NHL subtypes are classified based on clinical and histologic characteristics, as well as lymphocyte developmental stage. NHL sub-types include diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL),mantle cell lymphoma (MCL), Burkitt lymphoma (BL), primary effusion lymphoma (PEL) to name just a few. Many lymphomas such as DLBCL, MCL, and AIDS-associated lymphomas are very aggressive and rapidly fatal if unresponsive to therapy. Indolent lymphomas, such as FL are also many times not curable with conventional chemotherapy. Clearly, novel treatment paradigms are needed to improve clinical outcomes of patients with NHL.
We had previously shown that the phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) pathway was important for NHL survival and that single and dual inhibitors of this path way effectively inhibited NHL proliferation in vitro and in mouse xenograft models of NHL including FL and PEL1,2.
Another pathway that is linked to tumorigenesis is the mitogen activated protein kinase (MAPK), and many sub-types of NHL display an activated MAPK pathway3–5.
Although conventional chemotherapeutic drugs are useful in some cases, a significant number of NHL patients will eventually relapse and develop resistance to chemotherapy, which necessitates determining additional therapeutics. Since both PI3K and MAPK pathways are activated in NHL, we decided to investigate combination therapy involving inhibition of both pathways. It has been demonstrated that such therapy is effective against certain solid tumors 6,7.
In this study we used a combination of NVP-BEZ234 and AZD6244. NVP-BEZ235 is an imidazo[4,5-c]quinoline derivative compound, shown to inhibit PI3K and mTOR kinase activity both in vitro and in vivo including PEL and FL8,9. AZD6244is an inhibitor of MEK1 and MEK2 that does not compete with ATP binding. It is a potent and selective MEK1/2 inhibitor that demonstrates high activity in solid tumor models in vitro and in vivo10.
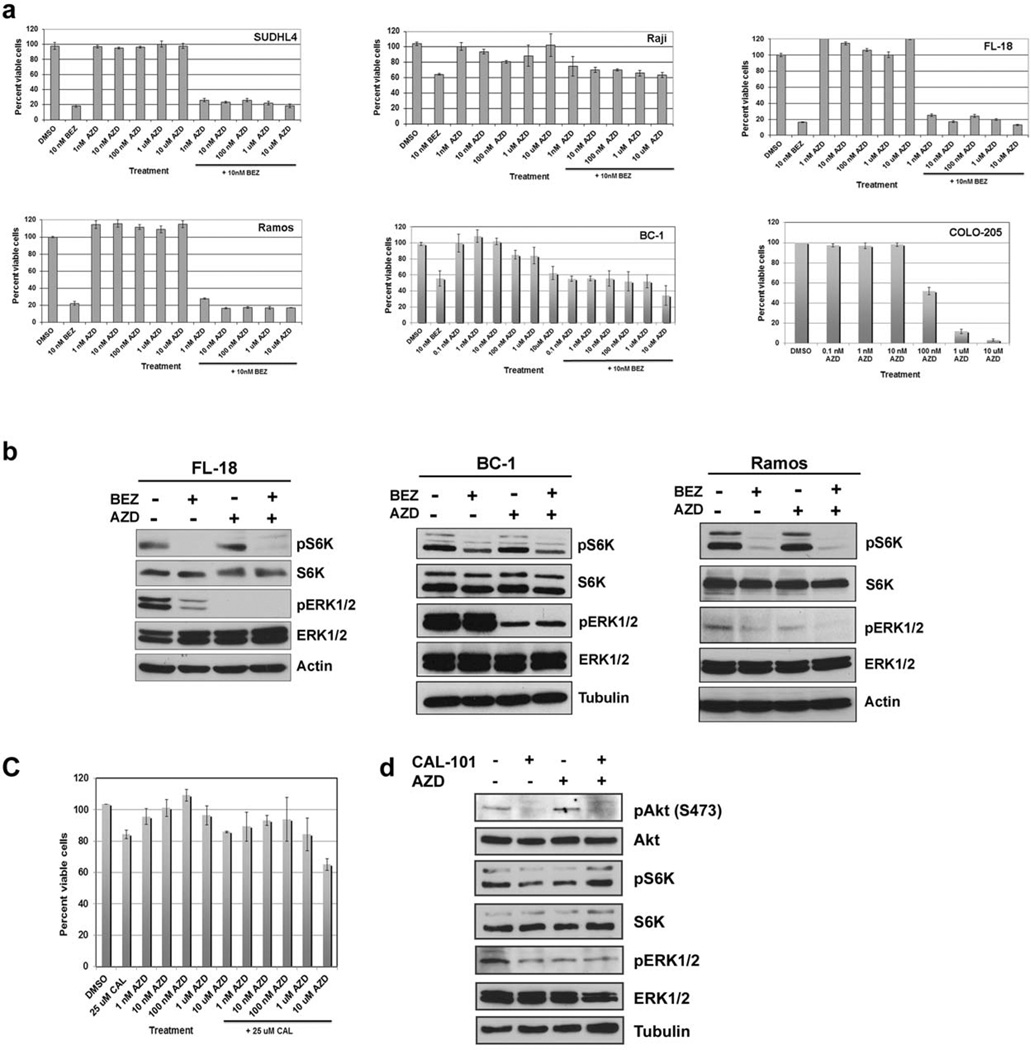
Representative types of NHL cells, includingSUDHL4 (DLBCL), FL-18 (FL), Raji (BL), Ramos (BL), and BC-1 (PEL), were treated with 0.1 nM, 1 nM, 10 nM, 100 nM, 1000 nM (1 µM), and 10,000 nM (10 µM) of the MEK inhibitor, AZD6244, either alone or in combination with 10 nM of the PI3K/mTOR inhibitor, NVP-BEZ235 for 72 hours (Figure 1a). The cells were also treated with 10 nM NVP-BEZ235 alone. An MTS assay was performed similar to what we previously described 1,2. Each experiment was done in triplicate and standard deviation was calculated. The amount of NVP-BEZ235 was kept constant at 10 nM, and increasing doses of AZD6244 was tested in combination with NVP-BEZ235. In each case, we observed that AZD6244 by itself did not have any discernable effect on cell viability in cell culture, even at the highest dose of 10 µM AZD6244 (Figure 1a). Moreover, the combination of different amounts of AZD6244 with 10 nM NVP-BEZ235 did not affect cell viability when compared to 10 nM NVP-BEZ235 alone in each case (Figure 1a). A slight decrease in cell viability was only seen at the highest dose of AZD6244 (10µM) with 10 nM NVP-BEZ235. This demonstrates that the combined inhibition of the PI3K and MAPK pathways was neither additive nor synergistic.
Figure 1. Simultaneous inhibition of the PI3K/mTOR and MEK/ERK pathways fails to synergistically reduce NHL cell proliferation in vitro.
(a) Proliferation of NHL cell lines treated with drug(s) or vehicle were evaluated by MTS Assay using the Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s instructions. Cells (1 X 105/mL) were incubated with drug(s) or vehicle for 72 hours in a 12-well plate. Twenty microliters of MTS reagent was added to 100µL of cell suspension in a 96-well plate and incubated at 37°C for 1 hour. Percent of viable cells after 72 hours treatment is shown on the y-axis. The percent of viable cells for untreated cells was set as 100% for each sample. Elevated absorbance values are indicative of metabolically active cells. The COLO-205 cells (5 X 103 cells/100µL) were added to each well of a 96-well plate. Increasing concentrations of drug(s) or vehicle were added the following day. The MTS reagent was added after 72 hours following the addition of the drug(s) or vehicle, and incubated with the cells for 4 hours at 37°C. Absorbance was measured at 490 nm using a FLUOstar OPTIMA spectrophotometer. Percent viable cells after 72 hours treatment is shown on the y-axis. The percent of viable cells for vehicle treated was set as 100% for each sample. Elevated absorbance values are indicative of metabolically active cells.(b) Phosphorylation of ERK 1/2 and S6 kinase were determined by Western blot of NHL cell lines treated with AZD6244 (1µM) and/or NVP-BEZ235 (30 nM) for 24 hours. Equal volume of DMSO (0.1%) was added for vehicle control. (c) Proliferation of cells with 25 µM CAL-101 and increasing concentrations of AZD6244 was determined by MTS assay after 48 hours following the addition of the drug(s) or vehicle as described in panel a. (d) Phosphorylation of Akt, ERK 1/2 and S6 kinase were determined by Western blot of NHL cell lines treated with AZD6244 (1µM) and/or CAL-101 (25µM) for 24 hours.
To confirm that the drug AZD6244 used in our experiments had similar activity to that recorded in previous reports11, we evaluated the effect of AZD6244 on the COLO-205 colorectal cancer cell line by culturing these cells in increasing concentrations of AZD6244 or vehicle for 72 hours. We determined an IC50 of 107.18 ± 2.47 nM in COLO-205 (Figure 1a). Similar results were seen with another non-small cell lung cancer cell line Calu-6, that was previously shown to be inhibited by AZD6244 11 (data not shown). We also evaluated NHL cell viability in the presence of increasing concentrations of the chemically unrelated MEK inhibitor, PD18435212. We found that PD184352 was ineffective at reducing cell viability in various NHL cell lines except at the highest dose of 10 µM (data not shown). This data supports our observations made with the MEK inhibitor, AZD6244, and suggests that NHL cells are not addicted to the MEK/ERK pathway for survival.
To verify that each of the drugs was inhibiting their respective targets, we performed Western blot analysis on the NHL cells that were treated with the above therapies for 24 hours (Figure 1b). We used phosphorylated S6K as a downstream marker for active PI3K/mTOR as we previously described 1,2, and we used phosphorylated ERK1/2 as a downstream marker for MAPK activation 10. We found thatNVP-BEZ235 inhibited PI3K signaling as measured by reduced phosphorylated S6Klevels, and that AZD6244 inhibited MAPK signaling as measured by reduced ERK1/2 phosphorylation (Figure 1b).
To determine whether a PI3K inhibitor only would be synergistic with AZD6244, we evaluated the combination of 25 µM CAL-101 (a PI3Kδ inhibitor) with increasing doses of AZD6244 on NHL cell viability. CAL-101 (5-fluoro-3-phenyl-2-[(S)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one) is a small molecule inhibitor of the class I PI3K p110δ isoform13. CAL-101 has been found to have anti-tumor activity in patients with indolent NHL and hence we sought to investigate whether inhibiting PI3K p110δ and MAPK may be synergistic against NHL14. Figure 1c depicts a representative NHL cell line (Ramos) that was tested with CAL-101 and AZD6244. We did not observe a synergistic or additive effect when different amounts of AZD6244 was combined with 25 µM CAL-101 compared to 25 µM CAL-101 alone (Figure 1c), although each drug appeared to be effective in inhibiting downstream effectors of the pathway. Treatment with CAL-101 inhibited phosphorylation of Akt and S6K, and AZD6244 inhibited phosphorylation of ERK1/2 (Figure 1d). We found similar results for FL-18 and Raji cells (data not shown). Thus, neither a dual inhibitor of the PI3K/mTOR pathway (NVP-BEZ235) nor a single inhibitor of PI3K (CAL-101) appear to synergize with AZD6244 in our system.
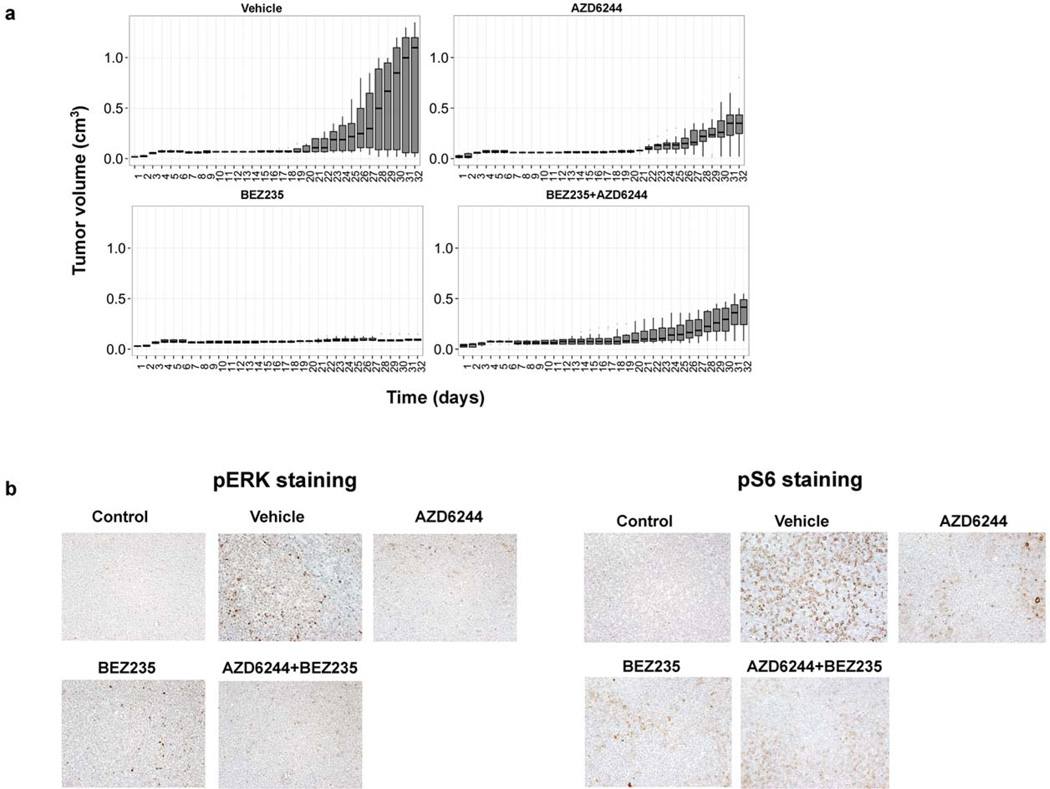
We next investigated the combination of NVP-BEZ235 and AZD6244 in a mouse xenograft model2. Four-to-five-week-old athymic nude-Foxn1^nu female mice were injected intraperitoneally with anti-asialo GM1 antibody (Wako). The following day, 1x106 FL-18 lymphoma cells were subcutaneously injected with growth-factor reduced matrigel as we previously described 2. Upon development of palpable tumors, the animals were split into four groups and treated by oral gavage6 days a week with either vehicle, 45 mg/kg NVP-BEZ235, 45 mg/kg AZD6244, or 25mg/kg each of NVP-BEZ235and AZD6244. While tumors grew in the mice treated with vehicle, mice treated with NVP-BEZ235 showed a significant inhibition of tumor growth as we previously reported 10. Interestingly, in the xenograft model, mice treated with AZD6244 alone did show a modest inhibition of tumor growth compared to vehicle treated mice. However, the combination of NVP-BEZ235 and AZD6244 did not have a synergistic or additive effect on tumor growth compared to NVP-BEZ235 or AZD6244 alone (Figure 2a).
Figure 2. Combined inhibition of the MEK/ERK and PI3K/mTOR pathways is no more effective at reducing tumor burden than single blockade of the PI3K/mTOR pathway in vivo.
(a) Athymic nude-Foxn1^nu 4-5-week-old female mice were injected subcutaneously into the right flank with 200 µL (1 X 106) of FL-18 cells. Five mice per group were treated with equal volume of vehicle, 45 mg/kg NVP-BEZ235, 45 mg/kg AZD6244, or 25 mg/kg each of NVP-BEZ235 and AZD6244 (combination therapy). Treatment involved oral gavage 6 times per week. Tumor volume was determined daily (L × W × D) and plotted over time. Shown for each time point is a box and whisker plot of the results per group. The horizontal bar indicates the median, the box indicates 25% and 75% of the data and the whiskers represent 1.5×the interquartile range, which encompasses 50% of the data.(b) Phosphorylation of ERK 1/2 or S6 protein from each treatment group was evaluated on paraffin-embedded tumors by immunohistochemical staining. Sections were counterstained with hematoxylin, and visualized at magnifications of 200X using a Leica DMLS microscope. Images were acquired using the Leica DFC480 camera and associated Leica Firecam software. Stored TIFF images were evaluated using Adobe Photoshop CS5.1.
The mice were sacrificed when maximal tumor size was reached. Tumors were excised, sectioned, and subjected to immunohistochemical analysis as we previously described 1. S6 was consistently phosphorylated in almost all cells in the vehicle control group, however we found reduced phosphorylated levels of S6, a downstream effector of the PI3K pathway in the NVP-BEZ235 treated grafts, and reduced phosphorylated ERK1/2, an effector of the MAPK pathway in the AZD6244 treated grafts (Figure 2b). Both markers were reduced in animals treated with both drugs. This suggests that both drugs were delivered to the tumors and inhibited their respective targets in the tumor cells in vivo. Overall, our data demonstrate that the combined inhibition of the PI3K and MAPK pathways was not synergistic or additive in preventing tumor growth of NHL cells in vitro or in vivo.
Currently, there are a number of clinical trials testing MEK and PI3K or mTOR inhibitors in patients. Our data suggest that combining inhibitors specific for these two path ways may not be an effective treatment option for NHL since there was no preclinical evidence showing any significant enhancement of cell death in vitro or inhibition of tumor growth in vivo when these inhibitors were combined. This is in direct contrast to reports in solid tumors such as melanoma and breast cancer in which the combination of these inhibitors showed a synergy in curtailing tumor growth in similar pre-clinical models 6,7. Davies et al. found that tumors with activating Ras mutations are more sensitive to AZD6244 than tumors with WT Ras11. Since activating mutations in Ras are infrequent in NHL 15, inhibition of the MEK/ERK pathway may not be as effective for NHL as for solid tumors despite the fact that ERK was highly phosphorylated in the cell lines tested.
Even though MEK/ERK and PI3K/mTOR pathways are active in NHL1,4,5, our data suggests that NHL are more reliant on the PI3K/mTOR pathway than the MEK/ERK pathway for cell survival. Normal B-lymphocyte survival is also dependent on the PI3K pathway16 and studies have shown that B-lymphocytes lacking BCR signaling do not survive while these cells can be rescued from apoptosis by being engineered to have constitutively active PI3K16.
Although the MEK/ERK pathway may be important in NHL pathology, we found that targeting this pathway alone is not sufficient to reduce NHL cell survival in vitro or in vivo. There are multiple arms of the MAPK pathway17 and to see additional benefit in NHL, we may need to modulate a different and/or multiple arms of this pathway along with PI3K/mTOR inhibition to see an enhancement in the reduction of NHL cell viability. Based on these results, we propose that further preclinical studies should be performed before a combination of MEK 1/2 and PI3K inhibitors is evaluated in a clinical trial setting for patients with NHL.
Acknowledgements
This work was supported by NIH grants CA096500, CA019014to BD, Leukemia & Lymphoma Society QFC grant to BD, CA163217to DD, and NIH/NCRR 1KL2RR025746 to SP.BD is a Leukemia & Lymphoma Society Scholar and a Burroughs Welcome Fund Investigator in Infectious Disease. PA is partially supported by T32-AI007419. We would like to acknowledge Novartis for providing NVP-BEZ235. We thank Charlene Ross and the Animal Models Core Facility for their help (CA016086).
Footnotes
Conflict of Interest Statement: The authors have no financial interests in relation to the work described in this manuscript and have nothing to disclose.
References
- 1.Bhatt AP, Bhende PM, Sin SH, Roy D, Dittmer DP, Damania B. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115(22):4455–4463. doi: 10.1182/blood-2009-10-251082. Prepublished on 2010/03/20 as DOI 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhende PM, Park SI, Lim MS, Dittmer DP, Damania B. The dual PI3K/mTOR inhibitor, NVP-BEZ235, is efficacious against follicular lymphoma. Leukemia. 2010;24(10):1781–1784. doi: 10.1038/leu.2010.154. Prepublished on 2010/08/13 as DOI 10.1038/leu.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elenitoba-Johnson KS, Jenson SD, Abbott RT, et al. Involvement of multiple signaling pathways in follicular lymphoma transformation: p38-mitogen-activated protein kinase as a target for therapy. Proc Natl Acad Sci U S A. 2003;100(12):7259–7264. doi: 10.1073/pnas.1137463100. Prepublished on 2003/05/21 as DOI 10.1073/pnas.1137463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogasawara T, Yasuyama M, Kawauchi K. Constitutive activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase in B-cell lymphoproliferative disorders. Int J Hematol. 2003;77(4):364–370. doi: 10.1007/BF02982645. Prepublished on 2003/05/31 as DOI. [DOI] [PubMed] [Google Scholar]
- 5.Ding H, Gabali AM, Jenson SD, Lim MS, Elenitoba-Johnson KS. P38 mitogen activated protein kinase expression and regulation by interleukin-4 in human B cell non-Hodgkin lymphomas. J Hematop. 2009;2(4):195–204. doi: 10.1007/s12308-009-0049-5. Prepublished on 2009/01/01 as DOI 10.1007/s12308-009-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts PJ, Usary JE, Darr DB, et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res. 2012;18(19):5290–5303. doi: 10.1158/1078-0432.CCR-12-0563. Prepublished on 2012/08/09 as DOI 10.1158/1078-0432.CCR-12-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.She QB, Halilovic E, Ye Q, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18(1):39–51. doi: 10.1016/j.ccr.2010.05.023. Prepublished on 2010/07/09 as DOI 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7(7):1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 9.Serra V, Markman B, Scaltriti M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008;68(19):8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 10.Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13(5):1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. Prepublished on 2007/03/03 as DOI 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 11.Davies BR, Logie A, McKay JS, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6(8):2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. Prepublished on 2007/08/19 as DOI 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen TK, Jordan N, Friedberg J, Fisher RI, Dent P, Grant S. Inhibition of MEK/ERK1/2 sensitizes lymphoma cells to sorafenib-induced apoptosis. Leuk Res. 2010;34(3):379–386. doi: 10.1016/j.leukres.2009.07.013. Prepublished on 2010/02/02 as DOI 10.1016/j.leukres.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. doi: 10.1182/blood-2010-03-275305. Prepublished on 2010/10/21 as DOI 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. doi: 10.1056/NEJMoa1314583. Prepublished on 2014/01/24 as DOI 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahuja HG, Foti A, Bar-Eli M, Cline MJ. The pattern of mutational involvement of RAS genes in human hematologic malignancies determined by DNA amplification and direct sequencing. Blood. 1990;75(8):1684–1690. Prepublished on 1990/04/15 as DOI. [PubMed] [Google Scholar]
- 16.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. Prepublished on 1997/10/10 as DOI. [DOI] [PubMed] [Google Scholar]
- 17.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22):3291–3310. doi: 10.1038/sj.onc.1210422. Prepublished on 2007/05/15 as DOI 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]