Abstract
Rationale
Dopamine β-hydroxylase (DBH) catalyzes the conversion of dopamine to norepinephrine in the CNS and peripherally. DBH variants are associated with large changes in circulating DBH and implicated in multiple disorders; yet causal relationships and tissue-specific effects remain unresolved.
Objective
To characterize regulatory variants in DBH, effect on mRNA expression, and role in modulating sympathetic tone and disease risk.
Methods and Results
Analysis of DBH mRNA in human tissues confirmed high expression in the locus coeruleus (LC) and adrenal gland, but also in sympathetically innervated organs (liver>lung>heart). Allele-specific mRNA assays revealed pronounced allelic expression differences in the liver (2–11-fold) attributable to promoter rs1611115 and exon 2 rs1108580, but only small differences in LC and adrenals. These alleles were also associated with significantly reduced mRNA expression in liver and lung. Although DBH protein is expressed in other sympathetically innervated organs, mRNA levels were too low for analysis. In mice, hepatic Dbh mRNA levels correlated with cardiovascular risk phenotypes. The minor alleles of rs1611115 and rs1108580 were associated with sympathetic phenotypes including angina pectoris. Testing combined effects of these variants suggested protection against myocardial infarction in three separate clinical cohorts.
Conclusions
We demonstrate profound effects of DBH variants on expression in two sympathetically innervated organs, liver and lung, but not in adrenals and brain. Preliminary results demonstrate an association of these variants with clinical phenotypes responsive to peripheral sympathetic tone. We hypothesize that in addition to endocrine effects via circulating DBH and norepinephrine, the variants act in sympathetically innervated target organs.
Keywords: Dopamine beta-hydroxylase, regulatory genetic variants, genetic association, sympathetic tone, myocardial infarction, human, gene expression/regulation, genetic polymorphism
INTRODUCTION
Norepinephrine serves as a key hormone and neurotransmitter throughout the body. It is exclusively produced by the enzyme dopamine β-hydroxylase (DBH) catalyzing the conversion of dopamine to norepinephrine.
DBH protein expression is tissue specific. In the brain, DBH is transcribed in the locus coeruleus (LC), and DBH protein is found in brain regions innervated by noradrenergic neurons. In the periphery, DBH is predominantly concentrated in the adrenal glands and sympathetic nerve terminals. While DBH is robustly expressed in the adrenals, sympathetic nerves are a main source of plasma DBH. 1 Upon adrenergic stimulation, both DBH and norepinephrine are released from vesicles via exocytosis 2 accounting for substantial DBH levels in the circulation. Depletion of sympathetic nerve terminals reduces serum DBH activity by 50%.3 Blockade or depletion of DBH also increases the ratio of dopamine to norepinephrine in the circulation. 4, 5 DBH-positive nerve fibers have been observed in sympathetically innervated tissues including the liver 6 near portal triads and central veins. 7
Arising from both adrenals and sympathetically innervated organs, circulating DBH activity has been proposed as an index of sympathetic nervous system activity; however, results have been ambiguous. Initial studies suggesting acute stressors increase plasma DBH activity8 have not replicated in subsequent experiments. 9, 10 However, circulating DBH levels may be a useful measure of long-term noradrenergic function 11 as measurement of DBH and norepinephrine in organs is difficult and affected by removal into the circulation or by metabolism.
Rare cases of congenital DBH deficiency present with no detectable norepinephrine and a 10-fold increase in dopamine levels, 12, 13 orthostatic hypotension, hyperflexible joints, and hypoglycemia. Significant inter-individual variability in plasma DBH levels has been associated with genetic DBH variants. The minor allele (T) of the promoter single nucleotide polymorphism (SNP) rs1611115 (−1021C/T) is associated with lower serum DBH levels, accounting for 35–52% of the variation across populations, 11, 14–16 while individuals homozygous for the C allele have increased plasma norepinephrine levels. 19 However, it remains unknown how rs1611115-T reduces circulating DBH and norepinephrine levels. Reporter gene assays in rat PC12 chromaffin cells have yielded opposing results with rs1611115, 17–19 and whether this effect applies in vivo in human cell types remains uncertain.
Clinical association studies have examined the role of rs1611115 in addiction 20, 21,22, epilepsy 23, schizophrenia 24 and attention deficit hyperactivity disorder. 25 Other studies have focused on peripheral effects, such as blood pressure and glucose levels. Consistent with a lack of DBH causing hypotension, 12, 26 the major C allele has been associated with elevated blood pressure, both under normal conditions 27 and in response to stress, 17 and hypertension in the presence of increased fasting plasma glucose levels. 28
Fewer studies have examined the frequent rs1108580, located in exon 2 at the 5′ splice junction. This SNP is thought to alter RNA splicing, although this has not been demonstrated experimentally. 29, 30 The minor A allele has been associated with lower DBH in the serum and cerebrospinal fluid, 30 with mixed clinical correlations, 29, 31 or no association. 31–33 Recent GWAS results confirm a role of rs1611115 and suggest the presence of additional promoter/enhancer DBH variants, 34 but linkage disequilibrium (LD) to rs1108580 had not been considered.
This study characterizes two variants affecting DBH mRNA expression predominantly in peripheral sympathetic neurons, and tests associations with clinical phenotypes.
METHODS
Detailed Methods are available in the Online Supplement, which includes sources of human tissue; RNA, DNA and cDNA preparation; SNP genotyping; allelic mRNA expression analysis; quantitative real-time reverse transcriptase PCR; statistical analysis; 5′ RACE (rapid amplification of cDNA ends); measurement of cardiac pre-ejection period, and analysis of clinical cohorts, including phenome-wide association studies.
RESULTS
mRNA levels of DBH in human tissues
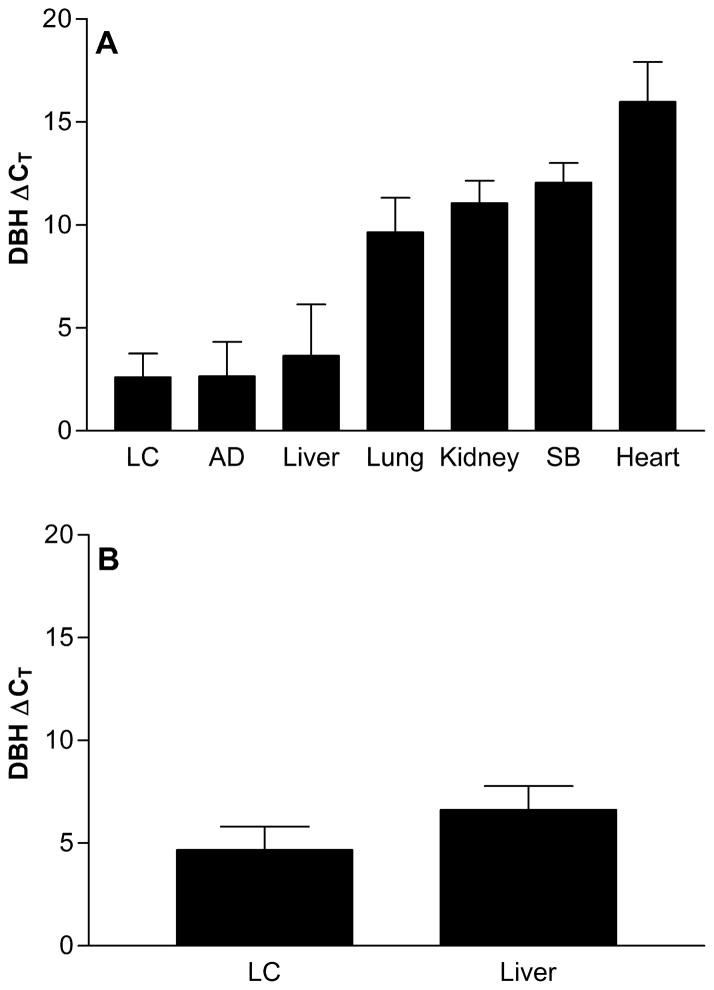
We measured DBH mRNA expression across several human tissues, including sympathetically innervated organs. As DBH protein is known to be axonally transported to sympathetic nerve terminals, 35 we hypothesized that mRNA could also be transported to tissues with sympathetic innervation, supporting local translation. The highest mRNA expression levels were detected in the LC, adrenal gland, and liver, followed by lower levels in lung, kidney, small bowel, and heart (Figure 1A). DBH mRNA detected in organs containing sympathetic nerve terminals but no neuronal nuclei was likely transported to target tissues, with strikingly high mRNA levels in the liver (Figure 1A).
Figure 1.
A. Comparison of DBH mRNA expression across tissues including locus coeruleus (LC), adrenal (AD) and small bowel (SB) using a Taqman assay targeting the exon 11–12 boundary, standardized to GAPDH. Each group contains 6–8 individual samples of each tissue type. B. DBH mRNA expression targeting the exon 2–3 boundary, standardized with PGK1 using qRT-PCR for 16 LC, and 41 liver samples. In both cases, the qRT-PCR cycle thresholds are standardized to the house keeping gene; a higher Ct denotes lower expression.
We then measured DBH mRNA expression in an expanded set of LC and liver tissues, with PCR primers spanning the exon 2–3 boundary to detect any influence of rs1108580 on RNA splicing (Figure 1B). The results did not differ significantly, arguing against the presence of splice isoforms. DBH mRNA was undetectable in the hepatocellular carcinoma cell line HepG2, consistent with immunohistochemistry experiments showing expression in nerve terminals. 7, 36
Sequencing of hepatic DBH mRNA, after conversion to cDNA, showed that the liver mRNA contained the full annotated DBH coding sequence according to the UCSC Genome Browser. 5′ RACE revealed the liver transcripts were capped and the 5′ untranslated region (UTR) was full-length (39 bases) matching the AceView and UCSC references.
Determination of Allelic DBH mRNA ratios as an indicator of regulatory polymorphisms
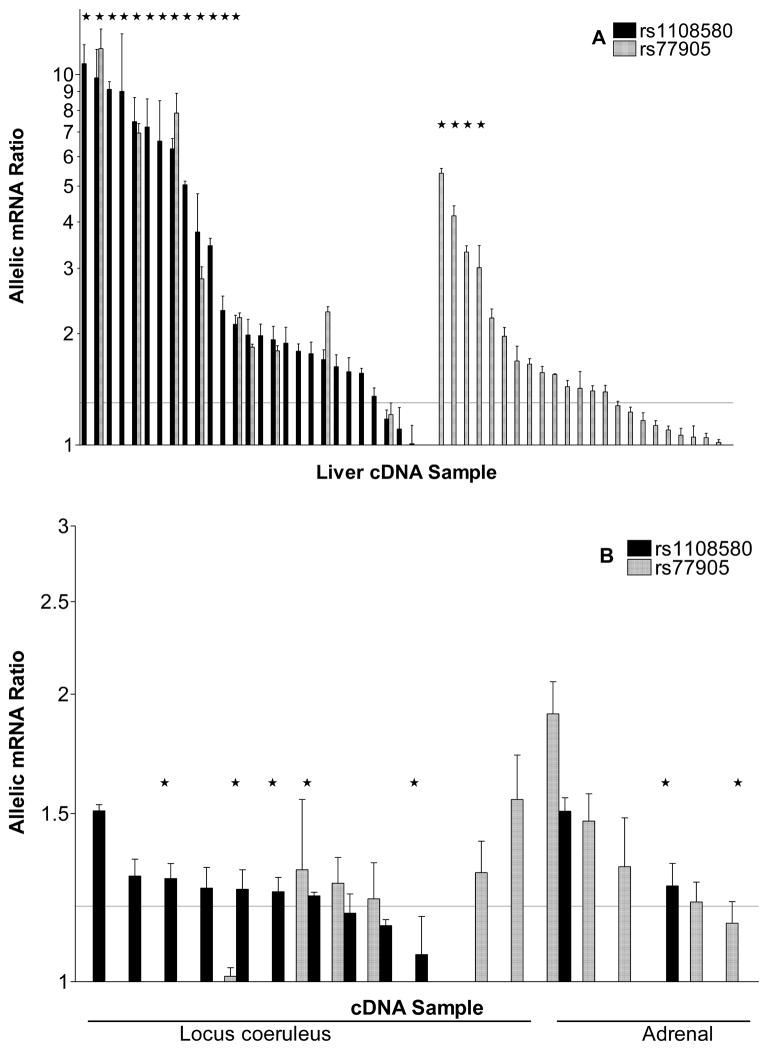
Allelic ratios in gDNA, measured at a SNP in heterozygous carriers, are assumed to be 1 for the autosomal DBH, while any significant deviation of allelic ratios in mRNA (allelic mRNA expression imbalance or AEI) reveals the presence of regulatory variants. 37 Two common SNPs located in the DBH coding region, rs1108580 and rs77905 were selected as markers to measure allelic mRNA ratios in human liver, LC, and adrenals. No sample displayed evidence of copy number variation. With three standard deviations of the gDNA ratio as the threshold, we considered an allelic mRNA ratio of <0.8 and >1.3 as significant evidence of AEI.
With marker SNP rs1108580, most tissues demonstrated AEI, with the main allele more abundant than the minor allele (ratios >1) in all tissues (Figure 2). Therefore, rs1108580 is likely causing this difference in allelic expression and/or shares a haplotype with one or more variants causing the change. Striking differences were observed in AEI ratios between tissues: less than 2-fold in LC and adrenals (Figure 2B) but up to 11-fold in the liver (Figure 2A). The large AEI ratios were replicated with marker rs77905, in a subset of livers heterozygous for both marker SNPs, yielding AEI ratios of similar magnitude, with a correlation of R2=0.96 (Online Figure II). Additional instances of AEI were revealed only with marker rs77905 (Figure 2A right panel). These results document a remarkable regulatory genetic effect specifically in liver, with lesser effects in the brain and adrenals. DBH mRNA levels in other tissues were too low for accurate AEI analysis.
Figure 2.
Allelic DBH mRNA expression ratios measured at marker rs1108580 (black) and rs77905 (grey) in liver (A) and locus coeruleus and adrenal gland (B). The values are plotted as a ratio of major over minor allele for rs1108580; all AEI ratios for rs110580 were >1. As rs77905 is not in LD with rs1108580, AEI ratios ranged from >1 to <1 with near equal distribution in either direction; for comparison, these ratios are shown as absolute values in only one direction (>1). Livers heterozygous for rs1611115 are indicated with ↔. The horizontal line demarks the threshold for significant AEI (1.3). Each bar is the mean AEI measurement from one subject.
Genotype association with instances and degree of allelic expression imbalance (AEI) in liver
To identify the DBH variants associated with AEI, 11 variants previously implicated in clinical association studies were genotyped in all samples. Online Table I lists the MAF and association with AEI, while Online Table II provides LD between the SNPs. AEI was significantly associated with rs1611115 genotype, measured at rs1108580 (p=2.0E-07) and at rs77905 (p=0.0016). Of the 17 livers with the highest AEI ratios, all were heterozygous for rs1611115 (Figure 2A), providing overwhelming evidence that this promoter SNP has strong effects on DBH mRNA expression. When measured with marker rs1108580 which is in partial LD with rs1611115, the minor allele was consistently associated with lower expression. Of 15 livers heterozygous for marker rs1108580 but not for rs1611115, 11 showed AEI with ratios above 1.3, indicating rs1108580 is causative itself. Moreover, livers with the highest ratios were heterozygous for both (Figure 2A). These results support the conclusion that both act to decrease mRNA expression of the variant allele, with rs1611115-T causing ~4-fold and rs1108580-A ~2-fold imbalance.
In contrast to the results in liver, rs1611115 genotype had no discernible effect in the brain or adrenal tissues, while rs1108580-A was associated with1.2–1.7-fold reduced DBH mRNA expression (Figure 2B), suggesting a small but potentially relevant effect of the minor allele of rs1108580 on mRNA abundance in these tissues.
Most other SNPs genotyped failed to achieve significance when tested for an association with AEI (see Online Table I for all values). SNP rs2519143 was statistically significant, but this can be accounted for by high LD with rs1611115 (Table 1); rs2519143 did not match the AEI pattern as well and was considered an unlikely causative variant. A subset of livers had AEI ratios of 1.5–2.5-fold measured at marker rs77905, but were homozygous for both rs1611115 and rs1108580. The entire DBH gene locus was sequenced in these samples, beginning 2 kb upstream of the coding region and extending 15 kb downstream from the end of the 3′UTR into the gene encoding sarcosine dehydrogenase (SARDH) with genotypes listed in Online Table III. However, no SNP accounted for these cases of AEI. Any additional regulatory variant would likely be in low LD with rs77905 because the allelic mRNA ratios ranged from below to above one. We hypothesize that this putative third variant is located outside of the gene region sequenced, and has a MAF of 15–20%
Table 1.
Minor allele frequencies and LD values (D′ and R2)
| D′ | R2 | D′ | R2 | D′ | R2 | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MAF | Marker | rs1611115 | rs1108580 | rs2519143 | |||
|
| |||||||
| (T): 0.21 | rs1611115 | . | . | . | . | . | . |
| (A): 0.46 | rs1108580 | 0.73 | 0.15 | . | . | . | . |
| (A):0.20 | rs2519143 | 0.91 | 0.79 | 0.77 | 0.16 | . | . |
SNPs in the liver cohort that were significantly associated with instances of AEI.
Effect of Genotype on DBH mRNA expression in human tissues
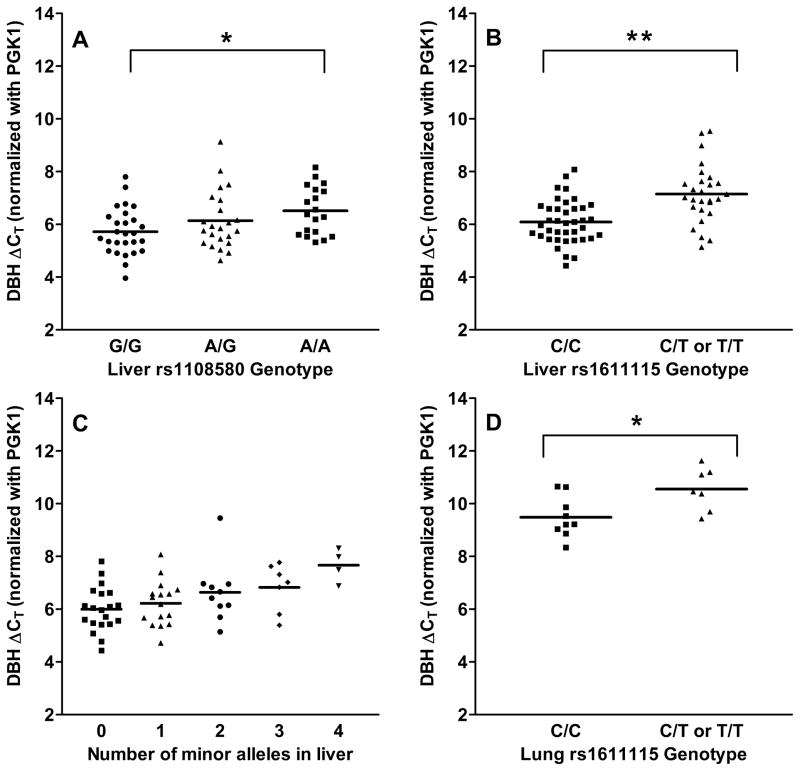
mRNA levels were measured in all available tissues and tested for association with genotypes (Figure 3). Expression was measured in liver, adrenal, brain and lung, while mRNA levels were close to the detection limit in heart and small bowel.
Figure 3. DBH mRNA levels grouped by genotype.
The data represent the mean (n=2, average standard deviation of 0.27) of qRT-PCR cycle thresholds standardized to PGK1 mRNA as the house keeping gene. There was a significant difference between groups (p=0.03) and between AA and GG genotypes for rs1108580 (*p=0.005) in liver (A), and a significant effect of rs1611115 in liver (**p=0.0001) (B), and lung (p=0.02) (D). A one-way ANOVA showed an overall significant difference between the groups (p=0.01) when liver expression was grouped by number of minor alleles of rs1108580 and rs1611115 (C).
The minor A allele of rs1108580 judged by AEI ratios was associated with lower expression in nearly all tissues tested, albeit with rather small effects in LC and adrenals. Accordingly, mRNA levels were significantly lower for AA livers compared to GG (Figure 3A; p=0.005; two-tailed t-test). The rs1108580 genotype was not significantly associated with mRNA levels in brain (p=0.6) nor lung tissues (p=0.3), possibly owing to small effect size (brain), large variability and low sample number.
Expression was not significantly associated with rs1611115 genotype in brain tissue (p=0.6), as expected from the absence of a discernible effect of rs1611115 on allelic mRNA expression. In livers, there was no significant difference between the expression in CT and TT (p=0.2), so these genotypes were combined. A two-tailed t-test showed a significant effect of rs1611115 genotype on total DBH mRNA expression in liver tissue (p=0.0001) (Figure 3B). The minor T allele was associated with decreased DBH expression. The CT difference between the means was 1.1, reflecting a 2-fold change in expression between the groups. Similarly, a significant effect of rs1611115 genotype on DBH expression was observed in the lung (p=0.02) with the T allele again associated with nearly 2-fold lower DBH expression (Figure 3D).
Since both rs1611115 and rs1108580 affected DBH mRNA expression in liver, we examined the combined effect on overall expression. A stepwise linear regression indicated that demographics (race, age and gender) had no significant effect on expression (Online Table V), and they were excluded as covariates. With increasing number of minor alleles per tissue, DBH mRNA levels decreased stepwise from 1–4 minor alleles, (Figure 3C) (ANOVA p=0.01), indicating that the joint effect of the two variants may be relevant for DBH associated phenotypes.
Association of DBH mRNA levels in the liver with phenotypes in inbred mouse strains
To determine whether DBH expression levels were associated with differences in sympathetic tone, we queried the Jackson Laboratory database which provides mRNA expression data in macrophages, brain, and liver, and a range of phenotypes. Dbh mRNA was detectable in all tissue types, and the query was not limited to sympathetic phenotypes. Expression of hepatic Dbh mRNA was associated with several phenotypes at p≤0.001 (Online Table VI), whereas no results were observed at p≤0.001 for Dbh expression in macrophages or brain. Inverse correlations included measures related to increased body size, weight, and length, whereas positive correlations involved lean mass. Hepatic Dbh mRNA was positively correlated with increased food and water intake and triglycerides. Higher Dbh expression was also associated with increased R wave electrocardiogram amplitude, a putative marker of increased cardiovascular risk in humans.
Myocardial contractility and sympathetic control measured by cardiac pre-ejection period (PEP)
As an index of myocardial contractility and sympathetic control we measured cardiac PEP under stress conditions in 130 human subjects. There was no significant genotype effect in females (p=0.77) while PEP values were also significantly higher than males under non-stress conditions (Online Figure III). In males, a t-test demonstrated a significant difference in stress PEP between subjects homozygous for the major allele of rs1611115 and carriers of at least one copy of the minor allele (p<0.05, effect size d = 0.54). CC males show significantly higher myocardial contractility during the stress condition as compared to male T carriers. No significant association was observed with rs1108580.
Clinical association studies with human phenotypes and diseases
To explore phenotypic associations in human subjects, we performed a phenome-wide association study (PheWAS) with rs1611115 and rs1108580. These analyses include de-identified electronic health record (EHR) data. 38–40 We used clinical and genotype information on 3,035 subjects from the Geisinger Clinic MyCode biorepository 41 and a replication sample of 4,027 from the Marshfield Personalized Medicine Research Project (PMRP).
The Geisinger study
Of the 482 phenotypes tested in the Geisinger dataset, single SNP analysis using an additive model revealed four associations with rs1108580 and three with rs1611115 at a cutoff of p<0.01 (Online Table VII). Notably, the minor allele of both SNPs, rs1611115-T (p=0.0002, OR=0.43, 95% CI=0.28–0.66) and rs1108580-A (p=0.007, OR=0.67, 95% CI=0.50–0.90) was associated with reduced risk of angina pectoris (code 413.9). Additionally, rs1611115-T was associated with risk for diabetes (p=0.002, OR=1.9, 95% CI=1.3–2.9). The most significant association for SNP rs1108580-A was with intervertebral disc disorders (p=3.1E-05) with a negative direction of effect. This may be related to clinical observations in which patients with congenital DBH deficiency have hyperflexible joints. In the dominant model, rs1611115-T was associated with reduced risk of angina pectoris, for the same diagnosis code (413.9), and in the same direction as in the additive model (p=0.001, OR=0.44, 95% CI=0.26–0.73). Also using a dominant model, rs1611115-T emerged as a risk factor for asthma with a large estimated effect size (p=0.007, OR=3.1, 95% CI=1.4–7.2).
Considering the molecular genetics results, we tested for statistical interactions between rs1611115 and rs1108580. Seven phenotypes had a likelihood ratio test (LRT) with p≤0.01 (Table 2). The combined effects of both SNPs were associated with reduced risk of myocardial infarction (code 410.1) (LRT p=0.001). At a lower level of significance (p=0.01), a potential risk effect with chronic ischemic heart disease (code 414.9) was apparent. Additional risk effects were observed for metabolic syndrome and hypoglycemia, and a potentially protective effect for obesity (Table 2).
Table 2.
PheWAS analysis results using an interaction model, in the Geisinger study
| fullV1 Beta | fullV2 Beta | fullV1.V2 Beta | fullMod pval | LRT pval | ICD-9 Code | ICD-9 Description | Cases | Ctrls |
|---|---|---|---|---|---|---|---|---|
| 2.3 | 0.80 | −3.5 | 0.0008 | 0.001 | 410.1 | Acute myocardial infarction: acute myocardial infarction of other anterior wall | 11 | 3024 |
| 1.2 | 0.43 | −1.3 | 1.5E-04 | 0.004 | 110.1 | Dermatophytosis: dermatophytosis of nail | 47 | 2988 |
| −3.5 | −0.39 | 2.3 | 1.1E-05 | 0.01 | 706.1 | Diseases of sebaceous glands: other acne | 16 | 3019 |
| −3.1 | −0.21 | 1.7 | 1.8E-06 | 0.01 | 277.7 | Other and unspecified disorders of metabolism: dysmetabolic syndrome X | 28 | 3007 |
| −1.9 | 0.031 | 0.98 | 2.8E-44 | 0.01 | 251.1 | Other disorders of pancreatic internal secretion: other specified hypoglycemia | 78 | 2957 |
| 2.3 | 1.3 | −1.7 | 3.1E-06 | 0.01 | 278.1 | Overweight obesity: localized adiposity | 18 | 2017 |
| −0.48 | −0.32 | 0.42 | 5.3E-50 | 0.01 | 414.9 | Ischemic heart disease: chronic ischemic heart disease | 283 | 2752 |
The model uses logistic regression and an interaction term to test the interaction of rs1611115 (V1) and rs1108580 (V2). This allowed us to identify whether the full model including the interaction was significant, and whether the full model (fullMod) results were significantly different when compared to the reduced model using a likelihood ratio test (LRT). Results with p≤0.01 are reported, and sorted by increasing LRT p-values.
The Marshfield study
To replicate these findings, all ICD-9 codes within any ICD-9 category that showed an association p<0.01 in the initial PheWAS analysis were also tested in the Marshfield EHR (92 phenotypes). We considered replication across EHR data within an ICD-9 category, even if the exact ICD-9 code is not implicated. While the two SNPs did not replicate with the same direction of effect for the main effect models (additive, dominant, and recessive), use of the two-SNP interaction model yielded similar results, with reduced risk for myocardial infarction (code 410.9) (p=0.0033), showing strong protective effect with 13 cases and 4,015 controls. We also found a protective effect with angina pectoris (code 413.9) (p=0.038) in 309 cases and 3,719 controls.
The Jackson Heart Study (JHS)
To specifically replicate cardiovascular associations and increase cohort diversity, we used data from the JHS. Among the 2,378 JHS participants, 62 incident MIs and 91 total CHD events were observed. Association of rs1611115 and rs1108580 with incident MI and CHD was tested by Cox Proportional Hazards (survival) analysis. Neither SNP alone was significantly associated with MI (rs1611115 p=0.11, rs1108580 p=0.077); however when considered together, there was a significant association between the SNPs and MI (p=0.039). When considered together, both SNPs were also significantly associated with CHD (p=0.033). Like the PheWAS analysis, the minor allele of both SNPs was protective, yielding a relative risk of 0.73 (95% CI 0.54–0.99) for each additional copy of a minor allele at either SNP with a cumulative relative risk for four copies of 0.28. Available data did not allow analysis of the relationship of these variants with either incident or prevalent angina.
DISCUSSION
This study identifies two regulatory DBH variants, rs1611115 and rs1108580, which robustly reduce DBH mRNA expression in two sympathetically innervated organs, the liver and the lung. In adrenals and brain the effect was undetectable (rs1611115) or less pronounced (rs1108580). We propose the hypothesis that rs1611115 and rs1108580 have tissue-specific effects at sympathetic nerve terminals, rather than altering stress response via the HPA axis. This hypothesis is supported by results from three clinical association studies, revealing effects on sympathetic phenotypes, including protection against MI.
Strong evidence indicates that rs1108580 affects circulating DBH levels 30 and rs1611115 genotype accounts for 35–52% of interindividual variability. 11, 14–16 The profound effect of these SNPs on circulating DBH levels is supported by their large effect on mRNA expression in liver and lung. We extrapolate from these results that genetic effects likely occur in all sympathetically innervated organs and thereby affect serum DBH.
A recent GWAS confirmed highly significant associations of circulating DBH levels with rs1611115 and rs1076150 34 (LD with rs1108580 of R2= 0.87, D′=0.96). However, enhancer region rs1076150 cannot fully account for the observed hepatic AEI ratios in our study, the most proximate phenotype for assessing the impact of regulatory variants. Although rs1108580 and rs1076150 are in high LD, one sample with two-fold AEI ratio was homozygous for rs1076150 and two samples lacking AEI were heterozygous for rs1076150 – arguing against a role of rs1076150. Therefore, we propose that rs1108580 is the likely functional variant acting on mRNA processing, with rs1076150 serving as a surrogate marker because of LD with rs1108580. Similarly, a GWAS reported significant association between a surrogate in high LD with rs1611115 (rs2519143, Table 1; see also 42), and DBH expression in the liver (p=2.31×10−18), 43 consistent with robust effects of rs1611115 on hepatic DBH mRNA expression.
The substantial level of DBH mRNA in the liver, and to a lesser extent in the lung, suggests that DBH mRNA is transported from sympathetic ganglia to nerve terminals in the target organ. Initial experiments in human celiac ganglia innervating the liver revealed a high level of DBH mRNA expression (data not shown), while others have demonstrated the presence of DBH mRNA in distal axons of sympathetic neuron cultures (N. Gervasi et al., 2012, 10th Int. Catecholamine Symposium, abstract). DBH mRNA transport in some tissues does not preclude protein migration, apparently the main mode in brain where we found low DBH mRNA levels outside the LC.
Selective effects of DBH variants on sympathetically innervated organs suggests that local release (together with norepinephrine) may have more phenotypic impact than circulating levels, with hepatic DBH mRNA expression serving as a possible indicator of paracrine effects across sympathetically innervated organs. The liver rapidly metabolizes norepinephrine upon local release and during passage, so that circulating norepinephrine only partially accounts for local exposure. 44 Subjects with rs1611115-C, associated with increased hepatic DBH mRNA in our study, had faster glomerular filtration rate decline, indicating decreased renal function, while the T allele was protective.19 Extrapolation to heart or kidney requires further study, but we hypothesize that rs1611115 affects phenotypes in these tissues via a paracrine mechanism. Further, our results reveal a DBH genotype effect on myocardial contractility, measured as PEP, a reflection of sympathetic tone. The minor allele of rs1611115 significantly reduces contractility in males under stress. It remains unclear why this effect is not observed in females. The PEP values did not change significantly with stress in the females, who differed substantially from baseline PEP values in males as well. Therefore, any genotype effects are likely not detectable in females under the test conditions. These results do not directly resolve the question whether the effect in males resulted from local or circulating sympathetic input, which will require additional studies.
Our results resolve a long-standing debate on the process by which the minor allele of rs1611115 is associated with strongly reduced circulating DBH activity. They also account for previous failures to resolve the promoter activity of rs1611115 in reporter gene assays in cell lines, out of context of the appropriate tissue environment. The majority of these experiments have been performed in PC12 rat pheochromocytoma cells17–19, 34 showing effects in vitro, contrary to our results in human adrenals lacking any rs1611115 effect. Reporter gene assays do not reproduce the in vivo conditions and can yield false positive results. Tissue-specific differences are frequently observed with regulatory variants, as observed with CETP45 and CYP3A4.46 Transcription factors can serve to suppress or enhance expression depending on context, with the DBH promoter/enhancer regions potentially containing several variants with distinct effects between tissues.
Our study established a non-detectable (rs1611115) or small (rs1108580) effect of these SNPs on mRNA in brain tissue. We also did not observe associations with CNS diseases (see “PheWAS” in Supplemental Material for phenotypes analyzed); however these results must be considered in the context of numerous positive findings from previous clinical association studies. It is possible that even small changes could affect noradrenergic function in the CNS. While norepinephrine and dopamine do not penetrate the blood-brain-barrier, circulating hormones have indirect effects on the CNS through the pineal gland or the pituitary, which are outside the barrier. They may also act at peripheral sites directly connected to the CNS, such as the vagus nerve, as its activity is regulated by peripheral catecholamines and has profound effects on the LC. 47, 48
In view of the single and combined effects of rs1611115 and rs1108580 on human tissues receiving sympathetic innervation, we have surveyed associations of DBH genotype with phenotypes in mice and humans. We present here evidence for the involvement of DBH in a range of peripheral sympathetic phenotypes, including metabolic disorders, cardiovascular disease, and asthma.
Effect of Dbh mRNA Levels in mouse liver on phenotypes
We found an association between Dbh mRNA expression in the liver and body size, metabolic processes, and cardiovascular phenotypes. While such broad phenotypic associations are expected from the multifaceted role of norepinephrine throughout the body, the association of increased hepatic Dbh mRNA levels with R wave amplitude, a prognostic indicator of myocardial hypertrophy, was of particular interest. Negative correlation of hepatic Dbh mRNA on body mass indicates a strong impact on metabolic activity. Although opposite effects were observed in the mouse model compared to the PheWAS, this may be a difference between localized adiposity and general fat distribution.
Effect of DBH genotype on clinical phenotypes
A PheWAS approach was employed to survey multiple phenotypes that could be affected by DBH variants. The adrenergic response to sympathetic activation in the liver is an increase in glycogenolysis, the breakdown of glycogen to glucose. Therefore, the association of rs1611115-T with type II diabetes risk is noteworthy. Also, Dbh knockout mice exhibit resistance to changes in glucose levels in an insulin tolerance test 49 and beta blockers have been associated with increased risk of type II diabetes.50 With decreased levels of DBH and less norepinephrine, rs1611115-T is a plausible risk factor for asthma, and should be investigated for responsiveness to adrenergic asthma medication, such as albuterol.
Consistent associations were observed with cardiovascular phenotypes. Angina pectoris was the most significantly associated phenotype with both rs1611115-T and rs1108580-A. Again along expectations for a reduced sympathetic tone, the minor alleles of both DBH variants were protective, paralleling the effect of beta blocker therapy.
Stipulating an interaction between rs1611115 and rs1108580 revealed a possible strong protective effect of the minor alleles against myocardial infarction in the Geisinger study, a result that was replicated in the Marshfield study. We speculate that the combined effect of two or more minor variants in these SNPs is needed to reach a threshold effect and thereby reveal this protective effect. In agreement with these results, rs1611115 and rs1108580 conveyed a protective effect against coronary heart disease attributable to an increasing number of minor alleles, while each SNP alone was not significant in the Jackson Heart Study of African Americans.
Limitations of the Study
While our results strongly support the finding that rs1611115 and rs1108580 affect expression of DBH mRNA specifically in liver and lung, but not adrenals and brain, extrapolation to cellular function and clinical phenotypes is exploratory at this point. On the basis of high congruence between allelic mRNA expression in the liver and profound genetic effects on circulating DBH levels that arise for all organs, and from the associated clinical phenotypes, it is reasonable to propose genetic effects involve all peripheral sympathetic neurons and their target organs. However, peripheral sympathetic ganglia may display different regulatory control. It appears that DBH mRNA and protein transport to nerve terminals differs between tissues, while the Human Protein Atlas shows medium to high protein staining in liver, testis, lung, stomach, colon, adrenals, and brain. Therefore, our proposal that the genetic variants in DBH act locally in a neurotransmitter or paracrine mode requires further study, but it does open new avenues for resolving clinically relevant phenotypes that often correlate poorly with circulating DBH and norepinephrine, possibly serving only as surrogate markers of robust local effects. Specifically, stress related processes involving the HPA axis may not be under genetic influence by DBH variants that have little effect on the adrenals.
Further confounding factors include the distinct biological effects of norepinephrine and dopamine that vary inversely with DBH genotype. However, most of the phenotypes observed in Dbh−/− mice are attributed to a lack of norepinephrine rather than increased dopamine, as DOPS rescue restores norepinephrine without affecting dopamine levels. 51
We are aware of the multiple hypothesis testing burden incurred from the number of phenotypes in the PheWAS analyses. However we reason Bonferroni correction is too stringent here. First, unlike a GWAS using an unbiased set of SNPs, we performed this PheWAS using two well characterized SNPs with established effects in target tissues. Further, as many of the diagnoses used in the PheWAS are correlated, not all outcomes are independent, reducing the number of independent variables. The clinical results require additional association testing and experimental work for full validation. These studies are observational and not designed a priori to test the specific associations observed here, with a relatively small number of cases. Despite the requirement for additional studies, our results highlight the potential of assessing sympathetic tone through DBH variants in pathophysiology and therapy of sympathetic dysregulation.
Supplementary Material
Novelty and Significance.
What Is Known?
Dopamine beta-hydroxylase (DBH) converts dopamine to norepinephrine in many tissues and is released from sympathetic nerve terminals upon stimulation.
Genetic DBH variants account for a significant portion of variability in plasma DBH levels and have been associated with multiple diseases, but the underlying mechanisms remain uncertain.
What New Information Does This Article Contribute?
Two single nucleotide polymorphisms (SNPs), rs1611115 and rs110850, are strongly associated with decreased DBH expression and must be considered jointly in association studies.
These effects of these variants was observed in sympathetically innervated organs such as the liver and lung, but not in brain or adrenal glands, suggesting that the DBH variants act locally in target organs.
When considered together, the two DBH SNPs were associated with protection against myocardial infarction.
Dopamine beta-hydroxylase mediates the sole pathway generating the neurotransmitter and hormone norepinephrine, critical to numerous body functions. Genetic variation of DBH has been under intense study revealing strong effects on DBH blood levels; yet, causative relationships and related phenotypes have remained enigmatic. We identify two frequent variants affecting mRNA expression with localized regulatory activity in sympathetically innervated organs such as liver and lung, rather than in adrenals and brain, tissues that are typically considered in DBH pathophysiology. We extended these studies in human genome-wide association studies, and identified an association between the two combined DBH variants and protection against myocardial infarction. These results implicate DBH effects in target organs such as lung (asthma), liver (diabetes), and kidneys (glomerular filtration), and the potential for developing a predictive clinical biomarker test and individualized drug therapies.
Acknowledgments
SOURCES OF FUNDING
This work was supported in part by NIH grant U01 GM092655 (WS), U01 GM074492 subcontract UF1011 (WS) and a Distinguished University Fellowship from The Ohio State University (ESB). Additional support was provided under award number UL1RR025755 from the National Center For Research Resources. Tissues were provided under NIH grant 5 U42 RR006042-20, UL1TR000427 and 1U01HG006389-01 and Emory P30NS055077. The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The PheWAS analyses were partially supported by NIH grants UL1TR000427, NIH 1U01HG006389-01, and NIH U01HL065962.
Nonstandard Abbreviations and Acronyms
- DBH
dopamine β-hydroxylase
- LC
locus coeruleus
- PheWAS
phenome-wide association study
- MAF
minor allele frequency
- qRT-PCR
quantitative real-time reverse transcriptase PCR
- RACE
rapid amplification of cDNA ends
- UTR
untranslated region
- AEI
allelic expression imbalance
- LD
linkage disequilibrium
- EHR
electronic health record
- PMRP
Marshfield Personalized Medicine Research Project
- PEP
pre-ejection period
Footnotes
DISCLOSURES
None.
References
- 1.Weinshilboum RM. Serum dopamine beta-hydroxylase. Pharmacol Rev. 1979;30:133–166. [PubMed] [Google Scholar]
- 2.Weinshilboum RM, Thoa NB, Johnson DG, Kopin IJ, Axelrod J. Proportional release of norepinephrine and dopamine-beta-hydroxylase from sympathetic nerves. Science. 1971;174:1349–1351. doi: 10.1126/science.174.4016.1349. [DOI] [PubMed] [Google Scholar]
- 3.Grobecker H, Roizen MF, Jacobowitz DM, Kopin IJ. Effect of prolonged treatment with adrenergic neuron blocking drugs on sympathoadrenal reactivity in rats. Eur J Pharmacol. 1977;46:125–133. doi: 10.1016/0014-2999(77)90248-5. [DOI] [PubMed] [Google Scholar]
- 4.Ohlstein EH, Kruse LI, Ezekiel M, Sherman SS, Erickson R, DeWolf WE, Jr, Berkowitz BA. Cardiovascular effects of a new potent dopamine beta-hydroxylase inhibitor in spontaneously hypertensive rats. J Pharmacol Exp Ther. 1987;241:554–559. [PubMed] [Google Scholar]
- 5.Bourdelat-Parks BN, Anderson GM, Donaldson ZR, Weiss JM, Bonsall RW, Emery MS, Liles LC, Weinshenker D. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- 6.Feher E. neuropeptide containing nerve fibres in the liver. Orv Hetil. 2004;145:1759–1763. [PubMed] [Google Scholar]
- 7.Pongor E, Ledo N, Altdorfer K, Lengyel G, Feher E. Distribution and possible origin of neuropeptide-containing nerve elements in the mammalian liver. Acta Vet Hung. 2010;58:177–187. doi: 10.1556/AVet.58.2010.2.4. [DOI] [PubMed] [Google Scholar]
- 8.Planz G, Palm D. Acute enhancement of dopamine-beta-hydroxylase activity in human plasma after maximum work load. Eur J Clin Pharmacol. 1973;5:255–258. [Google Scholar]
- 9.Laurian L, Oberman Z, Kisch E, Fitermann A. Changes in serum dopamine-beta-hydroxylase activity during cold pressor test in subjects with high and low basal activity of this enzyme. J Neural Transm. 1982;53:127–132. doi: 10.1007/BF01243404. [DOI] [PubMed] [Google Scholar]
- 10.Stone RA, Kirshner N, Gunnells JC, Robinson RR. Changes of plasma dopamine-beta-hydroxylase activity and other plasma constituents during the cold pressor test. Life Sci. 1974;14:1797–1805. doi: 10.1016/0024-3205(74)90281-1. [DOI] [PubMed] [Google Scholar]
- 11.Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, Cubells JF. A quantitative-trait analysis of human plasma-dopamine beta-hydroxylase activity: Evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet. 2001;68:515–522. doi: 10.1086/318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Man in ‘t Veld AJ, Boomsma F, Moleman P, Schalekamp MA. Congenital dopamine-beta-hydroxylase deficiency. A novel orthostatic syndrome. Lancet. 1987;1:183–188. doi: 10.1016/s0140-6736(87)90002-x. [DOI] [PubMed] [Google Scholar]
- 13.Robertson D, Goldberg MR, Onrot J, Hollister AS, Wiley R, Thompson JG, Jr, Robertson RM. Isolated failure of autonomic noradrenergic neurotransmission. Evidence for impaired beta-hydroxylation of dopamine. N Engl J Med. 1986;314:1494–1497. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]
- 14.Mustapic M, Pivac N, Kozaric-Kovacic D, Dezeljin M, Cubells JF, Muck-Seler D. Dopamine beta-hydroxylase (DBH) activity and -1021C/T polymorphism of DBH gene in combat-related post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1087–1089. doi: 10.1002/ajmg.b.30526. [DOI] [PubMed] [Google Scholar]
- 15.Garland EM, Black BK, Harris PA, Robertson D. Dopamine-beta-hydroxylase in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2007;293:H684–690. doi: 10.1152/ajpheart.01389.2006. [DOI] [PubMed] [Google Scholar]
- 16.Bhaduri N, Mukhopadhyay K. Correlation of plasma dopamine beta-hydroxylase activity with polymorphisms in DBH gene: A study on Eastern Indian population. Cell Mol Neurobiol. 2008;28:343–350. doi: 10.1007/s10571-007-9256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Wen G, Rao F, et al. Human dopamine beta-hydroxylase (DBH) regulatory polymorphism that influences enzymatic activity, autonomic function, and blood pressure. J Hypertens. 2010;28:76–86. doi: 10.1097/HJH.0b013e328332bc87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Zhang K, Wen G, Rao F, Sanchez AP, Wang L, Rodriguez-Flores JL, Mahata M, Mahata SK, Waalen J, Ziegler MG, Hamilton BA, O’Connor DT. Human dopamine beta-hydroxylase promoter variant alters transcription in chromaffin cells, enzyme secretion, and blood pressure. Am J Hypertens. 2011;24:24–32. doi: 10.1038/ajh.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasha DN, Davis JT, Rao F, et al. Heritable influence of DBH on adrenergic and renal function: Twin and disease studies. PLoS one. 2013;8:e82956. doi: 10.1371/journal.pone.0082956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freire MT, Marques FZ, Hutz MH, Bau CH. Polymorphisms in the DBH and DRD2 gene regions and smoking behavior. Eur Arch Psychiatry Clin Neurosci. 2006;256:93–97. doi: 10.1007/s00406-005-0610-x. [DOI] [PubMed] [Google Scholar]
- 21.Kalayasiri R, Sughondhabirom A, Gueorguieva R, Coric V, Lynch WJ, Lappalainen J, Gelernter J, Cubells JF, Malison RT. Dopamine beta-hydroxylase gene (DbetaH) −1021C-->T influences self-reported paranoia during cocaine self-administration. Biol Psychiatry. 2007;61:1310–1313. doi: 10.1016/j.biopsych.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kosten TR, Wu G, Huang W, Harding MJ, Hamon SC, Lappalainen J, Nielsen DA. Pharmacogenetic randomized trial for cocaine abuse: Disulfiram and dopamine beta-hydroxylase. Biol Psychiatry. 2013;73:219–224. doi: 10.1016/j.biopsych.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Depondt C, Cock HR, Healy DG, Burley MW, Weinshenker D, Wood NW, Goldstein DB, Sisodiya SM. The −1021C->T DBH gene variant is not associated with epilepsy or antiepileptic drug response. Neurology. 2004;63:1497–1499. doi: 10.1212/01.wnl.0000142092.16719.ad. [DOI] [PubMed] [Google Scholar]
- 24.Windemuth A, Calhoun VD, Pearlson GD, Kocherla M, Jagannathan K, Ruano G. Physiogenomic analysis of localized FMRI brain activity in schizophrenia. Ann Biomed Eng. 2008;36:877–888. doi: 10.1007/s10439-008-9475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhaduri N, Mukhopadhyay K. Lack of significant association between −1021C-->T polymorphism in the dopamine beta hydroxylase gene and attention deficit hyperactivity disorder. Neurosci Lett. 2006;402:12–16. doi: 10.1016/j.neulet.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Robertson D, Garland EM. Dopamine beta-hydroxylase deficiency. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. Genereviews. Seattle WA: University of Washington, Seattle; 1993. [Google Scholar]
- 27.Yeh TK, Yeh TC, Weng CF, Shih BF, Tsao HJ, Hsiao CH, Chuang FT, Hu CY, Chang CY. Association of polymorphisms in genes involved in the dopaminergic pathway with blood pressure and uric acid levels in Chinese females. J Neural Transm. 2010;117:1371–1376. doi: 10.1007/s00702-010-0492-6. [DOI] [PubMed] [Google Scholar]
- 28.Abe M, Wu Z, Yamamoto M, Jin JJ, Tabara Y, Mogi M, Kohara K, Miki T, Nakura J. Association of dopamine beta-hydroxylase polymorphism with hypertension through interaction with fasting plasma glucose in Japanese. Hypertens Res. 2005;28:215–221. doi: 10.1291/hypres.28.215. [DOI] [PubMed] [Google Scholar]
- 29.Wood JG, Joyce PR, Miller AL, Mulder RT, Kennedy MA. A polymorphism in the dopamine beta-hydroxylase gene is associated with “paranoid ideation” in patients with major depression. Biol Psychiatry. 2002;51:365–369. doi: 10.1016/s0006-3223(01)01367-1. [DOI] [PubMed] [Google Scholar]
- 30.Cubells JF, van Kammen DP, Kelley ME, Anderson GM, O’Connor DT, Price LH, Malison R, Rao PA, Kobayashi K, Nagatsu T, Gelernter J. Dopamine beta-hydroxylase: Two polymorphisms in linkage disequilibrium at the structural gene DBH associate with biochemical phenotypic variation. Hum Genet. 1998;102:533–540. doi: 10.1007/s004390050736. [DOI] [PubMed] [Google Scholar]
- 31.Kohnke MD, Kolb W, Kohnke AM, Lutz U, Schick S, Batra A. DBH*444G/A polymorphism of the dopamine-beta-hydroxylase gene is associated with alcoholism but not with severe alcohol withdrawal symptoms. J Neural Transm. 2006;113:869–876. doi: 10.1007/s00702-005-0365-6. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez F, Colson N, Quinlan S, MacMillan J, Lea RA, Griffiths LR. Association between migraine and a functional polymorphism at the dopamine beta-hydroxylase locus. Neurogenetics. 2009;10:199–208. doi: 10.1007/s10048-009-0176-2. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto K, Cubells JF, Gelernter J, Benkelfat C, Lalonde P, Bloom D, Lal S, Labelle A, Turecki G, Rouleau GA, Joober R. Dopamine beta-hydroxylase (DBH) gene and schizophrenia phenotypic variability: A genetic association study. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:33–38. doi: 10.1002/ajmg.b.10011. [DOI] [PubMed] [Google Scholar]
- 34.Mustapic M, Maihofer AX, Mahata M, Chen Y, Baker DG, O’Connor DT, Nievergelt CM. The catecholamine biosynthetic enzyme dopamine β-hydroxylase (DBH): first genome-wide search positions trait-determining variants acting additively in the proximal promoter. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu332. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahlstrom A. Axoplasmic transport (with particular respect to adrenergic neurons) Philos Trans R Soc Lond B Biol Sci. 1971;261:325–358. doi: 10.1098/rstb.1971.0064. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser MJ, Tiegs G, Neuhuber WL. Close apposition of dynorphin-positive nerve fibres to lymphocytes in the liver suggests opioidergic neuroimmunomodulation. Histochem Cell Biol. 2003;120:213–221. doi: 10.1007/s00418-003-0561-0. [DOI] [PubMed] [Google Scholar]
- 37.Wang D, Johnson AD, Papp AC, Kroetz DL, Sadee W. Multidrug resistance polypeptide 1 (MDR1, ABCB1) variant 3435C>T affects mRNA stability. Pharmacogenet Genomics. 2005;15:693–704. [PubMed] [Google Scholar]
- 38.Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, Wang D, Masys DR, Roden DM, Crawford DC. PheWAS: Demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26:1205–1210. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hebbring SJ, Schrodi SJ, Ye Z, Zhou Z, Page D, Brilliant MH. A PheWAS approach in studying HLA-DRB1*1501. Genes Immun. 2013;14:187–191. doi: 10.1038/gene.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pendergrass SA, Brown-Gentry K, Dudek SM, et al. The use of phenome-wide association studies (PheWAS) for exploration of novel genotype-phenotype relationships and pleiotropy discovery. Genet Epidemiol. 2011;35:410–422. doi: 10.1002/gepi.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottesman O, Kuivaniemi H, Tromp G, et al. The electronic medical records and genomics (EMERGE) network: Past, present, and future. Genet Med. 2013;15:761–771. doi: 10.1038/gim.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, de Bakker PI. SNAP: A web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schadt EE, Molony C, Chudin E, et al. Mapping the genetic architecture of gene expression in human liver. PLoS Biol. 2008;6:e107. doi: 10.1371/journal.pbio.0060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aneman A, Eisenhofer G, Olbe L, Dalenback J, Nitescu P, Fandriks L, Friberg P. Sympathetic discharge to mesenteric organs and the liver. Evidence for substantial mesenteric organ norepinephrine spillover. J Clin Invest. 1996;97:1640–1646. doi: 10.1172/JCI118590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papp AC, Pinsonneault JK, Wang D, Newman LC, Gong Y, Johnson JA, Pepine CJ, Kumari M, Hingorani AD, Talmud PJ, Shah S, Humphries SE, Sadee W. Cholesteryl ester transfer protein (CETP) polymorphisms affect mRNA splicing, HDL levels, and sex-dependent cardiovascular risk. PLoS one. 2012;7:e31930. doi: 10.1371/journal.pone.0031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J. 2011;11:274–286. doi: 10.1038/tpj.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol. 1987;393:213–231. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruffoli R, Giorgi FS, Pizzanelli C, Murri L, Paparelli A, Fornai F. The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanat. 2011;42:288–296. doi: 10.1016/j.jchemneu.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Ste Marie L, Palmiter RD. Norepinephrine and epinephrine-deficient mice are hyperinsulinemic and have lower blood glucose. Endocrinology. 2003;144:4427–4432. doi: 10.1210/en.2003-0561. [DOI] [PubMed] [Google Scholar]
- 50.Cooper-DeHoff RM, Bird ST, Nichols GA, Delaney JA, Winterstein AG. Antihypertensive drug class interactions and risk for incident diabetes: A nested case-control study. J Am Heart Assoc. 2013;2:e000125. doi: 10.1161/JAHA.113.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–2476. doi: 10.1046/j.1471-4159.1998.70062468.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.