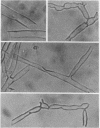
Abstract
A soluble elicitor of glyceollin accumulation was released from insoluble mycelial walls of Phytophthora megasperma var. sojae after incubation with soybean cotyledon tissue for as little as 2 minutes. Various enzymic and chemical treatments of the released elicitor indicated that the activity resided in a carbohydrate moiety, and gel filtration disclosed the presence of at least two active molecular species. Cell-free extracts from soybean cotyledons or hypocotyls also released soluble elicitors from fungal cell walls that were similar to those released by living cotyledon tissue. These results may suggest that contact of fungal pathogens with host tissues is required to release fungal wall elicitors which then initiate phytoalexin accumulation in the plant.
Full text
PDF
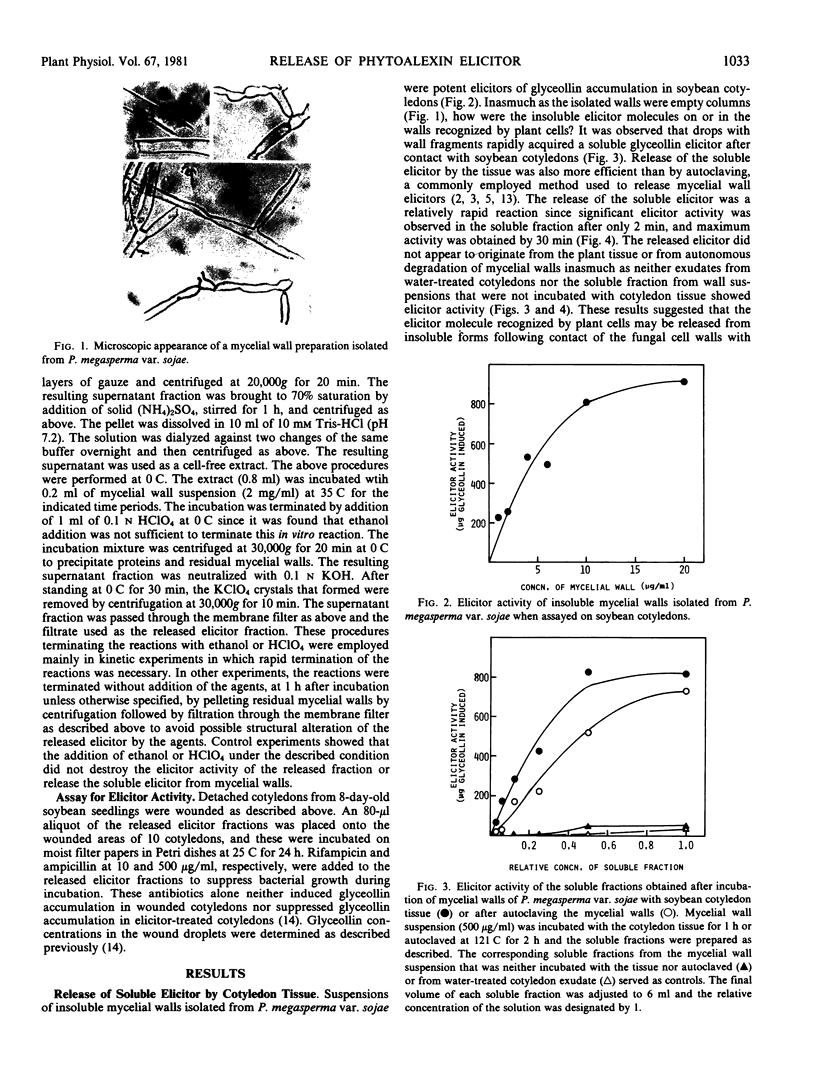
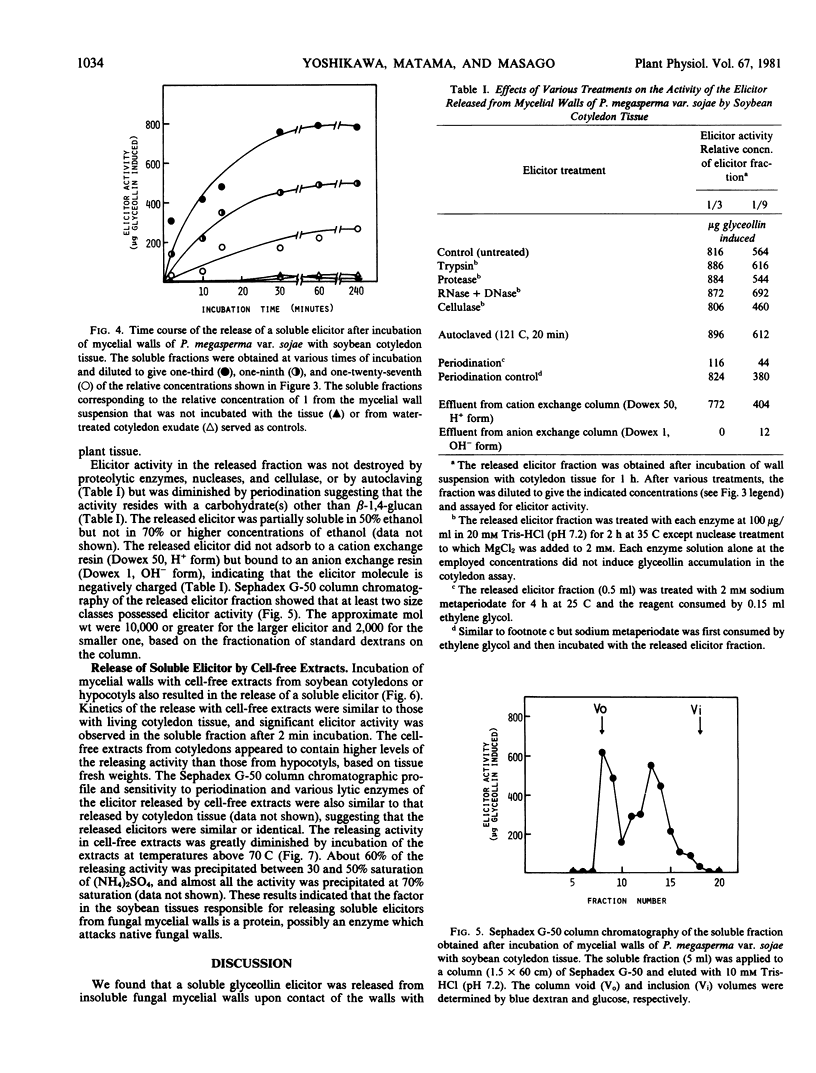
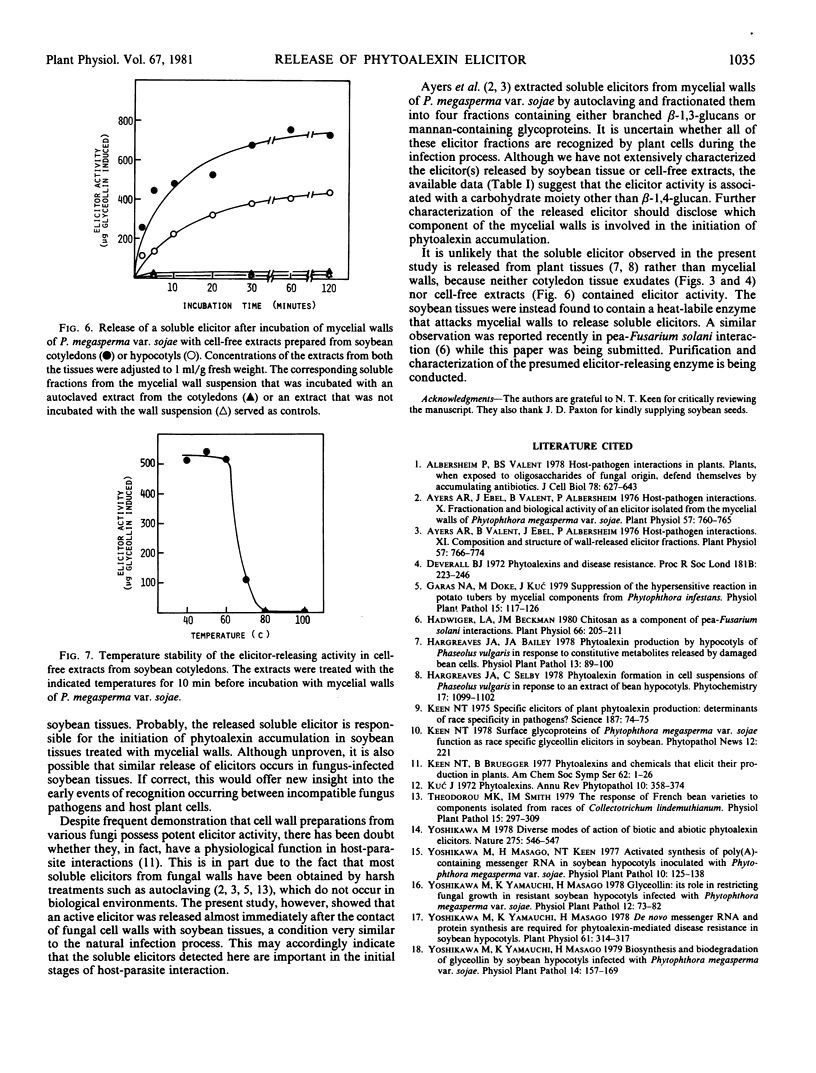
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Ebel J., Valent B., Albersheim P. Host-Pathogen Interactions: X. Fractionation and Biological Activity of an Elicitor Isolated from the Mycelial Walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976 May;57(5):760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers A. R., Valent B., Ebel J., Albersheim P. Host-Pathogen Interactions: XI. Composition and Structure of Wall-released Elicitor Fractions. Plant Physiol. 1976 May;57(5):766–774. doi: 10.1104/pp.57.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger L. A., Beckman J. M. Chitosan as a Component of Pea-Fusarium solani Interactions. Plant Physiol. 1980 Aug;66(2):205–211. doi: 10.1104/pp.66.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T. Specific elicitors of plant phytoalexin production: detenninants of race specificity in pathogens? Science. 1975 Jan 10;187(4171):74–75. doi: 10.1126/science.187.4171.74. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Yamauchi K., Masago H. De Novo Messenger RNA and Protein Synthesis Are Required for Phytoalexin-mediated Disease Resistance in Soybean Hypocotyls. Plant Physiol. 1978 Mar;61(3):314–317. doi: 10.1104/pp.61.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]