Abstract
Aim
Cross-sectional studies reported associations between short leucocyte telomere length (LTL) and measures of vascular and cardiac damage. However, the contribution of LTL dynamics to the age-related process of cardiovascular (CV) remodelling remains unknown. In this study, we explored whether the rate of LTL shortening can predict CV phenotypes over 10-year follow-up and the influence of established CV risk factors on this relationship.
Methods and results
All the participants from the MRC National Survey of Health and Development (NSHD) with measures of LTL and traditional CV risk factors at 53 and 60–64 years and common carotid intima-media thickness (cIMT), cardiac mass and left ventricular function at 60–64 years were included. LTL was measured by real-time polymerase chain reaction and available at both time points in 1033 individuals. While LTL at 53 years was not linked with any CV phenotype at 60–64 years, a negative association was found between LTL and cIMT at 60–64 years (β = −0.017, P = 0.015). However, the strongest association was found between rate of telomere shortening between 53 and 60–64 years and values of cIMT at 60–64 years (β = −0.020, P = 0.006). This association was not affected by adjustment for traditional CV risk factors. Cardiac measurements were not associated with cross-sectional or longitudinal measures of LTL.
Conclusion
These findings suggest that the rate of progression of cellular ageing in late midlife (reflected by the rate of LTL attrition) relates to vascular damage, independently from contribution of CV risk factor exposure.
Keywords: Ageing, Telomeres shortening, Cardiovascular diseases, Carotid artery
See page 3245 for the editorial comment on this article (doi:10.1093/eurheartj/ehu252)
Translational Perspective.
This is the first report to analyse the relationship between a marker of cellular ageing (LTL) and a full range of subclinical measures of cardiovascular disease. We found an association between the dynamic change of LTL and subclinical measures of atherosclerosis. This association remained significant following multiple adjustments for cardiovascular risk factors, behavioural, and socio-economic factors. In contrast, no associations were found with the cardiac phenotype. Our results support the hypothesis that biological pathways regulating cellular ageing can influence the evolution of cardiovascular disease in humans.
Introduction
Telomeres are multiple repetitions of standard DNA sequences (TTAGGG)n, which cap the ends of eukaryotic chromosomes and protect them from aberration and fusion.1 As DNA polymerase cannot fully replicate the 3′ end of linear DNA, somatic cell replication results in a progressive loss of telomeres repeats, a process eventually resulting in cellular senescence or apoptosis.2 The recent availability of high throughput assays to measure telomere length on peripheral leucocytes (leucocyte telomere length, LTL) has allowed investigation into the role of telomere length biology in the evolution of cardiovascular (CV) disease. This has led to several observational studies reporting cross-sectional associations between LTL, levels of CV risk factors3 and clinical4 or subclinical5 measures of CV damage. Consequently, LTL has been suggested as a novel marker of CV ageing, integrating the cumulative lifetime burden of genetic factors and environmental stressors involved in the evolution of CV damage. It remains unknown, however, whether CV phenotypes in mid and later life are influenced by the dynamic change of LTL, and whether any such associations are independent of traditional CV risk factors.
The MRC National Survey of Health and Development (NSHD, also known as the 1946 British Birth Cohort) is the oldest of the British Birth Cohort studies,6 and is unique in providing measures of LTL and CV risk factors at the ages of 53 and 60–64 years, together with a characterization of the cardiac and vascular phenotypes at the later time point. The aim of this study was to determine whether the rate of LTL shortening over 10 years predicts cardiac and vascular phenotypes independent of established CV risk factors.
Methods
Population and cardiovascular risk factors assessment
The MRC NSHD is a social class stratified sample of all singleton births to married parents in England, Scotland, and Wales during 1 week in March 1946. LTL measures were available in 1033 participants at both 53- and 64-year follow-up visits. The 53-year visit was performed at home by a team of trained nurses while at 60–64 year participants were invited to attend one of six clinical research facilities (CRFs) across Britain or, if they were unable or unwilling to travel, to have a research nurse visit them at home.7 More details on the assessment of CV risk factors are reported in the Supplementary material online. Ethical approval for the study was obtained from the Greater Manchester Local Research Ethics Committee and the Scotland A Research Ethics Committee for the 60–64 years collection and from the Multicentre Research Ethics Committee for the 53 years collection. Written, informed consent was obtained from the study member for each component of each data collection.
Leucocyte telomere length assay
At both ages, DNA was extracted from frozen EDTA blood samples using Puregene DNA isolation kits (Flowgen, Leicestershire, UK).8 LTL at both ages was measured in the same laboratory according to a previously validated real-time polymerase chain reaction technique in a blinded fashion.9 More details on the method used to measure LTL are reported in the Supplementary material online.
Vascular phenotype
The right and left common carotid arteries (cIMT) were imaged longitudinally, 1 cm proximal to the carotid bifurcation following a standardized protocol.10 All measures were undertaken using an ultrasound scanner (Vivid I or Vivid 7, GE Healthcare) with a high-resolution probe (12 MHz). More details on the methods used for image acquisition and analysis are reported in the Supplementary material online.
Cardiac phenotype
Echocardiography was performed by a trained, experienced sonographer using GE Vivid I machines. Echocardiographic images were obtained from parasternal long-axis and short-axis, apical five-chamber, four-chamber, three-chamber, two-chamber and aortic views along with conventional and tissue Doppler in the four-chamber view. Image analysis, including wall and chamber measurements for the evaluation of left ventricular mass, ejection fraction, and diastolic function, was undertaken in a single core laboratory according to ASE/EAE guidelines11 by three experienced readers blinded to patient identity using the GE EchoPac software (GE CT, USA). The left ventricular mass (LVM) was indexed to the body surface area (LVM/BSA).
Quality assurance of echocardiography was performed throughout the study and blind duplicate reading reproducibility studies were carried out to establish inter- and intra-reader reliability. These showed excellent reproducibility (intra-class correlation coefficients were >0.9).
Statistical analysis
Linear regression models were used to investigate the unadjusted association between LTL at 53 years, LTL at 60–64 years and their difference with each measure of vascular (cIMT) and cardiac phenotype at 60–64 years. A series of multiple regression models were then fitted to examine whether the traditional CV risk factors and other potential confounders influenced any associations observed between LTL and the vascular and cardiac phenotypes. More details on the statistical methods are reported in the Supplementary material online.
Results
Descriptive statistics
Characteristics of the study population at both time points are shown in Table 1. Not all individuals with LTL at 53 years had a measure of LTL at 60–64 years. However, the characteristics at age 53 years of the groups with and without an LTL measure at 60–64 years were similar. Overall, the population was overweight with relatively high levels of systolic blood pressure and tendency to high levels of cholesterol and HbA1c. However, values of cIMT and cardiac measures were consistent with a relatively healthy population with largely normal left ventricular systolic and diastolic function, normal cIMT, and normal cardiac structure. Table 1S (Supplementary material online) shows the characteristics of the population based on tertiles of LTL changes. Only HDL-cholesterol varied significantly across the categories of rate of change in LTL.
Table 1.
Demographic, anthropometric, and biochemical parameters
| At 53 years (n = 2611) | At 60–64 years (n = 1207) | At 53 years with LTL measures at follow-up (n = 1033) | At 53 years without LTL measures at follow-up (n = 1602) | |
|---|---|---|---|---|
| Gender, male (%) | 1297 (49.7) | – | 488 (47.2) | 809 (51.3) |
| BMI, kg/m2 | 27.34 ± 4.65 | 27.93 ± 4.88 | 27.20 ± 4.46 | 24.40 ± 4.47 |
| Systolic BP, mmHg | 136 ± 20 | 137 ± 8 | 136 ± 20 | 136 ± 20 |
| Diastolic BP, mmHg | 84 ± 12 | 18 ± 10 | 84 ± 12 | 84 ± 12 |
| HbA1c, %a | 5.63 ± 0.69 | 5.81 ± 0.71 | 5.61 ± 0.57 | 5.70 ± 0.76 |
| Cholesterol, mmol/La | 5.99 ± 1.08 | 5.53 ± 1.19 | 6.08 ± 1.07 | 6.09 ± 1.09 |
| Triglycerides, mmol/La | 1.78 ± 1.49 | 1.16 ± 0.78 | 2.08 ± 1.42 | 2.16 ± 1.53 |
| HDL, mmol/La | 1.59 ± 0.48 | 1.54 ± 0.41 | 1.67 ± 0.47 | 1.66 ± 0.49 |
| cIMT, mm | – | 0.683 ± 0.129 | – | – |
| R_cIMT, mm | – | 0.666 ± 0.134 | – | – |
| L_cIMT, mm | – | 0.692 ± 0.151 | – | – |
| Ejec. Fr., % | – | 68 ± 10 | – | – |
| LA Dia., mm | – | 3.81 ± 0.57 | – | – |
| LVM/BSA, g/m2 | – | 92.46 ± 27.12 | – | – |
| E/A ratio | – | 0.97 ± 0.27 | – | – |
| LTL (bp) | 5646 ± 1925 | 4285 ± 1309 | 5553 ± 1733 | 5555 ± 1755 |
Values are presented as mean ± standard deviation.
aGeometric mean ± standard deviation or absolute number of participants and percentages (%).
cIMT, average carotid artery; R_cIMT, right carotid artery; L_cIMT, left carotid artery; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; LVM/BSA, left ventricular mass indexed to body surface area; E/A ratio, ratio between early and late mitral inflow velocity.
Associations between leucocyte telomere length at 53 and 60–64 years and cardiovascular phenotypes
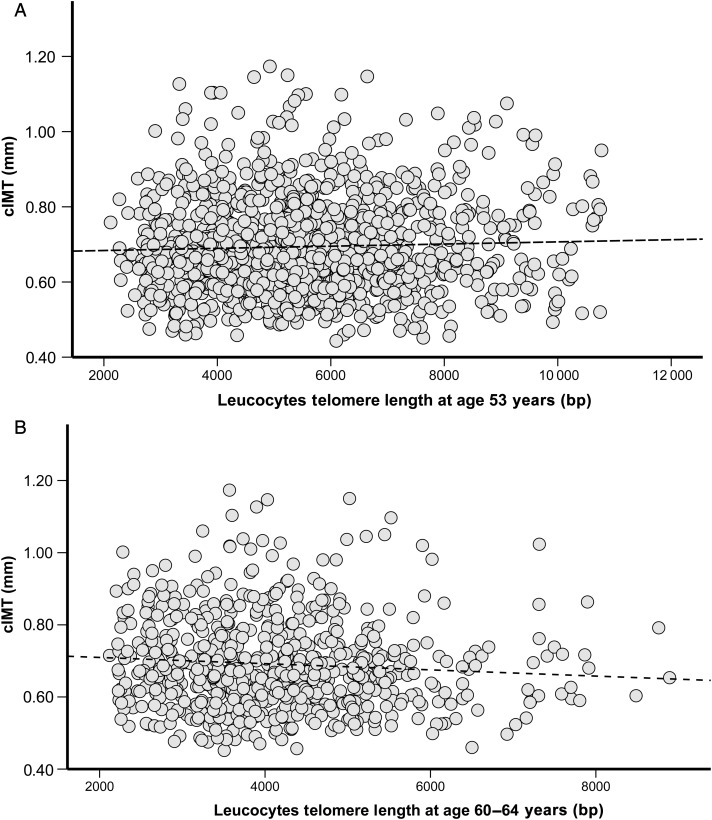
LTL at 53 years was not associated with vascular (cIMT) measures at 60–64 years (Figure 1A). Similarly, there was no evidence of associations between LTL at 53 years and any of the cardiac phenotypes at 60–64 years old (Supplementary material online, Table S1).
Figure 1.
Scatter plot of leucocyte telomere length (bp) measured at 53 years. (A) (R= 0.031, P= 0.271, n= 1261) and at 60–64 years (B) (R = −0.075, P= 0.046, n= 717) against values of carotid intima-media thickness (mm) measured at 60–64 years. Spearman rank correlation analyses were performed. The line represents the regression line for the unadjusted analysis.
At the age of 60–64 years, LTL was inversely associated with cIMT [regression coefficient (β) = −0.017 mm per 1 standard deviation (SD) LTL; 95% confidence interval (CI): −0.031, −0.003; P = 0.015; R2 = 0.005] (Figure 1B and Supplementary material online, Table S2). The association was greatly attenuated in the adjusted model (Model 2) (total cholesterol, systolic blood pressure at age 60–64, socio-economic status, medications use, clinic centre, and past history of myocardial infarction) (β = −0.008; 95% CI: −0.023, 0.006; P = 0.257; R2 = 0.07). Addition of LTL measure at 60–64 years in the fully adjusted model (model 2) did not substantially increase the overall model fit (R2 change = 0.005, P = 0.134). No associations were observed between LTL at 60–64 years and cardiac measurements (Supplementary material online, Table S2).
Associations between longitudinal change of leucocyte telomere length and cardiovascular phenotypes
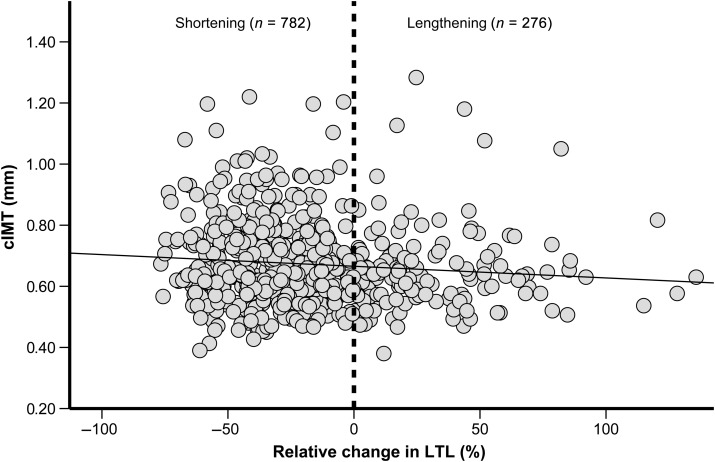
In 74% of participants, there was telomere shortening over the follow-up (Figure 2). A greater decrease in LTL change between the ages of 53 and 60–64 years was associated with thicker cIMT at 60–64 years old (unadjusted model: β = −0.020 mm per 1 SD decrease in LTL; 95% CI: −0.027, −0.005; P = 0.006; R2 = 0.008) (Table 2 and Figure 2). The strength of this association was not affected by multiple adjustment (model 3: β = −0.016, 95% CI: −0.029, −0.002; P = 0.022; R2 = 0.12) (Table 2). Addition of LTL change in the fully adjusted model (model 3) only slightly increased the overall model fit (R2 change = 0.007, P = 0.108). This association was linear so that the association with cIMT was observed in individuals with telomere elongation and those with telomere shortening. The right cIMT showed very similar findings, with the left showing a somewhat weaker association (Table 2). No associations were found between changes in LTL and cardiac phenotype measures of left ventricular mass, ejection fraction, and diastolic function (data not shown).
Figure 2.
Scatter plot of relative change in leucocyte telomere length (%) during the follow-up and values of carotid intima-media thickness at 60–64 years. Relative change in LTL = {[LTL (bp) at 60–64 years] – [LTL (bp) at 53 years]}/[LTL (bp) at 53 years] * 100. Values of carotid intima-media thickness are unadjusted and the reference dashed line separates individuals with telomere elongation from those with telomere shortening during the follow-up. Individuals with leucocyte telomere length shortening during the follow-up have increased values of carotid intima-media thickness at 60–64 years (R = −0.10; P = 0.01).
Table 2.
Mean difference (regression coefficient) of cardiovascular phenotypes per 1 SD higher change in leucocyte telomere length (change conditional to baseline leucocyte telomere length)
| cIMT (mm) |
R_cIMT (mm) |
L_cIMT (mm) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value | |
| Unadjusted | −0.020 (−0.027; −0.005) | 0.006 | −0.019 (−0.032; −0.006) | 0.004 | −0.014 (−0.027; −0.001) | 0.033 |
| Model 1 | −0.015 (−0.026; −0.003) | 0.011 | −0.019 (−0.032; −0.006) | 0.005 | −0.012 (−0.025; −0.001) | 0.068 |
| Model 2 | −0.017 (−0.030; −0.003) | 0.007 | −0.018 (−0.035; −0.005) | 0.009 | −0.014 (−0.029; −0.001) | 0.074 |
| Model 3 | −0.016 (−0.029; −0.002) | 0.022 | −0.017 (−0.033; −0.003) | 0.012 | −0.015 (−0.030; −0.001) | 0.052 |
LTL measures and all other confounders available in 672 participants at 53 and 64 years follow-up.
Generalized linear model; Model 1 = age difference in years, gender, smoking status 99, difference in BMI (99–09); Model 2 = Model 1+ cholesterol 2009, systolic BP 2009, socio-economic status, anti-inflammatory use, OAC, lipid lowering, clinic centre and history of MI; Model 3 = CVD risk SCORES (including age, gender, smoking, total- and HDL-cholesterol, systolic blood pressure) based on the SCORE charts, difference in BMI (99–09), socio-economic status, anti-inflammatory use, OAC, lipid lowering and clinic centre.
cIMT, average carotid artery; R_cIMT, right carotid artery; L_cIMT, left carotid artery.
Similarly, a greater shortening of LTL was associated with higher odds of increased cIMT [OR (95% CI) per one SD of LTL = 1.83 (1.03–3.25); P = 0.038]. Individuals diagnosed with carotid plaques had higher shortening of LTL during the follow-up compared with those without plaques (P < 0.01). Sensitivity analysis did not affect the results.
Discussion
This is the first study, to our knowledge, to explore the association between changes in LTL over 10 years and CV phenotypes. We found, using data from a nationally representative cohort of 60–64-year-old men and women, that a faster rate of decrease in LTL over 10 years was associated with increased cIMT. This association was seen in participants with both elongation and shortening of LTL and persisted after adjustment for potential confounders and mediators. Furthermore, single measures and longitudinal variations of LTL were not related with cardiac phenotypes. These results suggest that, over and above the contribution of classic CV risk factor exposure, a small proportion of the variation in vascular phenotype in late midlife relates to mechanisms regulating the rate of cellular ageing.
Several studies have reported cross-sectional associations between shorter LTL with subclinical and clinical markers of atherosclerosis.4,5 We have shown that the negative relationship between LTL and cIMT described in previous studies is likely to be consequent to a faster rate of LTL change during the lifespan. This suggests that, either regulation of LTL dynamics has a role in the evolution of vascular disease, or that the processes of cellular ageing and atherosclerosis are influenced by similar factors and proceed in parallel. A recent subanalysis of the Cardiovascular Health Study supports the first hypothesis, suggesting a causal relationship between ageing pathways and risk of CV disease.12 The ability of LTL to directly influence the evolution of vascular damage could lie on the unique feature of LTL to mirror telomere dynamics in haematopoietic stem cells (HSCs).13 As these cells also represent the haematological precursors of endothelial progenitor cells, a faster rate of LTL shortening may reflect a faster rate of telomere attrition in HSC reserves, ultimately resulting in a limited ability of the bone marrow to supply an adequate number of endothelial progenitor cells for effective reparation of vascular damage.14 On the other hand, several studies have supported the second hypothesis, suggesting oxidative stress as a potential mediator of the association between shorter LTL and higher levels of vascular damage. Oxidative stress exposure is currently considered the main driver of atherosclerosis.15 Similarly, an elevated burden of oxidative stress may result in a faster rate of LTL attrition by increasing the oxidative stress-mediated damage to the telomere sequence.16–18 Therefore, exposure to increased levels of oxidative stress may explain the parallel evolution of vascular damage and telomere attrition, potentially accounting for our findings.
Several studies have reported cross-sectional associations between LTL and smoking, obesity, and levels of insulin resistance.3 Consequently, researchers have suggested an increased CV risk factor burden could represent the primary mediator of the association between LTL and vascular phenotype, leading to a faster rate of LTL shortening and vascular remodelling. Although this hypothesis cannot be excluded, the few investigations that have analysed the determinants of LTL shortening in healthy and diseased populations found limited or no influence of CV risk factors levels on the rate of LTL attrition.19–21 This evidence raised doubts on the ability of traditional CV risk factors to effectively influence LTL dynamics and to mediate its association with CV phenotypes. Our study demonstrates that the association between LTL dynamics and markers of CV disease is unlikely to be dependent on traditional CV risk factor exposure, suggesting that other factors (such as oxidative stress or genetic mechanisms regulating the progression of cellular ageing) could drive this association.
Inconsistent associations have been reported between LTL and LVM or left ventricular function.22–24 We did not find associations between single measures and longitudinal measures of LTL and cardiac phenotypes. Our results are in line with current understanding of the LTL biology and the processes regulating the mechanisms of myocardial remodelling. Given that the haematopoietic system is the most proliferative among human tissues, age-dependent telomere shortening in HSCs, as expressed in LTL dynamics, is unlikely to reflect the evolution of the ageing process of cells with slow turnover rate, such as cardiomyocytes. Our findings support this hypothesis and provide the first epidemiological evidence of a possible different role of LTL biology in the evolution of cardiac and vascular remodelling. In keeping with our results, previously reported data from a meta-analysis including NSHD did not documented associations between LTL and clinical measures of muscular ageing, such as peripheral muscle strength.25
The MRC NSHD has a number of strengths for the investigation of LTL biology and its relationship with CV risk factors and phenotypes. Firstly, the 10-year follow-up between LTL measures and the relatively large number of participants included in the analysis represents the most important strengths. Indeed, the yearly rate of change of LTL is extremely low when compared to the ability of current LTL assays to detect small differences in telomere length.26 Consequently, changes in LTL over a long follow-up period and a large sample size are necessary to detect inter-individual variations in telomere attrition rates with confidence. Secondly, the acquisition of cIMT images was performed according to current guidelines.10 Furthermore, the use of automated edge detection programme has enabled a considerable reduction in differences between readers and has minimized the risk of change in reading behaviour over time (reader drift).27 The high interclass correlation between readers and the presence of an average cIMT in keeping with reference values confirm the quality of our cIMT acquisition and analysis protocols. While longitudinal studies have documented uncertain utility of repeated cIMT measures for prediction of CV outcome, a single measure of cIMT remains a useful tool to identify people at higher CV risk.28 The utility of this measure is strongly influenced by accuracy and standardization of the protocols for cIMT images acquisition and analysis.27 Finally, the availability of data on a wide range of established and novel CV risk factors and potential confounders at both ages, where LTL was measured allowed, for the first time, investigation of the independent contribution of LTL dynamics to CV phenotype.
Our report also has several limitations. The associations reported in this study are observational, and therefore no definitive conclusions can be made regarding causality. The proportion of cIMT variability explained by LTL dynamics is small and unlikely to be of clinical relevance. However, the aim of this paper was not to improve CV disease risk prediction by addition of a longitudinal measure of cellular ageing (such as LTL) to common CV risk factors, but to explore its relationship with a measure of vascular remodelling (surrogate outcome), to unravel possible new mechanisms of progression of atherosclerosis (i.e. biological pathways regulating cellular ageing). Furthermore, it should be highlighted that also the fully adjusted model including the continuous variable of future CVD risk (calculated with the SCORE charts) explains only a small proportion of the variability of cIMT in our population. This could be explained by the fact that our cohort comprises mainly CVD-free participants (as suggested by the normal average values of cIMT). Consequently, if LTL marks the residual ability to repair the vascular damage, a relatively low CV risk factor burden is likely to have underestimated the possible contribution of LTL dynamics to the cIMT variability in our analyses. Attrition is unavoidable in long-running studies such as NSHD, but our previous analyses have shown that the samples at 53 and 60–64 years remained broadly representative of the British born population of that age.29,30 Furthermore, we showed that the characteristics at age 53 years of the groups with and without an LTL measure at 60–64 years were similar and thus any drop out between the two ages is likely to have had a minimal effect on our findings. We did not measure telomere length in vascular cells but only in leucocytes. This is likely to have weakened the association between LTL dynamics and cIMT. However, Wilson et al.31 previously reported that LTL predicts vascular telomere length in humans with and without vascular disease and a strong synchronization between LTL and telomere length of other tissues was reported also by other authors.13,32,33 We used measures of cardiac remodelling that are strongly influenced by values of blood pressure. This might have reduced our ability to detect associations between LTL and cardiac phenotypes. However, our results raise further doubts on the associations between LTL and LVM, previously reported in cross-sectional studies.23,24 Finally, it is now well established that the delicate balance between injurious and repairing factors determines the level of genomic instability in each individual as well as his/her risk of developing age-related diseases, such as CV disease.34 In our paper, we measured a marker of cellular ageing which represents only one of the multiple pathways contributing to genomic instability. Larger longitudinal studies will need to confirm our results and address whether the combination of multiple ageing markers can better define the role of cellular ageing in the evolution of CV disease.
Conclusions
Our results suggest that previously reported cross-sectional associations between short LTL and measures of subclinical atherosclerosis are likely dependent upon a faster rate of LTL attrition. Importantly, the association was only partially confounded by exposure to CV risk factors. This suggests that, over and above chronological age and CV risk factors, a small proportion of vascular but not cardiac remodelling could be explained by mechanisms regulating the rate of progression of cellular ageing during lifespan.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the Medical Research Council (R.H., A.W., and D.K.) (programme numbers MC_UU_12019/1, MC_UU_12019/2) and by researchers at the National Institute for Health Research University College London Hospitals Biomedical Research Centre (S.M.) (fellowship number BRC135/CM/SG). S.M. is funded by ‘Il Circolo’, ‘Rosetrees Trust’ and holds the ‘European Research Grant in Hypertension’ from Servier and the European Society of Hypertension. F.D. holds a Clinical Senior Lectureship Award supported by the UK Clinical Research Collaboration. F.D., S.M., T.K., and J.D. work at UCL, which received a proportion of funding from the Department of Health's National Institute of Health Research (NIHR) Biomedical Research Centres funding scheme. J.D. is a British Heart Foundation Chair holder. A.H. received support from a Department of Health's National Institute of Health Research (NIHR) Biomedical Research Centre Award to Imperial NHS Healthcare Trust and a British Heart Foundation Research Centre Excellence Award to Imperial College London. Funding to pay the Open Access publication charges for this article was provided by the UK Research Council (RCUK).
Conflict of interest: none declared.
Acknowledgements
The authors are grateful to NSHD study members who took part in this latest data collection for their continuing support. We thank members of the NSHD scientific and data collection team at the following centres: MRC Unit for Lifelong Health and Ageing; MRC Lifecourse Epidemiology Unit, University of Southampton; MRC Human Nutrition Research, Cambridge; Wellcome Trust (WT) Clinical Research Facility (CRF) Manchester and the Department of Clinical Radiology at the Central Manchester University Hospitals NHS Foundation Trust; WTCRF and Medical Physics at the Western General Hospital in Edinburgh; WTCRF and the Department of Nuclear Medicine at University Hospital Birmingham; WTCRF and the Department of Nuclear Medicine at University College London Hospital; CRF and the Department of Medical Physics at the University Hospital of Wales; CRF and Twin Research Unit at St Thomas' Hospital London.
References
- 1.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 3.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- 4.Willeit P, Willeit J, Brandstatter A, Ehrlenbach S, Mayr A, Gasperi A, Weger S, Oberhollenzer F, Reindl M, Kronenberg F, Kiechl S. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30:1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell CJ, Demissie S, Kimura M, Levy D, Gardner JP, White C, D'Agostino RB, Wolf PA, Polak J, Cupples LA, Aviv A. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28:1165–1171. doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shetty P. Celebrating 65 years of the NSHD cohort. Lancet. 2011;377:802. doi: 10.1016/s0140-6736(11)60293-6. [DOI] [PubMed] [Google Scholar]
- 7.Kuh D, Pierce M, Adams J, Deanfield J, Ekelund U, Friberg P, Ghosh AK, Harwood N, Hughes A, Macfarlane PW, Mishra G, Pellerin D, Wong A, Stephen AM, Richards M, Hardy R. Cohort profile: updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol. 2011;40:e1–e9. doi: 10.1093/ije/dyq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau K, Vinall LE, Butterworth SL, Hardy RJ, Holloway J, Wadsworth ME, Swallow DM. MUC7 haplotype analysis: results from a longitudinal birth cohort support protective effect of the MUC7*5 allele on respiratory function. Ann Hum Genet. 2006;70(Pt 4):417–427. doi: 10.1111/j.1469-1809.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Ruiz CM, Gussekloo J, van HD, von ZT, Westendorp RG. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 10.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Burnett-Hartman AN, Fitzpatrick AL, Kronmal RA, Psaty BM, Jenny NS, Bis JC, Tracy RP, Kimura M, Aviv A. Telomere-associated polymorphisms correlate with cardiovascular disease mortality in Caucasian women: the Cardiovascular Health Study. Mech Ageing Dev. 2012;133:275–281. doi: 10.1016/j.mad.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura M, Gazitt Y, Cao X, Zhao X, Lansdorp PM, Aviv A. Synchrony of telomere length among hematopoietic cells. Exp Hematol. 2010;38:854–859. doi: 10.1016/j.exphem.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aviv A, Levy D. Telomeres, atherosclerosis, and the hemothelium: the longer view. Annu Rev Med. 2012;63:293–301. doi: 10.1146/annurev-med-050311-104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 16.von ZT, Serra V, Lorenz M, Saretzki G, Lenzen-Grossimlighaus R, Gessner R, Risch A, Steinhagen-Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80:1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- 17.von ZT. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 18.Masi S, Salpea KD, Li K, Parkar M, Nibali L, Donos N, Patel K, Taddei S, Deanfield JE, D'Aiuto F, Humphries SE. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med. 2011;50:730–735. doi: 10.1016/j.freeradbiomed.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS One. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collerton J, Martin-Ruiz C, Kenny A, Barrass K, von ZT, Kirkwood T, Keavney B. Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ study. Eur Heart J. 2007;28:172–176. doi: 10.1093/eurheartj/ehl437. [DOI] [PubMed] [Google Scholar]
- 23.Kuznetsova T, Codd V, Brouilette S, Thijs L, Gonzalez A, Jin Y, Richart T, van der Harst P, Diez J, Staessen JA, Samani NJ. Association between left ventricular mass and telomere length in a population study. Am J Epidemiol. 2010;172:440–450. doi: 10.1093/aje/kwq142. [DOI] [PubMed] [Google Scholar]
- 24.Vasan RS, Demissie S, Kimura M, Cupples LA, White C, Gardner JP, Cao X, Levy D, Benjamin EJ, Aviv A. Association of leukocyte telomere length with echocardiographic left ventricular mass: the Framingham heart study. Circulation. 2009;120:1195–1202. doi: 10.1161/CIRCULATIONAHA.109.853895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner MP, Martin-Ruiz C, Cooper R, Hardy R, Sayer AA, Cooper C, Deary IJ, Gallacher J, Harris SE, Shiels PG, Starr JM, Kuh D, von ZT, Ben-Shlomo Y. Telomere length and physical performance at older ages: an individual participant meta-analysis. PLoS One. 2013;8:e69526. doi: 10.1371/journal.pone.0069526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 27.Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S. Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J. 2013;34:2368–2380. doi: 10.1093/eurheartj/ehs380. [DOI] [PubMed] [Google Scholar]
- 28.O'Leary DH, Bots ML. Imaging of atherosclerosis: carotid intima-media thickness. Eur Heart J. 2010;31:1682–1689. doi: 10.1093/eurheartj/ehq185. [DOI] [PubMed] [Google Scholar]
- 29.Stafford M, Black S, Shah I, Hardy R, Pierce M, Richards M, Wong A, Kuh D. Using a birth cohort to study ageing: representativeness and response rates in the National Survey of Health and Development. Eur J Ageing. 2013;10:145–157. doi: 10.1007/s10433-013-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadsworth ME, Butterworth SL, Hardy RJ, Kuh DJ, Richards M, Langenberg C, Hilder WS, Connor M. The life course prospective design: an example of benefits and problems associated with study longevity. Soc Sci Med. 2003;57:2193–2205. doi: 10.1016/s0277-9536(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 31.Wilson WR, Herbert KE, Mistry Y, Stevens SE, Patel HR, Hastings RA, Thompson MM, Williams B. Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur Heart J. 2008;29:2689–2694. doi: 10.1093/eurheartj/ehn386. [DOI] [PubMed] [Google Scholar]
- 32.Butler MG, Tilburt J, DeVries A, Muralidhar B, Aue G, Hedges L, Atkinson J, Schwartz H. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogenet. 1998;105:138–144. doi: 10.1016/s0165-4608(98)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, Skurnick J, Awad G, Aviv A. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]