Abstract
Human factors and ergonomics approaches have been successfully applied to study and improve the work performance of healthcare professionals. However, there has been relatively little work in “patient-engaged human factors,” or the application of human factors to the health-related work of patients and other nonprofessionals. This study applied a foundational human factors tool, the systems model, to investigate the barriers to self-care performance among chronically ill elderly patients and their informal (family) caregivers. A Patient Work System model was developed to guide the collection and analysis of interviews, surveys, and observations of patients with heart failure (n=30) and their informal caregivers (n=14). Iterative analyses revealed the nature and prevalence of self-care barriers across components of the Patient Work System. Person-related barriers were common and stemmed from patients’ biomedical conditions, limitations, knowledge deficits, preferences, and perceptions as well as the characteristics of informal caregivers and healthcare professionals. Task barriers were also highly prevalent and included task difficulty, timing, complexity, ambiguity, conflict, and undesirable consequences. Tool barriers were related to both availability and access of tools and technologies and their design, usability, and impact. Context barriers were found across three domains—physical-spatial, social-cultural, and organizational—and multiple “spaces” such as “at home,” “on the go,” and “in the community.” Barriers often stemmed not from single factors but from the interaction of several work system components. Study findings suggest the need to further explore multiple actors, context, and interactions in the patient work system during research and intervention design, as well as the need to develop new models and measures for studying patient and family work.
Keywords: Work system, healthcare, patients, heart failure, aging
“[C]linicians are not the only actors in health care; patients also play an important role in their own care… the patient’s work must be examined in our efforts to reduce errors.”
(Unruh & Pratt, 2007, p.S236)
1. Introduction
The healthcare industry undeniably recognizes, even embraces, the human factors/ergonomics (HFE) discipline, its concepts, and methods (Carayon et al., 2014; Hignett et al., 2013; Russ et al., 2013). HFE approaches to safety management, human-computer interaction, teamwork training, and design have become valued tools in international campaigns to improve the safety and quality of healthcare delivery since the turn of the century (Carayon, 2012; Institute of Medicine, 2000; Vincent, 2006; World Health Organization, 2009) and in some cases earlier (Weinger et al., 1994; 1998).
In a recent paper, Holden and colleagues (2013) argued that maintaining HFE’s perceived value to an industry depends on the discipline’s ability to support the industry’s evolving practices and priorities. Addressing HFE in healthcare specifically, they and others (Unruh & Pratt, 2007; Vincent & Coulter, 2002) underscore the evolving role of the patient from passive recipient of care to “actor.” The authors accordingly promote a branch of HFE that they call patient-engaged human factors, or the application of human factors theories and principles, methods and tools, analyses, and interventions to study and improve work done by patients and families, alone or in concert with healthcare professionals (Holden, Carayon, et al., 2013; Holden & Mickelson, 2013).
1.1. Studying the patient work system: Toward patient-engaged human factors
A majority of HFE applications in healthcare target “professional work,” or “work in which a healthcare professional or team of professionals are the primary agents, with minimal active involvement of patients, family caregivers and other non-professionals” (Holden et al., 2013, p.1676). Nevertheless, there are many good examples of HFE applied to the work of unpaid individuals, including patients (Fisk et al., 2009; Lippa et al., 2008; Morrow et al., 2005; Pak & McLaughlin, 2011). This means that there are already HFE models and tools available to support patient-oriented research and interventions but that they need to be better advertised and more widely applied in the healthcare arena. In this paper, we apply one of HFE’s foundational tools, the systems model (Carayon, 2006), to investigate the factors shaping self-care performance among elderly heart failure patients and their informal caregivers.
1.1.1. Self-care in chronic illness and heart failure
Chronic illness is a controllable, but not curable illness lasting more than one year that often limits activities of daily living and requires continuous medical attention (National Center for Health Statistics, 2013). Chronic illness is globally prevalent, especially among the elderly. In the US, 80% of older adults have at least one chronic disease and 50% have two or more, accounting for 75% of healthcare expenditures (Centers for Disease Control, 2009). Annually over half of all deaths in the US are related to chronic illness (Kung et al., 2008). Controlling and managing the symptoms and progression of chronic illness is hardly a task for clinical professionals alone (Bodenheimer et al., 2002) because it depends critically on the performance of recommended self-care behaviors such as medication taking and nutrition management by patients or their informal (lay) caregivers (for an HFE-oriented review, see Mitzner et al (2013)).
This study focuses on those managing heart failure, a chronic illness described in Table 1. Heart failure is a prevalent, costly, progressive illness characterized by impairment of the pumping or filling functions of the heart. This impairs the delivery of oxygen to the body (causing shortness of breath and fatigue) and limits the body’s ability to expel wastes, particularly water, whose accumulation can cause harm. Multiple self-care activities are recommended to heart failure patients. Adherence is limited, despite the designation of self-care as a Class I recommendation—i.e., having the highest benefit-to-risk ratio—in professional guidelines for managing heart failure (Yancy et al., 2013). Non-adherence is estimated at 40–60% for medication taking, 12–92% for dietary and fluid restriction, 25–88% for daily weighing, and 41–58% for exercise (Moser & Watkins, 2008; van der Wal et al., 2005; Wu et al., 2008). This is problematic because excessive fluid congestion can lead to sudden death and non-adherence is associated with increased mortality and hospitalizations, reduced quality of life, and decline in health status (Ditewig et al., 2010; Jovicic et al., 2006; Lee et al., 2009).
Table 1.
Heart failure and heart failure self-care.
Summary (Remme & Swedberg, 2001; Rich, 2001; Yancy et al., 2013)
|
Prevalence and costs (Chaudhry et al., 2010; Curtis et al., 2008; Go et al., 2014)
|
Recommended self-care behaviors (Riegel et al., 2011; Riegel et al., 2009; Yancy et al., 2013)
|
Several studies identify barriers to performing recommended heart failure self-care (McEntee et al., 2009; Oosterom-Calo et al., 2012; Siabani et al., 2013; Zavertnik, 2014). Most of the studied barriers are patient-related factors such as age, lack of knowledge, and low self-efficacy (Oosterom-Calo et al., 2012). Person-level characteristics of the informal caregivers who help co-manage the disease are rarely considered and relatively few studies address barriers associated with healthcare professionals (Siabani et al., 2013). Characteristics of self-care tasks (e.g., treatment complexity, regimen side-effects) and tools (e.g., medication packaging, documentation systems) are less commonly studied but quite pertinent to self-care (Wu et al., 2008). Contextual or “environmental” barriers have been studied with variable regularity and often reveal self-care difficulties due to lacking social, financial, and community resources (e.g., transportation, access to care) (Arbaje et al., 2008; McEntee et al., 2009). The emphasis on barriers related to patient characteristics may explain why so many heart failure self-care interventions involve education, intensified contact with clinicians, or both (Ditewig et al., 2010; Molloy et al., 2012). Interventions focused on redesigning the patient’s work and work system (e.g., beyond educating the patient) are rare and could be promoted by considering self-care from a whole-systems human factors perspective.
Another limitation of the literature on heart failure self-care barriers is the relative shortage of studies with elderly patients (Zavertnik, 2014). Further, quantitative studies have been limited in scope (i.e., measuring fewer barriers, concurrently) and ability to understand how barriers operate in practice. Qualitative studies, in contrast, have used general probes to elicit a broader range of barriers (e.g., Riegel & Carlson, 2002; van der Wal et al., 2010); however, these rarely probed about specific categories of barriers nor provided reliable information about barrier prevalence. Critically, no single empirical study has used a systems model to elicit barriers to heart failure self-care. This is problematic because systematic reviews that have used systems frameworks to synthesize the barriers literature clearly demonstrated that self-care performance is shaped by multiple factors at and above the individual level of analysis (McEntee et al., 2009; Wu et al., 2008). Furthermore, conceptual models of geriatric self-care recognize that self-care is shaped by an interaction of patient characteristics, home and community factors, aspects of the healthcare system, and tool design (Murray et al., 2004). Indeed, applying a human factors framework depicting the entire system in a single study has the added benefit of showing how multiple system factors combine and interact to shape self-care performance (Carayon et al., 2014; Holden, Carayon, et al., 2013). Accordingly, heart failure self-care is a fitting target for our present application of a human factors whole-systems model to understand the barriers to patient (and family)-engaged work performance.
1.1.2. Study objectives and conceptual model
This study aimed to apply a systems model to investigate patient work performance and more specifically to use a human factors systems model to understand the nature and prevalence of barriers to self-care performance by elderly heart failure patients and their informal caregivers.
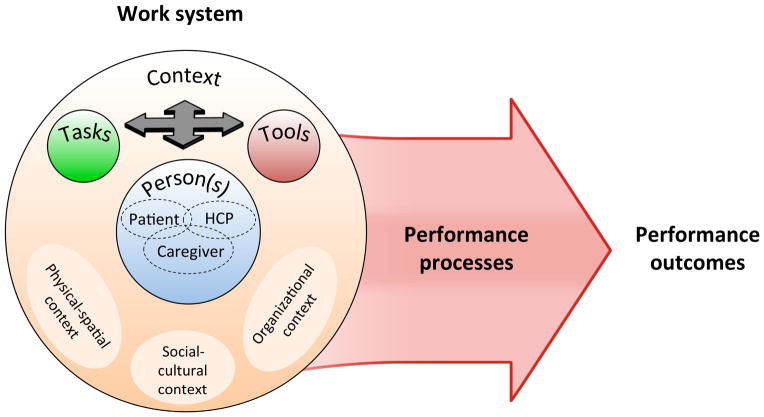
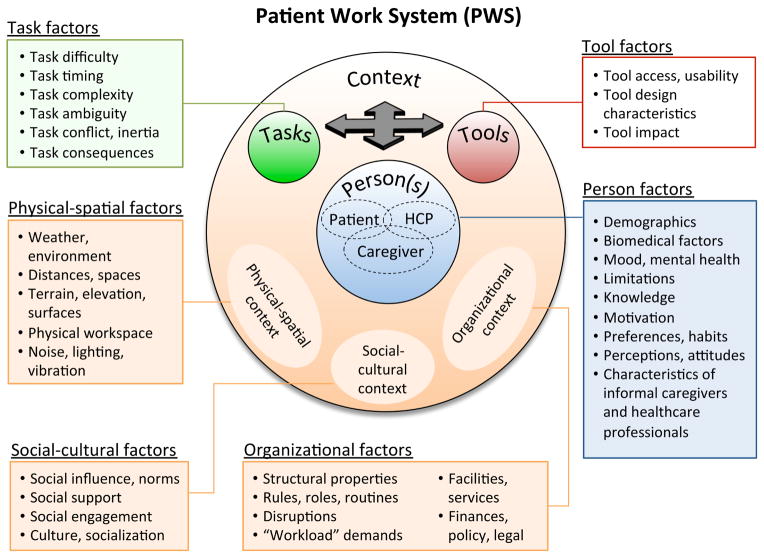
Figure 1 presents the Patient Work System model (PWS), integrating aging-specific (Fisk et al., 2009; Rogers & Fisk, 2010) and healthcare-specific (Holden, Carayon, et al., 2013; Karsh et al., 2006) human factors systems models. In PWS, work performance is shaped by four interacting components: Person(s); Tasks; Tools (or Technologies); and Context. Person(s) in PWS can be patients, healthcare professionals (HCPs), and informal caregivers—individuals who voluntarily carry out or assist patients in health- or disease-related activities. Context factors are ones that exist at higher levels of analysis, including physical-spatial, social-cultural, and organizational characteristics. Performance processes in the model refer to the physical, cognitive, and social-behavioral activities that are aimed at accomplishing a health-related goal or outcome. For patients, these might include purchasing, organizing, and taking medications; gathering information about a disease; communicating with a clinician; or purchasing groceries (Holden, Carayon, et al., 2013; Karsh et al., 2006).
Figure 1.
Patient Work System model (PWS) depicting interactions between person(s), tasks, tools, and context. Person(s) include patients, informal caregivers, and healthcare professionals (HCPs). Context includes physical-spatial, social-cultural, and organizational environments. In combination, the work system factors shape performance processes and outcomes.
It is important to go beyond this general model and further specify the nature and definitions of the factors in the PWS for specific patient groups (e.g., elderly heart failure patients) and instances of performance (e.g. self-care). Some have attempted to do so, drawing examples and suggesting definitions based on prior research (Henriksen et al., 2009; Holden, Carayon, et al., 2013; National Research Council, 2011; Zayas-Cabán & Valdez, 2012). In this study, we applied the general model in Figure 1 to gather and analyze empirical data from heart failure patients and their caregivers to further develop both the idea of the patient work system and to identify the work system factors specific to heart failure self-care. As a result, this paper produces a refined framework that is comprehensive, conceptually sound, empirically derived, and can be used across multiple studies. Notably, the framework is inclusive of individual-level and task, tool, and context-level factors, as well as the interactions between these factors. Therefore, when applied to study self-care performance, the framework and its development represent an advance over biomedical studies that have had a narrow focus on the factors shaping self-care (i.e., mostly individual-level ones) and have not used or produced a distinct framework of self-care barriers that can be used across multiple studies.
We note two additional novel aspects of this work. First, the notion of patients performing work shaped by a work system is not trivial: it represents a different way of thinking about who is involved (patients and caregivers), what they do (goal-driven work not compliance), the cause of patient outcomes (poor work system design not personal failings), and the useful avenues for intervention (whole-systems redesign to support work not individual-level reinforcement of knowledge or motivation) (Valdez et al., in press). Second, our research approach combines thick description with quantification of prevalence, rare in the heart failure self-care literature: one review reports 3% of studies (2/60) using mixed methods versus 82% (49/60) purely quantitative and 22% (13/60) purely qualitative (McEntee et al., 2009).
2. Methods
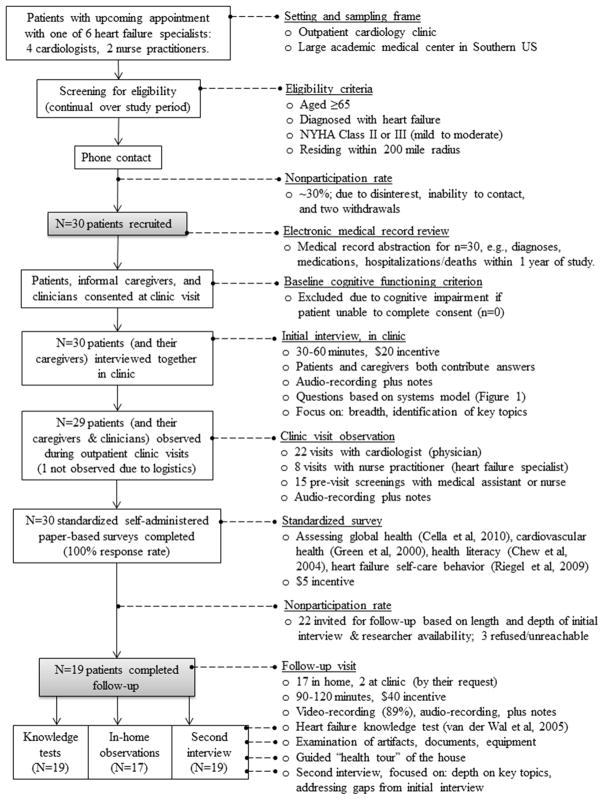
Data were collected from and about elderly heart failure patients (N=30) and their informal caregivers (N=14) using research interviews, observations, surveys, and medical record review (see Figure 2). Multiple and mixed methods were used to achieve a triangulated (i.e., multi-source, multi-perspective) understanding of the patient-engaged work system (Cresswell & Plano Clark, 2011). Semi-structured interviews yielded the largest and most useful set of data and therefore were the primary source for model development, with other data sources used in a complementary fashion. Initial interviews had the same general set of items, augmented with ad hoc probes, while follow-up interviews had some standard items and some questions that were created specifically for that participant, based on their initial interview responses (see Appendix A). In-clinic visit observations were focused on learning the content of self-care related information exchanged between patients, caregivers, and clinicians during standard follow-up cardiology clinic visits; observation notes included information not captured in audio-recordings (e.g., physical descriptions) and notes on topics to address in subsequent interviews. One or two researchers stood in the corner of the clinic room and made unobtrusive recordings. Surveys were handed to participants in-person. Most completed these at home and mailed back, although a small number of participants completed them during breaks in the first clinic visit. Patients could request help from others in completing the surveys, if they needed it. Knowledge tests were completed in front of the researcher during the follow-up interview.
Figure 2.
Flow diagram and details for study recruitment and data collection.
Data collection for each participant spanned on average one month, permitting: findings about changes; concurrent analysis; and exploration of key themes across encounters (Creswell, 2013). In particular, while initial interviews were used to cover a large range of topics and to identify those that were particularly important, follow-up interviews were able to focus on changes since the last interview, filling in any topics that were not yet covered, and probing deeper into topics of particular importance for that participant. Data collection spanned September 2012–March 2013. The Vanderbilt University Institutional Review Board (IRB) approved the study.
Professionally transcribed data files were entered into and analyzed in NVivo 10 (QSR International). Additional observation notes were added to the NVivo files, while surveys and medical record data were separately reviewed and integrated into the analysis. The goals of data analysis were to identify and describe barriers across the patient work system (Figure 1). Barriers were defined as “any work system property or condition that makes it impossible, difficult, or unsatisfying to perform self-care work in a timely and effective way” (adapted from Holden et al (2008; 2013). Barriers could be directly reported by participants or inferred from data. For each barrier category, we calculated the percent mentioning a barrier in that category as well as the number of references to that barrier category, defined as nonduplicative mentions of a barriers within that category across all participants. Distinct barriers in the same category (e.g., trouble hearing, trouble seeing) described by a single participant were each counted as references. However, an attempt was made to avoid counting multiple references to the exact same barrier for a given participant. Any additional information provided about a given barrier was added to sub-codes created specifically to house additional content but not double-counted.1 Patients and caregivers rarely openly disagreed about substantive content, though caregivers sometimes mentioned barriers that patients did not.
Table 2 further describes the analysis process. Analysts assigned and reassigned codes to segments of data judged as identifying a barrier (Miles et al., 2014). A stepwise analytic process was used, with earlier steps focused on identifying and broadly coding barriers, while later steps focused on further developing the conceptual model, identifying patterns, and then returning to the original data to revise the coding (Saldaña, 2013). Our data was the primary driver for decision making about how to define and group barrier subcategories, but existing work system models (Boehm-Davis, 2005; Fisk et al., 2009; Holden, Carayon, et al., 2013; National Research Council, 2011) were consulted when data were insufficient to guide those decisions.
Table 2.
Stepwise, iterative, and model-based data analysis process.
Step 1. Orientation to data
|
Step 2. Preliminary analytic model development
|
| Step 3. Preliminary codingLead author analyzes subset of data (initial interviews, n=15), assigning one or more codes, representing broad systems model categories, to each segment of data depicting a barrier. Lead author takes extensive notes on data content and coding challenges. |
Step 4. Further analytic model refinement
|
Step 5. Final coding of full data set
|
Step 6. Barrier category description and quantification
|
Three researchers carried out data analysis: the lead author and two (Master’s and PhD-level) co-authors. They represented diverse disciplines: human factors engineering and psychology (RJH), social science (CSS), and nursing (RSM). Several actions were taken to ensure convergence between analysts. First, the lead author trained the other analysts. Multiple pre-analysis readings and exercises were used to establish common theoretical grounding. Second, the lead researcher was present at most initial (60%) and follow-up (87%) interviews and observations and involved other researchers in 14 “tandem” interviews for convergence purposes. Third, during analysis, the lead author provided overall direction, took ultimate responsibility for analysis, possessed executive decision-making authority, coded the largest subset of data, and conducted periodic and final spot checks of analyses.
Finally, all analysts participated in four Coding Discussions, involving the intermittent review of (a) coding categories and definitions, (b) examples of coded content for each category, (c) difficult-to-code cases, (d) coding discrepancies, and (e) coding strategies/techniques. Unlike post hoc inter-rater reliability measures, coding discussions are a proven technique for facilitating, as opposed to simply measuring, analytic convergence (Barbour, 2001; Berends & Johnston, 2005). Prior to the first coding discussion, each analyst independently coded data from three participants; the discussion then focused on comparing the lead’s coding to that of the other two analysts, with correction of both and the updating of the coding scheme and definitions. For the second and third discussion, each analyst independently coded a new set of data and discussion focused on refining problematic definitions, coding anomalous or ambiguous passages, combining or splitting coding subcategories, and updating the coding book accordingly. The final discussion focused on review of coding categories used with relatively high or low frequency, fine-tuning of definitions, and other real-time minor adjustment. Finally, each analyst reviewed their coding to comply with analytic decisions made at the final coding discussion. Between discussion sessions, analysts consulted with one another on an ad hoc basis.
3. Results
Table 3 reports the demographic characteristics of patient participants and their self-reported self-care adherence.2
Table 3.
Patient (N=30) demographics and self-reported self-care adherence.
| Age | M=74.0 (SD=6.5) (range 65–86) |
| Gender | 17 male/13 female (57%/43%) |
| Racea | 18 White non-Hispanic (60%), 10 Black (33%), 2 Mixed-race (7%) |
| Education | 10 (33%) completing 12 years, 11 (37%) >12 years, 9 (30%) <12 years |
| Household incomeb | 7 (25%) ≤ $15,000, 15 (53%) ≤ $25,000, 21 (75%) ≤ $50,000 (annual) |
| Marital status | 16 (53%) married, 7 (23%) widowed, 5 (17%) separated or divorced, 2 (7%) single |
| Years diagnosed with heart failureb | M=6.84 (SD=5.5) (range 1 month – 17 years) |
| Employmentb | 26 (87%) retired, 3 (10%) disabled/unable to work, 1 (3%) part-time |
| Insuranceb | 100% Medicare, 17% Medicaid, 10% military, 87% private supplement |
| Self-reported global health | Physical health: 33% “very good,” 50% “good,” 7% “fair,” 0% “poor” Mental health: 7% “very good,” 47% “good,” 27% “fair,” 20% “poor” Quality of life: 20% “very good,” 43% “good,” 20% “fair,” 10% “poor” |
| Heart failure type | 9 (30% systolic, 13 (43%) diastolic, 8 (27%) systolic/diastolic |
| Heart failure severityb,c | NYHA Class: 11 (37%) II or “mild,” 18 (60%) III or “mild/moderate” BNP: M=465.1, SD=552.8 |
| Comorbidities | 80% hyperlipidemia, 83% hypertension, 53% diabetes mellitus |
| Health literacy | 47% get help reading hospital materials “all of the time” 57% with problems understanding written medical information “all the time” |
| Hospitalization/deathb | 23% hospitalized ≤30 days prior to enrollment (30% 60 days prior) 20% hospitalized ≤30 days after enrollment (30% 60 days after) 6 deaths (20%) and 54 total ED/hospital events within 1 year of enrollment |
| Informal caregivers | 14 consented to participate: 6 spouses, 8 adult children |
| Patients’ self-reported self-care adherence | 31% sometimes forget to take medicine 17% do not weigh daily; 48% do not check ankles for swelling daily 30% report no/rare 30 minutes of exercise; 10% report no exercise 43% do not avoid dietary sodium on daily basis; 40% never/rarely ask for low-sodium items when eating out or with others |
Compared to 81% White patients in hospital’s volunteer registry;
If known;
NYHA Class=New York Heart Association functional classification (Class II is “mild,” with slight physical limitation and symptoms from ordinary exertion; Class III is “moderate” with marked physical limitation and symptoms from less than ordinary exertion); BNP = B-type Natriuretic Peptide, a biomarker for heart failure with higher values indicate progressing illness.
Data about barriers are presented in the text below, with percentages referring to the proportion mentioning or otherwise demonstrating a category of barriers. Percentages in parentheses refer to barrier prevalence among the 30 patients (caregiver-reported barriers are associated with their respective patient). Tables 4 – 7 list major barrier categories, their prevalence, and provide longer, illustrative quotes for each. When reporting quotes, we provide sub-scripted patient identifiers using an AGE/SEX/RACE format, with race designations of White (Wh), Black (Bl), and Mixed (Mix). Thus, a 66-year old black female is 66/F/Bl.
Table 4.
Person barriers, their definitions, prevalence, and illustrative quotes.
|
Demographic characteristics (41 references over 63% of participants) Individual qualities representing membership to segments of the population (e.g., age, race, sex).
|
|
Biomedical characteristics (440 references over 100% of participants) States and events related to health, disease, and medical condition.
|
|
Mood and mental health (70 references over 68% of participants) Problems of psychological or emotional well-being, including depression, anxiety, and negative emotion.
|
|
Limitations (87 references over 100% of participants) Stable constraints on functional, physical, and cognitive-perceptual ability.
|
|
Knowledge (240 references over 100% of participants) Gaps in awareness or familiarity with the precepts of heart failure disease and related self-care.
|
|
Motivation (20 references over 47% of participants) Lack of initial or sustained drive to accomplish general or specific goals.
|
|
Personal preferences and habits (142 references over 87% of participants) Relatively stable and familiar personal preferences and tendencies developed and routinized over time.
|
|
Perceptions and attitudes (131 references over 93% of participants) Specific beliefs and general evaluations of health, disease, self-care, treatment, others, and oneself.
|
|
Informal caregiver characteristics (41 references over 63% of participants) Characteristics (e.g., availability, limitations, knowledge) or behaviors of informal caregivers.
|
|
Healthcare professional characteristics (48 references over 73% of participants) Characteristics (e.g., availability, ability) or behaviors of informal caregivers.
|
Table 7.
Examples of barriers across context domains and spaces.
| Context domains | |||
|---|---|---|---|
| Context spaces | Physical-spatial | Social-cultural | Organizational |
| “in the home” | “(stairs are) a little difficult. I’d rather have a house on the same level.”73/F/Bl | “(husband) likes fried chicken. And that kind of throws us off, ‘cause I eat fried chicken with him.”74/F/Bl | “I take care of my family…I have two living with me, and uh by the time I get that done, my day’s gone.”82/F/Wh |
| “at a social event” | “my wife likes to go on cruises and unfortunately I’m a party pooper…on the big ship cruise you have to walk quite a distance.”81/M/Wh | “Some of the things that were harder to change like…going out and have a beer or something like that with the fellows.”66/M/Bl | “being retired on a limited income it’s kind of stopped some of that (socializing).”66/M/Wh |
| “in the clinic” | “this place is too big for me to walk over” (referring to distances between two clinics).80/F/Bl | “I had a couple of doctors I didn’t like much. Every time we went to them with something they’d say well this pill right here will take care of it.”68/M/Wh | “I got no money. I keep …spending it on doctor bills.”70/F/Wh |
| “in the community” | “They want me to walk every day back and forth. That’s fine. But it’s cold.”65/F/Bl | “I live in the government projects, and everybody’s always trying to play a little game with you.”74/M/Bl | “the little drug store we use is (…) and it’s not open on weekends.”68/F/Wh |
3.1. Person barriers – Patient (Table 4)
Person-patient barriers arose from relatively stable characteristic of the patient. These were found for all patient participants, with an average 46.9 references per patient.
3.1.1. Demographic characteristics
Patients self-reported age (56%) and genetics (20%) as explanations for their conditions and limited self-care ability. Age was commonly given as a reason to discontinue exercise or indulge (“I’m 73 years old and I don’t plan to deprive myself too much of anything”73/F/Bl).
3.1.2. Biomedical characteristics
Many described heart failure symptoms that were barriers to self-care insofar as fatigue (67%) and shortness-of-breath (67%) impeded physical performance (exercise, shopping, cooking), sleeping difficulties (30%) impeded cognitive tasks (medication management), and nausea or lack of appetite (27%) impeded nutrition. In sixteen (54%), those symptoms had worsened over time and many experienced cardiovascular events such as acute fluid overload (50%), coronary bypass surgery (40%), stent and angioplasty procedures (27%), and pacemaker insertions (40%). These, as well as other medical events (mentioned by 73%) such as infections (27%), resulted in disrupted routines and worsening self-care ability. Twenty-five (83%) mentioned chronic comorbidities such as diabetes (37%) or arthritis (27%) interfering with heart failure self-care (see also Table 3). For example, one patient described heart failure exacerbations from consuming extra liquids to treat a urinary tract infection: “I ended up having blood in the urine… they say drink lots of water… [as a result] I couldn’t breathe and I mean I had a heck of a time. So you’re damned if you do and you’re damned if you don’t.”74/M/Wh
3.1.3. Mood and mental health
The majority of mood and mental health barriers involved fear, anxiety, stress, and worry about the future state of their disease and its effect on their lives (40%) (e.g., “having heart failure, it’s real scary … life seem like it’s gonna shut down for you,”65/F/Bl). Feeling down and depressed (23%) influenced motivation to engage in self-care activities (“I just don’t feel like doing anything,”72/F/Bl). Patients expressed frustration (27%) about limitations imposed by the disease, and the continuous demands of self-care. Medical record review revealed several participants were prescribed antidepressant or antianxiety medications (see also Table 3).
3.1.4. Personal limitations
The most commonly mentioned barrier-producing limitations were functional (93%), predominantly difficulty walking (63%), standing (37%), and getting up from a chair or out of bed (27%). Loss of functional abilities required dependence on caregivers (e.g., “[spouse] does everything for me, except breath. I wished he could take over that,”74/F/Wh). Physical limitations (67%) included lack of stamina and strength (37%) and limitations resulting from musculoskeletal injury or disease process (30%). Cognitive-perceptual limitations were commonly described (73%) and primarily involved memory problems (53%) and the inability to concentrate and comprehend information (17%). One patient described her difficulty remembering medications when going out, “I’ll forget to bring ‘em with me... I’ll forget ‘em when I get home. I just go to sleep and forgetting about the night pills.”74/F/Bl Vision and hearing limitations (27%) were described as barriers to receiving and processing medical information.
3.1.5. Knowledge
Patients’ knowledge about heart failure self-care was the most frequent knowledge barrier (87 references over 24 (80%) of participants). Sixteen (53%) indicated a knowledge-gap about how to comply with dietary restrictions. Several believed that not adding table salt to food would eliminate dietary sodium intake and one described drinking lots of water to “wash away”74/F/Bl sodium after consuming fast food. In interviews, eight (27%) demonstrated limited understanding of fluid restriction. Of the 19 completing a standardized knowledge test, seven (37%) could not select from three choices the recommend daily fluid intake and four responded that one should drink “as much fluid as possible.” Further, 21% taking the knowledge test did not know that they should weigh daily and 21% did not correctly endorse the statement that a sudden increase in weight (>5lbs over 2–3 days) warrants contacting a clinician.
Participants also lacked disease and symptom knowledge (78 references over 23 (77%) participants). Over a third (37%) who took the knowledge test could not select among three options the correct definition of heart failure. In interviews, a larger proportion (53%) indicated that they were not able to recognize heart failure symptoms or if they did, were unaware of the symptom’s relationship to their illness. For instance, one participant readily described having heart failure symptoms of fatigue and shortness-of-breath that he attributed to problems with his back, stating “my heart is just fine.”79/M/Mix Several references to knowledge barriers were medication-related, including 37% not understanding the purpose of their medications and 30% voicing uncertainty about medication colors and names, dosage, timing, and refills. Among a majority (53%) of participants, we discovered deficits in sensemaking and problem solving, meaning that in novel situations such as experiencing new symptoms, participants struggled to make sense of what was happening and how to act.
3.1.6. Motivation
Some individuals expressed a general lack of motivation, including difficulty getting out of bed in the morning (17%), no interest in life (13%), and resignation to their illness (10%). Specific motivational issues included lack of self-discipline and self-reported laziness with respect to exercise (27%) and splurging or giving in to temptation (53%) with respect to eating forbidden foods or drinking larger amounts of liquids (“So whenever I get a urge or something, I just really do it”73/F/Bl). Few clearly articulated what specifically motivated successful self-care.
3.1.7. Personal preferences and habits
Current, sometimes longstanding, preferences and habits in several cases acted as barriers to optimal self-care. This included preferences for consuming fluids (47%) and high-sodium foods (50%), for example, “that’s one of the hardest things… I used to cover it in salt and no plain tomato is good.”67/F/Wh Six (20%) participants displayed a preference for independence that conflicted with getting help with their self-care. A son described his father79/M/Mix as “a very independent man” whose desires for control manifested in “playing with” (i.e., skipping) daily medications or not attending appointments that required “begging people” for a ride.
3.1.8. Perceptions and attitudes
A common barrier (40% mentioning) was the perceived unimportance of self-care or the deprioritization of self-care activities, particularly low-sodium diet (23%) and daily exercise (20%). Perceived lack of control over health and disease was also common, with several stating that their health was up to a higher being (23%), their clinicians (13%), or no one (20%). Likewise, a third of participants described avoidant attitudes toward their disease, while three (10%) acknowledged the disease but preferred to focus on living life rather than extending it (“I’d rather live and do what I want to for three days than to live three months and not be able to enjoy my life”68/M/Wh). Nine (30%) expressed negative attitudes toward medicine and clinicians (“some doctors tell you anything, you have to be careful,”65/F/Bl) and twelve (40%) toward assistive technology, due to disinterest (23%), lack of skills (17%), or lack of perceived usefulness (17%).
3.2. Person barriers – Other actors (Table 4)
Observations and interviews revealed an important but underappreciated fact: “self-care work” is distributed across multiple individuals besides the patient, most commonly the (lay) informal caregiver (National Research Council, 2011). In our study, fourteen (47%) patients had one or more caregivers to help with or directly complete tasks such as medication management, food purchasing and preparation, symptom monitoring, transportation, and communication with clinicians. Most caregivers were adult children living with or close to the patient (57%) but in some cases it was a spouse, grandchild, or multiple family caregivers. While typically helpful, informal caregivers were sometimes a source of self-care barriers. Furthermore, certain characteristics of healthcare professionals were identified as barriers to self-care.
3.2.1. Informal caregiver characteristics
Adult child caregivers were often busy, working people with families of their own and thus described as unavailable (75%). Adult children rarely described feeling overburdened by the caregiver role (13%), yet many cohabitating spouses mentioned the caregiver role as stressful and tiring (67%) (e.g., “I didn’t have any (blood sugar issues) till he got sick. The doctor said it was stress,” wife of 79/M/Wh). Caregivers themselves also had physical and cognitive limitations (54%) such as memory issues due to advanced age and medical conditions. Caregivers, like patients, sometimes lacked self-care knowledge and skill (69%), especially involving dietary and fluid limitations (39%). Some caregivers had a negative effect on the patient’s self-care by modeling unhealthy behavior (30%) and putting the patient in bad circumstances (46%), for example, by bringing unhealthy food into the house (e.g., [husband] likes fried chicken…. and that kind of throws us off cause I eat fried chicken with him”74/F/Bl). One patient’s son described “sneaking” salt into his mother’s food to make it taste better for her. We identified as a typical caregiver challenge balancing the desire to help with not imposing too much control (“[daughter]’s like the warden, I feel like I’m in prison”65/F/Bl) and the resultant risk of fostering over dependence (38%) (e.g., “I drug him out of bed every morning. He didn’t want, ‘oh I don’t feel like getting up’ so I made him,”wife of 79/M/Wh).
3.2.2. Healthcare professional characteristics
There were relatively few mentions of clinicians acting as barriers to self-care and particularly few specific to current as opposed to past clinicians. Nevertheless, some participants described clinicians’ lack of access and availability (27%), including appointment scheduling problems (10%), inability to access them between appointments (17%), and being unavailable during appointments (7%). Seven (23%) mentioned problems receiving information from clinicians, including the use of medical jargon and not being clearly informed about a condition.
3.3. Task barriers (Table 5)
Table 5.
Task barriers, their definitions, prevalence, and illustrative quotes.
|
Task difficulty (151 references over 93% of participants) Real or perceived excessive load demands or quantities of tasks or sub-tasks, relative to one’s resources.
|
|
Task timing (42 references over 60% of participants) Frequency, periodicity, ubiquity, duration, or presence of delays in a performed or recommended task.
|
|
Task complexity (122 references over 90% of participants) Heterogeneity or variability, task turbulence (e.g., frequent changes), and need for precision in a task.
|
|
Task ambiguity (85 references over 93% of participants) Degree of difficulty in accurately assessing some aspect of the task such as its state or performance.
|
|
Task conflict and inertia (82 references over 70% of participants) Conflicts or different levels of inertia associated with the goals and performance of different tasks.
|
|
Task consequences (216 references over 100% of participants) Real or perceived undesirable or inadequate results of a task on a person’s mind, body, health, or life.
|
Task barriers were those arising from characteristics of self-care activities. These were found for all participants, with an average 23.8 references per patient.
3.3.1. Task difficulty
Participants described the general difficulty of self-care tasks (70%): “caring for the heart is a hard job”65/F/Bl and “a struggle.”74/M/Wh Eleven (37%) stated that adhering to a sodium-restricted diet was regarded as difficult and overly restrictive (“[2000mg of daily sodium] ain’t very much,”son of 85/F/Wh), given that “everything has huge amounts of salt in it.”81/M/Wh Four (13%) described the difficulty of physical tasks. Other difficult tasks were fluid restriction, getting health information, and monitoring for symptoms. Three (10%) stated that life itself sometimes seemed overwhelming (e.g., “I get nervous and tense and I just…I just wonder if I’m just going to make it through … this.”67/F/Wh Nine (30%) described how certain tasks such as organizing medications were too difficult to do alone.
Task quantity barriers involved multiple objects or subtasks, particularly the number of medications, which was mentioned by 21 (70%) patients (e.g., “I’m on a bunch and like, I’m a walking drugstore,”74/F/Wh). Patients had between 3 and 28 active prescriptions (M=15.0, SD=5.7), taken at different times of the day or week, and often described as problematic: “it all adds up and it’s very easy to get confused.”81/M/Wh Two patients described intentional nonadherence due to the quantity of medications. Five (17%) described numerous clinical encounters, procedures, and professionals (“I got too many doctors,”65/F/Bl). Two patients mentioned the vast amount of health information, including on television and the Internet: “there’s more information out there than you can possible read and there’s more advice … than you can possibly do.”74/M/Wh
3.3.2. Task timing
Task frequency, periodicity, and ubiquity were barriers commonly associated with recurring tasks (50%). Five (17%) described “going to the bathroom every 5 minutes or so”84/M/Wh and waking up multiple times at night to urinate, caused by diuretic medications. Periodic medication taking was commonly mentioned (23%), with most self-administering medications two to four times per day, and more for those on insulin. Others mentioned the high frequency of daily symptom documentation, medical appointments, and trips to the grocery store (to buy fresh produce to accommodate a low-sodium diet). Task duration (13%) and delays or lag (20%) were also mentioned.
3.3.3. Task complexity
In half the participants, there was evidence of general task complexity, including complex medication schedules with multiple medications having multiple names, shapes, and colors (mentioned by 57%). Changes in task (57%) were especially common in medication management (43%), including new prescriptions, discontinuation of prescriptions, name and pill appearance changes (e.g., from brand name to generic), and changes to dosage or schedule. In several cases such changes resulted from a hospitalization. Other barriers included conditional properties (53%) such as taking medication in sequence or conditional on specific circumstances (e.g., “Right before I eat,”79/M/Wh) and precision requirements or little tolerance for deviation (30%). Task complexity barriers were often related to—perhaps a function of—other task characteristics such as timing, ambiguity, and task conflict.
3.3.4. Task ambiguity
Ambiguity, or lack of clarity or transparency, was commonly present in self-monitoring for symptoms (77%). Eighteen (60%) described difficulty associating symptoms such as shortness of breath, dizziness, swelling, sudden weight gain, or chest pain with acute heart failure exacerbation versus other possible causes. This made it difficult to decide whether to be concerned, seek help, and take additional medications. Eleven (37%) also had difficulty self-assessing their health longitudinally because some signs of heart function were less “perceivable” (“I don’t have that much wrong that I can see that is wrong with me far as my heart. I can’t see what it’s doing inside,”74/F/Bl). Ambiguities sometimes resulted in patients “experimenting” with food and medication, including doubling or skipping doses.
Six (20%) referred to the ambiguity of monitoring dietary sodium. Participants spoke of “hidden” sodium and that “there’s more in that food than you really realize.”husband of 74/F/Wh Ambiguity was especially pronounced when eating out. Several patients resorted to guessing and trial and error to determine sodium content (“I can’t know until I take the first bite you know?”74/F/Wh).
3.3.5. Task conflict and inertia
Task conflict (60%) often involved needing to decide between or balance two or more self-care behaviors. Fourteen (47%) participants or their clinicians described balancing fluid restriction and diuretics, on the one hand, with avoiding dehydration, on the other. Among them, six (20%) described how fluid restriction conflicted with another medical condition, such as a urinary tract infection or diabetes, that caused thirst or required extra fluid intake (e.g., “I get thirsty, ‘cause I do have allergies and sinuses,”67/F/Wh). Ten (33%) described balancing heart failure related dietary restrictions with personal dietary preferences, dietary restrictions for other conditions (e.g., gout, diabetes, high cholesterol), and medication-related restrictions. Six (20%) mentioned the conflict between the self-care regimen and life, in general, e.g., the difficulty of striking a balance between exercise, appointments, sleep, and a busy personal schedule.
Task inertia (40%) occurred when a self-care task opposed a strongly routinized behavior, particularly food preparation and consumption habits (27%). Three others described difficulty restricting fluid (“mom drinks a lot of water and enjoys it,”son of 85/F/Wh), while others described the temptation of drugs and alcohol.
3.3.6. Task consequences
Consequences on the body, health, and mind (97%) produced by self-care tasks were commonly mentioned. Of the 118 such references, about half were related to medication side-effects, including effects on kidney function, bleeding, bruising, constant urination and resultant sleep disturbances, metabolic disturbances, appetite, dehydration, gastrointestinal problems, fatigue and weakness, hair loss, weight gain, headaches, pain, depression and violent thoughts, and risk of falling. Three individuals described severe allergic reactions and ten described general side effects, for instance, “for a day you feel blah,”81/M/Wh or “all the medications that [doctor’s] put me on, it makes me sick.”82/F/Wh One-third of respondents described undesired side-effects, particularly pain (20%) and fatigue or weakness (13%), resulting from daily physical exertion or exercise. Others (13%) mentioned problems due to dietary restrictions (e.g., “I just shut down if I don’t [eat regularly],”69/F/Wh) or fluid restriction (20%) (e.g., “I stay thirsty,”80/M/Mix).
Consequences on life and daily routine (70%) were primarily related to medications. Many (57%) described how diuretics, which caused frequent urination, controlled their lives and made it difficult to leave the house or get enough sleep (“I’m up all night. I mean, right now it’s killing me. I’m getting up four or five times a night,”74/M/Wh). Enjoyment of life was compromised by both medications (“since I been taking some medicine again, I ain’t been right,”68/M/Wh) and sodium restriction (“Bland, [food]’s too bland,”74/F/Wh).
Other task consequences included perceived personal costs or burden imposed by self-care tasks, including daily tracking of vitals (“I feel like a secretary,”80/M/Mix), appointments (“no matter what time you come, it’s an all day trip,”80/M/Mix), and health-related activities of daily living (“It just tires me out to go to that store,”74/F/Wh). Twelve (40%) patients raised concerns about the general effectiveness of following their self-care regimen. One patient who was not aware that the purpose of daily exercise was to strengthen cardiac muscle stated, “I don’t know whether that’s gonna benefit me or not because of the fact that I’m old,”74/F/Bl and several were skeptical about the effect of medications (e.g., “Well, can you tell me for sure that Coumadin [a blood-thinner] does more than aspirin does?”68/M/Wh).
3.4. Tools and technologies (Table 6)
Table 6.
Tool barriers, their definitions, prevalence, and illustrative quotes.
|
Tool access and usability (70 references over 73% of participants) Availability, cost, accessibility, and ease versus difficult of use of a potentially useful tool.
|
|
Tool design characteristics (61 references over 80% of participants) Aspects of the tool’s design not strictly related to ease of use, e.g., accuracy, portability, and durability.
|
|
Tool impact (33 references over 67% of participants) Undesirable effects on or response by the user caused by use of a tool.
|
Tool and technology barriers were those related to the availability, design, or consequence of using some artifact in the course of self-care. These were found for 26 (87%) participants, with an average of 5.5 references per patient.
3.4.1. Tool access and usability
Participants described unavailability of computers (17%), appropriate mobility devices (10%), tools to measure and track vital signs (10%), a telephone or wireless connection for transmitting data (10%), or other self-care related equipment such as a pillbox or exercise bike. Cost (e.g., of broadband internet) was mentioned as a barrier to tool use by five (17%) participants. In nine (30%) cases, participants owned tools that they were not accessible when and where they were needed. For example, asked whether she weighs herself when visiting friends overnight, a patient replied, “If they have a scale. If they don’t, I don’t.”73/F/Bl Two participants were not aware of electronic tools available to them for medical information and communication with clinicians. Usability problems (50%) were also common. For example, three mentioned trouble weighing daily due to poor weight scale usability such as numbers that were too small.
3.4.2. Tool design characteristics
Problematic design characteristics included inaccuracy (40%), size and portability issues (33%), problems with durability (23%), lack of transparency (23%), and inappropriate design for self-care tasks (23%). Ten (33%) participants were using paper artifacts with visible design problems, including paper lists that were not up to date, misplaced, or smudged. One participant, when asked about her heart failure dietary (i.e., sodium) restrictions brought out and read from an outdated list of post-stroke dietary (i.e., soft foods) recommendations. Another said he stopped writing down his daily weights because he ran out of paper diary sheets. In eight cases (27%), electronic information systems were problematic, including (1) difficulty of retrieving accurate and understandable information from the internet (“you find a lot of quack stuff,”81/M/Wh) and (2) inaccurate or incomprehensible information in the clinic’s electronic health record (EHR) and patient portal systems. Another seven (23%) described design problems with tools used for assessing vitals, including poorly calibrated or unreliable blood pressure cuffs and weight scales.
3.4.3. Tool impact
Ten (33%) mentioned negative consequence of tools or technology, including frustration and discomfort using computers and the internet. In another ten, we found evidence for potential over dependence on tools, particularly the use of potentially out-of-date paper documents as an “official record” of one’s medications.
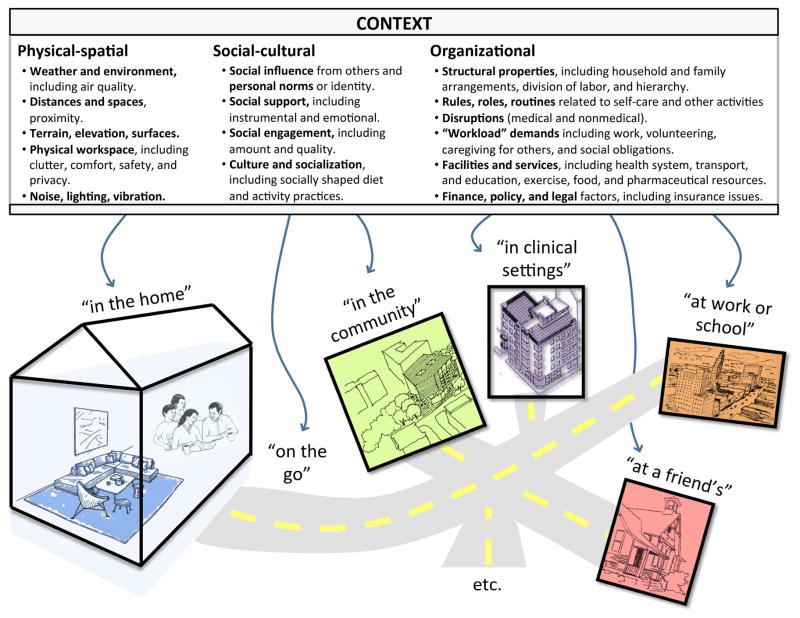
3.5. Context (Figure 3, Table 7)
Figure 3.
Context barriers, organized into three context domains and multiple context spaces.
Contextual or environmental barriers were coded in three “context domains”—physical-spatial (105 references over 87% of participants), social-cultural (149 references over 93% of participants), and organizational (603 references over 100% of participants)—defined in Figure 3.
We found that barriers in these categories separately and jointly influenced self-care in multiple “context spaces,” some of which are identified in Figure 3. Table 7 gives examples of how factors in the three context domains served as barriers in four context spaces: in the home, at a social event, in the clinic, and in the community.
3.6. Interactions
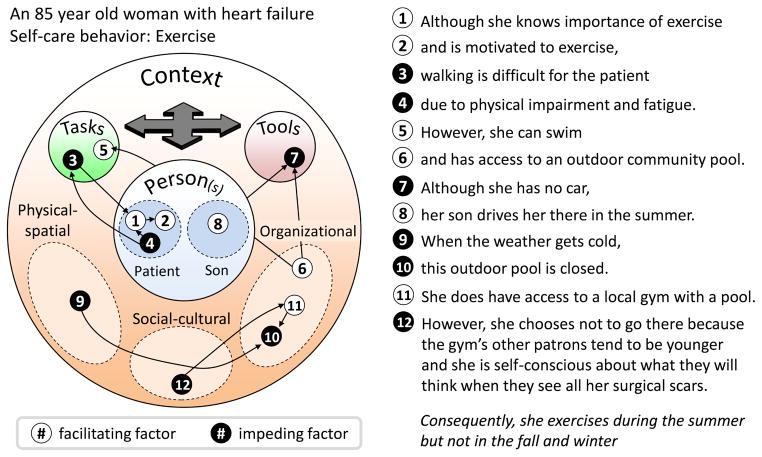
Interactions are the sine qua non of human factors systems models and in our analysis, many of the barriers listed under individual components such as “person” or “task” could in fact also be described as barriers arising from the combination of two or more factors. For example, barriers related to tool usability often represented tool design (small font) interacting with person factors (low visual acuity). Certain tasks characteristics such as walking distance and medication complexity were only barriers to self-care because of the presence of other factors such as physical or cognitive impairment. Sometimes many factors—hindering and facilitating—interacted to ultimately shape self-care performance, as seen in the case of one participant whose situation is depicted in Figure 4.
Figure 4.
An exemplar of a multi-factor interaction of facilitating and impeding conditions that ultimately result in difficulty performing self-care work. Arrows reflect interacting factors, while all the factors also interact to shape the ultimate behavioral outcome. Data, from an actual participant in this situation, are plotted on a “configural diagram,” adapted from Holden et al. (2013).
4. Discussion
This paper demonstrated one application of patient-oriented or patient-engaged human factors, an area of great importance as patients and families are expected to play a more active role in their health and healthcare (Dentzer, 2013). Many prior studies have applied work systems models to understand barriers to clinician performance (e.g., Carayon et al., 2014; Gurses & Carayon, 2007; Gurses et al., 2012; Holden, 2011; Pennathur et al., 2013; Wiegmann et al., 2010). However, ours applies a work system model or PWS to study person, task, tool, and context related barriers to the work performance of patients and informal caregivers. This is an important innovation as researchers increasingly acknowledge that what patients do resembles work activity (Granger et al., 2009) and can therefore be studied and supported using various ergonomic concepts, methods, and design strategies (Furniss et al., 2014). The PWS approach broaches the question, what kind of new design will be necessary, provided that patients and caregivers perform work? The approach also suggests consideration of the design implications of differences between professional work, including dissimilarity in supervision, accountability and legal responsibility, familial role distribution, reward and incentive structures, who suffers from performance breakdowns, control over design of the physical environment, the length of work “shifts” (e.g., 24 hours for patients), and more (Logan et al., 2013). For further discussion of the research and design implications of adopting a patient work lens, see Valdez et al. (in press) and Schubert et al. (in press).
Figure 5 presents a summary of the final Patient Work System model. The specific factors in the boxes in Figure 5 were derived based on empirical data but checked for consistency with existing definitions of professional and patient work systems and their subcomponents (Boehm-Davis, 2005; Fisk et al., 2009; Holden, Carayon, et al., 2013; National Research Council, 2011). Systems approaches have been called for in heart failure research (Retrum et al., 2013) and they promote systemic interventions in addition to educating, motivating, or reminding patients (Ditewig et al., 2010). Consequently, the model in Figure 5 can serve as the basis for such research and interventions, both in the area of heart failure self-care and for other activities carried out by patients and caregivers.
Figure 5.
The Patient Work System model (PWS), based on prior human factors systems models and adapted to depict the factors shaping patient work.
4.1. Person factors – beyond the individual patient
By taking a systems approach, our findings about barriers not only confirm but extend prior research on barriers to heart failure self-care performance. For example, Riegel and Carlson’s (2002) seminal interview-based study identified mostly person-level self-care barriers such as physical limitations, difficulty coping, knowledge deficits, distressed emotions, and multiple comorbidities, but fewer task, tool, or context barriers (e.g., difficulty affording medications). In particular, task complexity and related barriers (e.g., ambiguity, timing, conflict) identified here have not been well described in the past and the same is true of tool characteristics and organizational context barriers such as performance-compromising rules, roles, routines, and “workload” demands.
Like other prior studies, we found many person-level barriers. For example, the classic interview study by van der Wal, et al. (2010) also found several patient-related barriers such as forgetting and lack of knowledge, but also emphasized many task-related barriers related to the complexity or disruptiveness of the self-care regimen. Our analysis also identified many task-related barriers, as well as barriers related to tool and context factors that were not found in the above two studies. The innovation of looking across the entire patient work system to identify barriers to heart failure self-care is clearly illustrated by looking at two recent systematic reviews of predictors of heart failure self-care. In Oosterom-Calo et al.’s (2012) review of 26 quantitative studies, 180 variables were tested, but among them 74% (133) were related to disease or individual characteristics such as age, gender, knowledge, or disease etiology, 13% (24) were related to household living arrangements, marital status, or caregiver characteristics, and only 7% (12) were related to the task or context conditions such as regimen complexity or family income. In a broader review by McEntee et al. (2009), including qualitative, quantitative, and mixed-methods studies of barriers to heart failure self-care, patient-level barriers care were found in 75% of reviewed studies (45/60), clinician barriers in 38% (23/60), and contextual (or “systems-level”) in only 22% (13/60). Few single studies have assessed barriers at multiple levels or explain how multiple barriers interact, as shown in the PWS framework and other systems models (Murray et al., 2004; Wu et al., 2008). Note that the PWS framework does not exclude person characteristics, but rather stresses how these might interact with other factors to shape self-care performance. Indeed, our findings suggest that there are many person-level factors shaping performance and that these may become progressively more important with age, cognitive decline, and disease progression: future work might even assess how changes to person-level characteristics over time might alter the nature or distribution of a person’s work system and barriers therein. For example, with age and disease progression, certain tasks may become especially complex, tools may become more difficult to use, and the physical environment might become a greater impediment to self-care.
We also found that disease management was a collective effort (see also, Mickelson & Holden, 2013; Palen & Aaløkke, 2006), with informal caregiver and clinician team members’ characteristics sometimes acting as barriers. Further research must examine what makes such collaborative chronic illness “co-management” problematic versus a source of resilience (Schubert et al., in press). For example, assuming the role of an informal caregiving can have detrimental effects on the caregiver’s own physical and emotional health (Beach et al., 2000; Pinquart & Sörensen, 2003), which can negatively impact the patient. In a study by Beach et al. (2005), caregiver depression was associated with increased risk of patient mistreatment by the caregiver. Schulz et al. (2007) suggest caregiver well-being is related to the patient’s suffering, and the caregiver’s ability to successfully reduce it. The intertwined interaction between the patient and informal caregiver requires further research.
4.2. Task factors – entanglements and trade-offs
Medication management tasks were particularly problematic due to factors such as difficulty and complexity; they are thus a good target for HFE research and interventions (Morrow et al., 2005). While we reported barriers related to attributes of discrete self-care tasks such as medication taking, our data suggested that self-care is entangled, i.e., intertwined with other life activities (Corbin & Strauss, 1985): for example, medication taking was inseparable from diet, activity, and sleep, as seen in the number of references to self-care task consequences (see also Simpson et al., 2000; van der Wal et al., 2010). Self-care tasks sometimes created conflicting goals, as when diuretic medication keeps patients homebound or awake at night. Another type of conflict was the workload imposed by multiple self-care tasks on top of other life activities (Shippee et al., 2012). Patients stated that goal and workload conflicts forced a choice between recommended self-care and other goals, with some choosing comfort or enjoyment over adherence. This raises an important question, framed by Greenhalgh (2009) and others (e.g., Forsythe, 1996; Reuben & Tinetti, 2012) as whose goals and views should dictate treatment decisions, judgments of treatment effectiveness, and design? While the present study assessed performance relative to the biomedical goal of managing chronic illness through self-care adherence, future research could take a more sociological view by assessing performance relative to how well patients cope with disease or how well they balance the multiple demands of life (Corbin & Strauss, 1985; Greenhalgh, 2009). Views that consider coping and managing trade-offs correspond with modern views of resilience and safety-critical performance (Hollnagel, 2009; Hollnagel et al., 2006).
Generally, the prevalence and importance of task barriers in this study indicate the need to adapt techniques such as workload measurement and cognitive task analysis to the patient and caregiver domain so that task characteristics can be more systematically assessed.
4.3. Tool factors – usability (and beyond)
Consistent with HFE principles, tool barriers were not simply the lack of tools but also problems with their design and usability. This finding urges the application of standard HFE and aligned approaches such as user-centered design processes, usability testing, human-system integration, and implementation science (Fisk et al., 2009; Jacko, 2012; Karsh, 2004). These approaches have gained acceptance in the design of medical devices (Weinger et al., 2011), are gaining traction in health information technology design (Schumacher & Lowry, 2010), and are recommended for the design of patient-facing technology (Agarwal et al., 2011).
4.4. Key directions for the future and preliminary implications
Although here we did not provide thorough treatment of context and interaction barriers, these were numerous. Context and interactions are two of the most fundamental concepts in HFE (Wilson, 2014) and therefore bear further investigation in the patient (and caregiver) domain. Our study laid the groundwork for representing context and interactions (Figures 3 and 4), but further work is needed.
Considering the findings of this study, an obvious practical consideration is to look more closely at existing self-care interventions. Relatively few interventions address contextual conditions such as the physical, social, or organizational characteristics of the patient’s home or access to resources. Instead, most focus on education or providing additional support or contact from a healthcare professional or case manager (Ditewig et al., 2010; Molloy et al., 2012). Further, our findings suggest the need to better assess the value of multi-component interventions (Molloy et al., 2012). Such interventions might combine education on heart failure self-care (person), introduction of self-care strategies or changes to the drug regimen (task), novel tools and the provision of symptom-monitoring equipment (tools), and instrumental support and increased contact with the healthcare system (context) (see e.g., DeWalt et al. (2006)). Another implication is that if there are interactions between system factors, some interventions could be targeted, for example, introducing different medication management applications (tools) depending on levels of literacy (person), medication regimen complexity (task), and internet availability (context). Consider the complex case in Figure 4; while some patients in this woman’s situation would benefit from a free gym membership, her social context suggests an intervention that either allows her to exercise in her home or connects her to other gym members her age. Because a patient experiences multiple, especially interacting systems barriers, present findings have inspired us to conduct further research on and prototyping of rapid methods for assessing barriers and visualizing them for busy clinicians. Other findings imply practical considerations such as: developing interventions that target the patient-caregiver dyad not the patient alone, help patients integrate self-care tasks into their existing clinical and personal tasks, and introduce mobile applications that can be used in many diverse settings.
4.5. Methodological strengths and limitations
The study’s main strengths included the use of a conceptual model to guide data collection and preliminary analysis; the iterative nature of analysis, permitting model and coding refinements; the use of multiple mixed methods; a team of investigators with complementary disciplinary backgrounds; and a relatively large sample size for such in-depth research (which yielded over 1500 pages of transcripts). For comparison, in recent reviews of studies of heart failure self-care, 76–79% of studies that included interviews had a patient sample size smaller than ours and 39–47% had a sample size smaller than 20 (Harkness et al., in press; Siabani et al., 2013).
In presenting the data we endeavored to present a balance of prevalence across researcher-generated analytic categories and rich quotes and examples using the participants’ own words and ideas. While using multiple analysts is challenging due to the need for common grounding and analytic convergence, we agree with others (Barry et al., 1999) that when done right, it can be a major strength. To this end we used multiple techniques including coding discussions to manage our multi-analyst approach. Furthermore, our study benefited from having both quantitative-prevalence and qualitative-descriptive components: while quantitative analyses revealed barriers (e.g., knowledge deficits, task difficulty and consequences) that warrant further research purely on the basis of their prevalence, qualitative analyses provide more insight into the complexities of barriers. For instance, knowledge deficits were most pronounced and problematic when patients encountered novel situations such as new foods or ambiguous symptoms and needed to apply learned knowledge or identify functional relationships under time constraints (Horowitz et al., 2004; Riegel et al., 2013).
Study limitations included the only partially longitudinal design, with participant contact usually spread over only one month. Although this is better than most studies of self-care, which include only a single encounter, future research should extend the timeline to capture major changes and transitions. Not all barriers were immediately volunteered in response to general questions, with some barriers elicited through probes; thus, it cannot be said that all of the barriers coded for an individual were equally salient or would have been mentioned in a more open-ended interview format. This paper examined barriers but not facilitators, coping, resilience, or adaptive strategies. Data collection yielded numerous examples of these and further analysis is needed to demonstrate which work system factors and strategies result in successful performance. Participants were all volunteers receiving care at an outpatient cardiology clinic of an academic medical center, making them potentially different from the general population. We used targeted sampling to ensure representativeness on age, income, race, gender, education, and location but recognize that this did not eliminate the selection of individuals willing to engage in research and show up to at least one appointment. Furthermore, patients were relatively healthy compared to all symptomatic heart failure patients (note that we excluded both asymptomatic NYHA Class I and high-severity NYHA Class IV patients). While even these patients were sometimes quite ill and likely to experience hospitalization and death (see Table 3), we have conducted additional research with similar-sized cohorts of elderly patients who have been recently hospitalized (n=30) and all-age (and thus sometimes uninsured) patients who presented to the emergency department with acute decompensated heart failure (n=30). Future papers will compare barriers across these groups. The above restrictions of range as well as our sample size are particularly important to consider when interpreting prevalence data. Lastly, it is possible that some of the interview answers provided were influenced by the presence of a caregiver, social desirability effects, or cognitive-perceptual limitations of participants’ verbal communication.
Lastly, this study was carried out with elderly heart failure patients. It remains to be seen to what extent the model is transferable to other patient and disease groups and what adaptations might be needed. For example, while one could begin an investigation of adolescent Type I diabetes patients using the model in Figure 5, some of the subcategories may need to be changed, for example, to distinguish between social influence from peers vs. authority figures (teachers, parents). Some categories such as functional limitations may also not be as relevant in some cases as they are with elderly heart failure patients.
5. Conclusion
There is now no shortage of advocates for patients to be involved in their health and healthcare. However, there is inadequate understanding of the actual “work” that patients and their informal caregivers do or the “work systems” that shape their work performance. The human factors discipline has the tools and expertise needed to better understand patient work systems and performance in a way that is comprehensive, theory-based, and methodologically rigorous. Importantly, human factors experts also have an opportunity to be involved in the design of work systems that better support patient work and engagement in health and healthcare.
This study demonstrates one application of patient-oriented or patient-engaged human factors—the conceptualization and data-driven refinement of the “Patient Work System.” For human factors to continue to make valued contributions to the fields of health and healthcare in a new era of “patient engagement,” we must build on this example by adapting other human factors approaches—including workload assessment, cognitive task analysis, user-centered design, usability testing, and participatory process redesign—to study and improve work done by patients.
Highlights.
Patients’ and informal caregivers’ work performance was shaped by system factors.
Person factors included biomedical, psychological, and physical characteristics.
Task factors included task difficulty, complexity, timing, and consequences.
Tool factors included tool accessibility, usability, impact, and design.
Context domains were physical-spatial, social-cultural, and organizational factors.
Acknowledgments
We thank the participants in this study—the many patients and their family members who graciously welcomed us into their lives and homes and the cardiologists, nurse practitioners, nurses, and medical assistants who let us into their clinics. We thank Dr. Matt Weinger for his mentorship and feedback, Dr. Doug Sawyer for his mentorship and clinical insights, and Drs. Kevin Johnson, Russell Rothman, and Jack Schnelle for their mentorship and assistance. Several clinicians helped us to understand the clinical domain: Melissa Smith and Connie Lewis. Thank you to Amanda McDougald Scott and Courtney Thomas for assisting with data collection and brainstorming. We thank the three reviewers for providing excellent feedback. This study and RJH were sponsored by grants from the National Institute on Aging (NIA) of the US National Institutes of Health (NIH) (K01AG044439) and grants UL1 TR000445 and KL2 TR000446 from the National Center for Advancing Translational Sciences (NCATS/NIH) through the Vanderbilt CTSA.
Appendix A. Topics and scripted questions for (a) the typical initial interview and (b) a sample follow-up interview
Table 8.
| (a) Scripted questions used for initial interviews | |
|---|---|
| Topic | Example questions (edited for interviewer instructions) |
| Introduction and scoping |
|
| Medical condition(s) and knowledge (Person) |
|
| Self-care activities and knowledge (Task, Person) |
|
| Use of technology (Tools & Technologies) |
|
| Virtual health tour (Whole System) |
|
| Physical, social, and organizational environments of self-care (Context) |
|
| Strategies (Whole System) |
|
| Barriers (Whole System) |
|
| Wrap-up and general attitude (Person) |
|
Table 9.
| (b) Scripted questions for sample follow-up interview participant | |
|---|---|
| Topic | Example questions (edited for interviewer instructions) |
| Updates |
|
| More on strategies (Whole System) |
|
| More on social environment and caregivers (Context) |
|
| More on self-care activities (Task) |
|
| More on medications (Task, Whole System) |
|
| More on barriers (Whole System) |
|
| More on typical and atypical days (Whole System) |
|
| Health tour (Whole System) |
|
| More on technology use (Tools & Technology) |
|
| Additional items (Whole System) |
|
| Wrap-up and knowledge test (Person) |
|
Footnotes
Some participants did indeed mention the same barrier several times, judged in many cases to be a sign of the importance or severity of the barrier. A separate analysis could be conducted to identify those barriers mentioned several times by participants.
We compared the age, sex, and race of the 30 participants to those of 15 screened and eligible nonparticipants who were not contacted due to a scheduling conflict (n=10) or declined participation or could not be reached (n=5). Age, sex, and race did not significantly differ between the two groups, as tested by t-test and chi-square test (p’s≥0.05).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Preliminary findings from the study were presented at the 2013 annual meeting of the Human Factors and Ergonomics Society.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
WORKS CITED
- Agarwal R, Anderson C, Crowley K, Kannan PK. Understanding development methods from other industries to improve the design of consumer health IT: Background report. Rockville, MD: Agency for Healthcare Research and Quality; 2011. (Prepared by Westat, under Contract No. HHSA290200900023I.) AHRQ Publication No. 11-00650-EF. [Google Scholar]
- Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Postdischarge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling medicare beneficiaries. The Gerontologist. 2008;48:495–504. doi: 10.1093/geront/48.4.495. [DOI] [PubMed] [Google Scholar]
- Barbour RS. Checklists for improving rigour in qualitative research: A case of the tail wagging the dog? British Medical Journal. 2001;322:115–117. doi: 10.1136/bmj.322.7294.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry CA, Britten N, Barber N, Bradley C, Stevenson F. Using reflexivity to optimize teamwork in qualitative research. Qualitative Health Research. 1999;9:26–44. doi: 10.1177/104973299129121677. [DOI] [PubMed] [Google Scholar]
- Beach SR, Schulz R, Williamson GM, Miller LS, Weiner MF, Lance CE. Risk factors for potentially harmful informal caregiver behavior. Journal of the American Geriatrics Society. 2005;53:255–261. doi: 10.1111/j.1532-5415.2005.53111.x. [DOI] [PubMed] [Google Scholar]
- Beach SR, Schulz R, Yee JL, Jackson S. Negative and positive health effects of caring for a disabled spouse: Longitudinal findings from the caregiver health effects study. Psychology and Aging. 2000;15:259–271. doi: 10.1037//0882-7974.15.2.259. [DOI] [PubMed] [Google Scholar]
- Berends L, Johnston J. Using multiple coders to enhance qualitative analysis: The case of interviews with consumers of drug treatment. Addiction Research & Theory. 2005;13:373–381. [Google Scholar]
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA-Journal of the American Medical Association. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- Boehm-Davis DA. Improving safety and effectiveness in the home. Reviews of Human Factors and Ergonomics. 2005;1:219–253. [Google Scholar]
- Carayon P. Human factors of complex sociotechnical systems. Applied Ergonomics. 2006;37:525–535. doi: 10.1016/j.apergo.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Carayon P, editor. Handbook of Human Factors and Ergonomics in Patient Safety. 2. Mahwah, NJ: Lawrence Erlbaum; 2012. [Google Scholar]
- Carayon P, Wetterneck TB, Rivera-Rodriguez AJ, Hundt AS, Hoonakker P, Holden RJ, Gurses AP. Human factors systems approach to healthcare quality and patient safety. Applied Ergonomics. 2014;45(1):14–25. doi: 10.1016/j.apergo.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. Healthy aging: Improving and extending quality of life among older Americans: National Center for Chronic Disease Prevention and Health Promotion. 2009 Retrieved from: http://www.cdc.gov/nccdphp/publications/aag/pdf/healthy_aging.pdf.
- Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. Journal of the American College of Cardiology. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J, Strauss A. Managing chronic illness at home: Three lines of work. Qualitative Sociology. 1985;8:224–247. [Google Scholar]
- Cresswell JW, Plano Clark VL. Designing and Conducting Mixed Methods Research. 2. Thousand Oaks, CA: SAGE; 2011. [Google Scholar]
- Creswell JW. Qualitative Inquiry & Research Design. 3. Thousand Oaks, CA: SAGE; 2013. [Google Scholar]
- Curtis LH, Greiner MA, Hammill BG, Kramer JM, Whellan DJ, Schulman KA, Hernandez AF. Early and long-term outcomes of heart failure in elderly persons, 2001–2005. Archives of Internal Medicine. 2008;168:2481–2488. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentzer S. Rx for the ‘blockbuster drug’ of patient engagement. Health Affairs. 2013;32:202. doi: 10.1377/hlthaff.2013.0037. [DOI] [PubMed] [Google Scholar]
- DeWalt DA, Malone RM, Bryant ME, Kosnar MC, Corr KE, Rothman RL, Pignone MP. A heart failure self-management program for patients of all literacy levels: A randomized, controlled trial. BMC Health Services Research. 2006;6(30) doi: 10.1186/1472-6963-6-30. http://www.biomedcentral.com/1472-6963/1476/1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: A systematic review. Patient Education and Counseling. 2010;78:297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Fisk AD, Rogers WA, Charness N, Czaja SJ, Sharit J. Designing for Older Adults: Principles and Creative Human Factors Approaches. 2. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- Forsythe DE. New bottles, old wine: Hidden cultural assumptions in a computerized explanation system for migraine sufferers. Medical Anthropology Quarterly. 1996;10:551–574. doi: 10.1525/maq.1996.10.4.02a00100. [DOI] [PubMed] [Google Scholar]
- Furniss D, Barber N, Lyons I, Eliasson L, Blandford A. Unintentional non-adherence: Can a spoon full of resilience help the medicine go down? BMJ Quality & Safety. 2014;23:95–98. doi: 10.1136/bmjqs-2013-002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Turner MB. Heart disease and stroke statistics--2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger BB, Sandelowski M, Tahshjain H, Swedberg K, Ekman I. A qualitative descriptive study of the work of adherence to a chronic heart failure regimen: Patient and physician perspectives. Journal of Cardiovascular Nursing. 2009;24(4):308–315. doi: 10.1097/JCN.0b013e3181a4be30. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T. Chronic illness: Beyond the expert patient. BMJ. 2009;338:629–631. doi: 10.1136/bmj.b49. [DOI] [PubMed] [Google Scholar]
- Gurses AP, Carayon P. Performance obstacles of intensive care nurses. Nursing Research. 2007;56:185–194. doi: 10.1097/01.NNR.0000270028.75112.00. [DOI] [PubMed] [Google Scholar]
- Gurses AP, Kim G, Martinez E, Marsteller J, Bauer L, Lubomski L, Thompson D. Identifying and categorizing patient safety hazards in cardiovascular operating rooms using an interdisciplinary approach: A multisite study. BMJ Quality & Safety. 2012;21:810–818. doi: 10.1136/bmjqs-2011-000625. [DOI] [PubMed] [Google Scholar]
- Harkness K, Spaling MA, Currie K, Strachan PH, Clark AM. A systematic review of patient heart failure self-care strategies. Journal of Cardiovascular Nursing. doi: 10.1097/JCN.0000000000000118. in press. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Joseph A, Zayas-Cabán T. The human factors of home health care: A conceptual model for examining safety and quality concerns. Journal of Patient Safety. 2009;5:229–236. doi: 10.1097/PTS.0b013e3181bd1c2a. [DOI] [PubMed] [Google Scholar]
- Hignett S, Carayon P, Buckle P, Catchpole K. State of science: Human factors and ergonomics in healthcare. Ergonomics. 2013;56:1491–1503. doi: 10.1080/00140139.2013.822932. [DOI] [PubMed] [Google Scholar]
- Holden RJ. What stands in the way of technology-mediated patient safety improvements? A study of facilitators and barriers to physicians’ use of electronic health records. Journal of Patient Safety. 2011;7:193–203. doi: 10.1097/PTS.0b013e3182388cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Alper SJ, Scanlon MC, Murkowski K, Rivera AJ, Karsh B. Challenges and problem-solving strategies during medication management: A study of a pediatric hospital before and after bar-coding; Paper presented at the Proceedings of the 2nd International Conference on Healthcare Systems Ergonomics and Patient Safety; Strasbourg, France. 2008. [Google Scholar]
- Holden RJ, Carayon P, Gurses AP, Hoonakker P, Hundt AS, Ozok AA, Rivera-Rodriguez AJ. SEIPS 2.0: A human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics. 2013;56(11):1669–1686. doi: 10.1080/00140139.2013.838643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Mickelson RS. Performance barriers among elderly chronic heart failure patients: An application of patient-engaged human factors and ergonomics. Proceedings of the Human Factors and Ergonomics Society. 2013;57(1):758–762. [Google Scholar]
- Holden RJ, Rivera-Rodriguez AJ, Faye H, Scanlon MC, Karsh B. Automation and adaptation: Nurses’ problem-solving behavior following the implementation of bar coded medication administration technology. Cognition, Technology & Work. 2013;15:283–296. doi: 10.1007/s10111-012-0229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollnagel E. The ETTO Principle: Efficiency-Thoroughness Trade-Off. Aldershot, UK: Ashgate; 2009. [Google Scholar]
- Hollnagel E, Woods DD, Leveson N, editors. Resilience Engineering: Concepts and Precepts. Aldershot, UK: Ashgate; 2006. [Google Scholar]
- Horowitz CR, Rein SB, Leventhal H. A story of maladies, misconceptions and mishaps: Effective management of heart failure. Social Science & Medicine. 2004;58(3):631–643. doi: 10.1016/s0277-9536(03)00232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. To Err is Human: Building a Safer Health System. Institute of Medicine Report on Medical Errors. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- Jacko JA, editor. The Human-Computer Interaction Handbook: Fundamentals, Evolving Technologies and Emerging Applications. 3. Mahwah, NJ: Lawrence Erbaum Associates; 2012. [Google Scholar]
- Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. doi: 10.1186/1471-2261-6-43. http://www.biomedcentral.com/1471-2261/6/43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsh B. Beyond usability for patient safety: designing effective technology implementation systems. Quality & Safety in Health Care. 2004;13:388–394. doi: 10.1136/qshc.2004.010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsh B, Holden RJ, Alper SJ, Or CKL. A human factors engineering paradigm for patient safety – designing to support the performance of the health care professional. Quality & Safety in Health Care. 2006;15:i59–i65. doi: 10.1136/qshc.2005.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. National Vital Statistics Report. 2008;56:1–120. [PubMed] [Google Scholar]
- Lee CS, Suwanno J, Riegel B. The relationship between self-care and health status domains in Thai patients with heart failure. European Journal of Cardiovascular Nursing. 2009;8:259–266. doi: 10.1016/j.ejcnurse.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa KD, Altman Klein H, Shalin VL. Everyday expertise: Cognitive demands in diabetes self-management. Human Factors. 2008;50:112–120. doi: 10.1518/001872008X250601. [DOI] [PubMed] [Google Scholar]
- Logan MK, Parker C, Gardner-Bonneau D, Treu D, Keller J, Winstel L, Rogers W. A roundtable discussion: Home healthcare--Not a hospital in the home. Biomedical Instrumentation & Technology, Suppl. 2013:10–15. doi: 10.2345/0899-8205-47.s1.10. [DOI] [PubMed] [Google Scholar]
- McEntee ML, Cuomo LR, Dennison CR. Patient-, provider-, and system-level barriers to heart failure care. Journal of Cardiovascular Nursing. 2009;24(4):290–298. doi: 10.1097/JCN.0b013e3181a660a0. [DOI] [PubMed] [Google Scholar]
- Mickelson RS, Holden RJ. Assessing the distributed nature of home-based heart failure medication management in older adults. Proceedings of the Human Factors and Ergonomics Society. 2013;57(1):753–757. [Google Scholar]
- Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. 3. Thousand Oaks, CA: SAGE; 2014. [Google Scholar]
- Mitzner TL, McBride SE, Barg-Walkow LH, Rogers WA. Self-management of wellness and illness in an aging population. Reviews of Human Factors and Ergonomics. 2013;8:277–333. [Google Scholar]
- Molloy GJ, O’Carroll RE, Witham MD, McMurdo MET. Interventions to enhance adherence to medications in patients with heart failure: A systematic review. Circulation: Heart Failure. 2012;5:126–133. doi: 10.1161/CIRCHEARTFAILURE.111.964569. [DOI] [PubMed] [Google Scholar]
- Morrow DG, Weiner M, Young J, Steinley D, Deer M, Murray MD. Improving medication knowledge among older adults with heart failure: A patient-centered approach to instruction design. The Gerontologist. 2005;45:545–552. doi: 10.1093/geront/45.4.545. [DOI] [PubMed] [Google Scholar]
- Moser DK, Watkins JF. Conceptualizing self-care in heart failure: A life course model of patient characteristics. Journal of Cardiovascular Nursing. 2008;23:205–218. doi: 10.1097/01.JCN.0000305097.09710.a5. [DOI] [PubMed] [Google Scholar]
- Murray MD, Morrow DG, Weiner M, Clark DO, Tu W, Deer MM, Weinberger M. A conceptual framework to study medication adherence in older adults. The American Journal of Geriatric Pharmacotherapy. 2004;2:36–43. doi: 10.1016/s1543-5946(04)90005-0. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2012: With Special Feature on Emergency Care. 2013 Retrieved February 13, 2014, from http://www.cdc.gov/nchs/data/hus/hus12.pdf. [PubMed]
- National Research Council. Health Care Comes Home: The Human Factors. Washington, DC: National Academies Press. Committee on the Role of Human Factors in Home Health Care, Board on Human-Systems Integration, Division of Behavioral and Social Sciences and Education; 2011. [Google Scholar]
- Oosterom-Calo R, van Ballegooijen AJ, Terwee CB, te Velde SJ, Brouwer IA, Jaarsma T, Brug J. Determinants of heart failure self-care: A systematic literature review. Heart Failure Reviews. 2012;17:367–385. doi: 10.1007/s10741-011-9292-9. [DOI] [PubMed] [Google Scholar]
- Pak R, McLaughlin A. Designing Displays for Older Adults. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- Palen L, Aaløkke S. Of pill boxes and piano benches: “Home-made” methods for managing medication; Paper presented at the Proceedings of the 2006 conference on Computer Supported Cooperative Work (CSCW); Banff, Alberta, Canada. 2006. [Google Scholar]
- Pennathur PR, Thompson D, Abernathy JH, 3rd, Martinez EA, Pronovost PJ, Kim GR, Gurses AP. Technologies in the wild (TiW): human factors implications for patient safety in the cardiovascular operating room. Ergonomics. 2013;56:205–219. doi: 10.1080/00140139.2012.757655. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. European Heart Journal. 2001;22:1527–1560. doi: 10.1053/euhj.2001.2783. [DOI] [PubMed] [Google Scholar]
- Retrum JH, Boggs J, Hersh A, Wright L, Main DS, Magid DJ, Allen LA. Patient-identified factors related to heart failure readmissions. Circulation: Cardiovascular Quality and Outcomes. 2013;6:171–177. doi: 10.1161/CIRCOUTCOMES.112.967356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, Tinetti ME. Goal-oriented patient care: An alternative health outcomes paradigm. New England Journal of Medicine. 2012;366:777–779. doi: 10.1056/NEJMp1113631. [DOI] [PubMed] [Google Scholar]
- Rich MW. Heart failure in the 21st century: A cardiogeriatric syndrome. Journal of Gerontology: Medical Sciences. 2001;56:M88–M96. doi: 10.1093/gerona/56.2.m88. [DOI] [PubMed] [Google Scholar]
- Riegel B, Carlson B. Facilitators and barriers to heart failure self-care. Patient Education and Counseling. 2002;46(4):287–295. doi: 10.1016/s0738-3991(01)00165-3. [DOI] [PubMed] [Google Scholar]
- Riegel B, Dickson VV, Topaz M. Qualitative analysis of naturalistic decision making in adults with chronic heart failure. Nursing Research. 2013;62:91–98. doi: 10.1097/NNR.0b013e318276250c. [DOI] [PubMed] [Google Scholar]
- Riegel B, Lee CS, Dickson VV. Self care in patients with chronic heart failure. Nature Reviews Cardiology. 2011;8:644–654. doi: 10.1038/nrcardio.2011.95. [DOI] [PubMed] [Google Scholar]
- Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Whellan DJ. State of the science: Promoting self-care in persons with heart failure: A scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- Rogers WA, Fisk AD. Toward a psychological science of advanced technology design for older adults. Journal of Gerontology: Psychological Sciences. 2010;65B:645–653. doi: 10.1093/geronb/gbq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ AL, Fairbanks RJ, Karsh B, Militello LG, Saleem JJ, Wears RL. The science of human factors: Separating fact from fiction. BMJ Quality & Safety. 2013 doi: 10.1136/bmjqs-2012-001450. forthcoming, http://dx.doi.org/10.1136/bmjqs-2012-002036. [DOI] [PMC free article] [PubMed]
- Saldaña J. The Coding Manual for Qualitative Researchers. 2. Thousand Oaks, CA: SAGE; 2013. [Google Scholar]
- Schubert CC, Wears RL, Holden RJ, Hunte G. Patients as source of resilience. In: Wears RL, Hollnagel E, Braithwaite J, editors. Resilience of Everyday Clinical Work. Aldershot, UK: Ashgate; in press. [Google Scholar]
- Schulz R, Hebert RS, Dew MA, Brown SL, Scheier MF, Beach SR, Nicols L. Patient suffering and caregiver compassion: New opportunities for research, practice, and policy. The Gerontologist. 2007;47:4–13. doi: 10.1093/geront/47.1.4. [DOI] [PubMed] [Google Scholar]
- Schumacher RM, Lowry SZ. NIST Guide to the Processes Approach for Improving the Usability of Electronic Health Records. Washington, DC: National Institute of Standards and Technology; 2010. http://www.nist.gov/itl/hit/upload/Guide_Final_Publication_Version.pdf. [Google Scholar]
- Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: A functional, patient-centered model of patient complexity can improve research and practice. Journal of Clinical Epidemiology. 2012;65:1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Siabani S, Leeder SR, Davidson PM. Barriers and facilitators to self-care in chronic heart failure: a meta-synthesis of qualitative studies. Springerplus. 2013;2 doi: 10.1186/2193-1801-2-320. http://www.springerplus.com/content/2/1/320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SH, Farris KB, Johnson JA, Tsuyuki RT. Using focus groups to identify barriers to drug use in patients with congestive heart failure. Pharmacotherapy. 2000;20:823–829. doi: 10.1592/phco.20.9.823.35205. [DOI] [PubMed] [Google Scholar]
- Unruh KT, Pratt W. Patients as actors: the patient’s role in detecting, preventing, and recovering from medical errors. International Journal of Medical Informatics. 2007;76:S236–S244. doi: 10.1016/j.ijmedinf.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Valdez RS, Holden RJ, Novak LL, Veinot TC. Transforming consumer health informatics through a patient work framework: Connecting patients to context. Journal of the American Medical Informatics Association. doi: 10.1136/amiajnl-2014-002826. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wal MH, Jaarsma T, Moser DK, van Gilst WH, van Veldhuisen DJ. Qualitative examination of compliance in heart failure patients in The Netherlands. Heart & Lung. 2010;39:121–130. doi: 10.1016/j.hrtlng.2009.07.008. [DOI] [PubMed] [Google Scholar]
- van der Wal MH, Jaarsma T, van Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? European Journal of Heart Failure. 2005;7:5–17. doi: 10.1016/j.ejheart.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Vincent C. Patient Safety. London: Elsevier; 2006. [Google Scholar]
- Vincent C, Coulter A. Patient safety: What about the patient? Quality & Safety in Health Care. 2002;11:76–80. doi: 10.1136/qhc.11.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger MB, Herndon OW, Paulus MP, Gaba D, Zornow MH, Dallen LD. Objective task analysis and workload assessment of anesthesia providers. Anesthesiology. 1994;80:77–92. doi: 10.1097/00000542-199401000-00015. [DOI] [PubMed] [Google Scholar]
- Weinger MB, Pantiskas C, Wiklund ME, Carstensen P. Incorporating human factors in the design of medical devices. JAMA-Journal of the American Medical Association. 1998;280:1484. doi: 10.1001/jama.280.17.1484-a. [DOI] [PubMed] [Google Scholar]
- Weinger MB, Wiklund M, Gardner-Bonneau D, editors. Handbook of Human Factors in Medical Device Design. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- Wiegmann DA, Eggman AA, ElBardissi AW, Parker SH, Sundt TM. Improving cardiac surgical care: A work systems approach. Applied Ergonomics. 2010;41:701–712. doi: 10.1016/j.apergo.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR. Fundamentals of systems ergonomics/human factors. Applied Ergonomics. 2014;45:5–13. doi: 10.1016/j.apergo.2013.03.021. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Patient Safety. 2009 Retrieved October 30, 2009, from http://www.who.int/patientsafety/en/
- Wu JR, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: A review of the literature. Nursing Clinics of North America. 2008;43:133–153. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- Zavertnik JE. Self-care in older adults with heart failure: An integrative review. Clinical Nurse Specialist. 2014;28:19–32. doi: 10.1097/NUR.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Zayas-Cabán T, Valdez RS. Human Factors and Ergonomics in Home Care. In: Carayon P, editor. Handbook of Human Factors and Ergonomics in Health Care and Patient Safety. 2. Boca Raton, FL: CRC Press; 2012. pp. 743–762. [Google Scholar]