SUMMARY
Penicillin and related beta-lactams comprise one of our oldest and most widely used antibiotic therapies. These drugs have long been known to target enzymes called penicillin-binding proteins (PBPs) that build the bacterial cell wall. Investigating the downstream consequences of target inhibition and how they contribute to the lethal action of these important drugs, we demonstrate that beta-lactams do more than just inhibit the PBPs as is commonly believed. Rather, they induce a toxic malfunctioning of their target biosynthetic machinery involving a futile cycle of cell wall synthesis and degradation, thereby depleting cellular resources and bolstering their killing activity. Characterization of this mode of action additionally revealed a quality-control function for enzymes that cleave bonds in the cell wall matrix. The results thus provide insight into the mechanism of cell wall assembly and suggest how best to interfere with the process for future antibiotic development.
INTRODUCTION
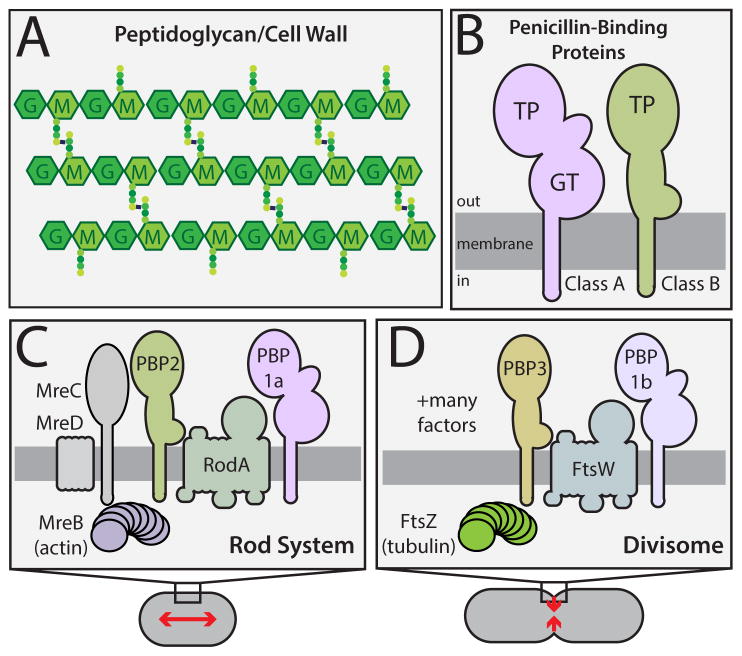
Penicillin and related beta-lactam drugs are one of our oldest and most widely used antibiotic classes. They have long been known to interfere with bacterial cell wall assembly as part of their mode-of-action (Park and Strominger, 1957). The cell wall is an essential polysaccharide structure that surrounds most bacterial cells and protects their cytoplasmic membrane from osmotic rupture. It is built from the polymer peptidoglycan (PG), which consists of glycan chains with attached peptides used to crosslink adjacent glycans to form a matrix structure (Figure 1A).
Figure 1. Peptidoglycan structure and the machines that synthesize it.
A. The PG matrix consists of glycan chains with the repeating unit of N-acetylmuramic acid (MurNAc, M) and N-acetylglucosamine (GlcNAc, G). Attached to the MurNAc sugars are peptides (colored circles) used to form crosslinks between adjacent glycans. B. Domain structure of the PG synthases. Both classes of PBPs have a single transmembrane domain with a large catalytic domain in the periplasm. C–D. Schematics highlighting the components of the two main PG synthetic systems in rod-shaped bacteria. Both systems require a dedicated bPBP (PBP2 or PBP3) and a SEDS (shape/elongation/division/sporulation) family protein (RodA or FtsW) for proper function. See text for details.
Beta-lactams disrupt PG biogenesis by inactivating enzymes called penicillin-binding proteins (PBPs) (Tipper and Strominger, 1965). Bacteria encode a variety of PBPs that participate in PG assembly (Sauvage et al., 2008). The high-molecular weight PBPs are the major PG synthases. They are subdivided into class A (aPBPs) and class B (bPBPs) enzymes (Fig. 1B). aPBPs are bifunctional and possess both glycosyltransferase (GT) activity for polymerizing the glycan strands and transpeptidase (TP) activity for crosslinking them. bPBPs, on the other hand, are only known to possess TP activity. The primary target of beta-lactams is the TP active site of the synthetic PBPs, which is covalently modified by the drug. In addition to the PG synthases, beta-lactams also inhibit the low-molecular weight PBPs. These factors belong to a large and diverse family of enzymes that cleave bonds in the PG matrix. Such enzymes, often referred to as PG hydrolases, are typically non-essential, but have been found to play important roles in morphogenesis (Uehara and Bernhardt, 2011).
The lethal activity of beta-lactams is thought to stem principally from the loss of wall integrity accompanied by cell lysis (Park and Strominger, 1957). According to the most widely accepted model, cell wall damage following beta-lactam treatment results from a drug-induced imbalance between the activities of cell wall synthases and hydrolases (Schwarz et al., 1969; Tomasz and Waks, 1975; Tomasz et al., 1970). This view is supported by the observation that PG hydrolase inactivation can prevent or delay beta-lactam-induced cell lysis (Chung et al., 2009; Heidrich et al., 2002; Tomasz, 1979; Tomasz and Waks, 1975; Tomasz et al., 1970; Uehara et al., 2009). However, surprisingly little mechanistic insight underlies this general framework for drug action. It remains largely unclear which PG hydrolases disrupt the wall following drug treatment, and whether these autolysins are “induced” to damage the wall or are simply carrying out their normal physiological function in the absence of TP activity. Clues suggesting a more complex mode-of-action for beta-lactams than simple TP inhibition have also been reported. Surprisingly, in Streptococcus pneumoniae mutants blocked for cell lysis, beta-lactam treatment still promoted cell death with kinetics similar to lysing cells (Moreillon et al., 1990). Additionally, in Escherichia coli, beta-lactams were unexpectedly shown to cause a depletion of lipid-linked cell wall precursors prior to cell lysis (Kohlrausch and Höltje, 1991).
The lysis-independent effects of beta-lactams indicate that much remains to be learned about the events following TP inhibition and how they contribute to the killing activity of this vital class of antibiotics. These events have been difficult to elucidate because the effect of drug treatment on cell growth and morphology varies depending on the organism studied and on the number of different PBPs inhibited by the beta-lactam derivative used (Spratt, 1975). We overcame this challenge by studying the activity of mecillinam, a beta-lactam specific for a single essential PBP in the model gram-negative bacterium Escherichia coli (Spratt, 1975). Our analysis revealed that, beyond simply inhibiting the TP activity of PBPs, mecillinam and other beta-lactams stimulate a deleterious futile cycle of cell wall synthesis and degradation by their target machineries that contributes to their lethal activity. Additional genetic analysis identified the enzyme responsible for beta-lactam-stimulated degradation of nascent PG. Characterization of the in vivo activity of this factor suggests a novel “quality control” function for cell wall cleaving enzymes in PG biogenesis. Our findings thus provide new insight into the cell wall assembly process in addition to uncovering an important mechanism by which beta-lactam antibiotics induce cell death.
RESULTS
Rationale
Like many rod-shaped bacteria, E. coli grows using two different PG biogenesis systems (Typas et al., 2012) (Fig. 1C–D). The actin-like MreB protein and its partners constitute the Rod system, which catalyzes the insertion of new PG material along the cell body to promote cell elongation (Typas et al., 2012) (Fig. 1C). The tubulin-like FtsZ protein, on the other hand, organizes the divisome to synthesize PG for the new daughter cell poles (de Boer, 2010) (Fig. 1D). Each of these machineries requires an essential bPBP for their activity: PBP2 for the Rod system and PBP3 for the divisome (Typas et al., 2012) (Fig. 1B–D). Proper PG biogenesis by these systems in E. coli is also thought to require the aPBPs, PBP1a and PBP1b, to promote glycan chain polymerization and crosslinking (Typas et al., 2012) (Fig. 1B–D).
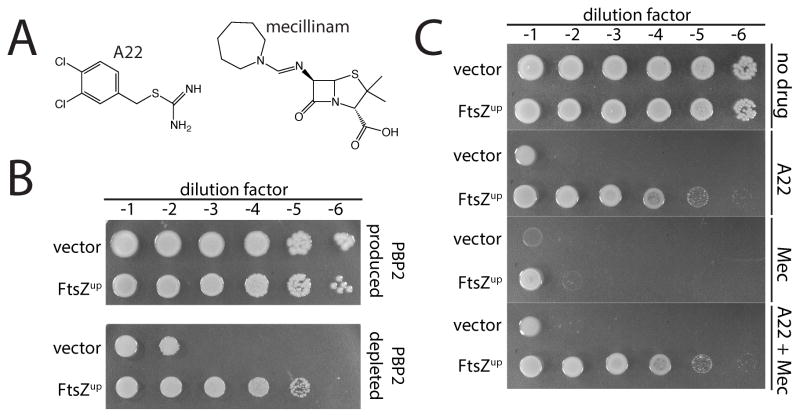
Rod system function is normally required for viability (Bendezú and de Boer, 2008; Kruse et al., 2004). Inactivation of any of the essential components (MreB, MreC, MreD, PBP2, or RodA), causes cells to form spheres that fail to divide and eventually lyse. This lethal phenotype can be suppressed by overproduction of the division protein FtsZ, with the Rod− FtsZup cells growing and dividing as small spheres (Bendezú and de Boer, 2008; Kruse et al., 2004; Vinella et al., 1992). However, the mechanism by which increased FtsZ concentration suppresses the lethality of Rod system inactivation is not clear. In addition to genetic inactivation, Rod system function can also be blocked with the small molecules A22 (Gitai et al., 2005) or mecillinam (Spratt, 1975) (Fig. 2A). A22 inactivates MreB (Gitai et al., 2005), and mecillinam is a beta-lactam that is specific for the TP active site of PBP2 (Spratt, 1975). Because PBP2 can be rendered non-essential by the overproduction of FtsZ (Vinella et al., 1993) (Fig. 2B), we reasoned that studies of mecillinam presented a unique opportunity to study the consequences of PBP inhibition by beta-lactams under conditions where the target is not required for growth.
Figure 2. Mecillnam induces a lethal malfunctioning of the Rod system.
A. Shown are the chemical structures of A22 and mecillinam. B. Conditional essentiality of PBP2. Cultures of strain HC439/pHC817 [ΔpbpA Plac::pbpA] harboring either a vector control (pSC101, vector) or a derivative (pTB63, FtsZup) were serially diluted and spotted on LB agar with either 1 mM IPTG (PBP2 replete) or agar without inducer (PBP2 depletion). Plates were incubated overnight at 30°C and photographed. C. Cultures of strain MG1655 [WT] harboring either pSC101 or pTB63 serially diluted and spotted on LB agar as in part B. Agar was supplemented with A22 (10 μg/ml), mecillinam (1 μg/ml), or both drugs as indicated.
Mecillinam induces a lethal malfunctioning of the Rod system
The lethal effects of inactivating MreB with A22 are suppressed by the low copy vector pTB63, from which the ftsQAZ operon is expressed via its native promoters (a condition we will henceforth designate as FtsZup) (Bendezú and de Boer, 2008) (Fig. 2C). Surprisingly, however, FtsZup cells remained sensitive to mecillinam (Fig. 2C). Thus, blocking PBP2 function with mecillinam is lethal even under conditions where the protein itself is not essential (Fig. 2B). This observation suggested that, beyond inactivation of the TP activity of PBP2, mecillinam is also conferring a dominant-negative function to the inactivated protein. We reasoned that this toxic activity of inhibited PBP2 may stem from the malfunctioning of the Rod system. To test this hypothesis, we treated cells with both mecillinam and A22 to simultaneously inactivate the Rod system and the TP domain of PBP2. Indeed, inactivation of MreB with A22 in FtsZup cells completely suppressed the toxicity of mecillinam (Fig. 2C). Genetic inactivation of the Rod system was also found to restore growth to FtsZup cells treated with mecillinam (Fig. S1). The contribution of Rod system malfunction to the lethal action of mecillinam was also apparent for cells lacking pTB63. Addition of mecillinam to a culture of wild-type cells led to a drop in culture OD600 and a loss of viability that was reduced in severity by co-treatment with A22 (Fig. S2A–B). The production of a PBP2 variant with a defective TP active site [PBP2(S330A)] also killed FtsZup cells (Fig. S2C), and this toxicity could also be suppressed by A22 treatment (Fig. S2C). Thus, the toxicity induced by mecillinam is specific for PBP2 inhibition, and not the result of an off-target activity. We conclude that blocking the TP activity of PBP2 causes the Rod system to malfunction in a manner that contributes to the lethal activity of mecillinam.
A futile cycle of PG synthesis and turnover is responsible for mecillinam toxicity
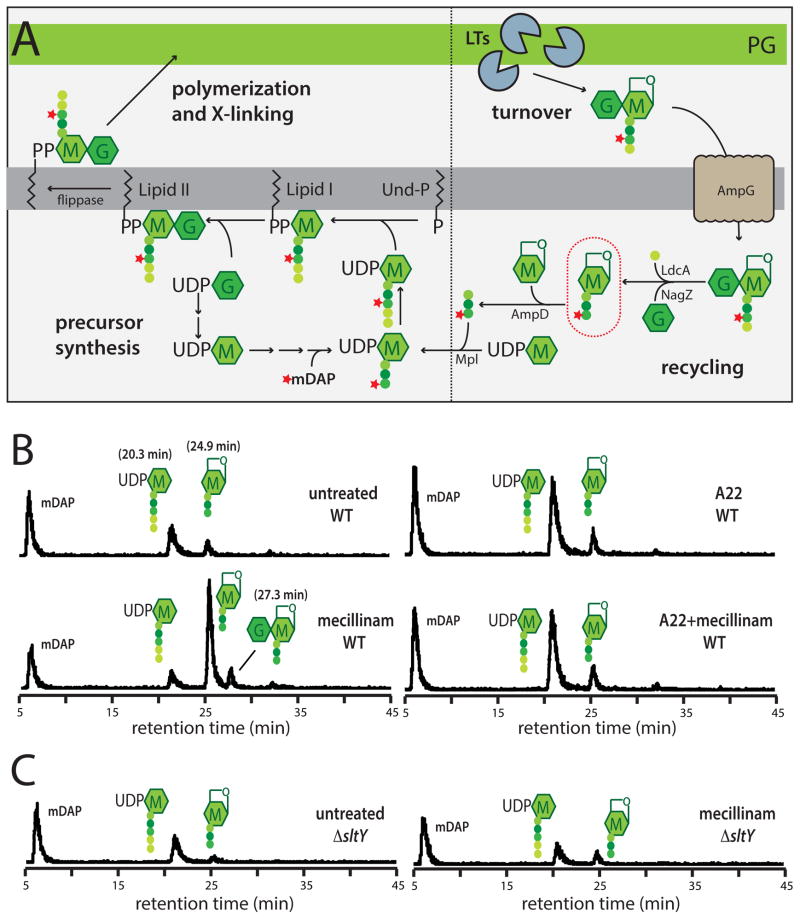
Results from Uehara and Park (2008) previously found that mecillinam treatment induced the degradation of newly synthesized PG while A22 blocks synthesis. We suspected that the observed turnover of nascent PG was key to the lethal mode-of-action of mecillinam. We therefore monitored PG synthesis and turnover using similar procedures to those of Uehara and Park (2008). Cells were metabolically labeled with [3H]-meso-diaminopimelic acid (mDAP), an amino acid unique to the peptide moiety of PG (Fig. 3A), following which, PG synthesis can be readily measured by monitoring the production of the major cytoplasmic precursor UDP-MurNAc-pentapeptide (UDP-MurNAc-pep5) and the incorporation of label into the PG matrix. [3H]-mDAP labeling can also be used to quantify PG turnover. The major degradation products of PG are disaccharide fragments liberated by lytic transglycosylases (LTs) (Fisher and Mobashery, 2014) (Fig. 3A). Rather than hydrolyzing the glycosidic bond, these enzymes promote the formation of an anhydro linkage between the C1 and C6 positions of MurNAc to generate GlcNAc-anhydro-MurNAc-peptides (GlcNAc-anhMurNAc-peptide). These degradation products are normally imported and processed by a number of enzymes in the cytoplasm for recycling (Fisher and Mobashery, 2014) (Fig. 3A). We therefore used a strain lacking the amidase AmpD to trap PG turnover products and allow the quantification of PG degradation without complications of complete recycling (Uehara and Park, 2008) (Fig. 3A).
Figure 3. Measurement of PG synthesis and turnover following mecillinam treatment.
A. Schematic summarizes the PG synthesis and recycling pathways. Sugars and peptides are represented as in Figure 1A. The composition of the pentapeptide of the cytoplasmic precursors is L-Ala-gamma-D-Glu-meso-diaminopimelic acid (mDAP)-D-Ala-D-Ala. UDP-sugars are first made in the cytoplasm before being transferred to the lipid carrier undecaprenol-phosphate (Und-P). The final precursor lipid II is the substrate used by the PBPs to form PG. As a result of the crosslinking mechanism and the activity of carboxypeptidase enzymes that remove the terminal D-Ala, the PG layer consists primarily of glycans with tetrapeptides. The turnover product circled in red will accumulate in a ΔampD strain if nascent PG is degraded during a pulse labeling experiment. See text for details. B–C. Measurement of PG synthesis and turnover by the Rod system. Cells of TU278 [ΔlysA ΔampD] (B) or its Δslt derivative (HC419) (C) producing SulA to block cell division were pulse labeled with [3H]-mDAP following treatment with the indicated drug(s) (10 μg/ml each when added). Soluble metabolites were separated by HPLC and detected using an in-line radiodetector. The resulting chromatograms are shown. Peaks are labeled with schematics of the corresponding compounds. The identity of each peak is based on the results shown in Figure S3. See text for details.
To specifically monitor the effect of drug treatment on PG synthesis and degradation by the Rod system, divisome activity was blocked during the radiolabeling by expression of the FtsZ antagonist SulA prior to the addition of antibiotic(s). After 5 minutes of growth in the presence of drugs, [3H]-mDAP was added to the medium and growth was continued for 10 minutes (1/10th of a mass doubling). Cells were then harvested and extracted with hot (90°C) water. Soluble compounds (PG precursors and degradation products) were separated and quantified by HPLC and radiodetection. Material bincorporated into the PG matrix was quantified as radioactive material released from the cell pellet by lysozyme treatment.
In untreated cells, robust incorporation of label into the PG matrix was observed (Fig. 4). Very little of the newly synthesized material was degraded as evidenced by the small amount of labeled anhMurNAc-peptides detected relative to the precursor pools (Fig. 3B, 4, and S3). As reported by Uehara and Park (2008), we found the effects of A22 treatment differed dramatically from those induced by mecillinam. In cells treated with A22, incorporation of radiolabel into mature PG was significantly reduced as expected. This defect was accompanied by increased pools of the PG precursors mDAP and labeled UDP-MurNAc-pep5 (Fig. 3B, 4, and S3), suggestive of a late stage block in the synthesis pathway. Only a modest increase in anhMurNAc-peptide accumulation was observed following A22 treatment, indicating that the drop in label incorporation into the PG matrix resulted primarily from the inhibition of PG synthesis. Similarly, in cells treated with mecillinam, incorporation of label into PG was also greatly reduced (Fig. 3B and 4). However, as expected from the results of Uehara and Park (2008), a large pool of labeled turnover products was detected in mecillinam-treated samples (Fig. 3B and 4). This observation confirms the conclusion that, rather than blocking PG synthesis by the Rod system like A22, mecillinam induced the degradation of nascent PG material produced by the machine. Given that A22 suppressed the toxicity of mecillinam, we wondered if it would also block the apparent futile cycle of nascent PG synthesis and turnover induced by this beta-lactam. This indeed proved to be the case (Fig. 3B and 4), thus linking the induction of nascent PG turnover with the lethal mode-of-action of mecillinam.
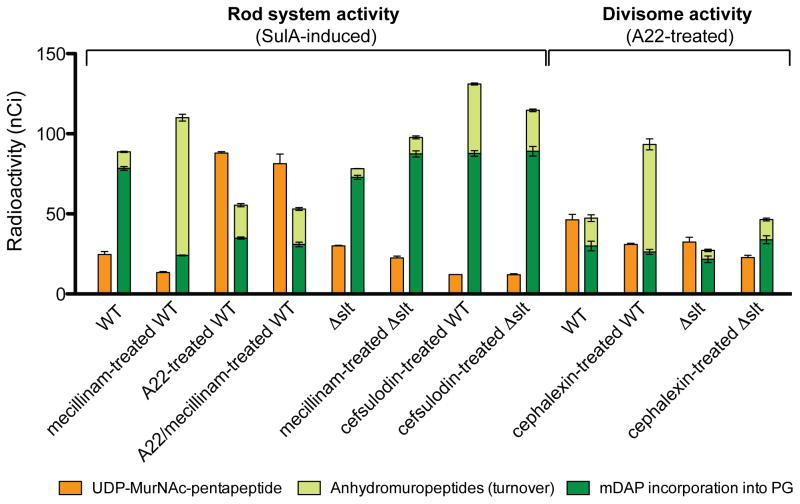
Figure 4. Quantification of PG synthesis and turnover following beta-lactam treatment.
Cells of TU278 [ΔlysA ΔampD] or its Δslt derivative (HC419) were grown, radiolabeled, and analyzed as in Figure 3. The amount of UDP-MurNAc-pentapeptide and total anhydromuropeptides produced were quantified from the area under the peaks in HPLC chromatograms. Radiolabel incorporation into PG was determined by quantifying the amount of label released from cells by lysozyme. Antibiotic concentrations used were: mecillinam (10 μg/ml), A22 (10 μg/ml), cephalexin (10 μg/ml), and cefsulodin (100 μg/ml). Results are the average of three independent experiments with the error bars representing the standard deviation. The drop in precursor levels between untreated and mecillinam-treated WT cells is significant (p < 0.005). See text for details.
PBP inhibition by beta-lactams was previously found to decrease cellular levels of the final PG precursor, lipid II (Kohlrausch and Höltje, 1991). Based on in vitro PBP assays, it was recently suggested that the decline in lipid II concentration may result from the stimulation of glycan strand synthesis when TP activity is blocked (Banzhaf et al., 2012). A dual mode of action for beta-lactams was thus proposed where TP inhibition and substrate depletion act synergistically to promote efficient bacterial killing (Banzhaf et al., 2012). Our results indicate that the futile-cycle of PG synthesis and turnover induced by beta-lactams is likely to be the main driver of the PG precursor depletion observed here and by others (Kohlrausch and Höltje, 1991; Uehara and Park, 2008) (Fig. 3B and 4). To investigate the contribution of precursor depletion to the lethal activity of mecillinam, we generated strains that overexpress uppS or murA, which encode enzymes required for the synthesis of the lipid-carrier Und-P or UDP-MurNAc, respectively (Fig. 3A). Importantly, both strains gained partial resistance to mecillinam (Fig. S2D). Additionally, studies by D’Ari and colleagues (Vinella et al., 1993) showed that high-level overproduction of FtsZ (a 7x increase from pZAQ (Begg et al., 1998), as opposed to a 2x increase from pTB63 (Bernhardt and de Boer, 2005)), a condition where the divisome may more effectively compete with the Rod system for PG precursors, can partially suppress the lethal action of mecillinam. We conclude that mecillinam not only blocks the TP active site of PBP2, but by doing so, induces a futile cycle of cell wall synthesis and turnover by the Rod system that contributes to its bactericidal activity, at least in part, by depleting PG precursor pools.
Futile cycle induction is a common property of beta-lactams
We investigated whether inhibiting PBPs other than PBP2 also stimulates the degradation of nascent PG. Cephalexin is a beta-lactam that preferentially targets PBP3, the cell division-specific bPBP (Spratt, 1975). To monitor the effect of cephalexin, we used a modified version of the labeling method described above. Instead of blocking division with SulA, cell cultures were treated with A22 to block Rod system activity prior to [3H]-mDAP addition. This procedure allowed us to focus the analysis on divisome-mediated PG synthesis. In the absence of cephalexin, robust incorporation of label into PG by the divisome was detected, but with a higher basal level of PG turnover than when the activity of the Rod system was monitored (Fig. 4 and S4A). This observation is consistent with previous results (Uehara and Park, 2008), and most likely reflects the activity of the cell separation PG hydrolases. Importantly, just as with mecillinam, cephalexin treatment led to a dramatic increase in the degradation of nascent PG (Fig. 4 and S4A).
The effect of blocking the TP activity of aPBPs was analyzed using cefsulodin, a beta-lactam that is specific for PBP1a and PBP1b (Curtis et al., 1979). Using labeling conditions identical to those for the mecillinam studies, we also observed a significant increase in nascent PG turnover upon cefsulodin treatment (Fig. 4 and S4B). However, this increase was not accompanied by a corresponding drop in label incorporation into the PG matrix, presumably because PBP2 and other components of the Rod system remain functional when the TP activity of aPBPs is blocked. We conclude that the induction of nascent PG degradation is a common property of beta-lactams regardless of the growth process or class of PBP they target.
Slt is responsible for nascent PG turnover following beta-lactam treatment
We reasoned that a genetic selection for mutants resistant to mecillinam might reveal the cell wall degrading enzyme responsible for the nascent PG turnover induced by beta-lactams. Notably, selections for mecillinam-resistance were performed many years ago by several laboratories (Spratt, 1975; Vinella et al., 1992; Wachi et al., 1987). This genetic analysis was instrumental in the initial identification of the genes encoding the Rod system (mreBCD, pbpA, and rodA). In retrospect, however, these selections were more complicated than originally appreciated because they were carried out in genetic backgrounds with wild-type FtsZ levels. Thus, based on our current understanding, these early mecillinam-resistance selections were actually demanding mutants with two changes: (i) those that increase FtsZ levels to render the Rod system non-essential, and (ii) those that generate defects in the Rod system or other factors in order to alleviate the toxicity of the futile cycle of PG synthesis and degradation. Consistent with this view, the classic mecillinam-resistant mutants mre-129 and mre-678 with lesions in the mreBCD operon (Wachi et al., 1987) were shown to produce elevated levels of FtsZ (Bendezú and de Boer, 2008). Therefore, to simplify the genetic analysis, we revisited the selections for mecillinam resistance in an FtsZup genetic background.
MG1655/pTB63 cells were mutagenized with a transposon and plated on LB agar supplemented with mecillinam. Similar to prior studies, two classes of resistant mutants were isolated: those that remained spherical in the absence of mecillinam and those that regained rod shape when the drug was removed. As expected, all of the permanently spherical mutants possessed transposon insertions that mapped to genes encoding components of the Rod system (data not shown). Mutants in the second class, however, retained rod-shaped without mecillinam and therefore must have a functional Rod system. We suspected that a subset of these mutants were likely to harbor insertions in genes important for the degradation of nascent PG following mecillinam treatment. One such mutant had an insertion in the slt gene encoding a periplasmic cell wall-degrading enzyme called Soluble Lytic Transglycosylase (Slt).
Because LTs cleave glycan strands to generate the anhMurNAc-peptide products that accumulate following beta-lactam treatment, we focused on the slt mutant. To test whether Slt was responsible for beta-lactam-induced turnover of nascent PG, we performed the radiolabeling assay in a Δslt strain. Without treatment, the loss of Slt function did not affect net PG synthesis by either the Rod system or the divisome, but the basal rate of turnover in each case was reduced (Fig. 3B and 4). Significantly, following mecillinam or cephalexin treatment, the Δslt strain displayed a dramatically reduced level of nascent PG turnover relative to the WT strain. Moreover, inactivation of Slt restored wild-type levels of PG incorporation into the matrix of mecillinam-treated cells (Fig. 4). Slt also appeared to be important for PG turnover in cefsulodin-treated cells, but unlike the situation with drugs that inactivate bPBPs, a significant proportion of the turnover following aPBP inhibition was found to be Slt-independent (Fig. 4). Notably, the turnover products produced upon cefsulodin treatment had a much greater anhMurNAc-pentapeptide content (25%) than those observed following mecillinam treatment (4%) (Fig. 3B and S4B). This observation suggests that uncrosslinked, nascent PG with a relatively high proportion of pentapeptides is formed by the cefsulodin-treated cells and that such material is subject to destruction by LTs other than Slt. Nevertheless, we conclude that Slt is likely to be the major enzyme responsible for nascent PG turnover following beta-lactam treatment.
Differential effect of Slt inactivation on mecillinam sensitivity depending on FtsZ levels
Curiously, in contrast to our results, Slt inactivation was previously found to result in mecillinam hypersensitivity rather than resistance (Templin et al., 1992). However, in a separate study, loss of Slt function was shown to increase mecillinam resistance in a Salmonella typhimurium mutant already partially resistant to the drug (Costa and Antón, 2006. Because the original observation of mecillinam hypersensitivity for a Δslt mutant was made with otherwise wild-type cells, and our selection for resistance was performed with FtsZup cells, we suspected that the the apparent contradiction was related to cellular FtsZ concentration. Indeed, we confirmed that for cells lacking Slt and producing normal levels of FtsZ, the minimal inhibitory concentration (MIC) of mecillinam is almost ten times lower (0.04 μg/ml) than that of wild-type cells (MIC = 0.3 μg/ml). Conversely, as expected from our genetic analysis, FtsZup cells defective for Slt showed much higher resistance to mecillinam (MIC > 10 μg/ml) than those with functional Slt (MIC = 0.3 μg/ml). The change in mecillinam sensitivity of Δslt cells relative to wild-type as a function of FtsZ levels was readily visualized on solid medium (Fig. 5A). Importantly, Δslt cells are not generally more sensitive to agents that target the Rod system; wild-type cells and a strain inactivated for Slt displayed the same A22 sensitivity (Fig. S5A).
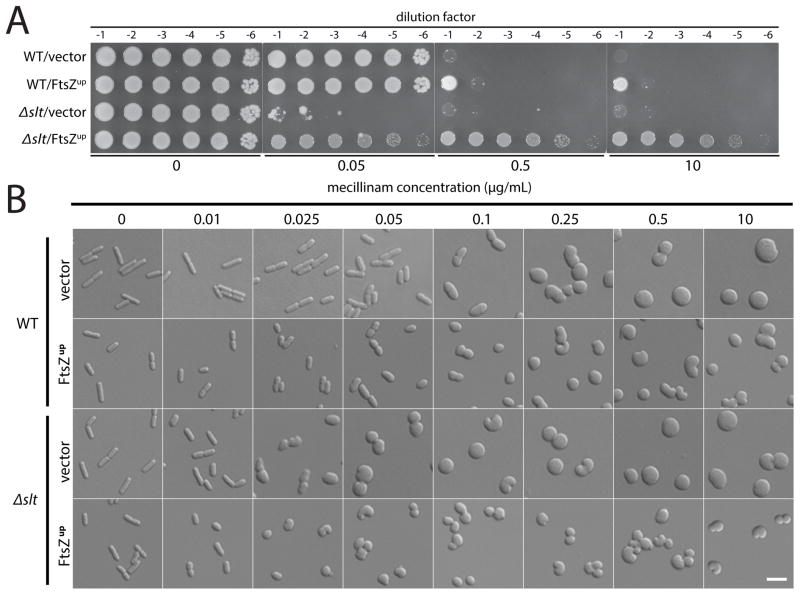
Figure 5. Effect of mecillinam on the growth and morphology of cells defective for Slt.
A. Cells of MG1655 [WT] or HC408 [Δslt] harboring either pSC101 (vector) or pTB63 (FtsZup) were grown overnight, diluted, and spotted on agar containing the indicated concentration of mecillinam as described for Figure 2B. B. The same cells were grown to exponential phase and diluted to an OD600 of 0.025 into LB medium containing the indicated concentration of mecillinam. Growth was continued for an additional 3 hours and the cells were fixed. The fixed cells were then imaged on agarose pads using DIC optics. Bar equals 4 microns.
How can Slt inactivation result in both hypersensitivity and resistance to mecillinam? To investigate this conundrum, we compared the effect of mecillinam on the growth and morphology of wild-type and Δslt cells with and without increased FtsZ production. Microscopic analysis following treatment with mecillinam for 3 hours revealed that cells lacking Slt undergo a morphological change at a much lower mecillinam concentration than wild-type cells. Moreover, the concentration of drug that leads to this shape defect was strongly correlated with the concentration that induces a growth defect in Δslt cells (Fig. 5 and S6). As with the growth phenotype, the morphology of Δslt cells was only hypersensitive to mecillinam; wild-type and Δslt cells were found to undergo shape changes at equivalent A22 concentrations (Fig. S5). It has been well documented that the loss of rod shape results in cell division defects and poor growth unless FtsZ is overproduced (Bendezú and de Boer, 2008). We therefore conclude that the mecillinam hypersensitivity of Δslt cells with normal FtsZ levels results from the morphological changes and consequent division inhibition induced by low doses of drug. On the contrary, when Δslt cells have elevated FtsZ levels, they overcome the adverse effects of the shape alterations just as mutants with defects in the Rod system are suppressed by FtsZ overproduction.
Aberrant PG crosslinking in Δslt cells treated with mecillinam
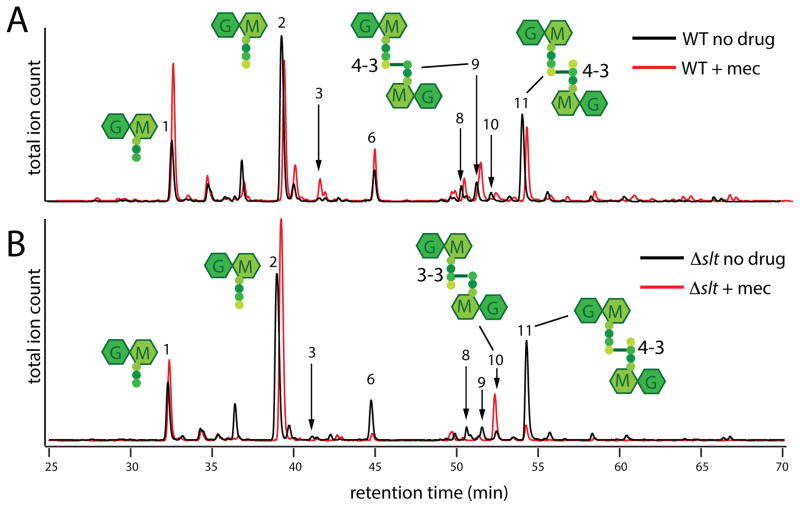
The shape change observed for Δslt cells at low doses of mecillinam suggests that when uncrosslinked PG produced by beta-lactam-targeted synthetic complexes is not destroyed by Slt, it can be misincorporated into the PG matrix to alter morphology. To investigate this possibility, we analyzed the composition of purified cell walls (sacculi) isolated from wild-type or Δslt cells following mecillinam treatment. In wild-type cells, the total amount of PG isolated following mecillinam treatment was reduced as expected, but the composition of the isolated material, including the relative degree of crosslinking, did not change dramatically (Figure 6A, Table S1). The muropeptide profile of sacculi from untreated Δslt cells was similar to that observed for wild-type PG. However, following mecillinam treatment, major alterations were observed (Figure 6B, Table S1). Strikingly, muropeptides with PBP-generated crosslinks, those linked via the fourth residue of one peptide and the third residue of the other (a 4-3 crosslink), were almost completely depleted from mecillinam-treated Δslt sacculi. This loss was accompanied by a corresponding increase in muropeptides with an alternative 3-3 (mDAP-mDAP) crosslink formed by L,D-transpeptidases. Thus, the failure to degrade uncrosslinked PG produced by beta-lactam-targeted synthetic machines indeed results in the aberrant incorporation of PG into the matrix. We conclude that the observed misincorporation is most likely responsible for the shape defects induced in Δslt cells treated with low levels of mecillinam. Furthermore, the accompanying drug hypersensitivity displayed by these mutants suggests that, in cells with wild-type levels of FtsZ, the shape changes induced by PG misincorporation are worse for viability than the consequences of the futile cycle, at least at low drug concentrations. Accordingly, the growth of Δslt cells with the vector control is more adversely affected by low doses of drug than the corresponding WT cells (Fig. S6). At higher drug concentrations, however, functional Slt becomes detrimental, presumably due to the increased burden of the futile cycle as a higher proportion of Rod complexes become targeted (Fig. S6).
Figure 6. Muropeptide composition of PG following mecillinam treatment.
Cells of MG1655 [WT] (A) or HC408 [Δslt] (B) were grown to an OD600 of approximately 0.5 and diluted 1:20 into fresh LB medium with or without mecillinam (10 μg/ml) as indicated. Growth was continued for an additional 3 hours and PG sacculi were prepared from cells of each culture. The purified PG was then digested with the muramidase mutanolysin and the resulting muropeptides were reduced and analyzed by LC/MS. Total ion count chromatograms are shown with the chromatograms of the mecillinam-treated samples off-set for clarity. Note that the scales of each chromatogram are not identical. They were scaled to show the relative muropeptide composition rather than the total amount of material. For example, the total peak area in the chromatogram from mecillinam-treated WT cells is one third that of the corresponding untreated sample even though an equivalent of twice the volume was injected. Schematics of several major muropeptide products are shown near their corresponding peaks with the numbers referring to the type of crosslink (4-3 v.s. 3-3). The identities for all labeled peaks and the quantification of their relative amounts are listed in Table S1. The results were reproducible over two biological replicates of each sample and three technical replicates for each biological replicate.
DISCUSSION
A common theme for the mode-of-action of bactericidal drugs
Bactericidal drugs promote cell death while bacteriostatic agents merely stop bacterial growth. Antibiotics belonging to both general categories interfere with essential cellular pathways or enzymes. However, studies of the mode-of action of clinically important drugs in the aminoglycoside and fluoroquinolone classes provide examples of how bactericidal agents typically do more than just inhibit their target enzymes. Aminoglycosides disable the proofreading capacity of the ribosome, causing the production of mistranslated proteins that are ultimately thought to cause irreversible membrane damage and death (Davis, 1987). Similarly, fluoroquinolones bind to DNA gyrase and topoisomerase IV, leading to the formation of stable drug-enzyme-DNA complexes that block DNA replication and promote the formation of double-strand breaks (Hooper, 2001). Thus, rather than simply inhibiting an essential activity, these drugs disrupt key functions of their target such that the activity of the crippled enzyme or multi-component machine becomes toxic and reduces viability. Whether beta-lactams elicit a similar toxic malfunctioning of their target has remained unclear.
The widely accepted view of the mode-of-action of beta-lactams is that by inhibiting the TP activity of the PBPs, these drugs simply disrupt the balance between PG synthases and the action of PG hydrolases working to expand the cell wall matrix (Tomasz, 1979). Here, we show that beta-lactams derange the process in a more direct and insidious manner than this general framework suggests. Our results indicate that TP inhibition induces the turnover of nascent PG material produced by the targeted complex. In the case of PBP2 targeting by mecillinam, we clearly show that this turnover contributes significantly to the lethal action of the drug by inducing the depletion of cellular PG precursor pools such that even non-targeted PG synthetic complexes are likely to be adversely affected by beta-lactam treatment. Our findings thus connect the killing mechanisms of three important and widely used antibiotics (aminoglycosides, fluoroquinolones, and beta-lactams); they all stimulate a dominant-negative activity in their target pathway to induce systemic toxicity. Furthermore, all three drug classes typically function against multiple targets: aminoglycosides target ribosomes made from rRNA encoded by several genetic loci per genome (Davis, 1987), fluoroquinolones target gyrase and topoisomerase IV (Hooper, 2001), and beta-lactams typically target several different PBPs in a given organism (Curtis et al., 1979). It has been discussed previously that interfering with the activity of multiple cellular targets is an important property of monotherapies needed to reduce the incidence of mutational resistance (Silver, 2011). However, the potential benefit of such therapies inducing a dominant-negative activity in the affected pathway in addition to hitting multiple targets has been under appreciated. The combination of these properties likely enhances antibiotic effectiveness because, even if one of the targets is mutationally altered to block drug binding, the sensitivity and lethal malfunctioning of the other targets will be dominant over the lone resistant allele. Thus, the principles learned from this and other studies of the mode-of-action of our classic antibacterial therapies indicate that, rather than searching for simple inhibitors of essential enzymes as has been common practice, antibiotic discovery efforts should ideally be seeking new molecules that induce a lethal malfunctioning of multiple cellular targets.
A potential role for Slt as a quality control enzyme in PG biogenesis
In addition to revealing novel features of the beta-lactam killing mechanism, our findings also shed significant new light on the process of PG biogenesis. Pulse-labeling studies of PG assembly indicate that nascent PG material is rapidly crosslinked into the mature matrix (Burman and Park, 1983; Glauner and Höltje, 1990), indicating a tight coupling between GT and TP activities. Such an orderly insertion mechanism is likely critical for the maintenance of proper cell shape and integrity. Here, we show that the inhibition of TP activity by beta-lactams functions to uncouple glycan polymerization from crosslinking, leading to the generation of uncrosslinked glycan strands (Figure 7). Our results indicate that this nascent material is rapidly degraded in an Slt-dependent manner, setting up a futile cycle of synthesis and degradation (Figure 7). Mutants defective for other PG cleaving enzymes were not isolated in selections for mecillinam resistance. Additionally, nascent PG turnover was largely eliminated in the absence of Slt. We therefore conclude that Slt is likely to be directly responsible for destroying the uncrosslinked material produced by the beta-lactam-targeted synthetic machinery.
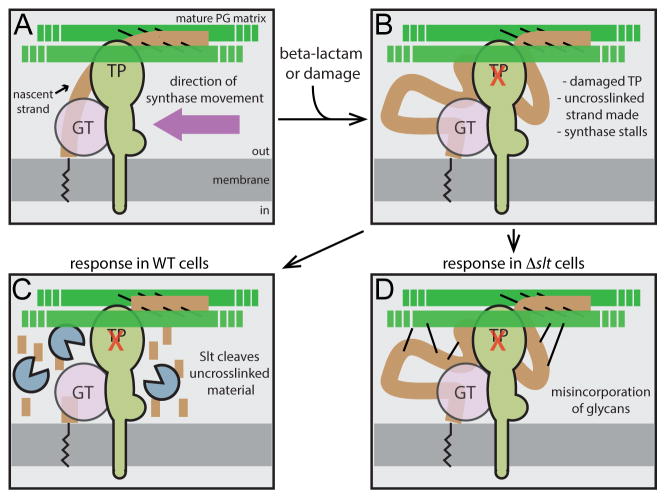
Figure 7. Role of Slt in maintaining coordination between GT and TP activity during PG biogenesis.
Shown is a schematic highlighting the role of Slt in PG biogenesis. Panel (A) shows the normal synthetic process with properly coupled GT and TP activities. The new glycan strands (brown) are polymerized from lipid-linked precursors (black zig-zag line) by an enzyme with GT activity (pink) that may either be the canonical domain of an aPBP or an as yet unidentified GT enzyme. The newly polymerized glycans are rapidly crosslinked into the mature matrix (green) by an associated TP activity shown here as a bPBP, but it could also be the TP domain of an aPBP. Other components of the putative synthetic complex including cytoskeletal elements were omitted for simplicity. When the TP active site is damaged or targeted by a beta-lactam (panel B), GT activity continues to produce glycan chains that are no longer crosslinked into the matrix. In WT cells (panel C), such strands are targeted for destruction by Slt. Conversely, in cells inactivated for Slt (panel D), the uncrosslinked glycans produced by the damaged/targeted machinery are not degraded, but are instead aberrantly incorporated into the matrix by an alternative crosslinking enzyme. In cells with normal FtsZ levels, the resulting morphological changes are lethal, leading to beta-lactam hypersensitivity of Slt-defective cells.
How Slt is activated to degrade uncrosslinked PG is not known. Importantly, affinity chromatography studies identified a potential interaction of Slt with PBPs 1b, 2, and 3 in E. coli (Rechenberg et al., 1996; Romeis and Höltje, 1994), suggesting that the turnover we observe may be stimulated by association with the beta-lactam-targeted synthases. Additionally, Slt is known to have a doughnut-like structure with the active site oriented towards a central hole wide enough to accommodate only a single uncrosslinked glycan chain (Thunnissen et al., 1994). Thus, the enzyme may have a strong substrate preference for uncrosslinked glycan chains, a possibility that is supported by the observation that pretreatment of purified cell wall sacculi with an endopeptidase stimulates the release of soluble material by Slt (Romeis and Höltje, 1994). This apparent substrate preference may contribute to its role in the futile cycle in addition to, or independent of, an interaction with the PBPs. Notably, Slt functions primarily as an exoglycosidase (Beachey et al., 1981) with some detectable endoglycosidase activity (Lee et al., 2013). Although it has been argued that Slt degrades glycan chains from an anhydro-MurNAc end (van Asselt et al., 1999), the structural data are also consistent with Slt processing glycan strands from the non-reducing end as has been demonstrated biochemically. We therefore envision that Slt may either recognize an existing, free nonreducing end of an uncrosslinked glycan, or in the case of partially crosslinked material, it could create such an end with its weak endoglycosidase activity, followed by the degradation of the remaining uncrosslinked glycan chain.
The physiological role of Slt has remained mysterious for some time. It has been implicated in the turnover of mature cell wall material and cell separation (Heidrich et al., 2002; Kraft et al., 1999). However, these functions have been ascribed to Slt based on the phenotypes of mutants lacking multiple PG processing enzymes, suggesting that they represent secondary activities. Clues to the likely primary physiological function of Slt were provided by our analysis of the response of Δslt cells to mecillinam treatment. In the absence of Slt, PG produced by mecillinam-targeted synthetic complexes was found to be aberrantly incorporated into the PG matrix via the formation of alternative (3-3) crosslinks by non-PBP transpeptidases. Additionally, the morphology of Δslt cells was dramatically altered following exposure to low doses of mecillinam while the same treatment had little effect on the shape of wild-type cells. Because these drug concentrations are well below the IC50 for PBP2 (Curtis et al., 1979), only a fraction of the cellular PBP2 pool is predicted to be inhibited in cells treated with this level of mecillinam. Thus, the morphological changes displayed by Δslt cells exposed to low concentrations of mecillinam are unlikely to be due to general inhibition the Rod system. Instead, we conclude that misincorporation of uncrosslinked PG produced by mecillinam-targeted machines is likely to be responsible for the shape change of the mutant, most likely because the aberrant material creates a local defect in the matrix that interferes with the proper assembly or remodeling of PG by other (untargeted) machines. By extension, we infer that a major function of Slt is to prevent the stable accumulation of uncrosslinked glycan strands so that they are not aberrantly crosslinked into the mature matrix. Such an activity is consistent with a novel “quality-control” or “repair” function that helps maintain cell shape and integrity.
In our experimental system, low drug concentration most likely serves to mimic environmental damage to the synthetic machinery or other natural conditions resulting in the uncoupling of GT and TP activities. For example, uncrosslinked glycan strands might accumulate if synthetic complexes enter an area of the PG matrix with few peptides available to accept crosslinks. In these cases, we envision that glycan chain degradation by Slt might facilitate the exchange of a damaged TP for a functional one, and/or allow the release of stalled complexes tethered to a partially crosslinked glycan strand so that they can restart synthesis at other locations. In support of the former possibility, recent studies indicate that bPBPs are likely to be dynamically associated with the synthetic machinery (Lee et al., 2014).
An additional phenotype consistent with a quality control function for Slt is the rapid lysis displayed by Δslt cells treated with cephalexin (Templin et al., 1992). PBP3 inactivation normally results in a division block and the formation of filamentous cells that lyse many mass doublings after cephalexin addition (Spratt, 1975). However, cells defective for Slt, lyse rapidly following cephalexin treatment (Templin et al., 1992). Rapid lysis under these conditions is known to require the activity of the cell separation amidases (Heidrich et al., 2001). By analogy with mecillinam-treated cells, addition of cephalexin to Δslt cells likely results in the production of stable uncrosslinked glycan strands that can be misincorporated by alternative crosslinking enzymes. Lysis may result because this misincorporated material mimics productive PG synthesis by the divisome such that the cell separation enzymes are activated to process the malformed material resulting in a localized breach in the PG. Thus, in addition to shape maintenance, the proposed quality control function of Slt may also enforce the coupling of GT and TP activities to prevent the misactivation of PG hydrolases by damaged PG synthases. Taking this line of reasoning a step further, it is interesting to speculate that it may be the failure of Slt to process certain types of glycan strands (e.g. those with high pentapeptide content) that underlies the ability of some beta-lactam drugs to induce rapid cell lysis via the misactivation of autolytic PG hydrolases.
Slt, beta-lactamase induction, and potential combination therapies
Many gram-negative bacteria encode a beta-lactamase (AmpC) produced in response to beta-lactam treatment (Fisher and Mobashery, 2014). In some organisms, expression of the ampC gene is controlled by the transcriptional regulator AmpR, which normally represses ampC expression (Jacobs et al., 1997). Mutants defective for the AmpD amidase involved in cell wall recycling were found to overexpress ampC even in the absence of beta-lactams (Jacobs et al., 1994). This finding along with supporting in vitro transcription experiments has led to a model in which beta-lactam treatment leads to the accumulation of anhMurNAc-peptide turnover products in the cytoplasm that serve as a signal for ampC induction (Fisher and Mobashery, 2014; Jacobs et al., 1997). It has been widely assumed that these recycling intermediates increase in concentration because beta-lactams induce general cell wall damage. Here, we have shown that targeting PG synthetic machines with beta-lactams causes nascent PG to be degraded by Slt, generating high levels of anhMurNAc-peptides. Thus, rather than generic insults to the PG matrix, the ampR-ampC regulatory system is perfectly tuned to detect the futile cycle of PG synthesis and degradation induced upon TP inhibition. Importantly, based on the critical role observed for Slt in ampC induction (Kraft et al., 1999) and the hypersensitivity of Slt-defective mutants to beta-lactams (Templin et al., 1992), it has been proposed that Slt inhibitors may be useful in combination therapies with beta-lactams (Kraft et al., 1999). Our results provide a mechanistic picture of what such combinations would likely accomplish. The downside of blocking Slt activity simultaneously with beta-lactam treatment would be loss of the toxic effects of the futile cycle and subsequent precursor depletion. However, what would be gained is an alternative malfunction of PG biogenesis caused by the accumulation of uncrosslinked glycan strands that can cause lysis or lethal shape changes as described above. Thus, inhibiting Slt function should indeed be an effective way to stymie beta-lactamase induction systems in gram-negative pathogens while still allowing beta-lactams to derange the PG biogenesis machinery to promote cell death.
Conclusion
Beta-lactams are arguably one of the most important therapeutic classes in the history of medicine. In this report, we present data that changes the view of how these drugs work. They are not simple inhibitors of cell wall synthesis as is commonly believed. Instead, they derange the activity of the PG biogenesis machinery to induce a futile cycle of PG synthesis and degradation that depletes cellular resources. While investigating the nature of the futile cycle, we uncovered what appears to be a new role for PG-cleaving enzymes as quality control factors that intervene when problems arise during PG synthesis. Overall, these new insights provide a mechanistic foundation that will not only help us better understand the process of cell wall biogenesis, but also how best to interfere with it for the development of novel antibacterial treatments.
EXPERIMENTAL PROCEDURES
Media, bacterial strains, plasmids, and culture conditions
Cells were grown in LB or minimal M9 medium supplemented with 0.2% casamino acids and carbon source (0.4% glycerol or 0.2% maltose) as indicated. The bacterial strains and plasmids used in this study are listed in Tables S2 and S3, respectively.
Measurement of the turnover of newly synthesized PG
The effect of beta-lactams and A22 on the turnover of nascent PG was monitored by using ΔlysA ΔampD strains essentially as described previously (Uehara and Park, 2008). Cells of the relevant strains were grown overnight in M9-glycerol medium with 0.2% casamino acids. The resulting cultures were then diluted to an OD600 of 0.04 in the same medium and grown to an OD600 between 0.3–0.4 at 30°C. For experiments measuring PG synthesis and turnover by the Rod complex, sulA expression was induced from a chromosomally integrated plasmid (pHC739) for 30 min before drug treatment. The cultures were then treated with the indicated beta-lactams and/or A22 for 5 min after adjusting the culture OD600 to 0.3. Following drug treatment, [3H]-mDAP (1 μCi) was added to 1mL of each culture for 10 min (1/10 of the doubling time) to label the newly synthesized PG and its turnover products. After the labeling, cells were pelleted, resuspended in 0.7 ml water, and heated at 90°C for 30 min to extract water-soluble compounds. After the hot water extraction, insoluble material was pelleted by ultracentrifugation. The resulting supernatant was then removed, lyophilized, and resuspended in 0.1% formic acid for HPLC analysis. To determine [3H]-mDAP incorporated into the PG matrix, the pellet fraction was washed and treated with lysozyme. The suspensions were incubated overnight at 37°C. Insoluble material was then pelleted by centrifugation, and the resulting supernatant was subjected to scintillation counting. Similar procedures were used for measuring PG synthesis and turnover by the divisome following cephalexin treatment except that Rod system activity was suppressed by treating the cultures with A22. See Supplementary Information for a detailed protocol.
Muropeptide analysis of PG sacculi isolated from drug-treated cells
Cultures of MG1655 and its Δslt derivative, HC408, were diluted in 1 liter of LB or LB containing 10 μg/mL mecillinam to OD600 of 0.025 and incubated at 30°C for 3 hrs with shaking. The OD600 of each culture reached approximately 0.6 at the end of the growth period. PG was then purified from each culture and analyzed by LC/MS as described in Supplemental Information.
Supplementary Material
Cells of TU233 [ΔmreBCD], AG7 [ΔmreD], TU232 [ΔmrdAB], and TU231 [ΔmrdB] all containing the pTB63 [ftsQAZ] plasmid were grown to saturation in LB broth, diluted, and plated on LB with mecillinam (1 μg/ml). Plates were also incubated overnight at 30°C and photographed.
A–B. An overnight culture of MG1655 was diluted 1:100 in LB broth and grown at 30°C to exponential phase. They were then diluted to an OD600 of 0.02 in LB broth supplemented with A22, mecillinam, or both drugs as above. Where appropriate, an equivalent amount of DMSO was added to mimic the addition of A22. Growth was then continued at 30°C and OD600 (A) and viability (colony forming units, cfu) (B) were monitored for several hours. Error bars in (B) represent the range of cfu measured in three independent experiments. C. Production of a PBP2 variant defective for TP activity mimics mecillinam treatment. Cells of TU232/pTB63 [ΔpbpA-rodA/ftsQAZ] harboring the medium-copy vectors pRY47 (vector), pHC857 (Plac::pbpA-rodA), or pHC858 (Plac::pbpA(S330A)-rodA) were grown overnight in M9-Maltose-chloramphenicol (Cam, 25 μg/ml), diluted, and spotted on LB-Cam agar with 1 mM IPTG and A22 (10 μg/ml) as indicated. Notice that production of PBP2(S330A) is lethal unless the Rod complex is inhibited with A22. D. Cells of MG1655/pTB63 harboring either a vector control (pHC800), pHC808 (Ptac::uppS), or pHC851 (Ptac::murA) as indicated were grown to saturation in LB supplemented with Cam (25 μg/ml), tetracycline (5 μg/ml), and IPTG [10 μM (left) or 100 μM (right)]. Serial dilutions were then prepared and spotted onto LB medium containing IPTG [10 μM (left) or 100 μM (right)] and mecillinam (1 μg/ml). The plates were photographed after two days at 30°C.
Pulse labeling studies were performed in our WT labeling strain TU278 [ΔlysA ΔampD] or its ΔldcA, ΔdacA, or ΔnagZ derivatives exactly as described for Figure 3. Peaks labeled with purple stars were identified based on the retention times of unlabeled authentic standards analyzed by LC/MS with identical separation conditions. Other peaks were identified based on the change in migration of the observed peaks in samples prepared from mutant cells. For example, the peak at 24.9 min observed in mecillinam treated WT cells was identified as anhMurNAc-tripeptide because this peak shifts to anhMurNAc-tetrapeptide (30 min) in mutants defective for LdcA, which converts anhMurNAc-tetrapeptide to the tripeptide species. Similarly, pentapeptide or anhydro-disaccharide species were identified based on their accumulation in strains lacking the DacA carboxypeptidase or the NagZ glucosaminidase, respectively.
Representative HPLC chromatograms for the indicated pulse labeling experiments are shown. Cells in (A) were treated with A22 (10 μg/ml) to focus the analysis on PG synthesis and turnover at the division site, whereas cells in (B) were treated exactly as those in Figure 3 with the exception that cefsulodin (100 μg/ml) replaced mecillinam. Replicates of each experiment were used to generate Figure 4. See text for details.
A. Cells of MG1655 [WT] or HC408 [Δslt] harboring either pSC101 (vector) or pTB63 (FtsZup) were grown overnight, diluted, and spotted on agar containing the indicated concentration of A22 as described for Figure 2B. B. The same cells were grown to an OD600 of approximately 0.5 and diluted 1:20 into LB medium containing the indicated concentration of A22. Growth was continued for an additional 3 hours and the cells were fixed. The fixed cells were then imaged on agarose pads using DIC optics. Bar equals 4 microns.
Cells of MG1655 [WT] or HC408 [Δslt] harboring either pSC101 (vector) or pTB63 (FtsZup) were grown to an OD600 of approximately 0.5 and diluted 1:20 into LB medium containing the indicated concentration of mecillinam and growth was monitored by following culture OD600.
Table S1. Identity of muropeptide species and changes in cell wall composition following mecillinam treatment. Lists the identity of the muropeptides corresponding to the labeled peaks in the chromatograms shown in Fig. 6. Also indicates the change in the abundance of the indicated species following drug treatment.
Table S2. Bacterial strains used in this study.
Table S3. Plasmids used in this study.
Acknowledgments
The authors would like to thank Christina Montero and Alex Godfrey for help with mutant characterization and members of the Bernhardt, Rudner, and Walker laboratories for helpful discussions. We also thank Tom Dougherty, Mary-Jane Tsang, Anastasiya Yakhnina, and David Rudner for comments on the manuscript. Special thanks to Suzanne Walker and her laboratory their help with LC/MS. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01-AI099144 and U19-AI109764).
Footnotes
AUTHOR CONTRIBUTIONS
H.C. designed, performed, and interpreted all experiments. T.U. designed, performed and interpreted critical foundational studies for this work. T.G.B. designed and interpreted the experiments. The paper was written and edited by T.G.B., H.C., and T.U.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banzhaf M, van den Berg van Saparoea B, Terrak M, Fraipont C, Egan A, Philippe J, Zapun A, Breukink E, Nguyen-Distèche M, den Blaauwen T, et al. Cooperativity of peptidoglycan synthases active in bacterial cell elongation. Mol Microbiol. 2012;85:179–194. doi: 10.1111/j.1365-2958.2012.08103.x. [DOI] [PubMed] [Google Scholar]
- Beachey EH, Keck W, de Pedro MA, Schwarz U. Exoenzymatic activity of transglycosylase isolated from Escherichia coli. Eur J Biochem. 1981;116:355–358. doi: 10.1111/j.1432-1033.1981.tb05342.x. [DOI] [PubMed] [Google Scholar]
- Begg K, Nikolaichik Y, Crossland N, Donachie WD. Roles of FtsA and FtsZ in activation of division sites. J Bacteriol. 1998;180:881–884. doi: 10.1128/jb.180.4.881-884.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú FO, de Boer PAJ. Conditional lethality, division defects, membrane involution, and endocytosis in mre and mrd shape mutants of Escherichia coli. J Bacteriol. 2008;190:1792–1811. doi: 10.1128/JB.01322-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt TG, de Boer PAJ. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over Chromosomes in E. coli. Mol Cell. 2005;18:555–564. doi: 10.1016/j.molcel.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman LG, Park JT. Changes in the composition of Escherichia coli murein as it ages during exponential growth. J Bacteriol. 1983;155:447–453. doi: 10.1128/jb.155.2.447-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Yao Z, Goehring NW, Kishony R, Beckwith J, Kahne D. Rapid-lactam-induced lysis requires successful assembly of the cell division machinery. Proc Natl Acad Sci USA. 2009;106:21872–21877. doi: 10.1073/pnas.0911674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa CS, Antón DN. High-level resistance to mecillinam produced by inactivation of soluble lytic transglycosylase in Salmonella enterica serovar Typhimurium. FEMS Microbiol Lett. 2006;256:311–317. doi: 10.1111/j.1574-6968.2006.00133.x. [DOI] [PubMed] [Google Scholar]
- Curtis NA, Orr D, Ross GW, Boulton MG. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979;16:533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BD. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987;51:341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer PAJ. Advances in understanding E. coli cell fission. Curr Opin Microbiol. 2010;13:730–737. doi: 10.1016/j.mib.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JF, Mobashery S. The sentinel role of peptidoglycan recycling in the β-lactam resistance of the Gram-negative Enterobacteriaceae and Pseudomonas aeruginosa. Bioorg Chem. 2014;56C:41–48. doi: 10.1016/j.bioorg.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitai Z, Dye NA, Reisenauer A, Wachi M, Shapiro L. MreB actin-mediated segregation of a specific region of a bacterial chromosome. Cell. 2005;120:329–341. doi: 10.1016/j.cell.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Glauner B, Höltje JV. Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem. 1990;265:18988–18996. [PubMed] [Google Scholar]
- Heidrich C, Templin MF, Ursinus A, Merdanovic M, Berger J, Schwarz H, de Pedro MA, Höltje JV. Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol Microbiol. 2001;41:167–178. doi: 10.1046/j.1365-2958.2001.02499.x. [DOI] [PubMed] [Google Scholar]
- Heidrich C, Ursinus A, Berger J, Schwarz H, Höltje JV. Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J Bacteriol. 2002;184:6093–6099. doi: 10.1128/JB.184.22.6093-6099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC. Mechanisms of action of antimicrobials: focus on fluoroquinolones. Clin Infect Dis. 2001;32(Suppl 1):S9–S15. doi: 10.1086/319370. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Huang LJ, Bartowsky E, Normark S, Park JT. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C, Frère JM, Normark S. Cytosolic Intermediates for Cell Wall Biosynthesis and Degradation Control Inducible β-Lactam Resistance in Gram-Negative Bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- Kohlrausch U, Höltje JV. Analysis of murein and murein precursors during antibiotic-induced lysis of Escherichia coli. J Bacteriol. 1991;173:3425–3431. doi: 10.1128/jb.173.11.3425-3431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AR, Prabhu J, Ursinus A, Höltje JV. Interference with murein turnover has no effect on growth but reduces beta-lactamase induction in Escherichia coli. J Bacteriol. 1999;181:7192–7198. doi: 10.1128/jb.181.23.7192-7198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse T, Bork-Jensen J, Gerdes K. The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex. Mol Microbiol. 2004;55:78–89. doi: 10.1111/j.1365-2958.2004.04367.x. [DOI] [PubMed] [Google Scholar]
- Lee M, Hesek D, Llarrull LI, Lastochkin E, Pi H, Boggess B, Mobashery S. Reactions of all Escherichia coli lytic transglycosylases with bacterial cell wall. J Am Chem Soc. 2013;135:3311–3314. doi: 10.1021/ja309036q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Tropini C, Hsin J, Desmarais SM, Ursell TS, Gong E, Gitai Z, Monds RD, Huang KC. A dynamically assembled cell wall synthesis machinery buffers cell growth. Proc Natl Acad Sci USA. 2014;111:4554–4559. doi: 10.1073/pnas.1313826111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreillon P, Markiewicz Z, Nachman S, Tomasz A. Two bactericidal targets for penicillin in pneumococci: autolysis-dependent and autolysis-independent killing mechanisms. Antimicrob Agents Chemother. 1990;34:33–39. doi: 10.1128/aac.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Strominger J. Mode of Action of Penicillin. Biochemical Basis for the Mechanism of Action of Penicillin and for Its Selective Toxicity. Science. 1957;125:99–101. doi: 10.1126/science.125.3238.99. [DOI] [PubMed] [Google Scholar]
- von Rechenberg M, Ursinus A, Höltje JV. Affinity chromatography as a means to study multienzyme complexes involved in murein synthesis. Microb Drug Resist. 1996;2:155–157. doi: 10.1089/mdr.1996.2.155. [DOI] [PubMed] [Google Scholar]
- Romeis T, Höltje JV. Specific interaction of penicillin-binding proteins 3 and 7/8 with soluble lytic transglycosylase in Escherichia coli. J Biol Chem. 1994;269:21603–21607. [PubMed] [Google Scholar]
- Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- Schwarz U, Asmus A, Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969;41:419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Silver LL. Challenges of antibacterial discovery. Clin Microbiol Rev. 2011;24:71–109. doi: 10.1128/CMR.00030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt BG. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci USA. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin MF, Edwards DH, Höltje JV. A murein hydrolase is the specific target of bulgecin in Escherichia coli. J Biol Chem. 1992;267:20039–20043. [PubMed] [Google Scholar]
- Thunnissen AM, Dijkstra AJ, Kalk KH, Rozeboom HJ, Engel H, Keck W, Dijkstra BW. Doughnut-shaped structure of a bacterial muramidase revealed by X-ray crystallography. Nature. 1994;367:750–753. doi: 10.1038/367750a0. [DOI] [PubMed] [Google Scholar]
- Tipper DJ, Strominger JL. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci USA. 1965;54:1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A, Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci USA. 1975;72:4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A, Albino A, Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970;227:138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Typas A, Banzhaf M, Gross CA, Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol. 2012;10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Bernhardt TG. More than just lysins: peptidoglycan hydrolases tailor the cell wall. Curr Opin Microbiol. 2011;14:698–703. doi: 10.1016/j.mib.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Park JT. Growth of Escherichia coli: significance of peptidoglycan degradation during elongation and septation. J Bacteriol. 2008;190:3914–3922. doi: 10.1128/JB.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Dinh T, Bernhardt TG. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J Bacteriol. 2009;191:5094–5107. doi: 10.1128/JB.00505-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asselt EJ, Thunnissen AM, Dijkstra BW. High resolution crystal structures of the Escherichia coli lytic transglycosylase Slt70 and its complex with a peptidoglycan fragment. J Mol Biol. 1999;291:877–898. doi: 10.1006/jmbi.1999.3013. [DOI] [PubMed] [Google Scholar]
- Vinella D, D’Ari R, Jaffé A, Bouloc P. Penicillin binding protein 2 is dispensable in Escherichia coli when ppGpp synthesis is induced. EMBO J. 1992;11:1493–1501. doi: 10.1002/j.1460-2075.1992.tb05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinella D, Joseleau-Petit D, Thévenet D, Bouloc P, D’Ari R. Penicillin-binding protein 2 inactivation in Escherichia coli results in cell division inhibition, which is relieved by FtsZ overexpression. J Bacteriol. 1993;175:6704–6710. doi: 10.1128/jb.175.20.6704-6710.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi M, Doi M, Tamaki S, Park W, Nakajima-Iijima S, Matsuhashi M. Mutant isolation and molecular cloning of mre genes, which determine cell shape, sensitivity to mecillinam, and amount of penicillin-binding proteins in Escherichia coli. J Bacteriol. 1987;169:4935–4940. doi: 10.1128/jb.169.11.4935-4940.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells of TU233 [ΔmreBCD], AG7 [ΔmreD], TU232 [ΔmrdAB], and TU231 [ΔmrdB] all containing the pTB63 [ftsQAZ] plasmid were grown to saturation in LB broth, diluted, and plated on LB with mecillinam (1 μg/ml). Plates were also incubated overnight at 30°C and photographed.
A–B. An overnight culture of MG1655 was diluted 1:100 in LB broth and grown at 30°C to exponential phase. They were then diluted to an OD600 of 0.02 in LB broth supplemented with A22, mecillinam, or both drugs as above. Where appropriate, an equivalent amount of DMSO was added to mimic the addition of A22. Growth was then continued at 30°C and OD600 (A) and viability (colony forming units, cfu) (B) were monitored for several hours. Error bars in (B) represent the range of cfu measured in three independent experiments. C. Production of a PBP2 variant defective for TP activity mimics mecillinam treatment. Cells of TU232/pTB63 [ΔpbpA-rodA/ftsQAZ] harboring the medium-copy vectors pRY47 (vector), pHC857 (Plac::pbpA-rodA), or pHC858 (Plac::pbpA(S330A)-rodA) were grown overnight in M9-Maltose-chloramphenicol (Cam, 25 μg/ml), diluted, and spotted on LB-Cam agar with 1 mM IPTG and A22 (10 μg/ml) as indicated. Notice that production of PBP2(S330A) is lethal unless the Rod complex is inhibited with A22. D. Cells of MG1655/pTB63 harboring either a vector control (pHC800), pHC808 (Ptac::uppS), or pHC851 (Ptac::murA) as indicated were grown to saturation in LB supplemented with Cam (25 μg/ml), tetracycline (5 μg/ml), and IPTG [10 μM (left) or 100 μM (right)]. Serial dilutions were then prepared and spotted onto LB medium containing IPTG [10 μM (left) or 100 μM (right)] and mecillinam (1 μg/ml). The plates were photographed after two days at 30°C.
Pulse labeling studies were performed in our WT labeling strain TU278 [ΔlysA ΔampD] or its ΔldcA, ΔdacA, or ΔnagZ derivatives exactly as described for Figure 3. Peaks labeled with purple stars were identified based on the retention times of unlabeled authentic standards analyzed by LC/MS with identical separation conditions. Other peaks were identified based on the change in migration of the observed peaks in samples prepared from mutant cells. For example, the peak at 24.9 min observed in mecillinam treated WT cells was identified as anhMurNAc-tripeptide because this peak shifts to anhMurNAc-tetrapeptide (30 min) in mutants defective for LdcA, which converts anhMurNAc-tetrapeptide to the tripeptide species. Similarly, pentapeptide or anhydro-disaccharide species were identified based on their accumulation in strains lacking the DacA carboxypeptidase or the NagZ glucosaminidase, respectively.
Representative HPLC chromatograms for the indicated pulse labeling experiments are shown. Cells in (A) were treated with A22 (10 μg/ml) to focus the analysis on PG synthesis and turnover at the division site, whereas cells in (B) were treated exactly as those in Figure 3 with the exception that cefsulodin (100 μg/ml) replaced mecillinam. Replicates of each experiment were used to generate Figure 4. See text for details.
A. Cells of MG1655 [WT] or HC408 [Δslt] harboring either pSC101 (vector) or pTB63 (FtsZup) were grown overnight, diluted, and spotted on agar containing the indicated concentration of A22 as described for Figure 2B. B. The same cells were grown to an OD600 of approximately 0.5 and diluted 1:20 into LB medium containing the indicated concentration of A22. Growth was continued for an additional 3 hours and the cells were fixed. The fixed cells were then imaged on agarose pads using DIC optics. Bar equals 4 microns.
Cells of MG1655 [WT] or HC408 [Δslt] harboring either pSC101 (vector) or pTB63 (FtsZup) were grown to an OD600 of approximately 0.5 and diluted 1:20 into LB medium containing the indicated concentration of mecillinam and growth was monitored by following culture OD600.
Table S1. Identity of muropeptide species and changes in cell wall composition following mecillinam treatment. Lists the identity of the muropeptides corresponding to the labeled peaks in the chromatograms shown in Fig. 6. Also indicates the change in the abundance of the indicated species following drug treatment.
Table S2. Bacterial strains used in this study.
Table S3. Plasmids used in this study.