Abstract
The vertebrate inner ear is composed of multiple sensory receptor epithelia, each of which is specialized for detection of sound, gravity or angular acceleration. Each receptor epithelium contains mechanosensitive hair cells, which are connected to the brainstem by bipolar sensory neurons. Hair cells and their associated neurons are derived from the embryonic rudiment of the inner ear epithelium, but the precise spatial and temporal patterns of their generation, as well as the signals that coordinate these events, have only recently begun to be understood. Gene expression, lineage tracing, and mutant analyses suggest that both neurons and hair cells are generated from a common domain of neural and sensory competence in the embryonic inner ear rudiment. Members of the Shh, Wnt and FGF families, together with retinoic acid signals, regulate transcription factor genes within the inner ear rudiment to establish the axial identity of the ear and regionalize neurogenic activity. Close-range signaling, such as that of the Notch pathway, specifies the fate of sensory regions and individual cell types. We also describe positive and negative interactions between basic helix-loop-helix and SoxB family transcription factors that specify either neuronal or sensory fates in a context-dependent manner. Finally, we review recent work on inner ear development in zebrafish, which demonstrates that the relative timing of neurogenesis and sensory epithelial formation is not phylogenetically constrained.
Introduction
The vertebrate inner ear is a sensory organ dedicated to the detection of sound and motion. It comprises a series of fluid-filled chambers known collectively as the labyrinth, and contains six epithelial sensory structures (Fig. 1A). The organ of Corti runs along the length of the cochlear duct and is dedicated to hearing; it is known as the papilla in non-mammalian vertebrates. Fluid motion in the three semicircular canals caused by angular movements of the head is detected by cristae positioned at the base of each canal, while linear acceleration and gravity are detected by two sensory organs, the maculae, housed in two epithelial chambers called the utricle and saccule. Detection of sound and motion in each sensory organ is mediated by an array of mechanosensitive hair cells and associated supporting cells. Hair cells receive afferent innervation from sensory neurons of the VIIIth cranial or cochleo-vestibular ganglion (CVG), which is sub-divided into regions that innervate either the cochlea (the spiral ganglion in mammals) or the vestibular system (Fig. 1B).
Figure 1. Inner ear sensory regions and their innervation by spiral (cochlear) and vestibular ganglia.
(A) An embryonic day 15.5 mouse inner ear that has been fixed, cleared and its cavity filled with paint (Kiernan, 2006) to reveal the three-dimensional interior of the epithelial labyrinth. Sensory structures of the epithelium are shaded as shown in the accompanying key: three ampullae (am) contain sensory cristae (magenta); the utricle (ut) and saccule (sa) each contain a sensory macula (red), and the cochlea (co) contains the sensory organ of Corti (cyan). The panel is modified from (Groves and Fekete, 2012). (B) Space filling models offering lateral and medial views of an embryonic day 13.5 inner ear epithelial labyrinth and VIIIth ganglion (CVG) components. The CVG comprises the vestibular ganglion (VG), which innervates cristae and maculae, and the spiral ganglion (SpG), which innervates the organ of Corti. A portion of the panel is modified from (Raft, et al., 2004). Scale bars in (A) and (B) = 100 micrometers.
Both the mechanosensory regions of the inner ear labyrinth and the sensory neurons that innervate them are derived from a common primordium, the otic placode (Groves, 2005, Ohyama, et al., 2007, Riley and Phillips, 2003, Streit, 2001). This arises from primitive embryonic ectoderm on either side of the hindbrain in response to inducing signals, and then thickens and invaginates to form an otocyst. Many studies over the past 20 years suggest that the otocyst has already received much spatial patterning information by the time invagination is complete, and distinct sets of genes have been identified that divide the ear into broad territories in the anterior-posterior, dorso-ventral and medio-lateral axes (Fekete, 1996, Fekete and Wu, 2002, Wu and Kelley, 2012). In amniotes, the first indication of cell fate differentiation within the otic epithelium is the delamination of neuroblasts from a ventral region (Alsina, et al., 2004, Alsina, et al., 2009, Raft, et al., 2004, Wu and Kelley, 2012). In the mouse, this process begins at the anterior-posterior midline of the invaginating placode and subsequently expands to encompass the entire ventral face of the otocyst (Raft, et al., 2004). After roughly two embryonic days of neurogenesis, this region – sometimes referred to as the neural-sensory competent domain – begins producing the prosensory cells that will differentiate as hair cells or supporting cells. Neurogenesis and the production of sensory patches continue together for several days until neurogenesis is extinguished (Raft, et al., 2007). However, sensory tissue continues to differentiate for days and sometimes weeks: for instance, the mouse utricular macula does not finish adding hair cells until two weeks after birth (Burns, et al., 2012).
The coordinated production of hair cells and associated neurons requires that a precise series of signals induce or inhibit transcription factors specific to the neural or sensory lineages. In this review, we describe recent findings on how these signals are spatially and temporally regulated during development of the inner ear and its associated CVG.
1. The evolutionary origins of hair cells and the transcription factors that specify them
Vertebrate hair cells have an apical stereociliary bundle, an elaborate tuft of elongated actin-rich microvilli (Nayak, et al., 2007). A true cilium, the kinocilium, develops in all vertebrate hair cells, although it may disappear in some hair cell types as they mature. Vertebrate hair cells are secondary receptor cells: they do not elaborate either axons or dendrites, but are innervated by axons of bipolar sensory neurons that also send processes to brainstem nuclei. Many non-vertebrate taxa possess mechanoreceptive cells with similar apical specializations; these are also sometimes referred to as hair cells, although their degree of homology to vertebrate hair cells is still actively debated (Burighel, et al., 2011, Burighel, et al., 2008, Burighel, et al., 2003, Fritzsch and Straka, 2014, Manley and Ladher, 2008). These non-vertebrate “hair cells” cells can be either secondary receptors, or primary sensory neurons with intrinsic mechanosensitive specializations (Burighel, et al., 2011). Some non-vertebrate groups use both kinds of receptor cells in their sensory organs. For example, urochordate ascidians have ciliated primary sensory cells in their cupular and capsular organs that are thought to detect water flow (Burighel, et al., 2011, Caicci, et al., 2007, Gasparini, et al., 2013). More recently it has been shown that some ascidians possess a distinct sensory organ at the base of the oral siphon, the coronal organ, which also contains secondary receptor cells bearing stereocilia (Burighel, et al., 2003). Cephalopod mollusks have evolved very elaborate sensory systems, including statocysts that detect linear and angular acceleration and a lateral line system analogous to those seen in fish and amphibians (Budelmann, et al., 1997, Burighel, et al., 2011). These organs can also contain both secondary and primary sensory receptor cells with varying numbers of stereocilia and kinocilia. In contrast, insects appear to have lost secondary receptor cells, and instead have a wide range of mechanoreceptive sensory neurons arranged in clusters all over their bodies (Kernan, 2007). One class of stretch receptive organs, chordotonal organs, are deployed in different ways in different insect species to detect a variety of stimuli in addition to stretch, such as sound, gravity, and air flow (Eberl, 1999, Eberl, et al., 2000, Kamikouchi, et al., 2009, Yorozu, et al., 2009). Chordotonal organ sensory neurons have a single ciliated dendrite believed to contain mechanosensitive ion channels but have no microvillous specializations analogous to vertebrate stereocilia (Boekhoff-Falk, 2005, Eberl and Boekhoff-Falk, 2007, Kernan, 2007).
Vertebrate hair cells and CVG neurons are specified by distinct basic helix-loop-helix (bHLH) class transcription factors (Fritzsch, et al., 2007, Fritzsch, et al., 2010, Fritzsch, et al., 2006). Atoh1 (formerly known as Math1) is the first known transcription factor to be expressed in hair cell progenitors (Bermingham, et al., 1999, Cai, et al., 2013, Chan, et al., 2007, Driver, et al., 2013, Woods, et al., 2004). Atoh1 is both necessary and sufficient for hair cell development and survival: hair cell progenitors rapidly die in Atoh1 mutant mice (Bermingham, et al., 1999, Cai, et al., 2013, Chen, et al., 2003, Pan, et al., 2011), and the ectopic expression of Atoh1 in other parts of the inner ear is able to generate ectopic hair cells capable of attracting afferent innervation (Izumikawa, et al., 2005, Kawamoto, et al., 2003, Kelly, et al., 2012, Liu, et al., 2012a, Woods, et al., 2004, Zheng and Gao, 2000). Neurog1 (formerly Ngn1) is a closely related bHLH factor expressed in progenitor cells of the ventral otocyst shortly before they delaminate as neuroblasts (Ma, et al., 2000). Its expression is then extinguished and replaced by another closely related bHLH gene, Neurod1 (Kim, et al., 2001, Liu, et al., 2000), and both genes are necessary for the generation of VIIIth ganglion neurons. The Atoh, Neurogenin and NeuroD bHLH transcription factor families are evolutionarily ancient, and representatives of each family are present even in diploblastic animals and sponges (Simionato, et al., 2008, Simionato, et al., 2007). All three families have experienced both gene duplication and loss in the course of bilaterian evolution. For example, the Neurogenin and NeuroD families appear to have been lost in flies (Fritzsch, et al., 2010), whereas the Drosophila Atoh1 orthologue atonal has been duplicated to give two additional orthologues: amos (absent multidendritic and olfactory sensilla) and cato (cousin of atonal; (Jarman and Groves, 2013).
Despite the fact that these three bHLH families are more closely related to each other than to other bHLH genes, they retain a striking specificity of function. For example, Drosophila atonal is capable of functionally replacing Atoh1 in mice, and vice versa (Ben-Arie, et al., 2000, Wang, et al., 2002). In contrast, Neurog1 can only rescue loss of Atoh1 in mice to an extremely modest degree (Jahan, et al., 2012), suggesting that Neurog1 is only able to activate a subset of Atoh1 target genes. It has been known for many years that related bHLH factors are capable of binding to similar but distinct E-box DNA motifs (Kewley, et al., 2004). With the advent of better tools for investigating transcription factor binding in vitro and vivo, it has been possible to better define the range of motifs bound by a given bHLH factor. In the case of Atoh1, analysis of its targets in the cerebellum (Klisch, et al., 2011) has identified a consensus Atoh1 binding motif based on an extended E box variant that is reminiscent of, but not identical to, that identified for Drosophila atonal (G/A,C/A,CA,G/T,C/A,TG,G/T,C/T). This Atoh1 E-box associated motif, or AtEAM, is present close to the coding regions of over 65% of genes with sequences bound by Atoh1 in the cerebellum. It is likely that Neurog1 and Neurod1 also have their own conserved extended E-box DNA binding motifs that are similar to, but distinct from, the AtEAM motif, and that subtle differences in the binding affinities of these factors have far-reaching consequences on the range of genes each factor can activate. Differences in the range of targets will thus determine the cell fate – neuron or hair cell – that can be specified by different bHLH factors. This topic is reviewed elsewhere in this volume by Fritzsch and colleagues.
2. Lineage relationships between neurons and sensory cells of the inner ear
Morphologic and genetic homologies between the sensory organs of the vertebrate ear and insects described in the previous section raise the question of whether neurons, hair cells, and sensory supporting cells derive from a common progenitor cell type (Fritzsch, et al., 2007, Fritzsch, et al., 2010). Tracing the progeny of Neurog1-expressing cells with tamoxifen-inducible Neurog1-CreER transgenic mice reveals that these cells can give rise to neurons, hair cells and supporting cells of the utricular and saccular maculae, and to non-sensory cells surrounding the maculae (Koundakjian, et al., 2007, Raft, et al., 2007). Loss of Neurog1, either in mouse mutants or by morpholino knockdown in zebrafish, increases the number of macular hair cells at the expense of neurons (Raft, et al., 2007, Sapede, et al., 2012), suggesting that neuronal progenitors are transformed into sensory progenitors under these experimental conditions. Although these studies suggest that the neural-sensory competent population can generate both cell types, they cannot address whether bipotential progenitors exist in this region. However, retroviral lineage tracing experiments in the chicken shows that vestibular and spiral (auditory) neurons of the VIIIth ganglion can be clonally related to sensory and non-sensory utricular epithelial cells (Satoh and Fekete, 2005, Satoh and Fekete, 2009). Similar results have recently been observed using single cell photo-conversion of genetically encoded fluorescent tracers (Sapede, et al., 2012). Another recent study involving single cell retroviral lineage tracing in mouse failed to find common progenitors for neurons and hair cells (Jiang, et al., 2013); however it should be stressed that the age of labeling in this study (embryonic day 11) was quite late relative to the appearance of the neural-sensory competent domain, and that the number of retroviral clones analyzed in this study was quite small and may have been insufficient to capture rare clones containing both cell types. These studies show that the progenitors for sensory derivatives (hair cells and supporting cells) and neurons can differentiate in close proximity to one another, and that multi-potent cells capable of generating neurons, hair cells, or supporting cells can be identified at low frequencies.
Gene expression studies in both chicken and mouse indicate that neurogenesis occurs within a larger sensory-competent domain of the otocyst defined by overlapping expression of Jagged1 and Sox2 (Adam, et al., 1998, Cole, et al., 2000, Daudet, et al., 2007, Fekete and Wu, 2002, Kiernan, et al., 2006, Morsli, et al., 1998, Neves, et al., 2007, Neves, et al., 2011) (Fig. 2). In mice, the early-stage distribution of neurogenesis along the entire ventral face of the otocyst is progressively restricted to an antero-ventral region (Supplemental video), and these dynamics are paralleled by those of Lunatic Fringe expression (Raft, et al., 2004). The antero-ventral neurogenic region, as described above, ultimately forms sensory and non-sensory regions of the utricle and saccule (Koundakjian, et al., 2007, Raft, et al., 2007). The neurogenic region is adjacent and complementary to another portion of otocyst epithelium expressing Bmp4, the T-box gene Tbx1, Hairy1, and Lmx1 (Abello, et al., 2007, Morsli, et al., 1998, Nichols, et al., 2008, Raft, et al., 2004). This region is non-neurogenic and generates sensory and non-sensory regions of the cochlea and vestibular canals. At later developmental stages, neurogenesis and sensory cell generation coincide for a period of several days at the nascent utricular and saccular maculae, and neurogenesis is progressively extinguished as developing sensory (macular) territories expand (Raft, et al., 2007). In the following sections, we describe the determinants and interactions from within and outside the otocyst that pattern neurogenic and adjacent non-neurogenic tissue.
Figure 2. Neurogenic patterning in the otocyst epithelium.
Schematized lateral surface views of the otocyst show developmentally relevant domains of gene expression across a 36-hour period in mouse mid-gestation. Features of the medial otocyst are not represented, except for the posterior ‘tail’ of the neurogenic region (beige), shown as a slim crescent in the E9.5 schematic. Mouse developmental stages are indicated at top. Genes expressed within or outside the region of neurogenesis are color-coded to the diagrams. Genes are culled from published data on both mouse and chicken (see text for details). Lack of a gene’s depiction at any particular stage does not imply its absence, but rather that its expression cannot be correlated to the scheme shown here given currently available data. Inner ear derivatives of the E11 otocyst are shown at right.
3. Neurogenic patterning: Indirect effects of Shh and other factors on ventral otic neurogenesis
A view of the otocyst as a paraxial structure analogous to the somite has uncovered extrinsic signals influencing otic neurogenesis. Studies of the mouse and chicken offer compelling evidence that a source of Sonic Hedgehog (Shh) at the ventral midline (notochord or floorplate of the neural tube) promotes ventral otocyst Neurog1/NeuroD expression and CVG formation (Bok, et al., 2005, Riccomagno, et al., 2002). This signaling had been viewed as instructive with respect to otic Neurog1, since Neurog1 is induced locally by ectopic expression of Shh in the mouse dorsal otocyst, and ectopic/heterochronic implantation of Shh-producing cells near the chicken otocyst up-regulates NeuroD. A caveat to this interpretation is that Shh loss-of-function in the mouse dysregulates gene expression along the entire dorso-ventral axis of the otocyst (Bok, et al., 2007, Brown and Epstein, 2011, Riccomagno, et al., 2002), such that dorsally expressed transcription factor genes are ectopically activated in ventral regions (Fig. 3). It is therefore possible that in the Shh−/− embryo, ventral mis-localization of a neurogenic suppressor causes a loss of Neurog1 expression, in which case Shh should be viewed as regulating Neurog1 permissively. This appears to be the case, as Shh−/− mouse embryos ectopically express Tbx1, encoding a T-box family transcription factor and known repressor of otic Neurog1 and neurogenesis (Bollag, et al., 1994, Brown and Epstein, 2011, Raft, et al., 2004). Complementation analysis using Shh−/−;Tbx1−/+ embryos finds a recovery of near normal Neurog1 expression in the ventral otocyst (Brown and Epstein, 2011). Pharmacological inhibition of hedgehog signaling also causes a concerted up-regulation of tbx1 and reduction of neurod expression in the zebrafish otic epithelium (Radosevic, et al., 2011).
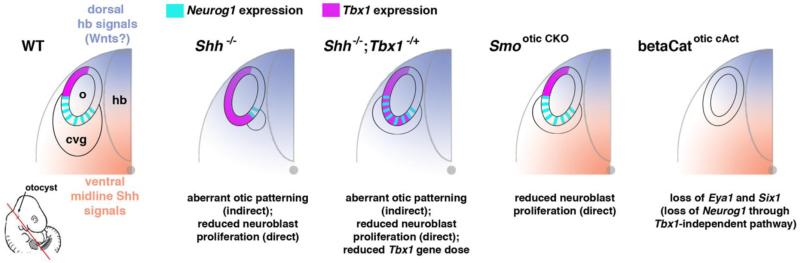
Figure 3. Direct and indirect effects of extrinsic signals on otic neurogenesis, as determined by phenotypes of targeted mouse mutants.
Graphic representations schematize the otocyst (o) and cochleo-vestibular ganglion (cvg) in relation to the hindbrain (hb) and notochord (dot), as seen in transverse section through the embryo (bottom left). Graded colors represent presumptive gradients of Shh and Wnt proteins emanating from the ventral and dorsal midline, respectively, and their alteration in Shh−/− embryos. Neurog1 and Tbx1 expression in the otocyst epithelium is color coded according to the key at top. The Smootic CKO mouse conditionally inactivates the Shh transducer Smoothened in the otocyst epithelium. The betaCatotic cAct mutant conditionally expresses an activated form of the Wnt signaling transducer beta-catenin in the otocyst epithelium. Interpretations of phenotypes are specified below each graphic. Results (Brown and Epstein, 2011; Freyer and Morrow, 2010; and Ohyama, et al., 2006) are discussed in greater detail in the text.
A phenotypic comparison of Shh−/− mice and mice specifically lacking otocyst expression of the Shh signaling transducer Smoothened suggests that ventral midline Shh has dual effects on otic neurogenesis: it promotes proliferation of otic neural precursors through a direct, long-range effect on the ventral otocyst and affects otic Tbx1 and Neurog1 expression indirectly, most likely through the dorso-ventral patterning of diffusible signals generated within the neural tube (Brown and Epstein, 2011) (Fig. 3). Wnt signaling from the dorsal neural tube induces a dorsal otic fate (Ohyama, et al., 2006, Riccomagno, et al., 2005), and wild-type mouse embryos exposed to the canonical Wnt signaling agonist LiCl up-regulate otic Tbx1 and lose otic Neurog1 expression (Brown and Epstein, 2011). However, the effects of Wnt signaling on otic Tbx1 appear to be complex, as other studies find that conditional activation of beta-catenin, a transcriptional mediator of canonical Wnt signaling, in the early mouse otic epithelium down-regulates otic Tbx1 expression, as well as that of Neurog1 and Neurod1 (Freyer and Morrow, 2010, Ohyama, et al., 2006). Other evidence indicates that hindbrain rhombomere mis-patterning in the kreisler (Krml1/MafB) mouse mutant causes ectopic neurogenesis in the dorsal otocyst with no apparent change in Tbx1 expression (Vazquez-Echeverria, et al., 2008), suggesting that other unknown factors also regulate patterning of neurogenesis along the otocyst dorso-vental axis.
Nuclear factors that are expressed within the otocyst and required for neurogenesis also appear to exclude Tbx1 from the ventral otocyst. Mice deficient in the ventrally expressed phosphatase-transactivator Eya1 show reduced otic neurogenesis and ventral expansion of Tbx1 expression (Friedman, et al., 2005) although other evidence indicates that Eya1 is a positive and direct activator of otic neurogenesis through complex formation with Six1, Sox2, and SWI/SNF chromatin-remodeling subunits (Ahmed, et al., 2012b). Interestingly, embryos lacking the ventrally expressed ATP-dependent chromatin-remodeling enzyme Chd7, which is required for Neurog1 expression, show expansion of Tbx1 into the ventral otocyst, although current evidence suggests that Eya1 and Chd7 repress Tbx1 expression through distinct pathways (Hurd, et al., 2010). It is important to note that otocysts of both Eya1 and Chd7 loss-of-function mutants are considerably hypoplastic (Hurd, et al., 2010, Zou, et al., 2004, Zou, et al., 2006), and this may account, wholly or in part, for the observed mis-pattering of Tbx1; by contrast, the effects of Shh on otocyst proliferation and Tbx1 patterning have been dissociated by genetic analyses, as described above (Brown and Epstein, 2011). Nevertheless, multiple extrinsic and intrinsic signals controlling otic neurogenesis appear to act through Tbx1.
4. Neurogenic patterning: Retinoic acid-dependent activation of Tbx1 represses neurogenesis and controls antero-posterior polarity of the otocyst
The first evidence that Tbx1 represses otic neurogenesis came from a study of germ-line Tbx1 null homozygotes, heterozygotes, and transgenic mice over-expressing human TBX1 (Raft, et al., 2004); it was found that Tbx1/TBX1 copy number correlates negatively with size of the otocyst neurogenic region and CVG, and coherently alters the position of a sharp interface between neurogenic and non-neurogenic regions of the otocyst (Fig. 4A). Normally, the Tbx1 and NeuroD gene expression domains are dynamic and strongly complementary; developmentally, the neurogenic region contracts as the Tbx1 domain expands relative to total otocyst size (Supplemental video) (Raft, et al., 2004). Tbx1 transcript is not found in the CVG, but genetic fate mapping of Tbx1 null homozygote otocyst cells, in which Cre recombinase replaces Tbx1 coding sequence, reveals many labeled cells in an ectopic portion of the CVG (adjacent to normallyTbx1-positive posterior otocyst regions) and few labeled cells in other parts of the mutant CVG; by contrast, fate mapping of Tbx1 heterozygous cells finds small numbers of labeled cells throughout the CVG (Xu, et al., 2007), which can be interpreted as further evidence for gene dose-dependent effects of Tbx1/TBX1 on otic neurogenesis (Raft, et al., 2004). This comparison of labeling profiles clearly indicates that Tbx1 null homozygosity results in the neuronal differentiation of cells that would normally remain within the otocyst and form the inner ear epithelium. Since effects of Tbx1/TBX1 on otic neurogenesis are gene-dose dependent and fate mapping data have been obtained using a Tbx1Cre/+ ‘knock-in’ mouse model, the efficiency or absoluteness of Tbx1-mediated neural fate suppression in normal development remains unknown. Tissue-specific ablations of Tbx1 unequivocally localize the neurogenic repressor activity to the otic epithelium (Arnold, et al., 2006, Xu, et al., 2007), and this may well occur at slightly overlapping borders of adjacent Tbx1 and NeuroD domains (Vazquez-Echeverria, et al., 2008).
Figure 4. Tbx1 is an otocyst-intrinsic repressor of neurogenesis and mediator of posteriorizing signals.
(A) Lateral surface views of E11 otocysts in whole mount-hybridized wild-type and Tbx1−/− embryos. The beige asterisk highlights Neurod1 signal within the normal otocyst epithelium, which has well-defined borders and is complementary to the domain of otocyst Tbx1 expression. The developing cochleo-vestibular ganglion (cvg) is seen as a dense ovoid adjacent to the anterior otocyst. The Tbx1−/− cvg is duplicated about the otocyst antero-posterior midline, and the otocyst is severely hypoplastic. Scale bar = 50 micrometers. Panels are modified from (Raft, et al., 2004) (B) A source and ‘sink’ model of RA availability to the normally-developing chicken otic epithelium (top) and one example of a perturbation supporting the conclusion that RA induces Tbx1 and promotes posterior otic character (bottom) (Bok, et al., 2011). At left are depictions of the anterior chicken embryo in dorsal view; experimental (bead) and presumptive endogenous (somites) sources of RA are highlighted in blue. At middle, Neurod1 and/or Tbx1 expression patterns in the otocyst are depicted. At right are depictions inner ear morphology from end-point analyses. Cristae are highlighted in magenta to futher underscore the anatomical symmetry resulting from placing an RA-soaked bead anterior to the otic cup.
A recent study using zebrafish indicates that tbx1 suppresses neurogenesis through a rapid and Notch-independent activation of her9, the zebrafish ortholog of Hes1 (Radosevic, et al., 2011). As in zebrafish, the chick Hes1 ortholog Hairy1 is expressed in the non-neurogenic region of the otocyst (Abello, et al., 2007). Hairy and enhancer of split (Hes) genes, which are most commonly activated by Notch signaling, encode basic helix-loop-helix proteins that repress transcription and antagonize proneural activites of the Neurogenin, Atonal, and Achaete scute families of basic helix-loop-helix proteins (Fischer and Gessler, 2007). Interestingly, her9 represses transcription of otocyst neurod and neurod4, but not neurog1, whereas the tbx1 null homozygous zebrafish mutant van gogh shows altered expression of otocyst neurog1 (Radosevic, et al., 2011), which lies upstream of neurod. How tbx1 suppresses neurog1 remains unknown. Zebrafish her9 is also required for normal levels of cell proliferation, and this may occur through inhibition of the cyclin dependent kinase inhibitor cdkn1bl; both her9 and tbx1 loss-of-function causes smaller zebrafish otocysts (Radosevic, et al., 2011). In the mouse otocyst, conditional Tbx1 loss-of-function reduces cell proliferation through an unknown cell-autonomous mechanism (Xu, et al., 2007).
Tbx1/tbx1 homozygous null otocysts in mouse and zebrafish embryos are severely hypoplastic, and inner ear morphogenesis fails in these mutants (Arnold, et al., 2006, Jerome and Papaioannou, 2001, Piotrowski, et al., 2003, Raft, et al., 2004, Vitelli, et al., 2003, Xu, et al., 2007). This has been attributed to deficient proliferative expansion of the otocyst epithelium (Vitelli, et al., 2003, Xu, et al., 2007), as well as to altered gene expression and loss of otic progenitors by aberrant fate switching to a delaminating neural precursor phenotype (Raft, et al., 2004). Aberrantly symmetric gene expression in the early otocyst and apparent duplication of the CVG about the antero-posterior (A-P) midline of the developing ear (Fig. 4A) led to the suggestion that Tbx1 is an intrinsic determinant of A-P patterning in the early-stage otic epithelium (Raft, et al., 2004). More recent evidence from studies of zebrafish, chick, and mouse embryos suggests that Tbx1 directly mediates an A-P polarizing activity of retinoic acid (RA; (Bok, et al., 2011, Radosevic, et al., 2011). Evidence indicates that this activity is independent of the hindbrain, and may derive from a posterior mesodermal/somatic RA source and anterior ectodermal RA ‘sink’, which flank the early-stage otic placode (Bok, et al., 2011). Using various experimental manipulations of the chick in ovo, Bok and colleagues (Bok, et al., 2011) provided evidence that RA induces Tbx1 rapidly and independently of protein translation in the otic epithelium, suppresses neurogenic gene expression, and confers posterior identity to the inner ear (Fig. 4B). Interestingly, and in support of the idea that the otocyst is polarized by either a concentration gradient of RA or a differential time exposure of anterior and posterior otic epithelia to RA, focal pharmacologic depletion of RA anterior to the otic placode reduces Lfng and NeuroD signals in the anterior otocyst. This suggests that low concentrations or short exposures to RA are required under normal conditions to induce or maintain anterior gene expression and neurogenesis (Bok, et al., 2011).
Results just described support a long-held suspicion that the inner ear rudiment is at first equipotential along its A-P axis and later becomes compartmentalized about its A-P midline (Harrison, 1936). Tbx1 may be one intrinsic component of a complex mechanism for converting a continuous gradient of RA into a binary state of the otocyst, namely its compartmentalization into anterior (neurogenic) and posterior (non-neurogenic) domains. This is not unlike the developmental activity of optomotor-blind (omb), encoding a Drosophila T-box protein most closely related to Tbx2/3 (Agulnik, et al., 1996). Each Drosophila larval segment is divided into anterior and posterior compartments, and the anterior compartment is itself subdivided into anterior (At, for anterior tergite) and posterior (Pt) regions based on differences in derived cell types and differential responses of At and Pt to a similarly shaped hedgehog gradient (Kopp, et al., 1997, Struhl, et al., 1997a, Struhl, et al., 1997b). Omb is expressed in Pt, specifies Pt identity, and is required for differential responses of At and Pt to the hedgehog gradient (Kopp and Duncan, 1997). Whether other features of the classical compartment and boundary model of Drosophila developmental genetics (Lawrence and Struhl, 1996) drive differentiation of the amniote ear (Fekete, 1996, Fekete and Wu, 2002) remains to be determined. To date, evidence exists for limited intermingling of cells across the interface of neurogenic and non-neurogenic regions (Abello, et al., 2007), as well as induction of new cell states at this same interface (Raft, et al., 2007), as described in the following section.
To summarize, the segregation of neurogenic otic tissue and adjacent otic tissue that will later form sensory or structural elements of the cochlea and vestibular canals appears to involve, at a minimum, the integrative effects of extrinsic Shh, Wnt, and RA signals onTbx1; this restricts neurogenesis to an antero-ventral portion of the otocyst and promotes Tbx1-dependent growth and morphogenesis of the cochlea and vestibular canals (Funke, et al., 2001, Raft, et al., 2004). Other otic-expressed transcription factors of the Lmx and Irx classes may also contribute, by means of suppression, to the patterning of neurogenic activity (Abello, et al., 2010; Bosse, et al., 1997; Koo, et al., 2009). More recent studies indicate that spiral ganglion-derived Shh influences the timing of hair cell differentiation in the organ of Corti (Liu et al., 2010; Tateya et al, 2013; Bok et al., 2013), and provide an example of a developmental effect brought about by signaling between neuronal and sensory components after they have been patterned and segregated into distinct structures. In the next section, we discuss genetic determinants and interactions that extinguish neurogenesis at the utricle and saccule during early stages of sensory organ development.
5. Decisions at close range: Interactions between Neurog1 and Atoh1 convert the neurogenic domain into sensory maculae
As stated above, new cell states emerge at a putative compartment boundary of the late-stage mouse otocyst: a small number of Atoh1-positive cells are first detected within and very near neurogenic domain borders (Raft, et al., 2007) (Fig. 5A). Two discrete stripes of Atoh1-positive cells appear, and over a period of three gestational days, these expand to form maculae of the utricle and saccule. The Neurog1/NeuroD domain contracts in complementary fashion (Raft, et al., 2007), much like the contraction of neurogenic domain area observed in concert with expansion of the Tbx1 domain during an earlier phase of development. Raft and colleagues also found that Atoh1 null homozygosity abolishes contraction of neurogenic activity in the developing utricle and saccule, and Atoh1 heterozygosity attenuates it. Effects of Neurog1 null homozygosity on saccular development are complicated by growth abnormalities, but expansion of the Atoh1+ utricular domain is accelerated in Neurog1−/− mutants, and increased numbers of Atoh1+ cells are found in the Neurog1 heterozygote utricle and saccule compared to control.
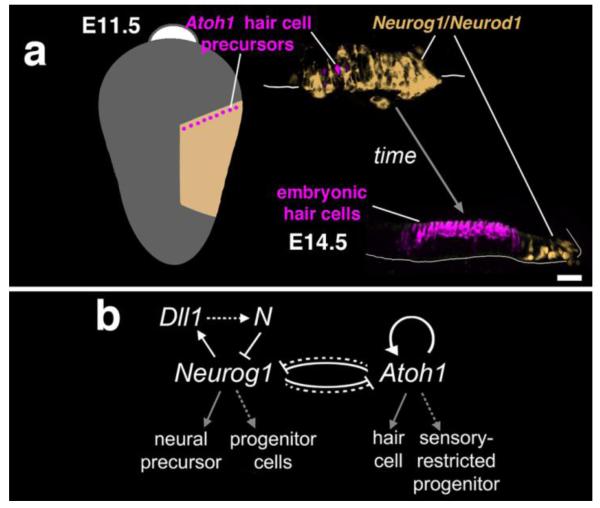
Figure 5. Transformation of the neurogenic region into sensory maculae, bHLH gene cross-inhibition, and differential autoregulation.
(A) At left, a schematized E11.5 otocyst, viewed laterally, shows a stripe of Atoh1-positive cells (magenta) at a border of the neurogenic domain (beige). At right is a cross-section through the stripe (E11.5) and a cross-section through a corresponding region of the E14.5 ear (utricular macula) showing embryonic hair cells (magenta) and a residual domain of neurogenesis (beige). Genetic fate mapping indicates that the region of embryonic hair cells was neurogenic at prior stages (Raft, et al., 2007). Scale bar = 25 micrometers. (B) Genetic interactions and cell fates associated with the neurogenic-to-sensory macular transformation, adapted from (Raft, et al., 2007). Dotted lines indicate non-cell autonomous interactions or outcomes; solid lines indicate cell autonomous interactions or outcomes. The precise mechanisms by which Neurog1 and Atoh1 cross-inhibit remain unknown. N, Notch receptor. See text for further details.
The findings just described are consistent with cross-inhibition among bHLH factors, which controls commitment of progenitor cells to alternative fates at various sites in the developing nervous system (Fode, et al., 2000, Gowan, et al., 2001). However, a puzzling feature of the present system is its developmental outcome: why does Atoh1 extinguish Neurog1 expression? Why do both genes’ inhibitory activities not lead to a steady state of complementary co-expression? Combining mouse mutant and genetic reporter analyses, Raft and colleagues (Raft, et al., 2007) confirmed previous evidence of a rapid and potent positive auto-regulatory activity of Atoh1 in the ear (Helms, et al., 2000, Lumpkin, et al., 2003). They also provided evidence for Notch-mediated negative auto-regulation of Neurog1 (lateral inhibition) in the region of interest (Raft, et al., 2007). These results suggested that progenitors expressing a high amount of Neurog1 (Neurog1high) have an inhibitory effect on Neurog1 accumulation in their immediate neighbors (Neurog1low) before delaminating from the epithelium as committed neural precursors. Since delamination is continuous, the attendant release of inhibition on Neurog1low epithelial progenitors allows for two possible outcomes: 1) continued competition amongst Neurog1low cells for a neural fate through Notch-mediated negative auto-regulation of Neurog1; or 2) negative regulation of Neurog1 by Atoh1 and rapid Atoh1 positive autoregulation. These considerations provide a possible explanation for the competitive advantage of Atoh1 over Neurog1 in the transition from neurogenesis to sensory macular development (Fig. 5B). In this model, Neurog1 is viewed as a proneural-type gene that cell-autonomously promotes neural fate and non-cell autonomously (through Notch-mediated lateral inhibition) preserves a population of progenitor cells for subsequent sensory epithelial development (Raft, et al., 2007).
6. Neural and sensory development by Notch signaling: pacing by inhibition, prevalence by induction
Notch-mediated lateral inhibition involves juxtacrine signaling, feedback loops, and transcriptional amplification, which drive a competitive process by which one cell amongst its immediate neighbors adopts a primary fate (Hori, et al., 2013). Nearly 25 years ago, it was proposed that a mechanism of this sort might generate the precise periodic spacing pattern of hair cells and supporting cells that is characteristic of the vertebrate ear (Corwin, et al., 1991, Lewis, 1991), and several recent reviews (Kiernan, 2013, Neves, et al., 2013a) have comprehensively discussed the influence of Notch signaling on development of inner ear sensory epithelia. By the late 1990s, homologues of the canonical inhibitory Notch receptor ligand Delta had been localized to regions of early-stage otic neurogenesis and sensory patch formation in the chick and zebrafish (Adam, et al., 1998, Haddon, et al., 1998, Riley, et al., 1999). At the same time, mouse Neurog1 was characterized as a positive regulator of the Delta homologue Dll1, an inhibitory target of Notch signaling, and an essential factor for neurogenesis at the otic and trigeminal placodes, thus fulfilling several criteria for consideration as a vertebrate proneural gene (Ma, et al., 1998). Proneural genes amplify lateral inhibition and serve as a ‘master switch’ for the primary cell fate (Anderson, 1997)
Evidence that lateral inhibitory Notch signaling controls the pace of neural and sensory cell differentiation was obtained in the first genetic proof that Notch signaling influences ear development. The zebrafish mutant mind bomb (Jiang, et al., 1996, Schier, et al., 1996), now known to be deficient in an E3 ubiquitin ligase required for Delta-induced Notch receptor activation (Itoh, et al., 2003), was found to have twice the normal number of CVG neurons, a gross overabundance of hair cells at the expense of supporting cells, and increased expression Dll1 and Jag2 Notch ligand homologues (Haddon, et al., 1998). Overabundance of hair cells occurs early in development of the mind bomb ear; these hair cells are subsequently extruded from the epithelium (Haddon, et al., 1999), and late-developing hair cells never form in the mind bomb mutant. This led Haddon and colleagues to suggest that one role of Delta-mediated lateral inhibitory Notch signaling is to maintain a population of competent progenitor cells for hair cell generation over a protracted period (Haddon, et al., 1998).
Since neurogenesis precedes hair cell formation in the mouse (Carney and Silver, 1983) and chick (Adam, et al., 1998), Haddon and colleagues also raised the question of how a gross overproduction of CVG neurons in mind bomb mutants can still preserve sensory-competent progenitors for precocious overproduction of hair cells. As discussed in a subsequent section, it is now known that temporal and spatial relationships between neurogenesis and sensory patch formation in the zebrafish differ from those in chicken and mouse embryos. Nevertheless, the question proved prescient, as a more recent study of Dll1 conditional inactivation in the mouse (Brooker, et al., 2006) has found the utricular and saccular maculae to be absent or hypoplastic, and early-stage CVG volume to be increased compared to wild-type. Of the six sensory patches in the Dll1 mutant ear, only maculae are deficient in hair cells. Hair cells in the Dll1 mutant organ of Corti (cochlea) are produced prematurely and in excess, and a hair cell:supporting cell ratio for the organ of Corti is increased compared to wild-type. Similar results are obtained by pharmacologic blockade of Notch signaling in the chicken embryo, which causes increased expression of the Delta homologue, an increase in the proportion of anterior otocyst cells that adopt a neural fate, and a focal disruption of epithelial structure in the anterior otocyst (Daudet, et al., 2007). These results are consistent with fate mapping of neurogenic epithelium solely to the maculae (among sensory patches) in mice (Koundakjian, et al., 2007, Raft, et al., 2007), and suggest that Delta-Notch-mediated lateral inhibition provides a pacing mechanism for sequential and partially overlapping phases of neurogenesis and sensory macular development.
Evidence suggests that an alternate mode of Notch signaling, lateral induction (Baker and Yu, 1997, Li and Baker, 2001), specifies a state of sensory competence in regions that will generate hair cells and supporting cells (Daudet, et al., 2007, Daudet and Lewis, 2005, Eddison, et al., 2000, Hartman, et al., 2010, Neves, et al., 2011). Lateral induction is a positive feed-forward mechanism in which ligand-mediated Notch activation up-regulates ligand expression in the activated cell. Ligand-Notch receptor juxtacrine signaling propagates activation to neighboring cells, which, in theory, allows for the creation of an equivalence group rather than the breaking of equivalence, as in the case of lateral inhibition. Studies in both chick and mouse implicate the ligand Jagged1/Serrate1 in this process: in both species, Jagged1/Serrate1 expression occupies the broad domain of neuro-sensory competence in the otocyst (Fig. 2), which then splits and foreshadows the positions of discrete sensory epithelia (Adam, et al., 1998, Cole, et al., 2000, Daudet, et al., 2007, Kiernan, et al., 2006); in both species, Notch-mediated positive regulation of Jagged1/Serrate1 expands regions of sensory competence (Daudet, et al., 2007, Daudet and Lewis, 2005, Eddison, et al., 2000, Hartman, et al., 2010); over-expression of human JAGGED1 in the chicken otocyst induces expression of Sox2, a mediator of sensory competence (see below), in a Notch-dependent manner and promotes ectopic sensory structures (Neves, et al., 2011); and conditional inactivation of Jagged1 in the mouse otocyst causes severe deficiencies or absence of hair and supporting cells (except in the saccular macula; (Brooker, et al., 2006, Kiernan, et al., 2001, Kiernan, et al., 2006). At otocyst stages, the normal domain of neurogenesis closely resembles the pattern of Lunatic Fringe (Lfng) expression (Raft, et al., 2004). The Jag1 conditional knock-out otocyst shows a reduced size of the Sox2 and Lfng domains, reduced numbers of Neurog1+ cells in the epithelium, and reduced CVG volume compared to wild-type, whereas the mutant otocyst is of normal size and shows no apparent defects in proliferation or apoptosis at the relevant stage (Pan, et al., 2010). Thus, for both otic neurogenesis and sensory epithelial generation, Notch signaling may first establish a region of developmental competence by lateral induction and then control the fine-grained patterning of cell types and/or pace of differentiation by lateral inhibition. Intriguingly, these roles are associated with different ligands, although other molecular differences undoubtedly remain to be discovered.
7: Sox2: A protagonist and antagonist of neuronal and sensory development
The previous sections have considered cell-intrinsic factors, long-range and contact-mediated signaling systems, and patterning mechanisms that regulate otic neurogenesis, with a particular emphasis on relationships between neurogenesis and sensory organ development. However, it is still an open question as to what factors provide the neural-sensory domain with competence to respond to neuronal or sensory-inducing signals at particular times in ear development. Accumulating evidence suggests that Sox2, an HMG box transcription factor belonging to the B1 family of Sox proteins, may be one such factor. During ear development, Sox2 is initially expressed in regions of the otocyst that will give rise to both sensory and non-sensory components (Neves, et al., 2007). It then becomes restricted to the neural-sensory competent domain, its expression persists in prosensory patches after neurogenesis has ceased (Adam, et al., 1998, Alsina, et al., 2009, Dabdoub, et al., 2008, Eddison, et al., 2000, Kiernan, et al., 2005, Kiernan, et al., 2006, Neves, et al., 2007, Neves, et al., 2011, Ohyama, et al., 2010, Puligilla, et al., 2010), and it continues to be expressed in supporting cells after hair cells differentiate (Hume, et al., 2007, Oesterle, et al., 2008). Sox2 mRNA and protein are down-regulated in differentiating neurons and hair cells (Evsen, et al., 2013, Neves, et al., 2007, Oesterle, et al., 2008), although unlike neurons, low levels of transient Sox2 can still be detected in nascent hair cells (Hume et al., 2007). Loss of Sox2 in the Sox2Lcc mutant causes an almost complete loss of both neuronal and sensory components of the inner ear (Kiernan, et al., 2005, Puligilla, et al., 2010), suggesting that it is necessary for the differentiation of both tissue types. However, further analyses of Sox2 function in the ear using gain of function approaches have provided a more nuanced understanding of its function in the neural-sensory competent domain, and we will first discuss its role in neurogenesis.
Down-regulation of Sox2 in delaminating otic neuroblasts suggests that Sox2 expression is inhibited by the neurogenic genes Neurog1 and NeuroD. Indeed, both bHLH factors can bind and negatively regulate a Sox2 enhancer, Nop1, which drives reporter gene expression in the chicken otocyst (Evsen, et al., 2013). Constitutive expression of Sox2 in the early chick otocyst can also block neurogenesis and leads to a markedly reduced VIIIth cranial ganglion (Evsen, et al., 2013). However, this negative regulation of neuronal production is very dependent on the precise cellular context, as similar over-expression in the chick inner ear just 24 hours later – once sensory cell differentiation is underway – results in a significantly enlarged ganglion (Neves, et al., 2011). Elevated expression of Sox2 in non-sensory regions of the cochlea can also promote neurogenesis, either alone (Puligilla, et al., 2010) or in concert with additional factors such as Six/Eya and elements of the SWI/SNF chromatin remodeling complex (Ahmed, et al., 2012b). Interestingly, the recent study by Evsen and colleagues (2013) and two other recent studies (Jeon, et al., 2011, Neves, et al., 2011) showed that although Sox2 over-expression inhibited the formation of delaminated neurons, it up-regulated expression of Neurog1, and data from ES cells suggest this up-regulation may be direct (Cimadamore et al, 2011). Together, these data suggest that in the early otocyst, Sox2 participates in the up-regulation of Neurog1, likely in co-operation with Six1 and Eya1 (Ahmed, et al., 2012b), and that Neurog1, together with its target NeuroD, exert negative feedback on the Sox2 locus to extinguish its expression in committed neuroblasts (Fig 6).
Figure 6. Common features of regulatory logic in neurogenic and prosensory differentiation.
During neurosensory differentiation, Sox2 operates with different partners to promote differentiation of neurons and sensory cells. In both cases, evidence suggests that Sox2 can directly induce differentiation genes (Neurog1 in neurons, Atoh1 in hair cells), but can also attenuate the degree or timing of their expression by inducing negative regulators, such as members of the Hes/Hey or Id gene families in an incoherent feed-forward loop (Neves et al., 2013b). During neurogenesis, Neurog1+ cells laterally inhibit their neighbors through expression of Dll1; in sensory regions, Atoh1+ cells laterally inhibit their neighbors through expression of Dll1 and Jag2. However, in prosensory tissue, Sox2 also induces the expression of Jag1, which then feeds back to maintain Sox2 through lateral induction. Differentiating hair cells and neurons also repress Sox2 through negative feedback loops; in the case of neurons, both Neurog1 and NeuroD directly inhibit Sox2 through the Nop1 enhancer (Evsen, et al., 2013). Green arrows represent positive genetic regulation through direct or indirect means; green circles indicate a positive signal through a Notch ligand and receptor; red bars indicate direct or indirect inhibition.
Sox2 is also necessary for the differentiation of hair cells and supporting cells and the specification of prosensory patches (Kiernan, et al., 2005). However, as seen during neurogenesis, ectopic expression of Sox2 can either promote or inhibit sensory cell formation depending on the context. For example, electroporation of Sox2 into the chicken otocyst can expand existing neurosensory patches and convert non-sensory tissue into neurosensory patches (Neves, et al., 2011). Similarly, electroporation or inducible transgenic activation of Sox2 in the mouse inner ear is also capable of generating small regions of hair cells and supporting cells from non-sensory tissue (Ahmed, et al., 2012a, Ahmed, et al., 2012b, Pan, et al., 2013). However, while Sox2 can promote Atoh1 expression, it also inhibits subsequent hair cell differentiation (Ahmed, et al., 2012a, Dabdoub, et al., 2008) – in other words, it appears to promote the formation of prosensory cells that can express Atoh1, but it inhibits their differentiation into bona fide hair cells. Interestingly, Sox2 and Six1 binding sites have recently been identified in the Atoh1 autoregulatory enhancer (Ahmed, et al., 2012a, Neves, et al., 2012), and the presence of these sites may explain why Sox2 and Six1 are able to co-operate to up-regulate Atoh1 expression. However, it appears that down-regulation of Sox2 in hair cell progenitors (Hume, et al., 2007) is necessary for their full differentiation, and it is likely that Atoh1 is one factor that leads to Sox2 extinction in hair cells (Dabdoub, et al., 2008). Sox2 thus appears to function as a pivotal factor in determining neurosensory competence: it can up-regulate genes involved in triggering neuronal and hair cell differentiation – i.e,, Neurog1 and Atoh1 – but these factors then feed back to inhibit Sox2 expression before neuronal or hair cell differentiation has commenced. Recently, a model for the action of Sox2 has been proposed that invokes Sox2 as a driver of incoherent feed-forward loops in both neuronal and sensory differentiation (Neves, et al., 2013b). In this model, Sox2 both activates expression of Neurog1 and Atoh1 and simultaneously activates transcriptional repressors of these two genes. A number of candidate repressors have been proposed, such as members of the Hes and Hey gene families, or the Id family of bHLH repressors (Neves, et al., 2013b). A slight imbalance between neurogenic and sensory signals allows some neuroblasts to begin expressing Neurog1, which in turn drives expression of Notch ligands such as Dll1, which act to keep Atoh1 repressed in neurosensory progenitors. The transition from neurogenic to sensory progenitors allows down-regulation of Sox2 and the activation of Atoh1 in differentiating sensory cells (Neves, et al., 2013b; Fig. 6).
What signals promote Sox2 expression in this model? Sox2 is initially expressed quite broadly in the otocyst before becoming refined to the neural-sensory competent domain (Neves, et al., 2007, Neves, et al., 2011). Sox2 can be induced by Sox3, which is one of the first markers of the developing otocyst (Abello, et al., 2010, Neves, et al., 2007) and the expression of which is established by both BMP and FGF family signals (Abello, et al., 2010). It is not clear whether Sox2 induction is simply an indirect consequence of Sox3 expression in response to these signals, or whether other signals can induce Sox2 expression independently. Regardless, it is very clear that once initiated, the maintenance of Sox2 in neurosensory progenitors is controlled by Notch signaling through the Jagged1 ligand (Alsina, et al., 2009, Kiernan, 2013, Neves, et al., 2013a). As evidence of this, inhibition of Notch signaling or inactivation of Jag1 down-regulates prosensory genes in the ear, leading to a reduction or loss of hair cells (Brooker, et al., 2006, Daudet, et al., 2007, Hayashi, et al., 2008, Kiernan, et al., 2006, Tsai, et al., 2001). The one exception to this appears to be the prosensory region of the future organ of Corti (Basch, et al., 2011, Yamamoto, et al., 2011). Conversely, activation of Notch signaling – for example by over-expression of its intracellular domain – results in an up-regulation of both Jag1 and Sox2 in a manner consistent with a lateral inductive mode of Notch activity (Daudet and Lewis, 2005, Hartman, et al., 2010, Neves, et al., 2011, Pan, et al., 2013, Pan, et al., 2010), although the ability of Notch activation to promote prosensory tissue is clearly age-dependent (Basch, et al., 2011, Liu, et al., 2012a, Liu, et al., 2012b, Pan, et al., 2013). It is possible that the maintenance of Sox2 expression by Notch signaling is direct, as has been shown in other systems (Ehm, et al., 2010).
What is Sox2’s role in the transition from neuronal production to hair and supporting cell production? As described above, the presence of co-operating factors such as the SWI/SNF chromatin remodeling complex can determine whether Sox2, Six1 and Eya1 promote neuronal differentiation over sensory cell production (Ahmed, et al., 2012a, Ahmed, et al., 2012b), and the down-regulation of this complex may explain why Sox2-expressing cells in the neural-sensory competent domain cease committing to a neural fate. A number of studies have shown that both Neurog1 and NeuroD can negatively regulate Atoh1 expression, and that loss of these factors can lead to ectopic up-regulation of Atoh1 in sensory regions of the ear (Raft, et al., 2007) or in the VIIIth ganglion itself (Jahan, et al., 2012). A recent demonstration of Atoh1 autoregulatory enhancer activity in the early chick otocyst (Neves, et al., 2013b) suggests that the competition between neurogenic and sensory transcriptional regulators is already present at this stage. It is also possible that additional factors co-operating with Sox2 in neurogenesis are lost as ear development proceeds. It is notable in this context that replacement of the Atoh1 coding region by Neurog1 in mice does not transform sensory progenitor cells into neurons (Jahan, et al., 2012), suggesting that these progenitors can no longer initiate a neurogenic program. One example of a possible co-operating factor is the related SoxB1 factor Sox3, which is also expressed in the early otocyst but is down-regulated as neurogenesis declines and is absent from prosensory patches (Abello, et al., 2010, Neves, et al., 2007). Since related SoxB1 factors can co-operate as homo- or heterodimers in a number of different systems (Archer, et al., 2011, Bernard, et al., 2003, Genzer and Bridgewater, 2007, Peirano and Wegner, 2000), it is possible that the combined action of Sox2 and Sox3 is necessary for neurogenesis and that down-regulation of Sox3 from the neural-sensory competent domain renders it unable to generate neurons.
8: Patterning neurons and sensory cells in the zebrafish – same players, different rules?
Although the mechanisms causing Sox2-expressing progenitors in the amniote otocyst to adopt a neural or sensory fate at different developmental stages are not fully understood, it is important to stress that the order of cell production – neurons before hair cells and supporting cells – is not constrained across phylogeny. In the final section of the review we discuss recent work on neurosensory differentiation in the zebrafish, in which the temporal sequence of neuronal and hair cell differentiation is reversed compared to amniotes. Complicating our understanding of the zebrafish ear is the fact that zebrafish have two atonal homologues, atoh1a and atoh1b (Adolf, et al., 2004, Itoh and Chitnis, 2001, Millimaki, et al., 2007, Whitfield, 2002, Whitfield, et al., 2002). Atoh1b is expressed widely and early in the otic placode at 10 hours post fertilization (hpf), prior to the differentiation of neurons and hair cells, and its expression is later restricted to two patches of future sensory epithelia (Millimaki, et al., 2007). Atoh1b is necessary for the development of tether cells, precocious hair cells that seed and localize the formation of otoliths (Millimaki, et al., 2007, Riley, et al., 1997), but Atoh1b is dispensible for development of the majority of later-forming hair cells in the ear and lateral line neuromasts (Millimaki, et al., 2007). Atoh1a is expressed later in development (at 14hpf) in the progenitors of the majority of hair cells and lateral line neuromasts (Itoh and Chitnis, 2001, Millimaki, et al., 2007, Whitfield, 2002, Whitfield, et al., 2002). Knockdown of both genes in zebrafish eliminates both early- and late-developing hair cells (Millimaki, et al., 2007), while ectopic expression of atoh1a causes expanded regions of hair cells in the zebrafish ear that would normally differentiate as non-sensory tissue (Millimaki, et al., 2007, Sweet, et al., 2011). In contrast with amniotes, neurons of the zebrafish statoacoustic (SAG) ganglion begin to differentiate shortly after the expression of atoh1a, at 16hpf. The first sign of SAG neurogenesis is the expression of neurog1, which is followed by the delamination of neuroblasts and their down-regulation of neurog1 and up-regulation of neurod. Neuroblast delamination occurs for the next 24 hours and is followed by a period during which these cells proliferate and differentiate into mature neurons (Radosevic, et al., 2011, Vemaraju, et al., 2012).
The most striking difference in molecular regulation of neurosensory differentiation between zebrafish and amniotes is in the expression and function of Sox2. Unlike amniotes, zebrafish sox2 is not expressed early and broadly in the otocyst – rather it is first up-regulated in the developing maculae after the induction of atoh1a and atoh1b (Millimaki, et al., 2010). As might be expected from such a reversal of expression timing, knockdown of zebrafish sox2 with morpholinos does not block the induction of sensory tissue or expression of atoh1a or b, although sox2 does appear to be necessary for the subsequent survival of hair cells (Millimaki, et al., 2010). Moreover, although heat shock activation of sox2 can increase the number of hair cells generated within sensory maculae, it does not lead to the production of ectopic regions of sensory cells (Millimaki, et al., 2010). In contrast, ectopic expression of atoh1a in the zebrafish ear is capable of generating sensory tissue and hair cells from regions of the otocyst that would normally produce non-sensory derivatives (Sweet, et al., 2011). This broad competence declines with age, although it can be augmented by co-activation of either sox2 or fgf8 (Sweet, et al., 2011).
These results suggest the intriguing possibility that the proneural functions of Atoh1 and Sox2 have been transposed in the course of amniote evolution. In fish, atoh genes act as bona fide proneural genes (Hassan and Bellen, 2000) – their expression precedes and coincides with the selection of sensory progenitors (Itoh and Chitnis, 2001, Millimaki, et al., 2007, Whitfield, 2002, Whitfield, et al., 2002), their expression is regulated by Notch signaling (Millimaki, et al., 2007) and their function is both necessary and sufficient for sensory cell development (Millimaki, et al., 2007, Sweet, et al., 2011). However, zebrafish sox2 fails to fulfill all these criteria. In amniotes, Sox2 seems to fulfill the criteria of a proneural gene better than Atoh1 in terms of its expression (Neves, et al., 2007, Neves, et al., 2011), regulation by Notch signaling (Hartman, et al., 2010, Neves, et al., 2013a, Pan, et al., 2013, Pan, et al., 2010), and its necessity and sufficiency for sensory cell development (Kiernan, et al., 2005, Neves, et al., 2012, Neves, et al., 2013b, Pan, et al., 2013). Atoh1, in contrast, is expressed in hair cell progenitors shortly before their differentiation, but not broadly in prosensory regions (Cai, et al., 2013, Driver, et al., 2013, Yang, et al., 2010). Although ectopic expression of Atoh1 can induce hair cells for an extended period of time during development (Gubbels, et al., 2008, Kelly, et al., 2012, Liu, et al., 2012a, Zheng and Gao, 2000), it cannot directly induce prosensory tissue or supporting cells. Given the observed cross-regulatory relationships between Atoh1 and Sox2 in amniotes, as well as evidence of Sox2 binding to the Atoh1 autoregulatory enhancer (Ahmed, et al., 2012a, Ahmed, et al., 2012b, Neves, et al., 2012), it will be interesting to determine to what extent these relationships are present in fish. For example, although Sox2 over-expression can directly inhibit Atoh1 expression and hair cell formation in mouse (Dabdoub, et al., 2008), current evidence suggests this may not be the case in fish (Millimaki, et al., 2010), although it is possible that the precise timing of over-expression may affect the outcome in these experiments (for example, (Neves, et al., 2013a, Neves, et al., 2013b).
Despite these clear and surprising differences in factors mediating neurosensory specification in amniotes and anamniotes, the function of other regulatory factors appears to be at least superficially conserved. For example, Six1 is expressed early in the developing amniote otocyst (Xu, et al., 1999, Zheng, et al., 2003, Zou, et al., 2004, Zou, et al., 2006) and is maintained in sensory regions later in development (Ahmed, et al., 2012a). In co-operation with Eya1 and Sox2, it is capable of promoting both neuronal and sensory cell formation depending on the cellular context and the presence of appropriate chromatin remodeling factors (Ahmed, et al., 2012a, Ahmed, et al., 2012b). Similarly, zebrafish six1 is also expressed broadly in the otic placode and later becomes restricted to the ventral region of the otocyst where neurosensory differentiation occurs (Bessarab, et al., 2004, Bricaud and Collazo, 2006). Knock down of six1 results in a reduction in hair cell numbers and an increase in the size of the statoacoustic ganglion; conversely, over-expression of six1 results in an increase in hair cells and fewer SAG neurons (Bricaud and Collazo, 2006). These changes are not due to alterations in cell fate; rather, six1 appears to regulate cell survival and cell proliferation. Interestingly, it affects these two processes differently in the two lineages: six1 regulates proliferation in hair cell progenitors and regulates survival of neuroblasts (Bricaud and Collazo, 2006). It does so by having a different mode of transcriptional regulation in each lineage: in the sensory lineage it partners with eya proteins to activate genes involved in cell proliferation, whereas in the neuronal lineage it partners with groucho co-repressors to properly restrict the domain of neurogenesis (Bricaud and Collazo, 2011). Other evidence suggests that transcriptional events occurring very early in zebrafish ear development biases the neuronal-sensory fate decision. For example, a recent study suggests that the transcription factors foxi1 and dlx3b may promote neural and sensory fates, respectively. Morpholino knockdown of either foxi1 or dlx3b/4b results in otocyst hypoplasia, and foxi1 morphants fail to generate neurons, whereas dlx3b/4b morphants fail to generate sensory cells (Hans, et al., 2013). Intriguingly, these results trace transcriptional inputs for neural and sensory cell generation to pre-placode stages of zebrafish ear development.
9. Conclusions and questions
The evolution of the vertebrate octavolateralis system – the inner ear and lateral line – has witnessed both addition and loss of sensory structures in different groups (Beisel, et al., 2005, Fritzsch and Straka, 2014), but commonalities across taxa are clear. All vertebrate inner ear sensory organs consist of secondary receptor (hair) cells specified by atonal class bHLH factors. Hair cells are innervated by sensory neurons that require the closely related but functionally non-redundant Neurog/NeuroD bHLH factors for their specification. Presently, we do not know whether a similar molecular scheme operates in non-vertebrate chordates that use primary and secondary mechanoreceptive cells to detect water flow (Burighel, et al., 2008), and it will be of great interest to determine whether neurons and mechanoreceptive cells in these animals are generated by the same developmental logic we have described here. The weight of current evidence suggests that although all vertebrate neurons and sensory cells derive from a common neural-sensory competent domain, only the nascent gravity-detecting organs – the utricular and saccular maculae – contain bipotential progenitor cells capable of forming both cell types. It is not yet clear whether maculae represent the most basal form among vertebrate inner ear sensory structures; cristae (and certainly the papilla of the amniote cochlea) may be more derived structures (Hammond and Whitfield, 2006, Maklad, et al., 2014) that co-opted an existing pool of neural progenitors to communicate with the CNS.
Although the factors regulating neurogenesis and sensory cell production are becoming better understood, mechanisms regulating the transition between these two programs of differentiation are less well understood. As we have described, a number of processes may be involved – for example, cessation of neurogenic competence may correlate with down-regulation of context-dependent factors that interact with Sox2, such as Sox3 and the Swi/SNF chromatin remodeling complex (Ahmed, et al., 2012b, Neves, et al., 2007), or with the inherent competitive disadvantage of neurally-determined cells as a result of their delamination from the sensory epithelium (Raft, et al., 2007). Mechanisms underlying the reverse transition from sensory cell to neuronal production in zebrafish are also currently unclear. The challenge for the immediate future is to better understand how changes in the external signaling environment of the developing otocyst interact with positive and negative transcriptional regulators and competence factors to switch from one developmental module to another.
Supplementary Material
Supplemental Material/Video: A video morph generated from serial section sets of mouse otocysts at three stages (24, 27, 30 somite stages; E9.5-E10) that were hybridized for detection of either Neurod1 (pink) or Tbx1 (blue) and reconstructed into space-filling models. The otocysts are viewed ventrally, as if through the second branchial arch, and oriented such that lateral is left, and anterior is up. The model at right renders the otocyst epithelium transparent to allow changes of Tbx1 expression in the dorsal otocyst to be visualized. Complementary patterning of Neurod1 and Tbx1 expression is maintained despite considerable changes in the extent of these domains over a 12-hour developmental period. Still images used to generate this morph, as well as methods for generating the space-filling models, are presented in (Raft, et al., 2004).
REFERENCES
- Abello G, Khatri S, Giraldez F, Alsina B. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech Dev. 2007;124:631–645. doi: 10.1016/j.mod.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F, Alsina B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol. 2010;339:166–178. doi: 10.1016/j.ydbio.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Adolf B, Bellipanni G, Huber V, Bally-Cuif L. atoh1.2 and beta3.1 are two new bHLH-encoding genes expressed in selective precursor cells of the zebrafish anterior hindbrain. Gene Expr Patterns. 2004;5:35–41. doi: 10.1016/j.modgep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Agulnik SI, Garvey N, Hancock S, Ruvinsky I, Chapman DL, Agulnik I, Bollag R, Papaioannou V, Silver LM. Evolution of mouse T-box genes by tandem duplication and cluster dispersion. Genetics. 1996;144:249–254. doi: 10.1093/genetics/144.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 2012a;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Xu J, Xu PX. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development. 2012b;139:1965–1977. doi: 10.1242/dev.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol. 2004;267:119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Alsina B, Giraldez F, Pujades C. Patterning and cell fate in ear development. Int J Dev Biol. 2009;53:1503–1513. doi: 10.1387/ijdb.072422ba. [DOI] [PubMed] [Google Scholar]
- Anderson DJJ. The determination of the neuronal phenotype. In: Cowan WMJ, Zipursky SL, editors. Molecular and cellular approaches to neural development. Oxford University Press; New York: 1997. pp. 26–63. Y.N. T.M. [Google Scholar]
- Archer TC, Jin J, Casey ES. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Dev Biol. 2011;350:429–440. doi: 10.1016/j.ydbio.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JS, Braunstein EM, Ohyama T, Groves AK, Adams JC, Brown MC, Morrow BE. Tissue-specific roles of Tbx1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum Mol Genet. 2006;15:1629–1639. doi: 10.1093/hmg/ddl084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Yu SY. Proneural function of neurogenic genes in the developing Drosophila eye. Curr Biol. 1997;7:122–132. doi: 10.1016/s0960-9822(06)00056-x. [DOI] [PubMed] [Google Scholar]
- Basch ML, Ohyama T, Segil N, Groves AK. Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J Neurosci. 2011;31:8046–8058. doi: 10.1523/JNEUROSCI.6671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel KW, Wang-Lundberg Y, Maklad A, Fritzsch B. Development and evolution of the vestibular sensory apparatus of the mammalian ear. J Vestib Res. 2005;15:225–241. [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, Bellen HJ, Zoghbi HY. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bernard P, Tang P, Liu S, Dewing P, Harley VR, Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- Bessarab DA, Chong SW, Korzh V. Expression of zebrafish six1 during sensory organ development and myogenesis. Dev Dyn. 2004;230:781–786. doi: 10.1002/dvdy.20093. [DOI] [PubMed] [Google Scholar]
- Boekhoff-Falk G. Hearing in Drosophila: development of Johnston’s organ and emerging parallels to vertebrate ear development. Dev Dyn. 2005;232:550–558. doi: 10.1002/dvdy.20207. [DOI] [PubMed] [Google Scholar]
- Bok J, Bronner-Fraser M, Wu DK. Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development. 2005;132:2115–2124. doi: 10.1242/dev.01796. [DOI] [PubMed] [Google Scholar]
- Bok J, Dolson DK, Hill P, Ruther U, Epstein DJ, Wu DK. Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development. 2007;134:1713–1722. doi: 10.1242/dev.000760. [DOI] [PubMed] [Google Scholar]
- Bok J, Raft S, Kong KA, Koo SK, Drager UC, Wu DK. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci U S A. 2011;108:161–166. doi: 10.1073/pnas.1010547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag RJ, Siegfried Z, Cebra-Thomas JA, Garvey N, Davison EM, Silver LM. An ancient family of embryonically expressed mouse genes sharing a conserved protein motif with the T locus. Nat Genet. 1994;7:383–389. doi: 10.1038/ng0794-383. [DOI] [PubMed] [Google Scholar]
- Bosse A, Zulch A, Becker MB, Torres M, Gomez-Skarmeta JL, Modolell J, Gruss P. Identification of the vertebrate Iroquois homeobox gene family with overlapping expression during early development of the nervous system. Mech Dev. 1997;69:169–181. doi: 10.1016/s0925-4773(97)00165-2. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26:10438–10451. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. Balancing cell numbers during organogenesis: Six1a differentially affects neurons and sensory hair cells in the inner ear. Dev Biol. 2011;357:191–201. doi: 10.1016/j.ydbio.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Brown AS, Epstein DJ. Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development. 2011;138:3967–3976. doi: 10.1242/dev.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budelmann BU, Schipp R, Von Boletzky S. Cephalopoda. In: Harrison EW, Kohn AJ, editors. Microscopic Anatomy of Invertebrates. Mollusca II. 6A. Wiley-Liss; New York: 1997. pp. 119–414. [Google Scholar]
- Burighel P, Caicci F, Manni L. Hair cells in non-vertebrate models: lower chordates and molluscs. Hear Res. 2011;273:14–24. doi: 10.1016/j.heares.2010.03.087. [DOI] [PubMed] [Google Scholar]
- Burighel P, Caicci F, Zaniolo G, Gasparini F, Degasperi V, Manni L. Does hair cell differentiation predate the vertebrate appearance? Brain Res Bull. 2008;75:331–334. doi: 10.1016/j.brainresbull.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Burighel P, Lane NJ, Fabio G, Stefano T, Zaniolo G, Carnevali MD, Manni L. Novel, secondary sensory cell organ in ascidians: in search of the ancestor of the vertebrate lateral line. J Comp Neurol. 2003;461:236–249. doi: 10.1002/cne.10666. [DOI] [PubMed] [Google Scholar]
- Burns JC, On D, Baker W, Collado MS, Corwin JT. Over Half the Hair Cells in the Mouse Utricle First Appear After Birth, with Significant Numbers Originating from Early Postnatal Mitotic Production in Peripheral and Striolar Growth Zones. J Assoc Res Otolaryngol. 2012;13:609–627. doi: 10.1007/s10162-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Seymour ML, Zhang H, Pereira FA, Groves AK. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. J Neurosci. 2013;33:10110–10122. doi: 10.1523/JNEUROSCI.5606-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicci F, Burighel P, Manni L. Hair cells in an ascidian (Tunicata) and their evolution in chordates. Hear Res. 2007;231:63–72. doi: 10.1016/j.heares.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215:359–369. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol. 2000;424:509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Jones JE, Katayama A, Kelley MW, Warchol ME. Hair cell regeneration: the identities of progenitor cells, potential triggers and instructive cues; Ciba Found Symp; 1991; pp. 103–120. discussion 120-130. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Driver EC, Sillers L, Coate TM, Rose MF, Kelley MW. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev Biol. 2013;376:86–98. doi: 10.1016/j.ydbio.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl DF. Feeling the vibes: chordotonal mechanisms in insect hearing. Current Opinion in Neurobiology. 1999;9:389–393. doi: 10.1016/S0959-4388(99)80058-0. [DOI] [PubMed] [Google Scholar]
- Eberl DF, Boekhoff-Falk G. Development of Johnston’s organ in Drosophila. Int J Dev Biol. 2007;51:679–687. doi: 10.1387/ijdb.072364de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci U S A. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm O, Goritz C, Covic M, Schaffner I, Schwarz TJ, Karaca E, Kempkes B, Kremmer E, Pfrieger FW, Espinosa L, Bigas A, Giachino C, Taylor V, Frisen J, Lie DC. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evsen L, Sugahara S, Uchikawa M, Kondoh H, Wu DK. Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. J Neurosci. 2013;33:3879–3890. doi: 10.1523/JNEUROSCI.4030-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM. Cell fate specification in the inner ear. Curr Opin Neurobiol. 1996;6:533–541. doi: 10.1016/s0959-4388(96)80061-4. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fischer A, Gessler M. Delta-Notch--and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007;35:4583–4596. doi: 10.1093/nar/gkm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode C, Ma Q, Casarosa S, Ang SL, Anderson DJ, Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- Freyer L, Morrow BE. Canonical Wnt signaling modulates Tbx1, Eya1, and Six1 expression, restricting neurogenesis in the otic vesicle. Dev Dyn. 2010;239:1708–1722. doi: 10.1002/dvdy.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RA, Makmura L, Biesiada E, Wang X, Keithley EM. Eya1 acts upstream of Tbx1, Neurogenin 1, NeuroD and the neurotrophins BDNF and NT-3 during inner ear development. Mech Dev. 2005;122:625–634. doi: 10.1016/j.mod.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Pauley S, Soukup G. Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol. 2007;51:663–678. doi: 10.1387/ijdb.072367bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Eberl DF, Beisel KW. The role of bHLH genes in ear development and evolution: revisiting a 10-year-old hypothesis. Cell Mol Life Sci. 2010;67:3089–3099. doi: 10.1007/s00018-010-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Beisel KW. Cells, molecules and morphogenesis: the making of the vertebrate ear. Brain Res. 2006;1091:151–171. doi: 10.1016/j.brainres.2006.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Straka H. Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology. 2014;200:5–18. doi: 10.1007/s00359-013-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke B, Epstein JA, Kochilas LK, Lu MM, Pandita RK, Liao J, Bauerndistel R, Schuler T, Schorle H, Brown MC, Adams J, Morrow BE. Mice overexpressing genes from the 22q11 region deleted in velo-cardio-facial syndrome/DiGeorge syndrome have middle and inner ear defects. Hum Mol Genet. 2001;10:2549–2556. doi: 10.1093/hmg/10.22.2549. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Caicci F, Rigon F, Zaniolo G, Burighel P, Manni L. Cytodifferentiation of hair cells during the development of a basal chordate. Hear Res. 2013;304:188–199. doi: 10.1016/j.heares.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Genzer MA, Bridgewater LC. A Col9a1 enhancer element activated by two interdependent SOX9 dimers. Nucleic Acids Res. 2007;35:1178–1186. doi: 10.1093/nar/gkm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Groves AK. The Induction of the Otic Placode. In: Popper AN, Kelley MW, Wu DK, editors. Development of the Inner Ear. Springer Handbook of Auditory Research. Springer Verlag; New York: 2005. pp. 10–42. [Google Scholar]
- Groves AK, Fekete DM. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Haddon C, Mowbray C, Whitfield T, Jones D, Gschmeissner S, Lewis J. Hair cells without supporting cells: further studies in the ear of the zebrafish mind bomb mutant. J Neurocytol. 1999;28:837–850. doi: 10.1023/a:1007013904913. [DOI] [PubMed] [Google Scholar]
- Hammond KL, Whitfield TT. The developing lamprey ear closely resembles the zebrafish otic vesicle: otx1 expression can account for all major patterning differences. Development. 2006;133:1347–1357. doi: 10.1242/dev.02306. [DOI] [PubMed] [Google Scholar]
- Hans S, Irmscher A, Brand M. Zebrafish Foxi1 provides a neuronal ground state during inner ear induction preceding the Dlx3b/4b-regulated sensory lineage. Development. 2013;140:1936–1945. doi: 10.1242/dev.087718. [DOI] [PubMed] [Google Scholar]
- Harrison RG. Relations of Symmetry in the Developing Ear of Amblystoma Punctatum. Proc Natl Acad Sci U S A. 1936;22:238–247. doi: 10.1073/pnas.22.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan BA, Bellen HJ. Doing the MATH: is the mouse a good model for fly development? Genes Dev. 2000;14:1852–1865. [PubMed] [Google Scholar]
- Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126:2135–2140. doi: 10.1242/jcs.127308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd EA, Poucher HK, Cheng K, Raphael Y, Martin DM. The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear. Development. 2010;137:3139–3150. doi: 10.1242/dev.047894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Chitnis AB. Expression of proneural and neurogenic genes in the zebrafish lateral line primordium correlates with selection of hair cell fate in neuromasts. Mech Dev. 2001;102:263–266. doi: 10.1016/s0925-4773(01)00308-2. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Calisto LE, Morris KA, Kopecky B, Duncan JS, Beisel KW, Fritzsch B. Expression of Neurog1 instead of Atoh1 can partially rescue organ of Corti cell survival. PLoS One. 2012;7:e30853. doi: 10.1371/journal.pone.0030853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarman AP, Groves AK. The role of Atonal transcription factors in the development of mechanosensitive cells. Semin Cell Dev Biol. 2013;24:438–447. doi: 10.1016/j.semcdb.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SJ, Fujioka M, Kim SC, Edge AS. Notch signaling alters sensory or neuronal cell fate specification of inner ear stem cells. J Neurosci. 2011;31:8351–8358. doi: 10.1523/JNEUROSCI.6366-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang L, Beier KT, Cepko CL, Fekete DM, Brigande JV. Lineage analysis of the late otocyst stage mouse inner ear by transuterine microinjection of a retroviral vector encoding alkaline phosphatase and an oligonucleotide library. PLoS One. 2013;8:e69314. doi: 10.1371/journal.pone.0069314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, Kane DA, Mullins MC, Odenthal J, van Eeden FJ, Nusslein-Volhard C. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal Mammalian cochlea in vivo. J Neurosci. 2012;32:6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. The international journal of biochemistry & cell biology. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Kiernan AE. The paintfill method as a tool for analyzing the three-dimensional structure of the inner ear. Brain Res. 2006;1091:270–276. doi: 10.1016/j.brainres.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Kiernan AE. Notch signaling during cell fate determination in the inner ear. Semin Cell Dev Biol. 2013;24:470–479. doi: 10.1016/j.semcdb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de, Angelis M. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci U S A. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch TJ, Xi Y, Flora A, Wang L, Li W, Zoghbi HY. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci U S A. 2011;108:3288–3293. doi: 10.1073/pnas.1100230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SK, Hill JK, Hwang CH, Lin ZS, Millen KJ, Wu DK. Lmx1a maintains proper neurogenic, sensory, and non-sensory domains in the mammalian inner ear. Dev Biol. 2009;333:14–25. doi: 10.1016/j.ydbio.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp A, Duncan I. Control of cell fate and polarity in the adult abdominal segments of Drosophila by optomotor-blind. Development. 1997;124:3715–3726. doi: 10.1242/dev.124.19.3715. [DOI] [PubMed] [Google Scholar]
- Kopp A, Muskavitch MA, Duncan I. The roles of hedgehog and engrailed in patterning adult abdominal segments of Drosophila. Development. 1997;124:3703–3714. doi: 10.1242/dev.124.19.3703. [DOI] [PubMed] [Google Scholar]
- Koundakjian EJ, Appler JL, Goodrich LV. Auditory neurons make stereotyped wiring decisions before maturation of their targets. J Neurosci. 2007;27:14078–14088. doi: 10.1523/JNEUROSCI.3765-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Lewis J. Rules for the production of sensory cells; Ciba Found Symp; 1991; pp. 25–39. discussion 40-53. [PubMed] [Google Scholar]
- Li Y, Baker NE. Proneural enhancement by Notch overcomes Suppressor-of-Hairless repressor function in the developing Drosophila eye. Curr Biol. 2001;11:330–338. doi: 10.1016/s0960-9822(01)00093-8. [DOI] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and deiters’ cells to immature hair cells by atoh1 ectopic expression. J Neurosci. 2012a;32:6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Owen T, Fang J, Srinivasan RS, Zuo J. In vivo Notch reactivation in differentiating cochlear hair cells induces Sox2 and Prox1 expression but does not disrupt hair cell maturation. Dev Dyn. 2012b;241:684–696. doi: 10.1002/dvdy.23754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Maklad A, Reed C, Johnson NS, Fritzsch B. Anatomy of the lamprey ear: morphological evidence for occurrence of horizontal semicircular ducts in the labyrinth of Petromyzon marinus. J Anat. 2014;224:432–446. doi: 10.1111/joa.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Ladher R. Phylogeny and evolution of ciliated mechanoreceptor cells. In: Dallos P, Oertel D, editors. The Senses: a Comprehensive Reference. Audition. Vol. 3. Academic Press; San Diego: 2008. pp. 1–34. [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Riley BB. Sox2 is required for maintenance and regeneration, but not initial development, of hair cells in the zebrafish inner ear. Dev Biol. 2010;338:262–269. doi: 10.1016/j.ydbio.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak GD, Ratnayaka HS, Goodyear RJ, Richardson GP. Development of the hair bundle and mechanotransduction. Int J Dev Biol. 2007;51:597–608. doi: 10.1387/ijdb.072392gn. [DOI] [PubMed] [Google Scholar]
- Neves J, Abello G, Petrovic J, Giraldez F. Patterning and cell fate in the inner ear: a case for Notch in the chicken embryo. Development, growth & differentiation. 2013a;55:96–112. doi: 10.1111/dgd.12016. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Neves J, Parada C, Chamizo M, Giraldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138:735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- Neves J, Uchikawa M, Bigas A, Giraldez F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS One. 2012;7:e30871. doi: 10.1371/journal.pone.0030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Vachkov I, Giraldez F. Sox2 regulation of hair cell development: incoherence makes sense. Hear Res. 2013b;297:20–29. doi: 10.1016/j.heares.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–358. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Basch ML, Mishina Y, Lyons KM, Segil N, Groves AK. BMP signaling is necessary for patterning the sensory and nonsensory regions of the developing mammalian cochlea. J Neurosci. 2010;30:15044–15051. doi: 10.1523/JNEUROSCI.3547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK, Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int J Dev Biol. 2007;51:463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–875. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2011;275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Chen J, Rottier RJ, Steel KP, Kiernan AE. Ectopic expression of activated notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J Neurosci. 2013;33:16146–16157. doi: 10.1523/JNEUROSCI.3150-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirano RI, Wegner M. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28:3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski T, Ahn DG, Schilling TF, Nair S, Ruvinsky I, Geisler R, Rauch GJ, Haffter P, Zon LI, Zhou Y, Foott H, Dawid IB, Ho RK. The zebrafish van gogh mutation disrupts tbx1, which is involved in the DiGeorge deletion syndrome in humans. Development. 2003;130:5043–5052. doi: 10.1242/dev.00704. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30:714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radosevic M, Robert-Moreno A, Coolen M, Bally-Cuif L, Alsina B. Her9 represses neurogenic fate downstream of Tbx1 and retinoic acid signaling in the inner ear. Development. 2011;138:397–408. doi: 10.1242/dev.056093. [DOI] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Raft S, Nowotschin S, Liao J, Morrow BE. Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development. 2004;131:1801–1812. doi: 10.1242/dev.01067. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ. Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 2002;16:2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BB, Chiang M, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- Riley BB, Phillips BT. Ringing in the new ear: resolution of cell interactions in otic development. Dev Biol. 2003;261:289–312. doi: 10.1016/s0012-1606(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev Biol. 1997;191:191–201. doi: 10.1006/dbio.1997.8736. [DOI] [PubMed] [Google Scholar]
- Sapede D, Dyballa S, Pujades C. Cell lineage analysis reveals three different progenitor pools for neurosensory elements in the otic vesicle. J Neurosci. 2012;32:16424–16434. doi: 10.1523/JNEUROSCI.3686-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Lineage analysis of inner ear cells using genomic tags for clonal identification. Methods Mol Biol. 2009;493:47–63. doi: 10.1007/978-1-59745-523-7_4. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H, Driever W. Mutations affecting the development of the embryonic zebrafish brain. Development. 1996;123:165–178. doi: 10.1242/dev.123.1.165. [DOI] [PubMed] [Google Scholar]
- Simionato E, Kerner P, Dray N, Le Gouar M, Ledent V, Arendt D, Vervoort M. atonal- and achaete-scute-related genes in the annelid Platynereis dumerilii: insights into the evolution of neural basic-Helix-Loop-Helix genes. BMC evolutionary biology. 2008;8:170. doi: 10.1186/1471-2148-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionato E, Ledent V, Richards G, Thomas-Chollier M, Kerner P, Coornaert D, Degnan BM, Vervoort M. Origin and diversification of the basic helix-loop-helix gene family in metazoans: insights from comparative genomics. BMC evolutionary biology. 2007;7:33. doi: 10.1186/1471-2148-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. Origin of the vertebrate inner ear: evolution and induction of the otic placode. J Anat. 2001;199:99–103. doi: 10.1046/j.1469-7580.2001.19910099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Barbash DA, Lawrence PA. Hedgehog acts by distinct gradient and signal relay mechanisms to organise cell type and cell polarity in the Drosophila abdomen. Development. 1997a;124:2155–2165. doi: 10.1242/dev.124.11.2155. [DOI] [PubMed] [Google Scholar]
- Struhl G, Barbash DA, Lawrence PA. Hedgehog organises the pattern and polarity of epidermal cells in the Drosophila abdomen. Development. 1997b;124:2143–2154. doi: 10.1242/dev.124.11.2143. [DOI] [PubMed] [Google Scholar]
- Sweet EM, Vemaraju S, Riley BB. Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Dev Biol. 2011;358:113–121. doi: 10.1016/j.ydbio.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H, Hardisty RE, Rhodes C, Kiernan AE, Roby P, Tymowska-Lalanne Z, Mburu P, Rastan S, Hunter AJ, Brown SD, Steel KP. The mouse slalom mutant demonstrates a role for Jagged1 in neuroepithelial patterning in the organ of Corti. Hum Mol Genet. 2001;10:507–512. doi: 10.1093/hmg/10.5.507. [DOI] [PubMed] [Google Scholar]
- Vazquez-Echeverria C, Dominguez-Frutos E, Charnay P, Schimmang T, Pujades C. Analysis of mouse kreisler mutants reveals new roles of hindbrain-derived signals in the establishment of the otic neurogenic domain. Dev Biol. 2008;322:167–178. doi: 10.1016/j.ydbio.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Vemaraju S, Kantarci H, Padanad MS, Riley BB. A spatial and temporal gradient of Fgf differentially regulates distinct stages of neural development in the zebrafish inner ear. PLoS Genet. 2012;8:e1003068. doi: 10.1371/journal.pgen.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli F, Viola A, Morishima M, Pramparo T, Baldini A, Lindsay E. TBX1 is required for inner ear morphogenesis. Hum Mol Genet. 2003;12:2041–2048. doi: 10.1093/hmg/ddg216. [DOI] [PubMed] [Google Scholar]
- Wang VY, Hassan BA, Bellen HJ, Zoghbi HY. Drosophila atonal fully rescues the phenotype of Math1 null mice: new functions evolve in new cellular contexts. Curr Biol. 2002;12:1611–1616. doi: 10.1016/s0960-9822(02)01144-2. [DOI] [PubMed] [Google Scholar]
- Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol. 2002;53:157–171. doi: 10.1002/neu.10123. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Riley BB, Chiang MY, Phillips B. Development of the zebrafish inner ear. Dev Dyn. 2002;223:427–458. doi: 10.1002/dvdy.10073. [DOI] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Wu DK, Kelley MW. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 2012;4:a008409. doi: 10.1101/cshperspect.a008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Viola A, Zhang Z, Gerken CP, Lindsay-Illingworth EA, Baldini A. Tbx1 regulates population, proliferation and cell fate determination of otic epithelial cells. Dev Biol. 2007;302:670–682. doi: 10.1016/j.ydbio.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Chang W, Kelley MW. Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev Biol. 2011;353:367–379. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Yang H, Xie X, Deng M, Chen X, Gan L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis. 2010;48:407–413. doi: 10.1002/dvg.20633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, Anderson DJ. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Fritzsch B, Xu PX. Eya1 and Six1 are essential for early steps of sensory neurogenesis in mammalian cranial placodes. Development. 2004;131:5561–5572. doi: 10.1242/dev.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Silvius D, Rodrigo-Blomqvist S, Enerback S, Xu PX. Eya1 regulates the growth of otic epithelium and interacts with Pax2 during the development of all sensory areas in the inner ear. Dev Biol. 2006;298:430–441. doi: 10.1016/j.ydbio.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material/Video: A video morph generated from serial section sets of mouse otocysts at three stages (24, 27, 30 somite stages; E9.5-E10) that were hybridized for detection of either Neurod1 (pink) or Tbx1 (blue) and reconstructed into space-filling models. The otocysts are viewed ventrally, as if through the second branchial arch, and oriented such that lateral is left, and anterior is up. The model at right renders the otocyst epithelium transparent to allow changes of Tbx1 expression in the dorsal otocyst to be visualized. Complementary patterning of Neurod1 and Tbx1 expression is maintained despite considerable changes in the extent of these domains over a 12-hour developmental period. Still images used to generate this morph, as well as methods for generating the space-filling models, are presented in (Raft, et al., 2004).