Abstract
Background
Chlamydia trachomatis (C. trachomatis) is the most prevalent cause of bacterial sexually transmitted infections (STI) recognized throughout the world. The aim of this study is to determine different genotypes of genital C. trachomatis and the association between the serological markers of inflammation and genotypes of C. trachomatis in sexually active women (n=80) attending Shahid Beheshti Hospital in Isfahan, Iran.
Materials and Methods
In this descriptive study, endocervical swabs were collected from 80 women. There were 17 endocervical samples that showed positivity for C. trachomatis by plasmid polymerase chain reaction (PCR) using KL1 and KL2 primers. The omp1 gene was directly amplified in 17 plasmid PCR positive samples and was used to differentiate the clinical genotypes by omp1 gene PCR-restriction fragment length polymorphism (PCR-RFLP). The levels of IgG and IgA specific to C. trachmatis and C-reactive protein (CRP) were evaluated.
Results
Based on restriction-digestion patterns, four genotypes were identified. Genotypes E (35.3%) and F (35.3%) were the most prevalent, followed by D/Da (23.5%) and K (5.9%). There was no significant association between genotypes and the presence of IgG and CRP. Patients infected with genotype E showed a serological marker of chronic inflammation, i.e. IgA seropositivity, significantly more than patients infected with other genotypes (p=0.042).
Conclusion
Nested PCR could increase the sensitivity of omp1 amplification. Based on the presence of IgA, chronic C. trachomatis infections were observed more frequently among genotype E-infected patients in our population.
Keywords: Chlamydia trachomatis, Genital Infection, Genotype, PCR, RFLP, Immune Markers
Introduction
Clamydia trachomatis (C. trachomatis) is the most common bacterial cause of sexually transmitted infections (STI). The World Health Organization estimates that approximately 92 million new cases of genital chlamydial infection occur worldwide annually (1, 2). Because 50% of infections in men and 80% in women are asymptomatic, the actual number of cases seems to be more than the estimated number (1, 3). Currently, 19 human genotypes and numerous variants including A, B/ Ba, C, D/Da, E, F, G/Ga, H, I/Ia, J, K, L1, L2/L2a, and L3 have been identified using polyclonal and monoclonal antibodies against the major outer membrane protein (MOMP) (4, 5). MOMP is a predominant antigen, which is different in various strains of C. trachomatis (6-8). Genotypes A-C (the trachoma biovars) cause conjunctivitis and lead to trachoma, the primary cause of preventable blindness in third world countries (8). Genotypes D-K are the most important cause of urogenital and neonatal infections. They are the most common cause of sexually transmitted genital infections, eliciting local acute epithelial infections, which can lead to pelvic inflammatory disease in women. Untreated infection or chronic infection can occasionally cause infertility, potentially fatal ectopic pregnancy, and premature delivery (7- 9). Genotypes L1-L3 cause the invasive disease known as lymphogranuloma venerum (7, 8).
Sequencing of the omp1 gene in C. trachomatis showed a significant difference among genotypes (10). Restriction fragment length polymorphism (RFLP) analysis of omp1 gene has been used to differentiate genotypes of C. trachomatis by using different enzymes (11-14).
Determination of the epidemiological relationship between the C. trachomatis genotype from different areas could be a suitable guideline for designing epidemiological programs for controlling chlamydial infections, and consequently controlling sexually transmitted diseases (STDs). Considering the importance of this issue, the aim of this study was to determine the prevalence of C. trachomatis genotypes in symptomatic cervical infections in Iranian women. A hypothesis currently under investigation states that genetic variations in the C. trachomatis genome may account for strain (genotype) differences in the course and outcome of infection with this bacteria (15-17). The chronic status in the course of a C. trachomatis infection is one of the most important aspects of this infection. It is associated with the persistence of the bacteria in the host cells that increases the risk of tubal factor subfertility (18). IgA antibodies are assumed to reflect chronic inflammation (19, 20). A level of C-reactive protein (CRP) >10 mg/l usually occurs in acute infections, and can be detected using common tests for CRP. A CRP level <1 mg/l indicates the absence of inflammation or infection (21). Because CRP is a general serological marker of acute inflammation, another object of this study is to detect the serological markers of acute and chronic infections in patients, with the intent to evaluate the differences among different genotypes.
Materials and Methods
Study population and sample collection
This descriptive study was approved by the Ethics Committee of Shahid Beheshti Hospital. Samples were collected after obtaining written informed consent from 80 patients who attended the Gynecology Outpatient Department of Shahid Beheshti Hospital in Isfahan, Iran in 2008. An endocervical swab from each individual was transferred to 5 ml of sterile phosphate buffered saline (PBS) and stored at -70˚C until DNA extraction. In addition, 5ml of peripheral blood was collected from each patient for serological investigation (22, 23).
DNA extraction
The endocervical swab sample was removed from the vial and the PBS collection tube was centrifuged at 2000 rpm for 15 minutes. The supernatant was discarded and the bottom was vortexed and transferred to a 1.5 ml microtube. This step was followed by centrifugation at 2000 rpm for 15 minutes. The supernatant was then removed. Then, 400 μl of tris-base-EDTA (TE) solution that contained 10 mM tris–HCl, pH= 8.0 and 1 mM EDTA, was added. 4 μl proteinase K (10 μg/ml) and 4 μl triton 10% (v/v) were added and incubated at 55˚C for 90 minutes, followed by 95˚C for 30 minutes. These samples were maintained at -20˚C until used (22-24).
Plasmid PCR
All 80 samples were examined by C. trachomatis plasmid-based PCR using KL1 and KL2 primers. Successful amplification of a 241 bp fragment of the bacterial endogenous plasmid genome was considered a positive result by polymerase chain reaction (PCR). The primers used for the C. trachomatis plasmid PCR were KL1 (5′-TCCGGAGCGAGTACGAAGA-3′) and KL2 (5′-AATCAATGCCCGGGATTGGT-3′; Metabion, Germany) (9, 22, 24). The final reaction mix contained 5 μl of the extracted DNA sample, 16 pM of each primers, 0.28 μM deoxynucleotide triphosphate (Cinnagen, Iran), 3 mM MgCl2 (Cinnagen, Iran), and 1 U of Taq polymerase (Cinnagen, Iran), for a total volume of 25 μl (9, 22, 24). The amplification protocol was 10 minutes of DNA denaturation at 94˚C followed by 40 cycles of amplification, with each cycle that consisted of denaturation at 94˚C for 1 minute, annealing at 55˚C for 1 minute, and extension at 72˚C for 1 minute. The final extension cycle at 72˚C was prolonged for 8 minutes (9, 22, 24). The PCR products were analyzed by 1.5% agarose gel electrophoresis. C. trachomatis genotype A was used as positive control and a sample that contained only distilled water was used as a negative control.
Omp1 PCR
We examined 17 plasmid PCR-positive samples for omp1 PCR. An approximately 1.2 kb fragment of the omp1 gene was amplified in the 17 plasmid-based PCR positive samples using three primers: CT1 (forward strand: 5′- GCCGCTTTGAGTTCTGCTTCCTC- 3′), CT5 (reverse strand: 5′- ATTTACGTGAGCAGCTCTCTCAT-3′), and PCTM3 (forward strand: 5′- TCCTTGCAAGCTCTGCCTGTGGGGAATCCT- 3′; Gene Fanavaran) (14). Primary PCR was performed on 10 μl of the extracted DNA in a final reaction mixture of 50 μl. The final reaction mixture contained 10 mM tris-HCl (pH= 8.3; Cinnagen), 50 mM KCl (Cinnagen), 1.5 mM MgCl2 (Cinnagen), 200 μM from each deoxynucleoside triphosphate (dATP, dTTP, dGTP, and dCTP; Cinnagen), 25 ρmol of each primer CT1, CT5, and 1U of Taq DNA polymerase (Cinnagen). The amplification protocol was 5 minutes of DNA denaturation at 95˚C followed by 35 cycles of amplification, with each cycle that consisted of denaturation at 95˚C for 1 minute, annealing at 55˚C for 1 minute, and extension at 72˚C for 1.5 minutes. The final extension cycle at 72˚C was prolonged for 4 minutes (14). In this study, an Eppendorf Master Cycler Epgradient was used. The semi-nested PCR was carried out in the following manner: 1 μl of the primary PCR product (as the DNA template) was added to a prepared PCR mixture that contained primer PCTM3, located 22 bp downstream of CT1 and the previous primer, CT5. The position of CT1 is at 34-56 bp and PCTM3 is at 55-84 bp. The amplification conditions of the semi-nested PCR were the same as the conditions of the primary PCR. The PCR products were analyzed by 1% agarose gel electrophoresis (14). For a 1% agarose gel we added 1 g of agarose to 100 ml of 1x electrophoresis buffer and 0.5 μg/ml ethtidium bromide to the molten agarose. In this study, C. trachomatis genotype E was used as a positive control and a sample that contained distilled water instead of DNA was used as a negative control.
RFLP
The omp1 seminested PCR products were digested with restriction enzymes according to the previous study (12, 14). Restriction digestion was performed in two manners: i. single digestion with restriction enzyme AluI; ii. triple digestion with three enzymes HpaII, EcoRI, and HinfI. The first digestion was carried out with 10 μl of amplified DNA on 4 U of AluI, using the assay buffer recommended by the manufacturer, at 37˚C for 4 hours. The second digestion was performed on 10 μl of amplified DNA, first with 4 U of HpaII in 10 mM tris HCl (pH=7.6) and 10 mM MgCl2 at 37˚C for 4 hours. HpaII was then added in a 10 minute incubation at 60˚C. Next, 2 μl of 200 mM tris HCl (pH=8) and 75 mM NaCl were added and samples were incubated overnight with 4 U of EcoRI and Hinfl at 37˚C (12). Rodriguez et al. reported the single digestion differentiates between ten genotypes: A, C, E, F, G, I, J, K, L1, and L2, while B, Ba, D, H, and L3 show similar patterns. The triple digestion differentiates 11 genotypes: D, E, F, G, H, I, J, K, L1, L2, and L3 but genotypes A, C and B, Ba have similar patterns (12,14). In our study, a triple digestion was carried out to discriminate genotypes D/Da from B/Ba. Genotype D was not differentiated from Da, because D and Da have similar patterns in both single and triple digestion (12). CfoI digestion can be used to differentiate genotype D from Da (25).
Digestion products were analyzed by a 6% polyacrylamide gel stained with 15 μg of ethidium bromide per ml. For identification of the clinical strains, the RFLP pattern of each sample was compared with the omp1 restriction fragment sizes (larger than 100 bp) presented in the previous study (12).
Serological tests
Blood samples were taken from 80 women and the sera were used to determine the level of IgG and IgA antibodies against C. trachomatis using p-ELISA kits (Medac, Germany). The p-ELISA was based on a synthetic peptide from the immunodominant region of the major outer membrane protein. The ELISA kit used in this study was very specific for detecting C. trachomatis without any cross activity with other species of Chlamydia. The level of CRP was determined by using a CRP kit (Omega, UK). The sensitivity of the CRP test was 6 mg/L. Both tests were performed according to the manufacturers’ instructions.
Statistical analysis
Analysis of the association of genotypes and the serological markers was carried out by a chisquare test. Data analysis was performed with SPSS statistical software version 15.0. P value less than 0.05 was considered significant.
Results
The successful amplification of a 241 bp fragment of the C. trachomatis plasmid genome was considered a positive result by plasmid-PCR. A total of 17 out of 80 samples (21.25%) were positive for C. trachomatis by KL1 and KL2 primers. omp1 was successfully amplified by CT1-CT5 PCR (primary-PCR) in 14 of 17 samples (82.3%). In the three remaining samples, omp1 was amplified after nested-PCR with PCTM3 and CT5 primers. AluI digestion resulted in four genotypes: D/ Da (or B/Ba), E, F and K. Digestion with HpaII, EcoRI, and HinfI were performed for distinguishing the D/Da and B/Ba genotypes from each other. The triple digestion patterns of these samples corresponded with genotype D/Da (Fig 1). Restriction patterns were compared with acquired restriction patterns in reference 12. Of 17 samples, there were 4 (23.5%) genotype D/Da, 6 (35.3%) genotype E, 6 (35.3%) genotype F, and 1 (5.9%) genotype K. RFLP patterns of omp1-seminested PCR products of four genotypes in single digestion and of one genotype (D/ Da) in triple digestion manner are shown in figure 1. Out of 17 patients, 5 (29.4%) showed positivity for IgG, 3 (17.6%) for IgA, and 6 (35%) for CRP. Table 1 shows the relationship between the serological characteristics of infections and genotypes.
Fig 1.
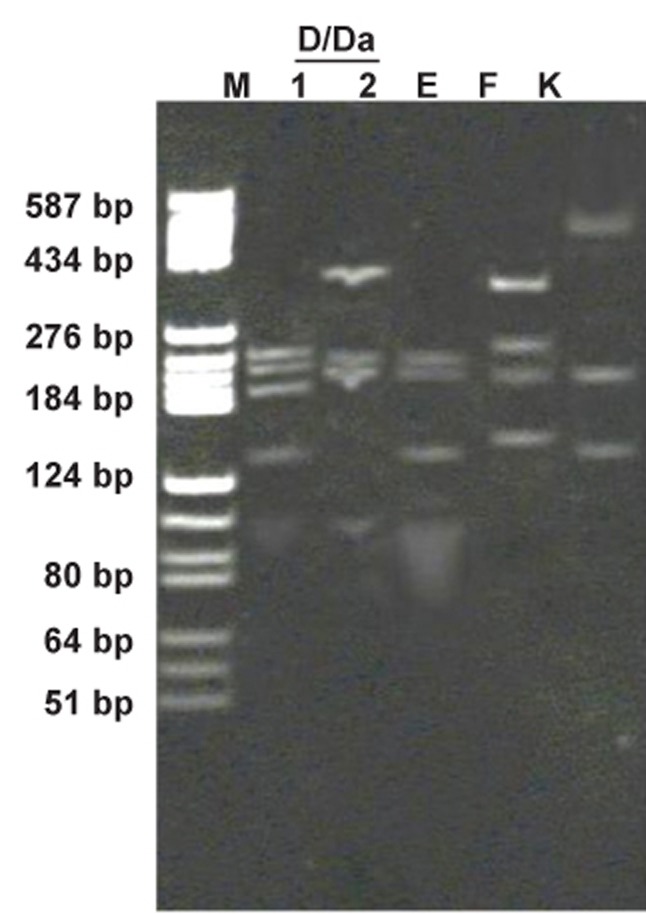
Restriction patterns were compared with acquired restriction patterns in reference 12. RFLP patterns of four clinical genital C. trachomatis strains (D/Da, E, F, and K) obtained by 6% polyacrylamide gel electrophoresis after restriction hydrolysis of the omp1 gene-PCR products. Lane 1: AluI digestion of genotype D/Da. Lane 2: HpaII-EcoRI-HinfI digestion of genotype D/Da. Lanes E, F, and K indicate AluI digestion of genotypes E, F, and K, respectively. Lane M: pBR322 digested with HaeIII as the DNA size marker.
Table 1.
Serological features of infections in relation to C. trachomatis genotypes
| Serological feature | Genotype D/Da | Genotype E | Genotype F | Genotype K | P value |
|---|---|---|---|---|---|
| IgG | 1/4 (25%) | 2/6 (33.3%) | 2/6 (333%) | 0(%) | 0.42 |
| IgA | 0/4 (0%) | 3/6 (50%) | 0 (0%) | 0(0%) | 0.04 |
| CRPa | 2/4 (50%) | 1/6 (16.7%) | 3/6 (50%) | 1/1 (100%) | 0.18 |
| Without immunological | 1 | 2b | 1 | 0 | |
| markers | |||||
| Total | 4 | 6 | 6 | 1 | |
a; C-reactive protein.
b; Two samples of genotype E were IgG and IgA double-seropositive. Out of 6 genotype E samples, 2 samples were without immunological markers.
Discussion
C. trachomatis is a common sexually transmitted infection with significant impact on public health. Therefore, effective epidemiological control as well as a correct and sensitive diagnostic method for C. trachomatis are required (22, 23). In order to develop epidemiological data and to detect various genotype infections, accurate and specific typing of C. trachomatis genotypes is necessary (12). Molecular techniques, like PCR-RFLP, are more sensitive and less time-consuming than immunofluorescence (12). Also, since immunotyping has serious limitations, a suitable and convenient tool that can be used to type and survey epidemiological studies is PCR-RFLP (12). In the current study, 17 out of 80 samples were positive with plasmid PCR. The plasmid-PCR showed 10x higher sensitivity than MOMP-PCR due to having ten copies of plasmid in the elementary body compared to the MOMP gene, which has only one (11, 24). Thus in this study plasmid-PCR was used as a gold standard.
Results of omp1 PCR showed that the nested PCR could increase the sensitivity of omp1 amplification, because the omp1 fragment was amplified in 14 out of 17 samples using primary PCR with CT1 and CT5 primers (82.3%), whereas omp1 was successfully amplified in the 3 remaining samples by using nested PCR with pCTM3 and CT5. This manner could be useful, particularly for direct PCR on crude suspensions of samples that contain low copy numbers of Chlamydia. RFLP patterns with fragments larger than 100 bp produced from the CT1-CT5 sequence of the omp1 gene for the 15 C. trachomatis genotypes in the previous study were considered as reference patterns in this study (12), but for optimizing omp1-PCR in the present study, the pCTM3- CT5 sequence was amplified and used for RFLP. Based on BLAST searching at the NCBI, the first restriction sites for AluI (AGCT) is located at 63-66 bp of the omp1 gene, and for HinfI (GAATC) at 78-82 bp of omp1 gene, and both are apparent on the pCTM3 primer. Therefore, RFLP patterns of products of CT1- CT5-PCR and PCTM3-CT5-PCR in single digestion are different in the 64 bp fragment and in triple digestion in a 78 bp fragme nt, which both are smaller than 100 bp.
In this study, genotypes E and F were the most prevalent genotypes, followed by genotype D/Da and K. The first investigation on genotyping of C. trachomatis was performed in Ahvaz, Iran. This study showed that the most prevalent genotype was E (31.5 %), followed by F (23.1 %), D/Da (13 %), K (9.2 %), I (8.3 %), G (7.5 %), H (5.5 %), and J (1.9 %) (14). In other parts of the world, genotypes E, F, and D were responsible for the most genital C. trachomatis infections (26-29).
The acute phase protein CRP is a general serological marker of inflammation. In this study, there was no significant association between the level of CRP, IgG antibodies, and genotypes (p=0.18 and 0.42, respectively). Serum IgG antibodies against microorganisms usually remain detectable for many years, even after antibiotic treatment (30).
Detecting merely IgA antibody by p-ELISA, indicating that C. trachomatis infection may be present at an early stage of acute infection (31). The presence of high titer of IgA antibodies is associated with chronic inflammation (19, 20). In this study, we have shown that genotype E samples were significantly related to IgA seropositivity (p=0.042), which indicated chronic C. trachomatis infections were observed more frequently among genotype E. Molano et al. reported that chronic C. trachomatis infections were observed more frequently among D and E genotypes, with a lower frequency among genotypes B, H, I, and K (27). These researchers have also shown that in a mouse model, the duration of lower genital tract infection was longest with D and E genotypes, and improvement in the upper genital tract occurred more often in mice infected with D genotype compared to mice infected with H genotype (27).
Our data and the above mentioned studies have indicated that the course of a C. trachomatis infection (such as whether the infection will be cleared or chronic) may be influenced by differences among various genotypes. However, there have not been enough studies undertaken in these areas to prove this theory. Serological responses to various genotypes might differ in different areas and populations. They can be justified with the role of genetic variations in immunologically important host genes in the course and outcome of infectious. To our knowledge, the evaluation of the relationship between C. trachomatis genotypes and features of the serological response has not been carried out in Iran. Further studies need to be done on larger populations in different parts of the world.
Conclusion
Our data showed that the nested PCR could increase the sensitivity of omp1 amplification. Genotypes E and F were the most prevalent genotypes in this cohort. It was also shown that there was no significant association between the levels of CRP, IgG antibodies, and different genotypes. Furthermore, genotype E samples were significantly related to IgA seropositivity.
Acknowledgments
The authors would like to thank the Vice Chancellor of Research of Shahid Chamran University and the University of Isfahan for their financial support. We are also thankful to the Head of the Biology and Biotechnology Research Center of Shahid Chamran University for providing laboratory facilities. There is no conflict of interest in this article.
References
- 1.World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infection: overview and estimates. Switzerland: WHO/HIV_AIDS; 2001. [Google Scholar]
- 2.Castellsague´ X, Peeling RW, Franceschi S, De Sanjose S, Smith JS, Albero G, et al. Chlamydia trachomatis infection in female partners of circumcised and uncircumcised adult men. Am J Epidemiol. 2005;162(9):907–916. doi: 10.1093/aje/kwi284. [DOI] [PubMed] [Google Scholar]
- 3.Gaydos CA, Theodore M, Dalesio N, Wood BJ, Quinn TC. Comparison of three nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens. J Clin Microbial. 2004;42(7):3041–3045. doi: 10.1128/JCM.42.7.3041-3045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu MC, Tsai PY, Chen KT, Li LH, Chiang CC, Tsai JJ, et al. Genotyping of Chlamydia trachomatis from clinical specimens in Taiwan. J Med Microbiol. 2006;55(Pt 3):301–308. doi: 10.1099/jmm.0.46262-0. [DOI] [PubMed] [Google Scholar]
- 5.Ngandjio A, Clerc M, Fonkoua MC, Thonnon J, Lunel F, Bebear C, et al. Restriction endonuclease patterns of omp1 gene of references Chlamydia trachomatis strains and characterization of isolates from cameroonian students. J Med Microbiol. 2004;53(Pt 1):47–50. doi: 10.1099/jmm.0.05333-0. [DOI] [PubMed] [Google Scholar]
- 6.Morre' SA, Ossewaarde JM, Lan J, van Doornum GJ, Walboomers JM, Maclaren DM, et al. Serotyping and genotyping of genital Chlamydia trachomatis isolates reveal variants of serovars Ba, G, and J as confirmed by omp1 nucleotide sequence analysis. J Clin Microbiol. 1998;36(2):345–351. doi: 10.1128/jcm.36.2.345-351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturm-Ramirez K, Brumblay H, Diop K, Gueye-Ndiaye A, Sankale JL, Thior I, et al. Molecular epidemiology of genital Chlamydia trachomatis infection in high-risk women in Senegal, West Africa. J Clin Microbial. 2000;38(1):138–145. doi: 10.1128/jcm.38.1.138-145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mygind P, Christiansen G, Persson K, Birkelund S. Detection of Chlamydia trachomatis-specific antibodies in human sera by recombinant major outer-membrane protein polyantigens. J Med Microbiol. 2000;49(5):457–465. doi: 10.1099/0022-1317-49-5-457. [DOI] [PubMed] [Google Scholar]
- 9.Jenab A, Golbang N, Golbang P, Chamani-Tabriz L, Roghanian R. Diagnostic value of PCR and ELISA for Chlamydia trachomatis in a group of asymptomatic and symptomatic women in Isfahan, Iran. Int J Fertil Steril. 2009;2(4):193–198. [Google Scholar]
- 10.Yuan Y, Zhang YX, Watkins NG, Caldwell HD. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immune. 1989;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J, Walboomers JM, Roosendaal R, van Doornum GJ, MacLaren DM, Meijer CJ, et al. Direct detection and genotyping of Chlamydia trachomatis in cervical scrapes by using polymerase chain reaction and restriction fragment length polymorphism analysis. J Clin Microbiol. 1993;31(5):1060–1065. doi: 10.1128/jcm.31.5.1060-1065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez P, Vekris A, De Barbeyrac B, Dutilh B, Bonnet J, Bebear C. Typing of Chlamydia trachomatis by restriction endonuclease analysis of the amplified major outer membrane protein gene. J Clin Microbiol. 1991;29(6):1132–1136. doi: 10.1128/jcm.29.6.1132-1136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayada C, Denaumur E, Orfia J, Catalan F, Elion J. Rapid genotyping of Chlamydia trachomatis major outer membrane protein by the polymerase chain reaction. FEMS Microbiol Lett. 1991;67(1):73–78. doi: 10.1016/0378-1097(91)90447-i. [DOI] [PubMed] [Google Scholar]
- 14.Taheri Beni B, Motamedi H, Ardakani MR. Genotyping of the prevalent Chlamydia trachomatis strains involved in cervical infections in women in Ahvaz, Iran. J Med Microbiol. 2010;59(Pt 9):1023–1028. doi: 10.1099/jmm.0.016717-0. [DOI] [PubMed] [Google Scholar]
- 15.Carlson JH, Hughes S, Hogan D, Cieplak G, Sturdevant DE, McClarty G, et al. Polymorphisms in the Chlamydia trachomatis cytotoxin locus associated with ocular and genital isolates. Infect Immun. 2004;72(12):7063–7072. doi: 10.1128/IAI.72.12.7063-7072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, et al. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28(6):1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read TD, Myers GS, Brunham RC, Nelson WC, Paulsen IT, Heidelberg J, et al. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of nichespecific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 2003;31(8):2134–2147. doi: 10.1093/nar/gkg321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiesenfeld HC, Hillier SL, Krohn MA, Amortegui AJ, Heine RP, Landers DV, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol. 2002;100(3):456–463. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 19.Falck G, Gnarpe J, Hansson LO, Svardsudd K, Gnarpe H. Comparison of individuals with and without specific IgA antibodies to Chlamydia pneumoniae.respiratory morbidity and the metabolic syndrome. Chest. 2002;122(5):1587–1593. doi: 10.1378/chest.122.5.1587. [DOI] [PubMed] [Google Scholar]
- 20.Wong BY, Gnarpe J, Teo KK, Ohman EM, Prosser C, Gibler WB, et al. Does chronic Chlamydia pneumonia infection increase the risk of myocardial injury?. Insights from patients with non-ST.elevation acute coronary syndromes. Am Heart J. 2002;144(6):987–994. doi: 10.1067/mhj.2002.126734. [DOI] [PubMed] [Google Scholar]
- 21.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 22.Jenab A, Roghanian R, Golbang N, Golbang P, Chamani- Tabriz L. Comparison of three methods of DNA extraction in endocervical specimens for Chlamydia trachomatis infection by spectrophotometry, agarose gel, and PCR. Arch Immunol Ther Exp (Warsz) 2010;58(3):227–234. doi: 10.1007/s00005-010-0076-z. [DOI] [PubMed] [Google Scholar]
- 23.Gopalkrishna V, Aggarwal N, Malhotra VL, Koranne RV, Mohan VP, Mittal A, et al. Chlamydia trachomatis and human papillomavirus infection in Indian women with sexually transmitted diseases and cervical precancerous and cancerous lesions. Clin Microbiol Infect. 2000;6(2):88–93. doi: 10.1046/j.1469-0691.2000.00024.x. [DOI] [PubMed] [Google Scholar]
- 24.Santos C, Teixeira F, Vicente A, Astolfi-Filho S. Detection of Chlamydia trachomatis in endocervical smears of sexually active women in Manaus-AM, Brazil, by PCR. Braz J Infect Dis. 2003;7(2):91–95. doi: 10.1590/s1413-86702003000200001. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez P, De Barbeyrac B, Persson K, Dutilh B, Bebear C. Evaluation of molecular typing for epidemiological study of Chlamydia trachomatis genital infections. J Clin Microbiol. 1993;31(8):2238–2240. doi: 10.1128/jcm.31.8.2238-2240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisler WM, Suchland RJ, Whittington WL, Stam WE. The relationship of serovars to clinical manifestation of urogenital Chlamydia trachomatis infection. Sex Transm Dis. 2003;30(2):160–165. doi: 10.1097/00007435-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Molano M, Meijer CJ, Weiderpass E, Arsalan A, Posso H, Franceschi S, et al. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow- up study. J Infect Dis. 2005;191(6):907–916. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 28.van Duynhoven YT, Ossewaarde JM, Derksen-Nawrocki RP, van der Meijden WI, Van De Laar MJ. Chlamydia trachomatis genotypes: correlation with clinical manifestations of infection and patients' characteristics. Clin Infect Dis. 1998;26(2):314–322. doi: 10.1086/516291. [DOI] [PubMed] [Google Scholar]
- 29.Ito JI Jr, Lyons JM, Airo-Brown LP. Variation in virulence among oculogenital serovars of Chlamydia trachomatis in experimental genital tract infection. Infect Immun. 1990;58(6):2021–2023. doi: 10.1128/iai.58.6.2021-2023.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Den Hartog JE, Morr`e SA, Land JA. Chlamydia trachomatis-associated tubal factor subfertility: Immunogenetic aspects and serological screening. Hum Reprod Update. 2006;12(60):719–730. doi: 10.1093/humupd/dml030. [DOI] [PubMed] [Google Scholar]
- 31.Komoda T. Kinetic study of antibodies (IgG, IgA) to Chlamydia trachomatis: importance of IgA antibody in screening test for C.trachomatis infection by peptide-based enzyme immunosorbent assay. JPN J Infect Dis. 2007;60(6):347–351. [PubMed] [Google Scholar]


