Abstract
Aim
High-density lipoproteins (HDLs) have several potentially protective vascular effects. Most clinical studies of therapies targeting HDL have failed to show benefits vs. placebo.
Objective
To investigate the effects of an HDL-mimetic agent on atherosclerosis by intravascular ultrasonography (IVUS) and quantitative coronary angiography (QCA).
Design and setting
A prospective, double-blinded, randomized trial was conducted at 51 centres in the USA, the Netherlands, Canada, and France. Intravascular ultrasonography and QCA were performed to assess coronary atherosclerosis at baseline and 3 (2–5) weeks after the last study infusion.
Patients
Five hundred and seven patients were randomized; 417 and 461 had paired IVUS and QCA measurements, respectively.
Intervention
Patients were randomized to receive 6 weekly infusions of placebo, 3 mg/kg, 6 mg/kg, or 12 mg/kg CER-001.
Main outcome measures
The primary efficacy parameter was the nominal change in the total atheroma volume. Nominal changes in per cent atheroma volume on IVUS and coronary scores on QCA were also pre-specified endpoints.
Results
The nominal change in the total atheroma volume (adjusted means) was −2.71, −3.13, −1.50, and −3.05 mm3 with placebo, CER-001 3 mg/kg, 6 mg/kg, and 12 mg/kg, respectively (primary analysis of 12 mg/kg vs. placebo: P = 0.81). There was also no difference among groups for the nominal change in per cent atheroma volume (0.02, −0.02, 0.01, and 0.19%; nominal P = 0.53 for 12 mg/kg vs. placebo). Change in the coronary artery score was −0.022, −0.036, −0.022, and −0.015 mm (nominal P = 0.25, 0.99, 0.55), and change in the cumulative coronary stenosis score was −0.51, 2.65, 0.71, and −0.77% (compared with placebo, nominal P = 0.85 for 12 mg/kg and nominal P = 0.01 for 3 mg/kg). The number of patients with major cardiovascular events was 10 (8.3%), 16 (13.3%), 17 (13.7%), and 12 (9.8%) in the four groups.
Conclusion
CER-001 infusions did not reduce coronary atherosclerosis on IVUS and QCA when compared with placebo. Whether CER-001 administered in other regimens or to other populations could favourably affect atherosclerosis must await further study.
Name of the trial registry: Clinicaltrials.gov; Registry's URL: http://clinicaltrials.gov/ct2/show/NCT01201837?term=cer-001&rank=2; Trial registration number: NCT01201837.
Keywords: Atherosclerosis, Coronary disease, High-density lipoproteins, Clinical trial
This paper was guest edited by Filippo Crea.
See page 3248 for the editorial comment on this article (doi:10.1093/eurheartj/ehu194)
Translational perspective.
Although there is extensive epidemiological and pre-clinical evidence supporting favourable cardiovascular effects of HDL, clinical trials of HDL-based therapies have often yielded disappointing results. Here, we report the results of the CHI-SQUARE study, the largest randomized clinical trial performed so far of serial HDL infusions in patients with a recent acute coronary syndrome. In this study, the HDL-mimetic agent CER-001 did not reduce coronary atherosclerosis on IVUS and QCA when compared with placebo. Whether CER-001 administered in other regimens or to other populations could favourably affect atherosclerosis is not known.
Introduction
Patients with a recent acute coronary syndrome are exposed to a high risk of recurrent events during the first year after the initial presentation despite intensive contemporary treatment.1 Atherosclerosis is the main underlying aetiology for the important cardiovascular disease burden in our societies.2 Therefore, further strategies to decrease atherosclerosis burden and improve cardiovascular outcomes are needed. There is an inverse association between high-density lipoprotein (HDL) cholesterol and risk of coronary heart disease complications in population-based epidemiological studies,3 although HDL particle number appears to be a better predictor of outcomes in the current era of aggressive statin use and very low LDL cholesterol levels.4 Yet recent data have indicated that this inverse relationship is substantially weakened in patients with manifest coronary heart disease.5 Several trials have reported disappointing results with medications affecting HDL such as niacin and cholesteryl ester transfer protein inhibitors, although some of these studies may have contained confounding factors.6–9 Three clinical studies have suggested benefits of HDL infusions on coronary plaque burden evaluated by intravascular ultrasonography (IVUS) when compared with baseline, but none established significance vs. placebo.10–12 Interpretation of the latter studies was limited by the small sample sizes10–12 and imbalances among groups in plaque burden at baseline.10
CER-001 is an engineered lipoprotein particle mimicking pre-beta HDL and consisting of a combination of recombinant human apolipoprotein A-I and two phospholipids. It has previously been shown to rapidly mobilize large amounts of cholesterol into the HDL fraction following its i.v. administration.13 The objective of the current study was to assess the safety and efficacy of CER-001 administered as a series of weekly infusions on coronary atherosclerosis as assessed by IVUS and quantitative coronary angiography (QCA).
Methods
Study design and population
Between March 2011 and August 2012, patients with a clinical indication for coronary angiography and a research-mandated IVUS recording approved by the IVUS core laboratory were randomized to receive either placebo or CER-001 infusions within 14 days of having an acute coronary syndrome defined as unstable angina, non-ST or ST segment elevation myocardial infarction. Eligible patients were women (without childbearing potential) and men up to 80 years of age with at least one narrowing of 20% or more on coronary angiography at baseline or history of percutaneous coronary intervention (PCI) (the infarct-related artery and all coronary arteries undergoing PCI were excluded from the imaging analysis). Patients with >50% stenosis in the left main coronary artery, a baseline IVUS recording determined to be of unacceptable quality by the IVUS core laboratory, renal insufficiency (serum creatinine >2.0 mg/dL), liver disease (enzymes greater than twice the upper limit of normal), uncontrolled diabetes mellitus (HbA1C >10%), triglycerides >500 mg/dL, uncontrolled hypertension, haemodynamic instability, class III or IV heart failure, known ejection fraction <35%, previous or planned coronary bypass surgery, valvular disease requiring cardiac surgery, or history of alcohol or drug abuse were excluded from study participation. Institutional ethics committees approved the protocol at all 51 participating study centres in the USA, the Netherlands, Canada, and France, and all trial patients provided written informed consent before any study procedure was performed.
The CHI-SQUARE study was a randomized, double-blinded, placebo-controlled, ascending dose trial. Qualifying patients were randomly assigned to receive six weekly volume-matched infusions of either CER-001 or placebo in a 3 : 1 ratio in three consecutive cohorts (CER-001 3 mg/kg vs. placebo, then 6 mg/kg vs. placebo, and finally 12 mg/kg vs. placebo), resulting in similar numbers of patients randomized to the four study arms (placebo, CER-001 3, CER-001 6, and CER-001 12 mg/kg). The randomization code used blocks of size 4 and was stratified according to site and cohort, and was generated by the Montreal Heart Institute Coordinating Center using the SAS procedure PROCPLAN and managed centrally via an interactive response system to ensure an ascending dose study design.
Prior to randomization, a baseline IVUS examination of the designated target coronary artery was performed and transferred to the Montreal Heart Institute core IVUS laboratory for a quality assessment. The proximal 4 cm of the target coronary artery in which IVUS was performed at baseline needed to have a reference diameter of 2.5 mm or more, be free of filling defects suggestive of thrombosis, not to have >50% reduction in lumen diameter by visual angiographic estimation at baseline, and not to have undergone previous PCI nor be a candidate for intervention at the time of the baseline catheterization or over the following 12 weeks. Three weeks after the last study infusion (2–5 week window), a follow-up IVUS examination was performed in the same segment of the target artery studied at baseline.
A follow-up visit occurred ∼6 months after the last dose of study medication to monitor for major adverse cardiovascular events and anti-apoA-I antibodies. Patient safety was monitored throughout the trial. Clinical blood laboratory data were evaluated on an ongoing basis throughout the study. An independent, unblinded safety monitoring committee met at intervals during the trial (including near the end of the recruitment of the first two cohorts to authorize dose escalation).
Intervention and blinding
CER-001 (Cerenis, France) is a negatively charged lipoprotein complex mimicking discoidal pre-beta HDL, consisting of recombinant human apolipoprotein A-I and a combination of two naturally occurring phospholipids. The apolipoprotein A-I component is expressed in mammalian CHO cells and purified by a three-step column chromatography process. The phospholipid component consists of egg sphingomyelin and 1,2-dihexadecanoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (dipalmitoylphosphatidyl-glycerol) in a 97 : 3 weight ratio. The ratio of protein to total phospholipids in the CER-001 complex is 1 : 2.7 weight/weight. The drug product is a solution of the CER-001 complexes in phosphate buffered sucrose/mannitol solution (10 mM phosphate buffer, 4.0% sucrose, 2.0% mannitol, pH 8.0). The concentration of CER-001 complexes in the formulation is expressed as the concentration of the apolipoprotein A-I component. Intravenous infusions of CER-001 or placebo (saline with all non-active ingredients of CER-001) were administered over 1 h.
The site pharmacist or designee dispensing study drug was unblinded to individual patient treatment allocation but had no other role in study conduct. All other site personnel, the patients, blinded study monitor, study management team, and sponsor, were blinded to treatment allocation. Study drug blinding was achieved by shrouding the i.v. container with an opaque bag, sealed by the pharmacist. Both the placebo saline solution and CER-001 solutions remained shrouded until the infusions were administered and returned to the pharmacy.
Intravascular ultrasonography and coronary angiography
The methods for IVUS image acquisition and measurement in atherosclerosis studies have been described previously.11,14–18 Intravascular ultrasonography examinations were performed using 40–45 MHz catheters at baseline and follow-up. The same type of IVUS catheter (Volcano Corp or Boston Scientific) was used and the same dose of intracoronary nitroglycerine (0.15 mg) was administered prior to automated IVUS pullbacks performed at both time-points. All IVUS examinations were analysed at the Montreal Heart Institute core laboratory by experienced technicians supervised by a cardiologist blinded to treatment assignment, according to published standards.19 The lumen and external elastic membrane borders were manually traced on 31 digitized cross-sections matched at baseline and follow-up (fewer if it was not possible to optimally match 31 cross-sections, but at least 16 matched frames were traced at both time-points in all cases) and selected throughout the segment of interest. The total atheroma volume was computed through the summation of plaque areas (the latter is equal to the external elastic membrane area minus the lumen area) of all traced cross-sections for the segment, and results were then indexed to a 30-mm segment to compensate for differences in segment length between subjects and to allow each patient to contribute equally to the overall result (indexation to a 30-mm segment was performed by dividing atheroma volume by the length of the reconstructed segment and multiplying by 30). Per cent atheroma volume was computed by dividing atheroma volume by external elastic membrane volume and then multiplying by 100%.
Care was taken to ensure identical conditions during the angiographic examinations at baseline and follow-up (catheters, contrast media, and projections). Intracoronary nitroglycerine (0.15 mg) was administered into each coronary artery before angiographic injection. The segments of interest were visualized in multiple transverse and sagittal views to clearly separate stenosis from branches, minimize foreshortening, and obtain views as perpendicular as possible to the long axis of the segments to be analysed. All angiograms were analysed at the MHI QCA core laboratory using the CMS system (MEDIS, Leiden, Netherlands).20,21 Quantitative coronary angiography was performed by experienced technicians supervised by an expert physician in matched projections from baseline and follow-up angiograms.17 For each lesion, an end-diastolic frame from both angiograms was selected with identical angulations that best showed the stenosis at its most severe degree with minimal foreshortening and branch overlap. All intervened coronary arteries were excluded from the analysis. The coronary artery segments analysed included all those with a reference diameter ≥1.5 mm and a stenosis ≥20% at baseline, and those with new lesions at follow-up. Computer software automatically calculated the minimum lumen diameter (MLD), reference diameter, and per cent diameter stenosis.
Efficacy parameters
The primary efficacy endpoint was the nominal change in the total atheroma volume (follow-up minus baseline) on IVUS. Secondary and exploratory efficacy measures included, respectively, the nominal change in per cent atheroma volume on IVUS, and the nominal changes in the coronary artery score (defined as the per-patient mean of MLD for all lesions measured) and in the cumulative coronary stenosis score (calculated as the summation of the per cent diameter stenosis of all lesions measured) on QCA. The cumulative coronary stenosis score is an index of the anatomic extension and severity of disease in all coronary arteries.22
Safety and clinical event evaluations
Patient safety was assessed by monitoring adverse events, physical examinations, electrocardiograms, and clinical laboratory results. All blood-related analyses were carried out centrally. A clinical endpoint committee adjudicated all major adverse cardiovascular events having occurred between the first administration of study drug and 6 months after the last administration of study drug, including death, resuscitated cardiac arrest, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization procedures (PCI and coronary bypass graft surgery), hospitalization for unstable angina and hospitalization for heart failure, according to established definitions.
Statistical analysis
The primary analysis was based on the modified intent-to-treat population, which included all randomized subjects with a post-randomization efficacy recording, irrespective of their protocol adherence. A sensitivity analysis was performed on the per-protocol population, which included subjects who were part of the modified intent-to-treat population and received all six infusions of study drug at the planned dosage without major protocol deviations. Safety results are presented for all patients who received at least one study infusion.
Parametric analyses were performed after basic assumptions were checked. The primary endpoint (nominal change in the total atheroma volume) was analysed using an analysis of a covariance model that included the treatment group and baseline value as a covariate. The adjusted mean in the CER-001 12 mg/kg group was compared with the adjusted mean in the placebo group at the 0.05 significance level and this comparison was considered as the primary analysis. Under the same analysis of the covariance model, the adjusted means in the other CER-001 groups (6 and 3 mg/kg) were compared with the adjusted mean in the placebo group; the change from baseline within each treatment group was also tested. These analyses of the primary endpoint were considered exploratory. Other IVUS and QCA endpoints expressed as a nominal change from baseline to follow-up were analysed as described above using an analysis of the covariance model adjusting for the baseline value of the parameter.
Sample size computation was based on an expected difference of at least 7 mm3 in change in the total atheroma volume between the CER-001 12 mg/kg and placebo groups. Assuming that the standard deviation of the change in the total atheroma volume would be 15 mm3 in both groups and using a two-sided 0.05 significance level, 98 subjects per treatment group were necessary to detect this difference with 90% power. To account for an attrition rate of 20–25%, 126 subjects per group were to be randomized for a total of 504 patients. Statistical analyses were performed using SAS version 9.3.
Results
Baseline demographics
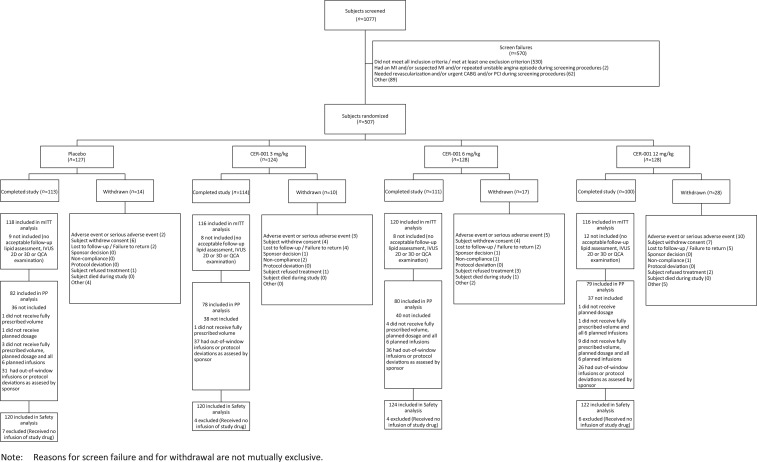
Five hundred and seven patients were randomized (Figure 1) and baseline patient characteristics were similar among groups (Table 1). There were 417 and 461 patients with paired IVUS and QCA measurements, respectively. The main reasons for the lack of IVUS analysis were early study termination and inability to obtain a matched coronary segment at both time points (Figure 1). The percentages of patients who received all six planned study drug infusions in the primary analysis (modified intent-to-treat) population were 97.5, 100, 96.7, and 91.4% in the placebo, CER-001 3, 6, and 12 mg/kg groups (P = 0.004).
Figure 1.
Disposition of patients in the trial.
Table 1.
Characteristics of patients in the modified intent-to-treat population (n = 470)
| Randomization group | Placebo (n = 118) | CER-001, 3 mg/kg (n = 116) | CER-001, 6 mg/kg (n = 120) | CER-001, 12 mg/kg (n = 116) |
|---|---|---|---|---|
| Age (years), means ± SD | 59.0 ± 9.0 | 57.3 ± 9.3 | 59.1 ± 9.3 | 60.5 ± 9.6 |
| Men, n (%) | 86 (72.9) | 93 (80.2) | 90 (75.0) | 85 (73.3) |
| Weight (kg), means ± SD | 90.2 ± 15.3 | 89.2 ± 17.8 | 89.8 ± 17.3 | 89.5 ± 19.5 |
| Current smoking, n (%) | 30 (25.4) | 34 (29.3) | 31 (25.8) | 31 (26.7) |
| Hypertension, n (%) | 89 (75.4) | 82 (70.7) | 82 (68.3) | 71 (61.2) |
| Diabetes, n (%) | 36 (30.5) | 27 (23.3) | 29 (24.2) | 25 (21.6) |
| Prior MI, n (%) | 26 (22.0) | 19 (16.4) | 16 (13.3) | 12 (10.3) |
| Prior PCI, n (%) | 42 (35.6) | 30 (25.9) | 31 (25.8) | 19 (16.4) |
| Lipid-lowering agent use, n (%) | 114 (96.7) | 114 (98.3) | 116 (96.8) | 113 (97.4) |
| Apo-B (mg/dL), Mean ± SD | 79.9 ± 21.5 | 78.7 ± 22.8 | 85.7 ± 25.8 | 81.1 ± 23.4 |
| Apo A-I (mg/dL), Mean ± SD | 130.2 ± 21.7 | 129.7 ± 22.2 | 131.1 ± 22.9 | 134.7 ± 22.9 |
| Presentation, n (%) | ||||
| Unstable angina | 65 (55.1) | 68 (58.6) | 53 (44.2) | 60 (51.7) |
| NSTEMI | 41 (34.7) | 37 (31.9) | 59 (49.2) | 50 (43.1) |
| STEMI | 12 (10.2) | 11 (9.5) | 8 (6.7) | 6 (5.2) |
Apo, apolipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Imaging efficacy results
Intravascular ultrasonography images were traced over a mean arterial segment length of 48 ± 15 mm (Table 2). The mean total atheroma volume at baseline was 155.24 ± 67.99 mm3. The adjusted means for change in the total atheroma volume were −2.71, −3.13, −1.50, and −3.05 mm3 in the placebo, CER-001 3, 6, and 12 mg/kg groups (P = 0.81 for the pre-specified primary analysis of 12 mg/kg vs. placebo). There were also no differences compared with placebo for the CER-001 6 mg/kg (nominal P = 0.45) and 3 mg/kg (nominal P = 0.77) groups. The change in per cent atheroma volume was similar among all study groups [0.02, −0.02, 0.01, and 0.19% in the placebo, CER-001 3 mg/kg (P = 0.86), 6 mg/kg (P = 0.95), and 12 mg/kg (P = 0.53) groups (nominal P-values vs. placebo)]. A sensitivity analysis conducted on the per-protocol population, which had 97% power to detect the target difference given the observed standard deviation, yielded similar results (Table 2).
Table 2.
Primary intravascular ultrasonography results
| Modified intent-to-treat population, total atheroma volume (mm3) | Placebo (n = 101) | CER-001, 3 mg/kg (n = 105) | CER-001, 6 mg/kg (n = 106) | CER-001, 12 mg/kg (n = 105) |
|---|---|---|---|---|
| Baseline (means ± SD) | 160.57 ± 59.99 | 141.01 ± 60.91 | 162.13 ± 78.86 | 157.37 ± 68.98 |
| Follow-up (means ± SD) | 157.70 ± 59.08 | 138.32 ± 59.43 | 160.41 ± 77.81 | 154.26 ± 67.43 |
| Nominal change (adjusted mean) (95% CI) | −2.71 (−4.89, −0.53) | −3.13 (−5.03, −1.24) | −1.50 (−3.76, 0.75) | −3.05 (−4.79, −1.30) |
| P-value vs. placebo | N/A | 0.77 | 0.45 | 0.81* |
| Per cent atheroma volume (%) | ||||
| Baseline (mean ± SD) | 38.03 ± 8.97 | 34.63 ± 9.13 | 37.37 ± 9.32 | 36.86 ± 9.09 |
| Follow-up (mean ± SD) | 38.01 ± 8.90 | 34.69 ± 8.91 | 37.35 ± 8.97 | 37.04 ± 9.22 |
| Nominal change (adjusted mean) (95% CI) | 0.02 (−0.31, 0.35) | −0.02 (−0.35, 0.31) | 0.01 (−0.31, 0.33) | 0.19 (−0.22, 0.60) |
| P-value vs. placebo | N/A | 0.86 | 0.95 | 0.53 |
| Per-protocol population, total atheroma volume (mm3) | (n = 82) | (n = 78) | (n = 80) | (n = 78) |
| Baseline (means ± SD) | 158.65 ± 58.98 | 141.66 ± 64.10 | 160.79 ± 77.61 | 153.86 ± 65.93 |
| Follow-up (means ± SD) | 156.17 ± 58.39 | 137.67 ± 61.93 | 159.14 ± 76.49 | 151.01 ± 65.34 |
| Nominal change (adjusted mean) (95% CI) | −2.34 (−4.71, 0.03) | −4.34 (−6.48, −2.19) | −1.45 (−4.17, 1.27) | −2.85 (−4.85, −0.85) |
| P-value vs. placebo | N/A | 0.22 | 0.62 | 0.74 |
| Per cent atheroma volume (%) | ||||
| Baseline (mean ± SD) | 37.59 ± 9.01 | 34.10 ± 8.71 | 37.37 ± 9.63 | 36.93 ± 9.00 |
| Follow-up (mean ± SD) | 37.58 ± 9.00 | 34.12 ± 8.58 | 37.30 ± 9.25 | 37.02 ± 9.27 |
| Nominal change (adjusted mean) (95% CI) | 0.02 (−0.34, 0.38) | −0.05 (−0.45, 0.34) | −0.05 (−0.42, 0.33) | 0.10 (−0.41, 0.61) |
| P-value vs. placebo | N/A | 0.78 | 0.80 | 0.80 |
*P-value for primary endpoint (in bold). All other P-values represent nominal values.
Quantitative coronary angiography results are described in Table 4. The change from baseline to follow-up in the coronary artery score was −0.022, −0.036, −0.022, and −0.015 mm in the placebo and CER-001 3, 6, and 12 mg/kg groups, respectively (vs. placebo, nominal P = 0.25, 0.99, 0.55, respectively). The change from baseline to follow-up in the cumulative coronary stenosis score was −0.51, 2.65, 0.71, and −0.77% in the placebo and CER-001 3, 6, and 12 mg/kg groups (nominal P = 0.01 for 3 mg/kg vs. placebo).
Table 4.
Quantitative coronary angiography results
| Modified intent-to-treat population, coronary artery score (mm) | Placebo (n = 116) | CER-001, 3 mg/kg (n = 115) | CER-001, 6 mg/kg (n = 119) | CER-001, 12 mg/kg (n = 111) |
|---|---|---|---|---|
| Baseline (means ± SD) | 1.961 ± 0.385 | 2.034 ± 0.392 | 2.003 ± 0.438 | 1.985 ± 0.499 |
| Follow-up (means ± SD) | 1.940 ± 0.384 | 1.997 ± 0.382 | 1.981 ± 0.436 | 1.970 ± 0.502 |
| Nominal change (adjusted mean) (95% CI) | −0.022 (−0.039, −0.006) | −0.036 (−0.052, −0.020) | −0.022 (−0.038, −0.006) | −0.015 (−0.032, 0.001) |
| P-value vs. placebo | N/A | 0.25 | 0.99 | 0.55 |
| Cumulative coronary stenosis score (%) | ||||
| Baseline (means ± SD) | 167.66 ± 100.73 | 169.27 ± 105.90 | 181.23 ± 109.01 | 165.81 ± 111.18 |
| Follow-up (means ± SD) | 167.13 ± 101.04 | 171.90 ± 108.72 | 182.00 ± 109.41 | 165.01 ± 111.20 |
| Nominal change (adjusted mean) (95% CI) | −0.51 (−2.42, 1.41) | 2.65 (1.00, 4.29) | 0.71 (−1.40, 2.82) | −0.77 (−2.66, 1.13) |
| P-value vs. placebo | N/A | 0.01 | 0.40 | 0.85 |
Given that the P-value for the primary endpoint was not significant, all P-values in this table represent nominal values.
Cardiovascular events
The number of patients with at least one major adverse cardiovascular event was 10 (8.3%) in the placebo group, and 16 (13.3%), 17 (13.7%), and 12 (9.8%) in the CER-001 groups, without statistically significant differences (Table 5).
Table 5.
Major adverse cardiovascular events (positively adjudicated) occurring between the first administration of study drug and 6 months after the last dose of study drug in patients who received at least one infusion of study drug
| Patients with at least one event | Placebo (n = 120) | CER-001, 3 mg/kg (n = 120) | CER-001, 6 mg/kg (n = 124) | CER-001, 12 mg/kg (n = 122) |
|---|---|---|---|---|
| Any MACE | 10 (8.3%) | 16 (13.3%) | 17 (13.7%) | 12 (9.8%) |
| P-value (log-rank) | N/A | 0.24 | 0.19 | 0.69 |
| Death | 0 | 0 | 0 | 0 |
| Cardiac arrest | 0 | 0 | 0 | 0 |
| Non-fatal MI (%) | 1 (0.8) | 1 (0.8) | 3 (2.4) | 4 (3.3) |
| Non-fatal stroke (%) | 0 (0) | 1 (0.8) | 1 (0.8) | 0 (0) |
| Coronary revascularization (%) | 8 (6.7) | 13 (10.8) | 12 (9.7) | 8 (6.6) |
| Hospitalization for unstable angina (%) | 5 (4.2) | 4 (3.3) | 2 (1.6) | 2 (1.6) |
| Hospitalization for heart failure (%) | 0 (0) | 1 (0.8) | 1 (0.8) | 1 (0.8) |
Given that the P-value for the primary endpoint was not significant, all P-values in this table represent nominal values. All MACEs were adjudicated by a clinical endpoint adjudication committee.
MACE, major adverse cardiovascular event; MI, myocardial infarction.
Safety results
CER-001 was generally well tolerated during the study (Table 6). There were a few infusion-type reactions during the study, which led to the temporary halting of patient recruitment (without interrupting ongoing study drug administration) to allow updating of the informed consent document as per the recommendation of the safety monitoring committee following its periodic review which included all subjects from the first two study cohorts and 128 subjects from the third cohort. Drug-related hypersensitivity reported as a serious adverse event occurred in 0, 1, 3, and 2 patients in the placebo and CER-001 groups. Treatment-emergent infusion-type reactions (with rigors, chills, nausea, and/or hypotension) occurred in 0, 0, 3, and 3 patients of the placebo, CER-001 3, 6, and 12 mg/kg groups, respectively. None of these patients had anti-apoA-I antibodies.
Table 6.
Selected adverse events in patients who received at least one infusion of study drug
| Safety population | Placebo (n = 120) | CER-001, 3 mg/kg (n = 120) | CER-001, 6 mg/kg (n = 124) | CER-001, 12 mg/kg (n = 122) |
|---|---|---|---|---|
| Any serious AE (%) | 8 (6.7) | 14 (11.7) | 14 (11.3) | 11 (9.0) |
| Any adverse event (%) | 96 (80.0) | 98 (81.7) | 86 (69.4) | 90 (73.8) |
| Infusion-type reaction (%) | 0 (0) | 0 (0) | 3 (2.4) | 3 (2.5) |
| Drug hypersensitivity (%) | 2 (1.7) | 1 (0.8) | 3 (2.4) | 6 (4.9) |
| Rash (%) | 0 (0) | 2 (1.7) | 1 (0.8) | 3 (2.5) |
| Dyspnoea (%) | 3 (2.5) | 3 (2.5) | 6 (4.8) | 7 (5.7) |
| Nausea (%) | 8 (6.7) | 11 (9.2) | 4 (3.2) | 2 (1.6) |
| Diarrhoea (%) | 3 (2.5) | 7 (5.8) | 4 (3.2) | 3 (2.5) |
AE, adverse event.
Treatment-emergent elevations in ALT (three times the ULN) occurred in 2, 2, 2, and 1 patients in the four study groups.
Post hoc re-analysis
At the end of the study, the sponsor requested a post hoc re-analysis of the IVUS recordings by a separate group, which also showed that the primary endpoint was not met (Table 3). The adjusted means for change in the total atheroma volume were −2.85, −4.76, −3.34, and −2.61 mm3 in the placebo, CER-001 3 mg/kg (P = 0.28), 6 mg/kg (P = 0.78), and 12 mg/kg (P = 0.89) groups (nominal P-values vs. placebo). A sensitivity analysis performed on the per-protocol population of these post hoc re-analysed data yielded similar results (Table 3).
Table 3.
Post hoc reanalysis of intravascular ultrasonography data
| Modified intent-to-treat population, total atheroma volume (mm3) | Placebo (n = 93) | CER-001, 3 mg/kg (n = 88) | CER-001, 6 mg/kg (n = 100) | CER-001, 12 mg/kg (n = 88) |
|---|---|---|---|---|
| Baseline (means ± SD) | 148.34 ± 57.06 | 133.54 ± 51.97 | 149.16 ± 72.19 | 146.21 ± 60.81 |
| Follow-up (means ± SD) | 145.32 ± 55.58 | 129.23 ± 50.92 | 145.63 ± 70.97 | 143.52 ± 59.09 |
| Nominal change (adjusted mean) (95% CI) | −2.85 (−5.27, −0.43) | −4.76 (−7.25, −2.26) | −3.34 (−5.67, −1.00) | −2.61 (−5.10, −0.13) |
| P-value vs. placebo | N/A | 0.28 | 0.78 | 0.89 |
| Per cent atheroma volume (%) | ||||
| Baseline (means ± SD) | 36.35 ± 9.11 | 34.54 ± 8.11 | 36.67 ± 9.09 | 35.87 ± 8.70 |
| Follow-up (means ± SD) | 36.16 ± 9.12 | 34.04 ± 7.81 | 36.23 ± 8.87 | 36.09 ± 8.95 |
| Nominal change (adjusted mean) (95% CI) | −0.17 (−0.66, 0.33) | −0.56 (−1.07, −0.05) | −0.41 (−0.89, 0.06) | 0.22 (−0.28, 0.73) |
| P-value vs. placebo | N/A | 0.27 | 0.48 | 0.28 |
| Per-protocol population, total atheroma volume (mm3) | (n = 67) | (n = 66) | (n = 72) | (n = 65) |
| Baseline (mean ± SD) | 150.35 ± 56.90 | 134.97 ± 53.82 | 146.44 ± 72.47 | 144.93 ± 61.52 |
| Follow-up (mean ± SD) | 146.41 ± 54.84 | 129.16 ± 52.13 | 143.50 ± 69.58 | 143.08 ± 60.07 |
| Nominal change (adjusted mean) (95% CI) | −3.63 (−6.34, −0.91) | −6.28 (−9.02, −3.54) | −2.83 (−5.44, −0.21) | −1.81 (−4.56, 0.95) |
| P-value vs. placebo | N/A | 0.18a | 0.68 | 0.36 |
| Per cent atheroma volume (%) | ||||
| Baseline (mean ± SD) | 36.91 ± 8.88 | 34.10 ± 7.44 | 36.92 ± 9.42 | 36.10 ± 8.41 |
| Follow-up (mean ± SD) | 36.59 ± 8.90 | 33.45 ± 7.21 | 36.40 ± 9.13 | 36.47 ± 8.78 |
| Nominal change (adjusted mean) (95% CI) | −0.28 (−0.87, 0.31) | −0.74 (−1.33, −0.14) | −0.48 (−1.04, 0.09) | 0.37 (−0.22, 0.97) |
| P-value vs. placebo | N/A | 0.28 | 0.63 | 0.13 |
aIn a non-parametric test not performed by the academic statistical centre, the nominal P was 0.03.
Discussion
This study did not demonstrate positive effects of the HDL-mimetic agent CER-001 on coronary atherosclerosis evaluated by IVUS and QCA. In the main modified intent-to-treat population, the differences in adjusted means of change in the total atheroma volume (active arm minus placebo) were −0.34 mm3 for CER-001 12 mg/kg (primary endpoint, P = 0.81), 1.20 mm3 for 6 mg/kg, and −0.42 mm3 for 3 mg/kg. In the per-protocol population, corresponding changes vs. placebo were −0.51, 0.90, and −2.00 mm3 for the CER-001 12, 6, and 3 mg/kg groups, respectively. The difference of −2 mm3 between the CER-001 3 mg/kg and placebo groups on IVUS in that sensitivity analysis was small and not nominally significant (nominal P = 0.22). The post hoc re-analysis of IVUS recordings requested by the sponsor yielded results similar to those of the pre-specified primary analysis.
This result on IVUS was accompanied by an increase of 3.15% of the cumulative coronary stenosis score on QCA in the CER-001 3 mg/kg group compared with placebo (nominal P = 0.01), which suggests that there was progressively greater obstruction of coronary arteries on QCA at this dose.22 The difference among groups for the change in the coronary artery score, however, did not reach statistical significance.
In the exploratory analysis of major adverse cardiovascular events, there was no statistically significant difference in the time to first event analysis or in the individual clinical endpoints, although this study was not powered for these outcomes.
Although CHI-SQUARE is much larger than the previous three clinical studies of HDL infusions,9–11 none of these trials has been able to demonstrate a therapeutic benefit on coronary atherosclerosis evaluated by IVUS compared with placebo using the intention-to-treat principle. Given that all four studies have focused on patients with a recent acute coronary syndrome, it is not known if CER-001 could be effective in other patient populations. Whether other dosing regimens (e.g. higher number of infusions, different dosage) could lead to a more favourable outcome is also unknown. It is also possible that the different lipoprotein compositions of these four HDL-related complexes may affect their effectiveness. The fact that the dose-related increase in cholesterol mobilization in the current study (estimated by the increase in plasma cholesterol after CER-001 infusion, see Supplementary Data) did not translate into progressively greater effects on IVUS underscores the lack of predictive value of this biomarker. Other properties of HDL particles, like their anti-inflammatory effects23 or changes in their proteome,24 could be of greater importance. Nevertheless, the plasma level of free cholesterol increased by ∼45% at 2 h after the start of the infusion of CER-001 12 mg/kg. The significance of this result obtained at a single time point after infusion is not entirely clear. Interestingly, in a previous phase I clinical study, significant increases in the plasma level of cholesteryl esters were observed after the infusion of CER-001 at dosages of 15 and 45 mg/kg.13 In that study, no clinically significant changes in laboratory red blood cell parameters were observed in humans with CER-001 doses up to 45 mg/kg. Similarly, no changes in red blood cell morphologic parameters were observed in in vitro compatibility studies using human red blood cells and CER-001 at a 30-fold concentration margin over the highest dose used in the current study. Although its source is not entirely certain, the majority of the cholesterol mobilized into circulation likely comes from the liver, as previously proposed.25
There were six patients treated with CER-001 who experienced non-fatal infusion reactions (3 with 6 mg/kg and 3 with 12 mg/kg). The pathophysiology of these reactions is uncertain, but not associated with anti-apoA-I antibodies or obvious complement depletion. An infusion reaction was also reported in the apoA-I Milano study.9
One study limitation was that it was not powered to detect differences in clinical outcomes among groups. Also, there was no assessment of potential changes in plaque quality by virtual histology, which may have concealed potentially favourable effects of this therapeutic approach in the long term. Increasing emphasis is indeed being placed on the stability or instability of atherosclerotic plaques and the associated risk of rupture.
In conclusion, when compared with placebo, infusions of CER-001 did not result in a significant reduction in coronary atherosclerosis as assessed by IVUS and QCA. Whether or not the non-significant reduction in coronary atheroma burden in those treated with CER-001 3 mg/kg reflects a beneficial effect of this lower dose will have to await further investigation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Funding for this study was provided by Cerenis, FRANCE. J-C.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Funding to pay the Open Access publication charges for this article was provided by the Montreal Heart Institute.
Role of the sponsor
The study steering committee was responsible for the design of the study in collaboration with the sponsor (Cerenis). The sponsor was not involved in the preparation or final approval of the manuscript but had the opportunity to review the article before submission.
Independent statistical analysis
All statistical analyses were performed by biostatisticians at the Montreal Heart Institute Coordinating Center (Marie-Claude Guertin PhD, Daniel Cournoyer MSc).
Acknowledgements
We acknowledge the post hoc re-analysis of IVUS data (requested by the sponsor) performed by Dr Stephen Nicholls.
Conflict of interest: Drs J-C.T., C.M.B., P.B., Z.A.F., J.J.P.K., W.K., T.F.L., A.T., and D.D.W. have received honoraria for their participation to the steering committee. Dr J.C.T. has received research grants and honoraria from Roche and Servier. T.F.L. has received research grants and honoraria from Merck and Roche.
Appendix
The following persons participated in the study: Safety Review Committee: M. Pfeffer (chair), V. Brown, J. Rouleau, P. Watkins, and L.J. Wei. Clinical Endpoint Adjudication Committee: G. Gosselin (chair), C. Chayer, S. Lanthier, G.B. Pelletier, N. Racine. Investigators (Institutions; screened, randomized): H. Agarwal (Alegent Health Heart and Vascular Specialists; 6, 2), E. Brilakis (Dallas VA Medical Center; 28, 16), L. Cannon (Cardiac and Vascular Research Center of Northern Michigan; 85, 55), D. Carrié (Centre Hospitalier Universitaire de Toulouse Rangueil; 21, 17), J. Corbelli (Buffalo Cardiology and Pulmonary Associates; 11, 6), P. Coste (Hopital Cardiologique de Bordeaux; 6, 5), R. de Winter (Academic Medical Center; 4, 1), A. Diaz (Centre Hospitalier Regional de Trois-Rivières; 26, 7), S. Eisenberg (Saint Joseph Hospital of Atlanta; 2, 1), B. Ennis (TCA Research; 3, 0), J. Fajadet (Clinique Pasteur; 8, 6), N. Fam (St Michael's Hospital; 12, 4), D. Fortuin (Mayo Clinic Arizona; 9, 4), C. Gessler (Heart Center Research; 116, 57), C. Grines (Detroit Medical Center Cardiovascular Institute; 2, 1), D. Guerra (MultiCare Health System - Research Institute; 3, 2), H. Gum (University of Michigan Health System; 1, 1), T. Haldis (Sanford Heart Center; 14, 6), T. Heestermans (Medisch Centrum Alkmaar; 13, 10), J.P. Herrman (Onze Lieve Vrouwe Gasthuis; 48, 33), T. Huynh (Montreal General Hospital; 13, 4), E. Kedhi (Maasstad Ziekenhuis; 6, 3), M. Koren (Jacksonville Center for Clinical Research; 48,19), S. Kouz (Centre Hospitalier Régional de Lanaudière; 36, 9), M. Krolick (Heart and Vascular Institute of Florida; 16, 4), G. Kumkumian (NIH at Suburban Hospital - John Hopkins; 12, 4), S. Lavi (London Health Sciences Center; 13, 7), R.J. Li (Penn Presbyterian Medical Center; 3, 2), ARZ Masud (Buffalo Heart Group; 22, 10), C. McAlhany (LeBauer Cardiovascular Research Foundation; 3, 1), F.A. McGrew (Baptist Memorial Hospital; 19, 4), C. O'Shaughnessy (North Ohio Research; 1, 0), A.J.M. Oude Ophuis (Canisius Wilhelmina Ziekenhuis; 62, 38), K. Parr (The Care Group; 9, 4), W. Penny (VA San Diego Health Care Center; 17, 8), Y. Pesant (St-Jerome Medical Research; 2, 0), H. Post (Catharina Ziekenhuis Eindhoven; 21, 9), S. Robinson (Victoria Heart Institute; 13, 7), J. Rodes-Cabau (Institut Universitaire de Cardiologie et de Pneumologie de Québec; 47, 11), A. Roy (Cité de la Santé de Laval; 15, 6), S. Schulman (John Hopkins Hospital; 1, 0), F. Spence (Foothills Medical Center; 9, 4), G. Stouffer (UHC Heart and Vascular Center; 10, 1), T. Stys (Sanford Research - USD; 4, 1), B. Sussex (St John Health Science Center; 8, 6), N. Tahirkheli (South Oklahoma Heart Research; 11, 5), J-C. Tardif and J. Grégoire (Montreal Heart Institute; 105, 38), J. ten Berg (St Antonius Ziekenhuis Nieuwegein; 10, 10), A.J. van Boven (Medisch Centrum Leeuwarden; 11, 10), C. von Birgelen (Medisch Spectrum Twente; 1, 0), D. Weinstein (Palm Beach Heart Research Institute; 111, 48).
References
- 1.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators: intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 2.US Burden of Disease Collaborators. The State of US Health, 1990–2010. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden WE. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High--Density Lipoprotein Intervention Trial. Am J Cardiol. 2000;86:19L–22L. doi: 10.1016/s0002-9149(00)01464-8. [DOI] [PubMed] [Google Scholar]
- 4.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silbernagel G, Schöttker B, Appelbaum S, Scharnagl H, Kleber ME, Grammer TB, Ritsch A, Mons U, Holleczek B, Goliasch G, Niessner A, Boehm BO, Schnabel RB, Brenner H, Blankenberg S, Landmesser U, März W. High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. Eur Heart J. 2013;34:3563–3571. doi: 10.1093/eurheartj/eht343. [DOI] [PubMed] [Google Scholar]
- 6.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 7.HPS-2 Thrive Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 10.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes. JAMA. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 11.Tardif JC, Grégoire J, L'Allier PL, Ibrahim R, Lespérance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodés-Cabau J Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 12.Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, Martin BD, Perlman TJ, Maltais JA, Weissman NJ, Fitzgerald PJ, Brewer HB., Jr A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55:2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 13.Keyserling CH, Hunt TL, Klepp HM, Scott RA, Barbaras R, Schwenderman A, Lalwani N, Dasseux JL. CER-001, a synthetic HDL-mimetic, safely mobilizes cholesterol in healthy dyslipidemic volunteers. Circulation. 2011;124:A15525. (abstract) [Google Scholar]
- 14.Tardif JC, Grégoire J, Lespérance J, Lambert J, L'Allier PL, Rodés J, Anderson T, Blue JW, Imus J, Heinonen T. Design features of the avasimibe and progression of coronary lesions assessed by intravascular ultrasound (A-PLUS) clinical trial. Am Heart J. 2002;144:589–596. doi: 10.1067/mhj.2002.125329. [DOI] [PubMed] [Google Scholar]
- 15.Tardif JC, Grégoire J, L'Allier PL, Anderson TJ, Bertrand O, Reeves F, Title LM, Alfonso F, Schampaert E, Hassan A, McLain R, Pressler ML, Ibrahim R, Lespérance J, Blue J, Heinonen T, Rodés-Cabau J Avasimibe and Progression of Lesions on UltraSound (A-PLUS) Investigators. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation. 2004;110:3372–3377. doi: 10.1161/01.CIR.0000147777.12010.EF. [DOI] [PubMed] [Google Scholar]
- 16.Tardif JC, Grégoire J, Schwartz L, Title L, Laramée L, Reeves F, Lespérance J, Bourassa MG, L'Allier PL, Glass M, Lambert J, Guertin MC Canadian Antioxidant Restenosis Trial (CART-1) Investigators. Effects of AGI-1067 and probucol after percutaneous coronary interventions. Circulation. 2003;107:552–558. doi: 10.1161/01.cir.0000047525.58618.3c. [DOI] [PubMed] [Google Scholar]
- 17.Berry C, L'Allier PL, Grégoire J, Lespérance J, Levesque S, Ibrahim R, Tardif JC. Comparison of intravascular ultrasound and quantitative coronary angiography for the assessment of coronary artery disease progression. Circulation. 2007;115:1851–1857. doi: 10.1161/CIRCULATIONAHA.106.655654. [DOI] [PubMed] [Google Scholar]
- 18.Côté G, Tardif JC, Lespérance J, Lambert J, Bourassa M, Bonan R, Gosselin G, Joyal M, Tanguay JF, Nattel S, Gallo R, Crépeau J. Effects of probucol on vascular remodeling after coronary angioplasty. Circulation. 1999;99:30–35. doi: 10.1161/01.cir.99.1.30. [DOI] [PubMed] [Google Scholar]
- 19.Mintz GS, Garcia-Garcia HM, Nicholls SJ, Weissman NJ, Tardif JC, Serruys PW. Clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound regression/progression studies. EuroIntervention. 2011;6:1123–1130. doi: 10.4244/EIJV6I9A195. [DOI] [PubMed] [Google Scholar]
- 20.Waters D, Higginson L, Gladstone P, Kimball B, Le May M, Boccuzzi SJ, Lespérance J. Effects of monotherapy with an HMG-CoA reductase inhibitor on the progression of coronary atherosclerosis as assessed by serial quantitative arteriography. The Canadian Coronary Atherosclerosis Intervention Trial. Circulation. 1994;89:959–968. doi: 10.1161/01.cir.89.3.959. [DOI] [PubMed] [Google Scholar]
- 21.Tardif JC, Cöté G, Lespérance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. N Engl J Med. 1997;337:365–372. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- 22.Solymoss BC, Bourassa MG, Campeau L, Sniderman A, Marcil M, Lespérance J, Lévesque S, Varga S. Effect of increasing metabolic syndrome score on atherosclerotic risk profile and coronary artery disease angiographic severity. Am J Cardiol. 2004;93:159–164. doi: 10.1016/j.amjcard.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 23.Barter PJ, Baker PW, Rye KA. Effect of high-density lipoprotein on the expression of adhesion molecules in endothelial cells. Curr Opin Lipidol. 2002;13:285–288. doi: 10.1097/00041433-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Lüscher TF, Landmesser U. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127:891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 25.Alam K, Meidell RS, Spady DK. Effect of Up-regulating individual steps in the reverse cholesterol transport pathway on reverse cholesterol transport in normolipidemic mice. J Biol Chem. 2001;276:15641–15649. doi: 10.1074/jbc.M010230200. [DOI] [PubMed] [Google Scholar]