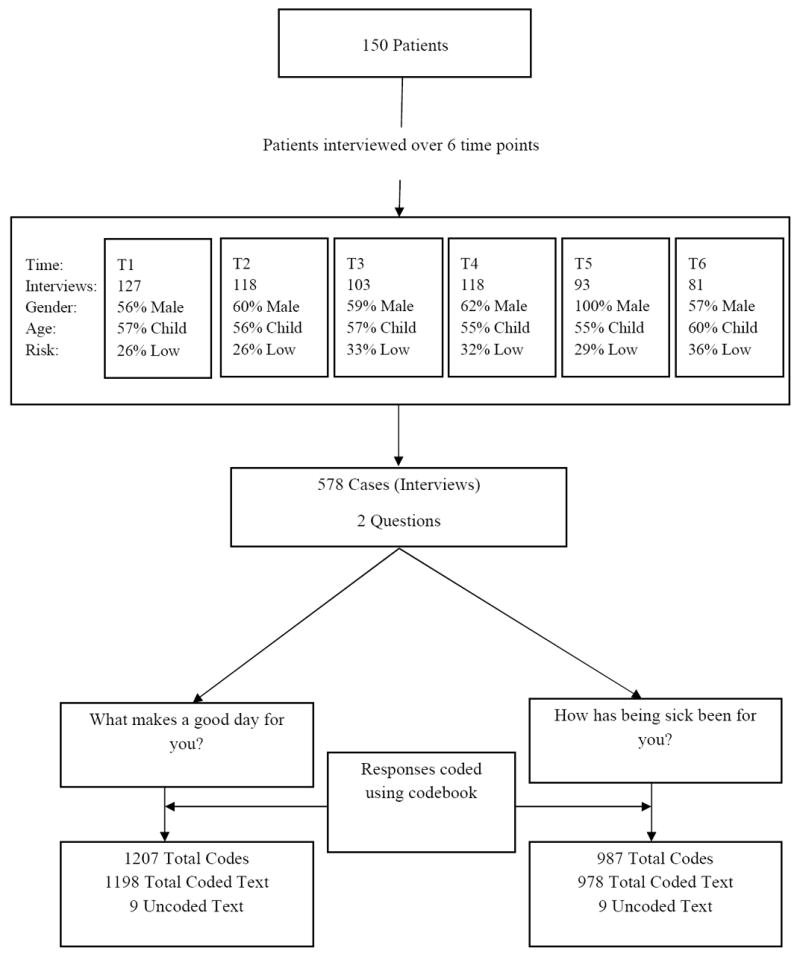
Study Procedures
Two open-ended questions were posed to children and adolescents at six time points (T1–T6) during the course of their treatment: (1) “What makes a good day for you?” (Question 1) and (2) “How has being sick been for you?” (Question 2). These questions were asked at day 40 of induction (T1), week 7 of consolidation (T2), week 48 of continuation (T3), week 120 of maintenance therapy (completion of therapy for females) (T4), week 146 of maintenance (males only) (T5), and 2 years after the completion of therapy (T6). These time points were chosen to represent clinically significant milestones in the therapeutic treatment protocol. Data were collected from 2002 through 2012.
Data Analysis
The creation of the coding dictionary used for the interview responses in the Good Day Study has been detailed in a pilot study previously reported by Hinds et al.4 In brief, in that study, coding was completed by five pediatric oncology nurses who participated in eight training sessions facilitated by two researchers. The initial interviews from the pilot study were coded jointly by all five nurses and a coding dictionary was generated. Subsequently, intercoder agreement was calculated and was found to range from 81% to 100% across coders and interview questions. None of the codes clustered with other codes, indicating that the code labels were sufficiently distinct from each other.
In the Good Day Study, all responses to the two interview questions were independently coded, using the coding dictionary from the pilot, by three researchers to ensure the reliability of coding. When coding was discrepant among coders, all coders met to discuss their interpretations and in each instance consensus was achieved on a final coding of the response. During the coding phase, the dictionary was revised to reflect the grouping of some first-level codes for their similarity of meaning and thus identified as a theme that captured the shared meaning. Several rarely reported first-level codes were excluded from the analysis as they occurred at low frequencies and could not be combined with other codes because of their conceptual uniqueness.
Coded qualitative responses were entered into the QDA miner program (version 4.0; Provalis Research, Montreal, Canada), an advanced qualitative data analysis software package for coding, annotating, retrieving, and analyzing small and large collections of documents and images.8 The codes were compared quantitatively for intrasample differences. Data were analyzed using descriptive frequencies and Chi-square tests, with the alpha level of significance set at 0.05. For time analysis, each time point was compared against the mean of all the other time points. No adjustment was made on the P-values for multiple comparisons. Descriptive variables included age (children 8–12 years vs. adolescents 13–18 years), gender, risk group (low vs. standard/high), and time (the 6 data collection points). Risk groups were categorized into low vs. standard/high for our analyses, as we had a limited number of patients in the high-risk arm. Some codes were repeated multiple times within the same interview and were treated as separately occurring codes for the same case in our quantitative analysis.
Of the 2194 responses coded during the study, only 18 responses (0.008%) could not be coded using our coding dictionary because they were categorized by two or more study team members as “not possible to comprehend the intended meaning” or as “not considered a response to the actual interview question.” We interpreted this very low rate of uncoded responses as indicating that the coding dictionary captured the spectrum of conceptual meanings reflected in the responses. Fifty-two codes were identified and included in our final coding dictionary. Codes were distributed across the six conceptual domains of HRQOL as described in our previous pilot study. 4 Table 2 presents examples of codes with definitions and exemplar corresponding responses.
Table 2.
Sample Codes, Definitions, and Examples of Patient Responses
| Code Word | Definition | Sample Responses |
|---|---|---|
| What Makes A Good Day For You? | ||
| Able Eat | Being able to eat | “If I can eat,” “Getting breakfast in bed,” “Having breakfast—especially good—biscuits,” “When I get to eat” |
| At Home | Being able to be at home | “The day they tell me I can go home,” “When I don’t have to come to the hospital,” “When I get to go home” |
| Be Normal | Being normal | “When I feel like a normal kid,” “When my life becomes close to normal or feels normal” |
| Do Usual | Doing my usual activities | “A good day is when we go out and do something like go to the zoo or the Fire Museum,” “Playing my video games and watching TV and running around playing with her [my little sister]” |
| Family | Being with family | “When I go see my grandparents,” “Whenever my family comes to visit me,” “Being with my mom and dad” |
| Friends | Feeling close with friends | “Talking to my girlfriend,” “Seeing my friends,” “When I go hang out with my friends,” “Having friends around” |
| Gd Mood | Positive mood | “When I get up in a good mood,” “When I’m relaxed and having a good day,” “Getting up in a good mood” |
| Go Places | Going places/being entertained | “Going to do something like the mall,” “Going to the movies,” “Going to sports games like the Grizzlies or Redbirds,” “Going fun places like the zoo or the fish store” |
| No Hosp | Not going to the hospital | “Not having to go to the hospital,” “When clinic visit is not scheduled for week” |
| No Sick | Not feeling sick | “When I feel good, when I can play, when I laugh a lot, not having to be here all the time,” “When I have no pain in my neck. Days like this—it just felt like a good day” |
| Smooth | Nothing goes wrong | “Just if nothing goes wrong—like no bad muscle cramps, no joint pain, no headaches. You know, just doing okay” |
| Spirit | Believing in a greater being | “The Lord makes my day really special” “To wake up and know there’s a god above” “Thanking the Lord for letting me be here” |
|
| ||
|
How Has Being Sick Been For You?
| ||
| Bad Hard | Illness experience hard | “Being sick really sucks, pain, all the things you can’t do like go home and have fun” |
| Bad Sick | Symptoms of being sick | “Bad, because I have been throwing up a lot,” “I get really scared when I pass out” “bad—especially nausea and ulcers” |
| Benefit | Receiving benefits | “It’s fun because I don’t have to go to school,” “I’ve met a lot of people to help me with my homework,” “People have been a lot nicer to me since I have been sick,” “Because I get all the attention” |
| Can’t Do | Can’t do what I want | “Not being able to do my normal stuff,” “Don’t feel like doing anything, can’t do the things I used to be able to do” |
| Gd Change | Changed life for the good | “It has actually made me a better person. I have always been tense, angry and selfish. I feel I need my family more. I’m more emotional, I cry more (teary eyed). I just have a better attitude towards life.” |
| Good | Positive | “Actually, it has been pretty good to me,” “Pretty good overall” “Everything has gone well for me,” “I still like coming here and seeing people” |
| New Exp | Change | “Being sick has really changed my life a lot. The medicine makes you very emotional so you cry a lot and have mood swings and have good days and bad days,” “An unforgettable experience” |
| No Friend | Missing friends and family | “I miss my normal life, friends, family, school and being at home.” |
| Not Bad | Not so bad | “It hasn’t really bothered me much, it really hasn’t” “Sick? I have not really been sick,” “Not as bad as I thought it would be so far” |
| Others | Being with others I like | “I’ve made a lot of friends here at and have met lots of cancer patients to talk to,” “Get to meet other children,” “I have met lots of new people that I think very dearly of” |
| Used To It | Getting used to it | “I’m used to it now,” “At first it was worse, but now it got better—I’m used to it,” “It’s upsetting, but I’ve come to terms with it” |
| Trust God | Trusting in God | “I know that there is some reason the Lord has put this burden on my family and me,” “I’m handling it good so far thanks to the good Lord” |
Results
Question 1: What makes a good day for you?
A total of 26 codes were included in the analysis of the responses to Question 1 and ranged from 0.5% to 34.9% of cases. For all time points combined, the most frequent response for what made a good day was “doing the usual,” followed by “being with friends.” Another commonly reported category was symptoms, with patients describing “not being sick” as making a good day.
Of the total codes, statistically significant differences were found with respect to age, gender, risk, and time (Table 3). The largest differences between children and adolescents were for the codes “having energy” and “not being sick”, both were being reported significantly more often by adolescents than children. Seven codes differed significantly by gender, with females reporting higher frequencies of codes than males. The largest differences were found in the codes of “having energy” and “not being sick,” both being reported more frequently by female patients. Three codes differed significantly by treatment risk group. Patients in the standard-/high-risk group were more likely to report a good day being “a short stay at the hospital” and “not being sick” than patients in the low-risk group, who were more likely to report a good day as “being at home.”
Table 3.
The 11 Most Frequent Codes Reported in Response to “What Makes A Good Day For You?”
| Category | Code | Count | % of Codes | Cases | % of Cases |
|---|---|---|---|---|---|
| Usual Acts | Do Usual | 286 | 23.7 | 202 | 34.9 |
| Social/Family Interactions | Friends | 160 | 13.3 | 153 | 26.5 |
| Symptoms | No Sick | 142 | 11.8 | 124 | 21.5 |
| Social/Family Interactions | Family | 85 | 7.0 | 80 | 13.8 |
| Health Status | No Hosp | 77 | 6.4 | 67 | 11.6 |
| Usual Acts | Go Places | 57 | 4.7 | 52 | 9.0 |
| Symptoms | Able Eat | 53 | 4.4 | 44 | 7.6 |
| Mood | Good Mood | 41 | 3.4 | 41 | 7.1 |
| Usual Acts | Be Normal | 32 | 2.7 | 29 | 5.0 |
| Social/Family Interactions | At Home | 30 | 2.5 | 29 | 5.0 |
| Symptoms | Smooth | 33 | 2.7 | 29 | 5.0 |
Over the six data collection time points, the number of significantly different codes decreased steadily from 11 (T1) to 6 (T4) (Table 4). At induction (T1), patients described a good day as not being at the hospital (no hosp); with this code becoming less frequent during treatment and not reported at all at the off-therapy time point (T6). Patients also expressed the importance of not being sick (no sick) as making a good day and continued to report the importance of not being sick 2 years after the completion of therapy. Both children and adolescents reported benefits associated with their illness, with this response being highest at T6. Across all time points, including off-therapy, not being sick (no sick) and being with friends (friends) made a good day. The importance of friends was the most commonly reported code to Question 1 at the 2-year off-therapy visit.
Table 4.
Significantly Different Code Percentages for “What Makes A Good Day for You?” by Age, Gender, Risk Group, and Time Point
| Age (No. of Codes)/%
|
Student’s F | P value | ||
|---|---|---|---|---|
| Child (n=662)/% | Adolescent (n=545)/% | |||
| Energy | 0.8 | 4.2 | 18.466 | .00 |
| No Sick | 7.1 | 17.4 | 31.025 | .00 |
| Do Usual | 29.6 | 16.5 | 11.025 | .01 |
| Not Sure | 2.7 | 0.6 | 7.605 | .01 |
| Be Normal | 1.5 | 4.0 | 7.448 | .01 |
| Just Live | 0.5 | 1.8 | 6.117 | .01 |
| Benefit | 3.2 | 1.1 | 5.212 | .02 |
| At Home | 3.5 | 1.3 | 4.882 | .03 |
| Good Mood | 2.6 | 4.4 | 4.117 | .04 |
| Short Stay | 0.9 | 2.2 | 4.100 | .04 |
| Take Care | 0.0 | 0.6 | 3.942 | .05 |
|
| ||||
|
Gender (Codes)/%
|
||||
| Female (n=458)/% | Male (n=749)/% | Student’s F | P value | |
|
| ||||
| Energy | 4.4 | 1.1 | 16.050 | .00 |
| No Sick | 15.3 | 9.6 | 9.695 | .01 |
| Do Usual | 17.5 | 27.5 | 5.672 | .02 |
| Take Care | 0.7 | 0.0 | 5.315 | .02 |
| Short Stay | 0.4 | 2.1 | 5.137 | .02 |
| Fine | 1.5 | 0.4 | 5.013 | .03 |
| Benefit | 1.1 | 2.9 | 3.898 | .05 |
|
| ||||
|
Risk Group (Codes)/%
|
||||
| Low (n=342)/% | Standard/High (n=865)/% | Student’s F | P value | |
|
| ||||
| Short Stay | 0.3 | 2.0 | 5.293 | .02 |
| No Sick | 8.8 | 12.9 | 4.892 | .03 |
| At Home | 4.1 | 1.8 | 3.962 | .05 |
|
Time (Codes)/%
|
||||||||
| T1 (n=290)/% | T2 (n=243)/% | T3 (n=213)/% | T4 (n=203)/% | T5 (n=110)/% | T6 (n=148)/% | Student’s F | P value | |
|
| ||||||||
| Short Stay | 4.1 | 0.8 | 0.9 | 1.0 | 0.0 | 0.0 | 4.706 | .00 |
| At Home | 2.1 | 6.6 | 1.4 | 0.5 | 3.6 | 0.0 | 5.020 | .00 |
| No Hosp | 10.7 | 9.9 | 6.1 | 3.9 | 0.9 | 0.0 | 6.164 | .00 |
| No Sick | 16.2 | 11.9 | 10.8 | 13.8 | 2.7 | 8.1 | 3.973 | .00 |
| Benefit | 0.3 | 0.8 | 3.3 | 2.0 | 3.6 | 6.1 | 3.264 | .01 |
| Visits | 1.7 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | 2.824 | .02 |
| Be Normal | 1.0 | 2.5 | 1.9 | 6.4 | 1.8 | 2.7 | 2.735 | .02 |
| Gift Mail | 1.7 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 | 2.552 | .03 |
| Friends | 7.2 | 11.9 | 16.9 | 14.8 | 16.4 | 17.6 | 2.432 | .03 |
| Go Places | 4.5 | 8.6 | 3.3 | 2.5 | 6.4 | 2.7 | 2.328 | .04 |
| Able Eat | 6.2 | 2.1 | 2.8 | 5.4 | 9.1 | 2.0 | 2.283 | .05 |
Question 2: How has being sick been for you?
A total of 26 codes were included for analyzing the responses to Question 2 and ranged from 0.3% to 39.4% of cases. The most frequently occurring codes are listed in Table 5. For all time points combined, the most frequent response was “bad hard.” Although patients viewed the experience as hard, they also expressed being sick as “not bad” and “good.”
Table 5.
11 Most Frequent Codes Reported in Response to “How Has Being Sick Been For You?”
| Category | Code | Count | (%) of Codes | Cases | % of Cases |
|---|---|---|---|---|---|
| Symptoms | Bad Hard | 244 | 24.7 | 228 | 39.4 |
| Health Status | Not Bad | 199 | 20.2 | 188 | 32.5 |
| Mood | Good | 86 | 8.7 | 81 | 14.0 |
| Symptoms | Bad Sick | 95 | 9.6 | 77 | 13.3 |
| Usual acts | Can’t Do | 59 | 6.0 | 46 | 8.0 |
| Meaning of Illness | Good Change | 39 | 4.0 | 33 | 5.7 |
| Meaning of Illness | New Exp | 27 | 2.7 | 27 | 4.7 |
| Meaning of Illness | Used To It | 27 | 2.7 | 26 | 4.5 |
| Social/Family Interactions | Others | 21 | 2.1 | 20 | 3.5 |
| Social/Family Interactions | No Friend | 22 | 2.2 | 20 | 3.5 |
| Meaning of Illness | Benefit | 25 | 2.5 | 20 | 3.5 |
The code “harder” was reported across all treatment groups, but was reported significantly more often among patients with standard-/high-risk disease. Over the six time points, the number of codes that differed significantly by time decreased from five to three (Table 6). Code frequencies also differed significantly between children and adolescents on seven codes, with the largest differences being for the codes “no home,” “good thought,” and “good change,” all being reported more frequently by adolescents than children. Children more often reported “benefit” from being sick. Females and males differed significantly on eight codes. Males reported being sick as “not bad” whereas females reported being sick as “bad hard,” “can’t do,” and “harder.” Although females reported being sick as a difficult experience, they were significantly more likely to report being sick as a “good change.” Females also expressed an inability to perform age-appropriate activities “can’t do” and missed friends “no friends” significantly more often than males.
Table 6.
Significantly Different Code Percentages For “How Has Being Sick Been For You?” by Age, Gender, Risk Group, and Time Point
| Age (No. of Codes)/%
|
Student’s F | P value | ||
|---|---|---|---|---|
| Child (n=516)/% | Adolescent (n=471)/% | |||
| No Home | 0.40 | 3.00 | 8.002 | .01 |
| Good Thought | 0.20 | 1.90 | 7.537 | .01 |
| Good Change | 2.50 | 5.50 | 6.435 | .01 |
| Benefit2 | 4.10 | 0.80 | 5.358 | .02 |
| New Exp | 1.90 | 3.60 | 4.419 | .04 |
| Be Worse | 0.60 | 1.70 | 3.932 | .05 |
| Trust God | 0.00 | 0.60 | 3.942 | .05 |
|
| ||||
|
Gender (Codes)/%
|
||||
| Female (n=397)/% | Male (n=590)/% | Student’s F | P value | |
|
| ||||
| Not Bad | 12.8 | 25.1 | 13.111 | .01 |
| Bad Hard | 27.5 | 22.9 | 10.028 | .01 |
| At Hosp | 0.0 | 2.2 | 7.664 | .01 |
| Can’t Do | 8.3 | 4.4 | 6.860 | .01 |
| Harder | 2.3 | 0.5 | 5.273 | .02 |
| Good Change | 5.5 | 2.9 | 5.089 | .02 |
| No Friend | 3.3 | 1.5 | 4.324 | .04 |
| Bad Sick | 11.6 | 8.3 | 4.292 | .04 |
|
Time (Codes)/%
|
||||||||
| T1 (n=240)/% | T2 (n=206) /% | T3 (n=180) /% | T4 (n=167) /% | T5 (n=85) /% | T6 (n=109) /% | Student’s F | P value | |
|
| ||||||||
| No Home | 5.8 | 0.5 | 0.6 | 0.0 | 0.0 | 0.0 | 5.236 | .01 |
| Can’t Do | 10.4 | 7.3 | 6.1 | 1.8 | 3.5 | 1.8 | 3.142 | .01 |
| No Friend | 4.6 | 3.4 | 1.1 | 1.2 | 0.0 | 0.0 | 2.837 | .02 |
| Used To It | 1.3 | 1.9 | 1.7 | 3.0 | 9.4 | 3.7 | 2.715 | .02 |
| Bad Sick | 11.3 | 14.1 | 11.1 | 6.6 | 3.5 | 4.6 | 2.499 | .03 |
When analyzed across time points, some codes decreased in frequency whereas others increased. As patients progressed in treatment, missing home “no home” and missing friends “no friends” were expressed less frequently. There were no responses for these two codes by time points 4 and 5. As patients progressed in treatment, they reported getting “used to it” and continued reporting this response 2 years after completion of treatment. Patients also increasingly reported “good change” as they progressed in time. Of note, across all time points, “bad sick” continued to be reported by patients 2 years after therapy.
Discussion
This study is the first to assess HRQOL using qualitatively induced codes from the responses of children and adolescents during and after treatment for ALL and to compare their responses by age, gender, risk group, and time in treatment by using a quantitative content analysis approach. Our study demonstrates how qualitative HRQOL data can be quantified to allow statistical inferences to be drawn across treatment variables in a clinical trial. This approach provides understanding and clarity to a large dataset of qualitative themes by quantifying and measuring differences in responses between groups. Our findings provide the basis for prioritizing future HRQOL interventions for children and adolescents with cancer during and after treatment.
Longitudinal assessment provides insights into the changing codes reported by children and adolescents as they progress through the trajectory of ALL therapy into survivorship. Patient responses to two questions revealed common codes in describing a good day and the illness experience as well as codes that are not elicited by commonly used HRQOL questionnaires. Commonly reported codes described a good day as being able to perform usual activities such as interacting with friends, playing, being outside, and attending school. From the perspective of both children and adolescents, responses to Question 1 emphasize the importance of integrating leukemia patients back into their usual or normal routines such as school and social interactions.
The benefit that the child or adolescent perceives from the cancer experience should also be considered. When children and adolescents were asked about the illness experience, the theme of benefit emerged and was expressed at all time points, including 2 years after the completion of therapy. Patients reported benefits such as getting additional attention from family and friends, extra help with homework, and not attending school. Finding benefit from one’s illness experience has been previously reported.9,10
Children and adolescents responded both positively and negatively to Question 2. They described their illness experience as being hard and the symptoms of the illness and treatment as bad. Two years after the completion of treatment, patients continued to express their illness experiences as having been bad, indicating a lingering negative recall of those aspects. However, another response was of viewing self as a changed individual after the illness and treatment experiences. Both children and adolescents described achieving some level of meaning in the negative illness experiences. One patient stated: “Although it has been hard, it has made me stronger and more mature. I have learned to enjoy and appreciate life more.” Previous studies have also reported positive changes in patients as they become more mature individuals and that they become sensitive and more caring for others.11,12 Despite several studies reporting this finding, commonly used instruments do not measure self-change or the meaning of illness as perceived by the child or adolescent.4 The coded HRQOL responses also represent the ability of children and adolescents to reflect about their illness experience during and after treatment and to see both positive and negative aspects. More adolescents and females reported both the positive and negative aspects of HRQOL, suggesting they may be more reflective or more expressive regarding the total treatment experience.
Codes varied significantly by age. Age has been an important factor in the development of HRQOL instruments in children and adolescents.1,13 As developmental tasks vary by age, patients differ in their illness experience. We found that both children and adolescents strive for “normalcy” in their life, with adolescents concerned with illness symptoms and children wanting to do the usual. However, most HRQOL instruments include age-universal items,14 which can limit the understanding of age-related experiences of the illness. Our findings highlight that efforts need to focus on addressing symptom control and well-being among adolescents while encouraging age-appropriate activities for both children and adolescents.
Several studies have found differences in HRQOL by gender, with females consistently reporting a lower HRQOL than males. 15-18 Our study found similar results, with females reporting negative codes more often than males. Females described the symptoms as being bad and the illness experience as hard and felt that the treatment did not allow them to perform their usual activities, including interacting with friends. Males described the experience as not bad, which may reflect social role expectations of males reacting to unpleasant experiences in a less affected manner.19 However, both female and male patients reported positive and negative aspects of the cancer treatment experience, suggesting that both can speak realistically about their experience in response to these questions. Ongoing discussions with males and females need to address the impact of their illness on their relationships, for which children and adolescents have been described as feeling marginalized or being outsiders in their social circle of friends.20 Discussions should also include body image, and for females their image-related interactions with males that may be affected by their body image. 21 Males may benefit from ongoing conversations that encourage expression of the illness experience.
We found statistically significant differences between risk groups in coded responses to Question 1, with the standard-/high-risk group reporting a good day as a short hospital stay and not being sick, and the low-risk group reporting being at home more frequently. Patients in the standard-/high-risk arm receive more intensive treatment and are thus more likely to be at the hospital or clinic; a brief appointment or stay at either location would be preferable than a longer stay. In response to Question 2, patients in the standard/high-risk group frequently reported that being sick was hard; however, the code did not differ significantly between standard-/high- and low-risk groups. Studies have shown that treatment intensity is consistently associated with a poorer HRQOL.15 A large-scale multisite cooperative trial needs to be undertaken to definitively address differences in HRQOL by risk group, with patients in the high-risk group being analyzed separately from those in other risk groups. Our findings provide the basis for hypothesis development for such a future trial.
As patients progress in their ALL treatment, the intensity of treatment subsides and the illness experience differs from the early months of therapy. Early in treatment, children and adolescents described a good day as not being in the hospital, being at home, and not being sick. At 2 years after completion of therapy, children and adolescents reflected on a good day as not being sick and that being sick was a bad experience. It is possible that the meaning of “being sick” changes over time from diagnosis (i.e., from a symptom-specific basis to an overall or total reflection on treatment in the responses); this possible conceptual change merits future study. Both children and adolescents expressed very frequently that being with friends made a good day; being with friends was the most frequent theme 2 years after the completion of therapy. As all patients were of adolescent age at T6, this finding of focus on social interactions is consistent with adolescent developmental milestones and provides support for clinicians to recognize the importance of clinical care interventions that foster social reintegration of patients with friends.
Codes of pain and spirituality are often associated with HRQOL among adults with cancer;22 however, these codes were not common among our patients’ responses. Although spirituality was included as a category in the pilot coding dictionary, codes of spirituality (Spirit, Trust God) were seldom expressed by children or adolescents in this study. Children and adolescents with cancer may view spirituality differently than adults with cancer, given that spirituality among adult oncology patients is the most frequently reported complementary and alternative therapy in the United States.23,24 This difference may reflect a developmental difference in the grasp of immortality between children and adults as well as the expectation of children for positive disease and treatment outcomes.25
Pain is a symptom that is often associated with the diagnosis and treatment of cancer. Hedstrom et al.26 reported that the most frequently mentioned reason for distress in children with cancer was pain from diagnostic procedures and treatments. Thus, we expected more frequent complaints of pain in children in response to Question 2. However, very few patient responses indicated pain as part of being sick. Instead, patients reported distressing symptoms such as nausea, vomiting, and fatigue. The few references to pain may be secondary to the setting’s standardized assessments of pain and pain intervention effectiveness. Routine symptom assessments and interventions may similarly reduce the commonly expressed symptoms of feeling sick and facilitate patients’ desire to feel normal.
This study addressed an important methodological concern of time constraint in assessing HRQOL among all patients. Obtaining even brief patient-reported HRQOL reports can be problematic in clinical settings,24 with clinicians not having sufficient time to assess and complete HRQOL questionnaires. However, when patient-reported outcomes are assessed and healthcare decisions are made according to reported symptoms, patients report an improved HRQOL.27 Our study demonstrates the feasibility of assessing HRQOL using only two questions and the clinical relevance of the responses. The two interview questions constitute a brief and useful assessment for clinicians, with the potential for guiding real-time interventions.
There are some limitations to our study. We relied upon the codebook previously developed from our pilot work and did not identify new codes in the interviewing process. This is likely a minor limitation given the low rate of uncoded responses and our ability to categorize most of the uncoded responses as uninterpretable. Nearly twice as many males participated as did females, and there were more than twice as many study patients on the standard-/high-risk arm than on the low-risk arm. To address these differences, we reported our findings descriptively and in terms of ratios, but such differences will influence quantitative analyses. Not all our quantitative comparisons could concurrently consider gender and risk arm. Also important in a quantitative analysis of qualitatively induced codes is that infrequently reported codes will result in no significant findings in certain comparisons. Our analysis could not address whether certain codes present at a particular time point predicted more positive or negative treatment outcomes for a study participant, but this will be an important consideration in future study designs about HRQOL in pediatric patients in treatment for ALL.
A unique aspect of this study is the method of qualitative analysis. Qualitative research aims to find meaning and to improve our understanding of a phenomenon. Thus, the use of quantification of qualitative data according to covariates can be viewed as beyond the qualitative research purpose and paradigm. Thus, this quantitative approach to a traditionally qualitative content analysis method has allowed us to gain a perspective on HRQOL in children with cancer according to age, gender, risk group, and time. We believe that this approach has offered strength to our findings, and resulted in refinement of the original pilot study.
Conclusions
The more commonly used measures for HRQOL do not adequately measure domains as expressed by our study participants, including the importance of feeling normal, of participating in normal activities, and the meaning of the illness experience. Our study findings support the domains that were identified from the initial pilot study as the defining domains of HRQL in children under active treatment for ALL. These domains are not consistently assessed in the more commonly used HRQL instruments. Our findings suggest that two interview questions are feasible for use in assessing HRQOL from the patient’s perspective in the clinical setting. In addition, this assessment can be readily administered by the nurse, advanced practice nurse, or physician at the point of care and can contribute in real time to interventions that improve the care and HRQOL of children and adolescents with cancer. The regular collection, documentation, and coding of responses can also help nurse researchers identify new trends or new issues that may arise with new therapies early in treatment.
Acknowledgments
Funding: This study was supported in part by a Cancer Center Support Grant, Grant number CA21765 from the National Cancer Institute (NCI), and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose
References
- 1.Hinds PS. Advances in Defining, Conceptualizing, and Measuring Quality of Life in Pediatric Patients With Cancer. Oncol Nurs Forum. 2006;33:23–29. doi: 10.1188/06.ONF.S1.23-29. [DOI] [PubMed] [Google Scholar]
- 2.Hinds PS. Progress in Quality of Life in Children and Adolescents with Cancer. Semin Oncol Nurs. 2010;26(1):18–25. doi: 10.1016/j.soncn.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Klassen A, Strohm S, Maurice-Stam H, Grootenhuis M. Quality of life questionnaires for children with cancer and childhood cancer survivors: a review of the development of available measures. Support Care Cancer. 2010;18(9):1207–1217. doi: 10.1007/s00520-009-0751-y. [DOI] [PubMed] [Google Scholar]
- 4.Hinds PS, Gattuso JS, Fletcher A, et al. Quality of Life as Conveyed by Pediatric Patients with Cancer. Qual Life Res. 2004;13(4):761–772. doi: 10.2307/4038916. [DOI] [PubMed] [Google Scholar]
- 5.Cantrell M. A Narrative Review Summarizing the State of the Evidence on the Health-Related Quality of Life Among Childhood Cancer Survivors. J Ped Oncol Nurs. 2011;28(2):75–82. doi: 10.1177/1043454210377901. [DOI] [PubMed] [Google Scholar]
- 6.Haas BK. Clarification and integration of similar quality of life concepts. Image J Nurs Sch. 1999;31(3):215–220. doi: 10.1111/j.1547-5069.1999.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 7.Eiser C, Morse R. A review of measures of quality of life for children with chronic illness. Arch Dis Child. 2001;84(3):205–211. doi: 10.1136/adc.84.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RB, Maas SM. QDA Miner 2.0: Mixed-Model Qualitative Data Analysis Software. Field Methods. 2007;19(1):87–108. [Google Scholar]
- 9.Eiser C, Eiser JR, Stride CB. Quality of life in children newly diagnosed with cancer and their mothers. Health Qual Life Outcomes. 2005;3:29. doi: 10.1186/1477-7525-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felder-Puig R, Gallo A, Waldenmair M, et al. Health-related quality of life of pediatric patients receiving allogeneic stem cell or bone marrow transplantation: results of a longitudinal, multi-center study. Bone Marrow Transpl. 2006;38(2):119–126. doi: 10.1038/sj.bmt.1705417. [DOI] [PubMed] [Google Scholar]
- 11.Haase JE, Rostad M. Experiences of completing cancer therapy: children’s perspectives. Oncol Nurs Forum. 1994;21(9):1483–1492. discussion 1493–1494. [PubMed] [Google Scholar]
- 12.Enskar K, Carlsson M, Golsater M, Hamrin E, Kreuger A. Life Situation and Problems as Reported by Children With Cancer and Their Parents. J Ped Oncol Nurs. 1997;14(1):18–26. doi: 10.1177/104345429701400104. [DOI] [PubMed] [Google Scholar]
- 13.Michel G, Bisegger C, Fuhr DC, Abel T The KIDSCREEN group. Age and gender differences in health-related quality of life of children and adolescents in Europe: a multilevel analysis. Qual Life Res. 2009;18(9):1147–1157. doi: 10.1007/s11136-009-9538-3. [DOI] [PubMed] [Google Scholar]
- 14.Wallander JL, Schmitt M, Koot HM. Quality of life measurement in children and adolescents: Issues, instruments, and applications. J Clin Psychol. 2001;57(4):571–585. doi: 10.1002/jclp.1029. [DOI] [PubMed] [Google Scholar]
- 15.Klassen A, Anthony S, Khan A, Sung L, Klaassen R. Identifying determinants of quality of life of children with cancer and childhood cancer survivors: a systematic review. Support Care Cancer. 2011;19(9):1275–1287. doi: 10.1007/s00520-011-1193-x. [DOI] [PubMed] [Google Scholar]
- 16.Sung L, Yanofsky R, Klaassen RJ, et al. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. Int J Cancer. 2011;128(5):1213–1220. doi: 10.1002/ijc.25433. [DOI] [PubMed] [Google Scholar]
- 17.Lund LW, Schmiegelow K, Rechnitzer C, Johansen C. A systematic review of studies on psychosocial late effects of childhood cancer: Structures of society and methodological pitfalls may challenge the conclusions. Ped Blood Cancer. 2011;56(4):532–543. doi: 10.1002/pbc.22883. [DOI] [PubMed] [Google Scholar]
- 18.Pud D. Gender differences in predicting quality of life in cancer patients with pain. Eur J Oncol Nurs. 2011;15(5):486–491. doi: 10.1016/j.ejon.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 19.McDonough P, Walters V. Gender and health: reassessing patterns and explanations. Social Science and Medicine. 2001;52(4):547–560. doi: 10.1016/s0277-9536(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee M-Y, Mu P-F, Tsay S-F, Chou S-S, Chen Y-C, Wong T-T. Body image of children and adolescents with cancer: A metasynthesis on qualitative research findings. Nurs Health Sci. 2012;14(3):381–390. doi: 10.1111/j.1442-2018.2012.00695.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones BL, Parker-Raley J, Barczyk A. Adolescent Cancer Survivors: Identity Paradox and the Need to Belong. Qual Health Res. 2011;21(8):1033–1040. doi: 10.1177/1049732311404029. [DOI] [PubMed] [Google Scholar]
- 22.Hinds PS, Martin J. Hopefulness and the self-sustaining process in adolescents with cancer. Nurs Res. 37(6):336–40. [PubMed] [Google Scholar]
- 23.Barnes LL, Plotnikoff GA, Fox K, Pendleton S. Spirituality, Religion, and Pediatrics: Intersecting Worlds of Healing. Pediatrics. 2000;106(Supplement 3):899–908. [PubMed] [Google Scholar]
- 24.Bradlyn AS. Health-Related Quality of Life in Pediatric Oncology: Current Status and Future Challenges. J Ped Oncol Nurs. 2004;21(3):137–140. doi: 10.1177/1043454204264376. [DOI] [PubMed] [Google Scholar]
- 25.Smith J, McSherry W. Spirituality and child development: a concept analysis. J Adv Nurs. 2004;45(3):307–315. doi: 10.1046/j.1365-2648.2003.02891.x. [DOI] [PubMed] [Google Scholar]
- 26.Hedström M, Haglund K, Skolin I, Essen L. Distressing Events for Children and Adolescents With Cancer: Child, Parent, an Nurse Perceptions. J Ped Oncol Nurs. 2003;20(3):120–132. doi: 10.1053/jpon.2003.76. [DOI] [PubMed] [Google Scholar]
- 27.Varni JW, Seid M, Knight TS, Uzark K, Szer IS. The PedsQLTM 4.0 Generic Core Scales: Sensitivity, Responsiveness, and Impact on Clinical Decision-Making. J Behav Med. 2002;25(2):175–193. doi: 10.1023/A:1014836921812. [DOI] [PubMed] [Google Scholar]


