Abstract
Introduction
Because of prognostic and therapeutic implications, the distinction between iRVOT and arrhythmogenic RV cardiomyopathy (ARVC) is clinically important. Over the last two decades multiple reports have identified RV abnormalities using CMR in patients with idiopathic VT, suggesting a link between these arrhythmias and ARVC. The purpose of this study was to assess for structural abnormalities in patients with idiopathic right ventricular outflow tract (iRVOT) tachycardia using contemporary cardiac magnetic resonance (CMR) imaging.
Methods and Results
CMR was performed in 46 patients with iRVOT tachycardia and 16 normal controls, with quantitative evaluation of right (RV) and left ventricular volumes and function, as well as assessment of myocardial fat and scar. iRVOT patients were similar to controls with respect to RV end-diastolic volumes (81±19 vs.79±18 mL/m2, p=0.77) and RV ejection fraction (57±8 vs. 59±7%, p=0.31). The prevalence of RV chamber dilation, defined using ARVC major task force criteria, was uncommon among iRVOT patients (9%) and controls (7%; p=1.0). Regional RV wall motion abnormalities were present in 2 iRVOT patients who had concomitant RV dilation or dysfunction. CMR tissue characterization demonstrated absence of both myocardial scar and fat infiltration in all patients and controls.
Conclusions
In patients with the clinical diagnosis of iRVOT tachycardia, CMR reveals RV structure, function, and myocardial tissue characteristics similar to normal controls. These findings suggest that the vast majority of patients with RVOT arrhythmias have a primary electrical disorder that is not a forme-fruste of ARVC.
Keywords: Idiopathic ventricular tachycardia, Ventricular premature beats, Cardiac magnetic resonance, right ventricular outflow tract
Introduction
Idiopathic right ventricular outflow tract (iRVOT) tachycardia is thought to occur in structurally normal hearts. Phenotypes of this arrhythmia include sustained monomorphic VT, repetitive monomorphic VT (RMVT), or frequent premature ventricular beats (PVCs)1; and the characteristic ECG morphologies of these arrhythmias reflect their origins near the pulmonic valve. The mechanism of iRVOT tachycardia is understood to be triggered activity, based on response to programmed stimulation and characteristic sensitivity to adenosine.2, 3 Consistent with this mechanism, these tachycardias arise from focal sources and respond to focal ablation.
VT originating from the right ventricular outflow tract can also occur in arrhythmogenic RV cardiomyopathy (ARVC), and distinction of iRVOT tachycardia from ARVC is clinically important.4, 5 The two entities differ in prognosis in that ARVC is associated with sudden death and right ventricular failure, whereas iRVOT tachycardia has a favorable prognosis. The distinction of iRVOT tachycardia from ARVC can be made on the basis of tachycardia characteristics (reentry versus focal triggered activity) and the presence of right ventricular structural disease. Conventional assessment of the RV with echocardiography, ECG, and cardiac catheterization may be insensitive for detecting disease in the early stages of ARVC.
Cardiac magnetic resonance (CMR) imaging is commonly used to screen patients for ARVC, as it provides high spatial resolution imaging of RV structure and myocardial tissue characteristics. Early use of CMR focused on identification of intramyocardial fat, which is a characteristic pathological finding in ARVC. However, the specificity of these CMR findings for ARVC has been questioned, as intramyocardial fat can be mimicked by image-artifact or epicardial adiposity.6 Several studies have reported CMR abnormalities such as intramyocardial fat and wall thinning in up to 70% of idiopathic RVOT tachycardia patients, despite the fact that this condition is not typically associated with structural heart disease.7-14 To address these inconsistencies and the enhanced sophistication of CMR imaging, consensus Task Force Criteria for diagnosis of ARVC were recently revised to include quantitative thresholds of RV volume and regional RV wall motion abnormalities as criteria for ARVC.15
Recent advances in CMR technology, including improved temporal resolution and ECG gating methods, have facilitated assessment of RV structure and contractile function. Beyond fat assessment, CMR also enables highly reliable assessment of myocardial scar/infarction, a known substrate for VT.16, 17 The objective of this study was to reevaluate CMR findings in patients with iRVOT tachycardia, in light of current diagnostic criteria, using state-of-the-art CMR methods for evaluation of myocardial structure, function, and tissue composition.
Methods
Patient populations
The study population comprised consecutive patients at Weill Cornell Medical College fulfilling established criteria for iRVOT arrhythmias based on invasive electrophysiology studies and who underwent CMR. Patients had VT or PVCs originating from the RV outflow or contiguous inflow regions, as determined by electroanatomical mapping and subsequent radiofrequency ablation. Patients with known structural heart disease (i.e., prior myocardial infarction, coronary revascularization, cardiac surgery), or with Task Force Criteria for ARVC (in the absence of CMR) were excluded from the study.
Healthy volunteers were recruited as controls for comparison to the iRVOT patient cohort. All volunteers had no known history of cardiovascular disease, valvular heart disease, or VT (as assessed based on dedicated screening evaluation).
This study was approved by the Institutional Review Board of the Weill Cornell Medical College.
CMR Protocol and Analysis
All participants (both patients and controls) underwent CMR for evaluation of myocardial structure, function, and tissue characteristics. Exams consisted of three components: (1) cine-CMR for function/morphology, (2) T1 weighted CMR for myocardial fat, and (3) delayed enhancement (DE)-CMR for myocardial infarction/scar. Figure 1 illustrates components of the CMR imaging protocol.
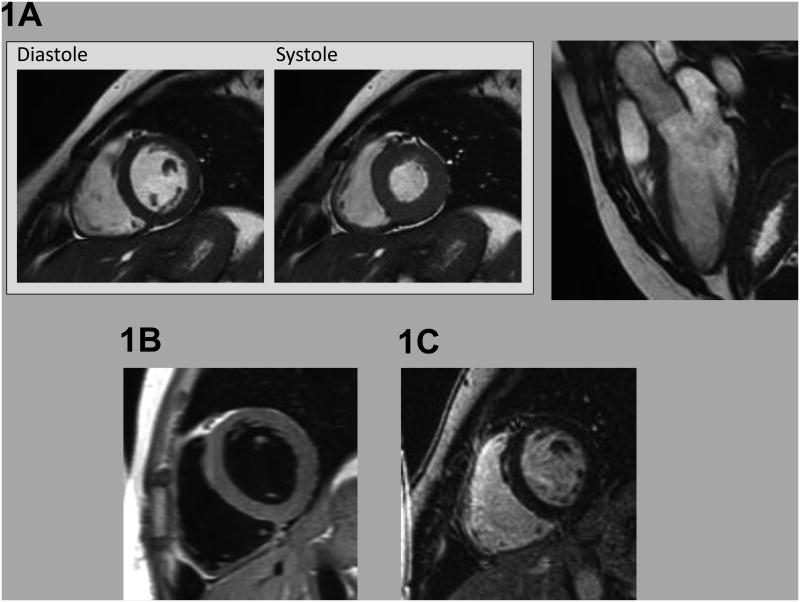
Figure 1. CMR Evaluation.
Representative example of CMR imaging approaches employed as part of the study protocol, including (1A) cine-CMR evaluation of cardiac structure/function (left: short axis images shown at end-diastole and end-systole, right: 3 chamber long axis at end-diastole), (1B) T1-weighted spin-echo CMR evaluation for myocardial fat infiltration, and (1C) delayed enhancement CMR evaluation for myocardial infarction/scar.
Cine-CMR was performed using a steady-state free precession sequence (typical parameters: repetition time (TR) 3.5 msec, echo time (TE) 1.6 msec, flip angle 60°, in-plane spatial resolution 1.9 mm × 1.4 mm). T1-weighted imaging was performed using an ECG gated black blood pulse sequence. Following cine- and T1- weighted imaging, gadolinium was intravenously administered (0.2 mmol/kg). DE-CMR was performed 10 minutes thereafter using both segmented and single shot pulse sequences. Cine- and DE-CMR were acquired in matching short and LV long axis (2-, 3, and 4-chamber) orientation.
CMR exams were interpreted by an experienced, ACC/AHA level III trained, investigator (JWW) blinded to clinical data and results of other imaging modalities. Cine-CMR was quantitatively analyzed for LV and RV size and function via planimetry of endocardial contours at end-systole and end-diastole. Cine-CMR images were also interpreted for presence of RV aneurysms, defined as a discrete dyskinetic bulge interrupting the normal RV contour in diastole and systole. 18 T1-weighted images were read for myocardial fat, identified based on localized increase in myocardial signal intensity; images were interpreted in accordance with established 5-point scoring system (1= definitely absent, 2=likely absent, 3=indeterminant, 4=likely present, 5=definitely present). 19 DE-CMR was graded for presence of myocardial scar/infarction, identified based on hyperenhancement in accordance with established criteria. 20
Electrocardiography
ECGs were acquired in patients and volunteers using a standard 12 lead surface electrode recording system (MAC 5000/550, General Electric, Waukesha WI); recording was performed at a sampling rate of 500 Hz. ECGs were analyzed for presence of right bundle branch block, right ventricular conduction delay, and T-wave inversions. The terminal activation delay was defined as the interval from the nadir of the S-wave to end of the QRS in leads V1 to V3, 21 in the absence of right bundle branch block, with the longest value tabulated for each study participant.
Electrophysiology Study and Catheter Ablation
All subjects with idiopathic tachycardia underwent an invasive electrophysiology study for the purpose of catheter ablation. VT induction was attempted using a standardized protocol, which included burst pacing and programmed stimulation with up to triple ventricular extrastimuli at two drive cycles from at least one right ventricular site. If VT was not inducible, programmed stimulation was repeated with burst pacing and up to triple extrastimuli on isoproterenol infusion. Mapping was performed with the CARTO system (Biosense, Daimond Bar, CA) and consisted of activation mapping to identify the earliest local bipolar electrogram with confirmation from a QS electrogram on the unipolar recording. Pace-mapping was performed with the goal of obtaining best possible pace maps (at least 11/12 match in QRS morphology). Ablation was performed with radiofrequency (Biosense Navistar 4 mm tip or Navistar Thermocool 3.5 mm tip); success was defined as complete elimination of the targeted morphology of the PVCs or tachycardia.
Adenosine was administered for cases of sustained VT. The initial dose was at the operator's discretion (6 or 12 mg), and the dose was increased by 6 mg until adenosine sensitivity of the ventricular arrhythmia was demonstrated or atrioventricular block occurred. “Sensitivity” to adenosine was defined as termination of sustained VT or elimination of RMVT during the period of adenosine effect. “Insensitivity” was defined as perpetuation of VT.
Statistical Methods
Comparisons of continuous variables were made using Student's t test (expressed as mean ± standard deviation). Categorical variables were compared using Chi-square or (when there were fewer than 5 expected outcomes per cell) Fisher's exact tests. Statistical analysis was performed with SPSS (version 20). A two-sided p-value of ≤0.05 was considered statistically significant.
Results
Population Characteristics
The study population included 46 patients with iRVOT arrhythmias and 16 normal controls. Patients with iRVOT and controls were similar with regard to age (46±13 vs. 46±15 years, p=0.98), gender (female: 54% vs. 69%, p=0.48) and body surface age (2.0± 0.3 vs. 1.9± 0.3 mˆ2; p=0.26).
In 35 patients, CMR was performed before the ablation procedure (median 13 days, interquartile range [IQR] 4-33). In 11 patients, CMR was performed after an ablation procedure (median 84 days, IQR 18-843). Five of these had prior unsuccessful ablations before undergoing CMR and subsequent successful ablation at our institution; the other 6 had CMR performed after the first successful ablation.
Tachycardia Characteristics
Over half (54%; 25/46) of iRVOT patients presented with single PVCs with or without runs of non-sustained VT; 26% (n=12) presented with RMVT and 9 with sustained monomorphic VT. Ninety-one percent (n=42) of ventricular arrhythmias originated from the RVOT and 7% (n=3) from the contiguous RV inflow region; 1 patient had two PVC morphologies – one was ablated from the RVOT and the other from the right coronary cusp. Based on Holter monitor data, the median PVC burden was 11% (IQR 3-29%, N=35).
Catheter ablation was successful in 96% (44/46) of patients. In one case, PVCs were mapped to the septal aspect of the RV inflow region, but ablation was not successful. In another patient, ablation in the posteroseptal RVOT decreased but did not eliminate clinical PVCs. In both cases, mapping from the coronary cusps did not identify appropriate ablation sites, and mapping in the coronary venous system was not successful due to anatomical constraints.
Eight patients received adenosine during induced sustained VT (mean dose 14±4 mg); all arrhythmias terminated and were thus classified as adenosine-sensitive.
Electrocardiography
Seven percent (3/46) of iRVOT patients had right ventricular conduction abnormalities on surface 12-lead ECG (1 right bundle branch block, 2 right ventricular conduction delay). Among controls, one subject had RV conduction delay on ECG and the other 15 had no conduction abnormalities. Two iRVOT patients and none of the control patients had anterior T-wave inversions beyond lead V2. The terminal activation delay in leads V1 to V3 was marginally longer in iRVOT patients vs. normal subjects (41±8 vs. 38±5 msec, p=0.070). Terminal activation delays were ≥55 msec in 4% (2/45) of iRVOT patients and no (0/16) controls.
Cardiac Magnetic Resonance
Chamber Geometry / Function
Table 1A compares quantitative RV indices between iRVOT patients and controls. As shown, RV geometry was similar between groups, whether assessed by chamber volumes or linear dimensions (all p=NS). Global RV function was also similar between RVOT patients and controls, whether measured via RV ejection fraction (EF) (p=0.31) or RV stroke volume (p=0.97). Regional RV dysfunction was uncommon, but exclusively associated with RVOT subjects (2 patients, no controls).
Table 1. Cardiac Structure and Function.
| 1A. Right Ventricular Indices | |||
|---|---|---|---|
| RV Tachycardia Patients | Controls | P | |
| RV End-Diastolic Volume (ml) | 159 ± 47 | 152 ± 48 | 0.64 |
| RV End-Diastolic Volume Index (ml/m2) | 81 ± 19 | 79 ± 18 | 0.77 |
| RV End-Systolic Volume (ml) | 69 ± 22 | 62 ± 29 | 0.32 |
| RV End-Systolic Volume Index (ml/m2) | 35 ± 10 | 33 ±12 | 0.54 |
| RV End-Diastolic Diameter (cm) | 4.3 ± 0.7 | 4.5 ± 0.7 | 0.46 |
| RV Stroke Volume (ml) | 91 ± 29 | 91± 29 | 0.97 |
| RV Ejection Fraction (%) | 57 ± 8 | 59 ± 7 | 0.31 |
| 1B. Left Ventricular Indices | |||
| RV Tachycardia Patients | Controls | P | |
| LV End-Diastolic Volume (ml) | 161 ± 56 | 134 ± 37 | 0.07 |
| LV End-Diastolic Volume Index (ml/m2) | 82 ± 24 | 73 ± 13 | 0.18 |
| LV End-Systolic Volume (ml) | 62 ± 30 | 45 ± 19 | 0.05 |
| LV End-Systolic Volume Index (ml/m2) | 31 ± 14 | 25 ± 8 | 0.12 |
| LV End-Diastolic Diameter (cm) | 5.6 ± 0.7 | 5.2 ± 0.3 | 0.01 |
| LV Mass (g) | 127 ± 42 | 114 ± 35 | 0.29 |
| LV Mass Index (g/m2) | 64 ± 16 | 62 ± 12 | 0.73 |
| LV Stroke Volume (ml) | 95 ± 28 | 89 ± 20 | 0.45 |
| LV Ejection Fraction (%) | 61 ± 9 | 67 ± 6 | 0.02 |
Table 1B reports LV structural and functional indices among the study population. LV chamber size, as measured based on end-diastolic diameter, was larger among iRVOT patients (p=0.01), with a similar trend for end-diastolic volume (p=0.07) and end systolic volume (p=0.05). Mean LVEF was lower iRVOT patients (p=0.02), although mean values were normal for both groups (61 ± 9 vs. 67 ± 6). As shown in Table 2, RVOT patients who presented with frequent PVCs or RMVT had significantly higher LV and RV volumes, and higher LV mass as compared to those who presented with sustained VT (all p<0.01). Consistent with this, LVEF tended to be lower among RVOT patients who presented with frequent PVCs or RMVT (p=0.09).
Table 2. Ventricular Structure and Function by Phenotype.
| 2A. Right Ventricular Indices | |||
|---|---|---|---|
| Frequent PVCs / RMVT | Sustained VT | P | |
| RV End-Diastolic Volume (ml) | 165 ± 46 | 132 ± 40 | 0.049 |
| RV End-Diastolic Volume Index (ml/m2) | 84 ± 18 | 68 ± 17 | 0.02 |
| RV End-Systolic Volume (ml) | 71 ± 22 | 62 ± 26 | 0.33 |
| RV End-Systolic Volume Index (ml/m2) | 36 ± 9 | 30 ± 10 | 0.14 |
| RV End-Diastolic Diameter (cm) | 4.3 ± 0.7 | 4.3 ± 0.4 | 0.88 |
| RV Stroke Volume (ml) | 94 ± 29 | 78 ± 27 | 0.19 |
| RV Ejection Fraction (%) | 57 ± 8 | 56 ± 11 | 0.78 |
| 2B. Left Ventricular Indices | |||
| Frequent PVCs / RMVT | Sustained VT | P | |
| LV End-Diastolic Volume (ml) | 171 ± 57 | 122 ± 24 | <0.001 |
| LV End-Diastolic Volume Index (ml/m2) | 87 ± 24 | 63 ± 10 | .005 |
| LV End-Systolic Volume (ml) | 66 ± 32 | 42 ± 5 | <0.001 |
| LV End-Systolic Volume Index (ml/m2) | 34 ± 15 | 22 ± 4 | <0.001 |
| LV End-Diastolic Diameter (cm) | 5.7 ± 0.7 | 5.0 ± 0.3 | 0.006 |
| LV Mass (g) | 133 ± 44 | 102 ± 24 | 0.007 |
| LV Mass Index (g/m2) | 67 ± 16 | 52 ± 7 | <0.001 |
| LV Stroke Volume (ml) | 97 ± 29 | 86 ± 20 | 0.38 |
| LV Ejection Fraction (%) | 60 ± 10 | 67 ± 3 | 0.09 |
Table 3 details electroanatomical and global volumetric characteristics of 2 iRVOT patients with RV contractile abnormalities. One of these patients demonstrated regional RV dysfunction accompanied by global impairment in RV performance (EF ≤40%), and the other had RV dilatation (RVEDVI ≥100mL/m2 (female)). Notably, aneurysms were not associated with the site of VT origin.
Table 3.
Patients with Right Ventricular Contractile Abnormalities.
| Age | Sex | Phenotype | TWI | RVCD/RBBB | TAD (msec) | PVC Burden | site of origin | LVEF (%) | RVEF (%) | LVEDVI (mL/m2) | RVEDVI (mL/m2) | Contractile Abnormalities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 51 | M | RMVT | No | No | 40 | 20% | anteroseptal RVOT | 57 | 39 | 56 | 60 | Focal dyskinesia, mid RV free wall |
| 55 | F | SMVT | No | No | 30 | 3% | posteroseptal RVOT | 70 | 55 | 79 | 105 | Focal dyskinesia, mid RV free wall |
LVEDVI indicates left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; RBBB, right bundle branch block; RMVT, repetitive monomorphic ventricular tachycardia; RVCD, right ventricular conduction delay; RVEDVI, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RVOT, right ventricular outflow tract; SMVT, sustained monomorphic VT; TAD, terminal activation delay; TWI, T-wave inversions in lead V2 or later.
ARVC Criteria
Table 4 reports prevalence of established CMR Task Force criteria for ARVC among RVOT tachycardia patients and controls. Consistent with absolute quantitative indices, application of volumetric cutoffs for ARVC was uncommon for both groups. With regard to major cutoffs for RV dilation, 4 patients (9%) and 1 control (7%) satisfied this criterion (p=1.0). Using minor cutoffs, 5 patients (11%) and 3 controls (21%) satisfied this criterion (p=0.38). Concerning aggregate ARVC criteria, both patients with regional RV dysfunction also fulfilled major criteria for ARVC based on global chamber volume (n=1) or RVEF (n=1). Figure 2 provides a representative example of imaging findings in a patient with RVOT tachycardia who fulfilled ARVC criteria based on CMR results.
Table 4. Prevalence of Established CMR Criteria for Arrhythmogenic Right Ventricular Cardiomyopathy.
| RV Tachycardia Patients | Controls | P Value | |
|---|---|---|---|
|
Major Volumetric Criterion:
* RVEDVI ≥110 mL/m2 (males), ≥100 mL/m2 (females) |
9% (4/44) | 7% (1/14) | 1.0 |
|
Minor Volumetric Criterion:
* RVEDVI ≥100 to <110 mL/m2 (males), ≥90 to <100 mL/m2 (females) |
11% (5/44) | 21% (3/14) | 0.38 |
| Any Volumetric Criteria (Minor or Major) | 20% (9/44) | 29%(4/14) | 0.71 |
| RVEF ≤40% † | 5% (2/39) | 0% (0/16) | 1.0 |
| RVEF >40% to ≤45% † | 0% (0/39) | 0% (0/16) | - |
|
RV wall motion abnormalities: regional akinesis or dyskinesis, |
4% (2/46) | 0% (0/16) | 1.0 |
ARVC Major Criteria
‡
|
4% (2/46) | 0% (0/16) | 1.0 |
ARVC Minor Criteria
‡
|
0% (0/46) | 0% (0/16) | - |
RVEDVI refers to right ventricular end diastolic volume index; RVEF, right ventricular ejection fraction
Indexed volumetric indices unavailable for 4 subjects (2 patients, 2 controls) due to absence of height/weight data; data presented for remainder of study population (n=58).
RV ejection fraction could not be calculated for 7/46 patients due to arrhythmia-related impaired ECG-gated imaging.
Classification required quantification of RVEF, RVEDVI, or both
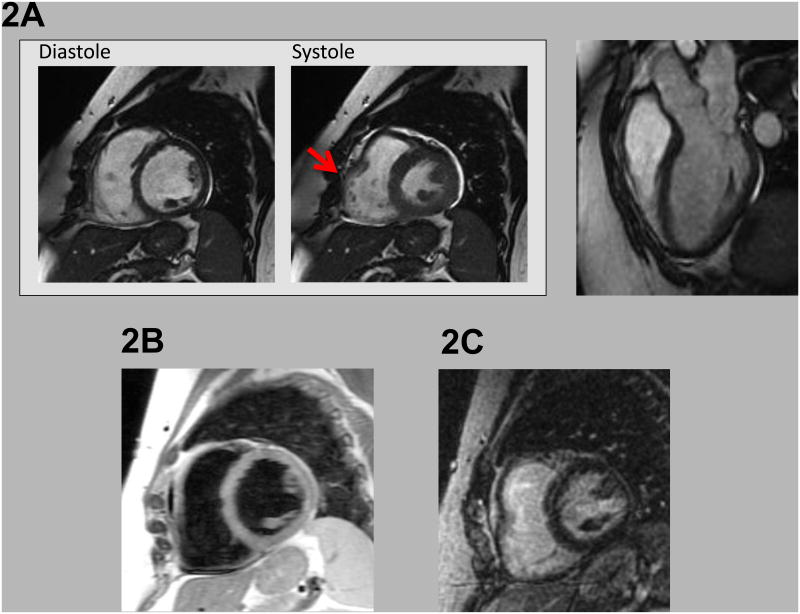
Figure 2. CMR Findings in an RV Tachycardia Patient with Contractile Dysfunction.
Regional systolic dysfunction within the RV free wall (red arrow) as evident on cine-CMR (2A) acquired dynamically during diastole and systole. Regional RV dysfunction was accompanied by global chamber dilation (RV end-diastolic volume = 105ml/mˆ2 [female subject]), thereby fulfilling CMR criteria for ARVC based on consensus task force criteria. Right panel shows 3 chamber long axis at end-diastole.
Note absence of accompanying myocardial tissue abnormalities, as evidenced by absence of myocardial fat on T1-weighted CMR (2B), or myocardial infarction/scar on delayed enhancement CMR (2C).
Because of the confounding effect of gating artifact in the presence of frequent PVCs, a sub-analysis was performed in 36 patients who had quantitative analysis of PVC frequency based on Holter monitor data. Of these, 24 had a PVC burden (high) ≥5% (23±13%) and 12 had a burden (low) <5% (1.6±1.6%). There was no difference in presence of focal wall motion abnormalities between these groups (1/24 patients with PVC burden ≥5% and 1/12 with PVC burden <5%).
Myocardial Tissue Characteristics
Neither RVOT patients nor controls demonstrated abnormal myocardial tissue characteristics on CMR. Regarding myocardial infarction/scar, delayed-enhancement CMR yielded uniformly normal results in all patients and controls (one control patient declined due to claustrophobia). Regarding myocardial fat (assessed in 84% [n=53/63] of the population), no exams were interpreted as definite or likely positive (13% were indeterminate due to arrhythmia-associated artifact and/or incomplete blood suppression).
Discussion
In contrast to several reports over the past decade,7-14 the findings of this study demonstrate that most patients with the clinical diagnosis of idiopathic RVOT tachycardia have structurally normal hearts as assessed by CMR. Using major and minor CMR criteria proposed for the diagnosis of ARVC, quantitative assessment of the RV demonstrated normal volume indices and right ventricular function in 80% of patients, compared to 71% of normal volunteers. If only major CMR criteria were applied, 9% of patients with idiopathic VT demonstrated RV dilatation compared to 7% of normal controls. These findings suggest that the vast majority of patients with RVOT arrhythmias have an electrical disorder not dependent on abnormal myocardial substrate, implying that they do not have a forme-fruste of ARVC.
CMR is highly sensitive for imaging myocardial abnormalities that may not be detected by other modalities such as echocardiography. While CMR is highly sensitive, it may be relatively nonspecific, especially if mild abnormalities are considered diagnostic of an RV cardiomyopathy. This is suggested by several studies over the past 2 decades, which showed CMR abnormalities in patients with RVOT tachycardias. In these studies, abnormalities were found in up to 70% of patients with RVOT arrhythmias. Of interest, several of these studies that identified abnormalities in RVOT patients were blinded and controlled. The significance of these abnormalities remained uncertain but were interpreted as a possible link between RVOT tachycardia and ARVC, suggesting that RVOT tachycardia might be a “limited” or “early” variant of ARVC in some patients. It is more likely these findings were nonspecific. While some of these studies may have included patients with different disease substrates, most of these included patients thought to have idiopathic arrhythmias. The prior studies predated technological advances in CMR, such as delayed contrast enhancement and more sophisticated image acquisition protocols. Quantitative analyses are not available for most of these earlier studies, and it is likely the abnormalities were minor. In contrast, a study of CMR in 20 patients with idiopathic RVOT tachycardia showed no differences in the incidence of qualitative MRI findings in patients compared with controls. 19 Our study confirms these results and extends these observations in a larger cohort of patients with idiopathic VT subjected to contrast-enhanced CMR and quantitative assessment of the RV. In this sense, our study serves as a corrective to the earlier literature.
To address the concerns of CMR non-specificity in the diagnosis of ARVC, new Task Force Criteria for ARVC have incorporated specific quantitative parameters with regard to RV volumes and function and distinguish major from minor CMR abnormalities based on the degree of chamber dilatation.15 In the revised criteria, RV volume and wall motion abnormalities are considered of primary importance, as opposed to wall thickness and intramyocardial fat, due to the difficulty of assessing these abnormalities with CMR in the normally thin-walled RV. Strict CMR criteria for ARVC require the combination of regional RV wall motion abnormalities (regional RV akinesia, dyskinesia or dyssynchronous RV contraction) together with RV dilatation or systolic dysfunction. Only 2/46 patients in this series met these combined criteria, but these patients had no other clinical or electroanatomical features of ARVC. Importantly, whereas a minority of our control group manifested RV chamber volumes that fulfilled volumetric cutoffs for ARVC, no controls fulfilled actual consensus diagnostic criteria for ARVC, which require that RV volumetric thresholds be accompanied by regional or global contractile dysfunction.
One notable finding of our study concerned an association between RVOT tachycardia and adverse LV remodeling. Specifically, quantitative CMR analysis demonstrated slight increases in both LV chamber size and mass, as well as decrements in LV ejection fraction, among RV arrhythmia patients compared to normal volunteers. These differences can be attributed to adverse LV remodeling related to frequent PVCs and RMVT. 22, 23 Consistent with this, our findings demonstrated that patients who presented with frequent PVC/RMVT phenotypes had more advanced LV and RV remodeling than did those who presented with sustained monomorphic VT. Follow-up CMR studies, which were not performed in these patients, would be needed to provide more definitive evidence of this association.
Clinical Implications
The distinction of idiopathic RVOT tachycardia from ARVC is an important clinical challenge. Patients with idiopathic VT generally have favorable prognoses and treatment does not involve implantation of defibrillators. Treatment in the form of medical therapy or catheter ablation is indicated for refractory symptoms or PVC-associated cardiomyopathy.22, 23 Conversely, ARVC imparts a risk of malignant ventricular arrhythmias and sudden death, and defibrillator implantation is required for high risk patients. Yet both conditions commonly present with VT or PVCs of a left bundle branch block morphology. In fact, a recent study of patients presenting with VT of a left bundle branch block and inferior axis, which were thought to be idiopathic, showed that 25% of patients had areas of RV scar, as determined by low voltage in electroanatomical maps and fibrosis in endomyocardial biopsies.4 Another recent study of patients with frequent PVCs of left bundle branch block morphology found quantitative and qualitative CMR abnormalities in 32%; yet, only a small minority fulfilled Task Force criteria for ARVC. In this study, those with CMR abnormalities had higher risks of having malignant arrhythmic events. 24 While biopsy and electrophysiological studies can aid in the differential diagnosis, there is a clinical need to distinguish these conditions with less invasive testing. Our findings show the absence of significant CMR abnormalities in the vast majority patients with RVOT tachycardia (who do not otherwise have Task Force Criteria ARVC), suggesting that the specificity of CMR in the diagnosis of ARVC is excellent and certainly better than previously thought, particularly if more rigorous criteria are used. We acknowledge that longer follow-up of these patients is warranted and a larger trial would be useful to determine the cost effectiveness of routinely obtaining CMR in this population.
Limitations
Several limitations should be noted. First, quantitative measurement of RV chamber size or function could not be measured in some patients, typically because of frequent ventricular ectopy, which resulted in impaired ECG gating. This technical consideration is not specific to our study, and constitutes a general limitation to the use of CMR imaging for assessment of ventricular arrhythmias. However, this limitation appears to have had little if any effect on our results since we did not observe a higher incidence of wall motion abnormalities in those with a higher PVC burden. Second, we cannot exclude the possibility that some patients had a concealed from of ARVC, in which arrhythmias precede the development of overt structural heart disease. Consistent with this notion, it is possible that the subgroup of iRVOT patients with RV contractile abnormalities (n=2) had early manifestations of ARVC. However, neither of these patients satisfied other task force criteria for ARVC. Finally, it is important to recognize that differences in LV size and function may in part be attributable to frequency of PVCs, which are a known cause of ventricular remodeling.22 Thus, our observed differences in LV geometry between patients and controls may reflect non-selective alterations in cardiac structure and function attributable to ventricular ectopy, rather than condition-specific remodeling attributable to iRVOT tachycardia. Additionally, selection bias must be considered in this retrospective review, in that patients had CMR imaging at the discretion of their treating physicians.
It is possible that frequent ventricular arrhythmias could cause functional abnormalities in the RV, including chamber dilatation and focal contractile dysfunction, which would be reversible after elimination of the arrhythmia. Thus, 6 patients in our series had CMR after a successful ablation, which could mask the existence of functional abnormalities. These patients were included in this analysis because the study was designed to test the hypothesis that patients suspected of having idiopathic RVOT arrhythmias have structurally normal hearts, and it was not designed to determine if RVOT arrhythmias cause reversible dysfunction. Also, we infer that frequent ventricular ectopy contributed to LV and RV functional abnormalities, but this conclusion is indirect because ventricular function was not assessed after successful ablation.
Conclusions
In patients with iRVOT arrhythmias, RV volumes and systolic function are no different on average than in normal controls. Strict CMR criteria, which require a combination of RV regional wall motion abnormalities and RV chamber dilatation or systolic dysfunction, appear to be relatively specific for ARVC. These findings indicate that the vast majority of patients with idiopathic RVOT arrhythmias do not have subtle structural abnormalities in the RV. Patients with frequent PVCs or RMVT may be prone to develop anatomical remodeling of the LV, even if they do not develop overt signs of tachycardia-related cardiomyopathy.
Acknowledgments
This work was supported in part by grants from the National Institute of Health (RO1 HL56139) and Medtronic Inc.
Dr. Markowitz received a research grant from Medtronic, Inc. to support this project.
Dr. Liu received a research grant from Biosense Webster for an unrelated project and speaking honoraria from St Jude Medical.
Footnotes
The other authors have no disclosures.
References
- 1.Kim RJ, Iwai S, Markowitz SM, Shah BK, Stein KM, Lerman BB. Clinical and electrophysiological spectrum of idiopathic ventricular outflow tract arrhythmias. J Am Coll Cardiol. 2007;49:2035–2043. doi: 10.1016/j.jacc.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 2.Lerman BB, Belardinelli L, West GA, Berne RM, DiMarco JP. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation. 1986;74:270–280. doi: 10.1161/01.cir.74.2.270. [DOI] [PubMed] [Google Scholar]
- 3.Lerman BB, Stein KM, Markowitz SM. Adenosine-sensitive ventricular tachycardia: a conceptual approach. J Cardiovasc Electrophysiol. 1996;7:559–569. doi: 10.1111/j.1540-8167.1996.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 4.Corrado D, Basso C, Leoni L, et al. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2008;51:731–739. doi: 10.1016/j.jacc.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmayer KS, Bhave PD, Marcus GM, et al. An electrocardiographic scoring system for distinguishing right ventricular outflow tract arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy from idiopathic ventricular tachycardia. Heart Rhythm. 2013;10:477–482. doi: 10.1016/j.hrthm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Tandri H, Macedo R, Calkins H, et al. Role of magnetic resonance imaging in arrhythmogenic right ventricular dysplasia: insights from the North American arrhythmogenic right ventricular dysplasia (ARVD/C) study. Am Heart J. 2008;155:147–153. doi: 10.1016/j.ahj.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Carlson MD, White RD, Trohman RG, Adler LP, Biblo LA, Merkatz KA, Waldo AL. Right ventricular outflow tract ventricular tachycardia: detection of previously unrecognized anatomic abnormalities using cine magnetic resonance imaging. J Am Coll Cardiol. 1994;24:720–727. doi: 10.1016/0735-1097(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz SM, Litvak BL, Ramirez de Arellano EA, Markisz JA, Stein KM, Lerman BB. Adenosine-sensitive ventricular tachycardia: right ventricular abnormalities delineated by magnetic resonance imaging. Circulation. 1997;96:1192–1200. doi: 10.1161/01.cir.96.4.1192. [DOI] [PubMed] [Google Scholar]
- 9.Globits S, Kreiner G, Frank H, Heinz G, Klaar U, Frey B, Gossinger H. Significance of morphological abnormalities detected by MRI in patients undergoing successful ablation of right ventricular outflow tract tachycardia. Circulation. 1997;96:2633–2640. doi: 10.1161/01.cir.96.8.2633. [DOI] [PubMed] [Google Scholar]
- 10.Proclemer A, Basadonna PT, Slavich GA, Miani D, Fresco C, Fioretti PM. Cardiac magnetic resonance imaging findings in patients with right ventricular outflow tract premature contractions. Eur Heart J. 1997;18:2002–2010. doi: 10.1093/oxfordjournals.eurheartj.a015212. [DOI] [PubMed] [Google Scholar]
- 11.Molinari G, Sardanelli F, Zandrino F, Parodi RC, Bertero G, Richiardi E, Di Donna P, Gaita F, Masperone MA. Adipose replacement and wall motion abnormalities in right ventricle arrhythmias: evaluation by MR imaging. Retrospective evaluation on 124 patients. Int J Card Imaging. 2000;16:105–115. doi: 10.1023/a:1006304626233. [DOI] [PubMed] [Google Scholar]
- 12.Gaita F, Giustetto C, Di Donna P, Richiardi E, Libero L, Brusin MC, Molinari G, Trevi G. Long-term follow-up of right ventricular monomorphic extrasystoles. J Am Coll Cardiol. 2001;38:364–370. doi: 10.1016/s0735-1097(01)01403-6. [DOI] [PubMed] [Google Scholar]
- 13.White RD, Trohman RG, Flamm SD, VanDyke CW, Optican RJ, Sterba R, Obuchowski NA, Carlson MD, Tchou PJ. Right ventricular arrhythmia in the absence of arrhythmogenic dysplasia: MR imaging of myocardial abnormalities. Radiology. 1998;207:743–751. doi: 10.1148/radiology.207.3.9609899. [DOI] [PubMed] [Google Scholar]
- 14.Krittayaphong R, Saiviroonporn P, Boonyasirinant T, Nakyen S, Thanapiboonpol P, Watanaprakarnchai W, Ruksakul K, Kangkagate C. Magnetic resonance imaging abnormalities in right ventricular outflow tract tachycardia and the prediction of radiofrequency ablation outcome. Pacing Clin Electrophysiol. 2006;29:837–845. doi: 10.1111/j.1540-8159.2006.00449.x. [DOI] [PubMed] [Google Scholar]
- 15.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, Reiber JH, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2:183–190. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 17.Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420. doi: 10.1016/j.jacc.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinsaft JW, Kim HW, Shah DJ, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol. 2008;52:148–157. doi: 10.1016/j.jacc.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 19.Tandri H, Bluemke DA, Ferrari VA, Bomma C, Nasir K, Rutberg J, Tichnell C, James C, Lima JA, Calkins H. Findings on magnetic resonance imaging of idiopathic right ventricular outflow tachycardia. Am J Cardiol. 2004;94:1441–1445. doi: 10.1016/j.amjcard.2004.07.150. [DOI] [PubMed] [Google Scholar]
- 20.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 21.Cox MG, Nelen MR, Wilde AA, Wiesfeld AC, van der Smagt JJ, Loh P, Cramer MJ, Doevendans PA, van Tintelen JP, de Bakker JM, Hauer RN. Activation delay and VT parameters in arrhythmogenic right ventricular dysplasia/cardiomyopathy: toward improvement of diagnostic ECG criteria. J Cardiovasc Electrophysiol. 2008;19:775–781. doi: 10.1111/j.1540-8167.2008.01140.x. [DOI] [PubMed] [Google Scholar]
- 22.Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, Tan V, Lerman BB, Mittal S. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–1097. doi: 10.1161/CIRCULATIONAHA.105.546432. [DOI] [PubMed] [Google Scholar]
- 23.Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010;7:865–869. doi: 10.1016/j.hrthm.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Aquaro GD, Pingitore A, Strata E, Di Bella G, Molinaro S, Lombardi M. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J Am Coll Cardiol. 2010;56:1235–1243. doi: 10.1016/j.jacc.2010.03.087. [DOI] [PubMed] [Google Scholar]