Abstract
Oxidative and reductive carbohydrate metabolism was studied in reaction mixtures based on chlorophyll-free stromal extracts from chloroplasts of Pisum sativum. A new assay system for the reductive pentose phosphate cycle was characterized.
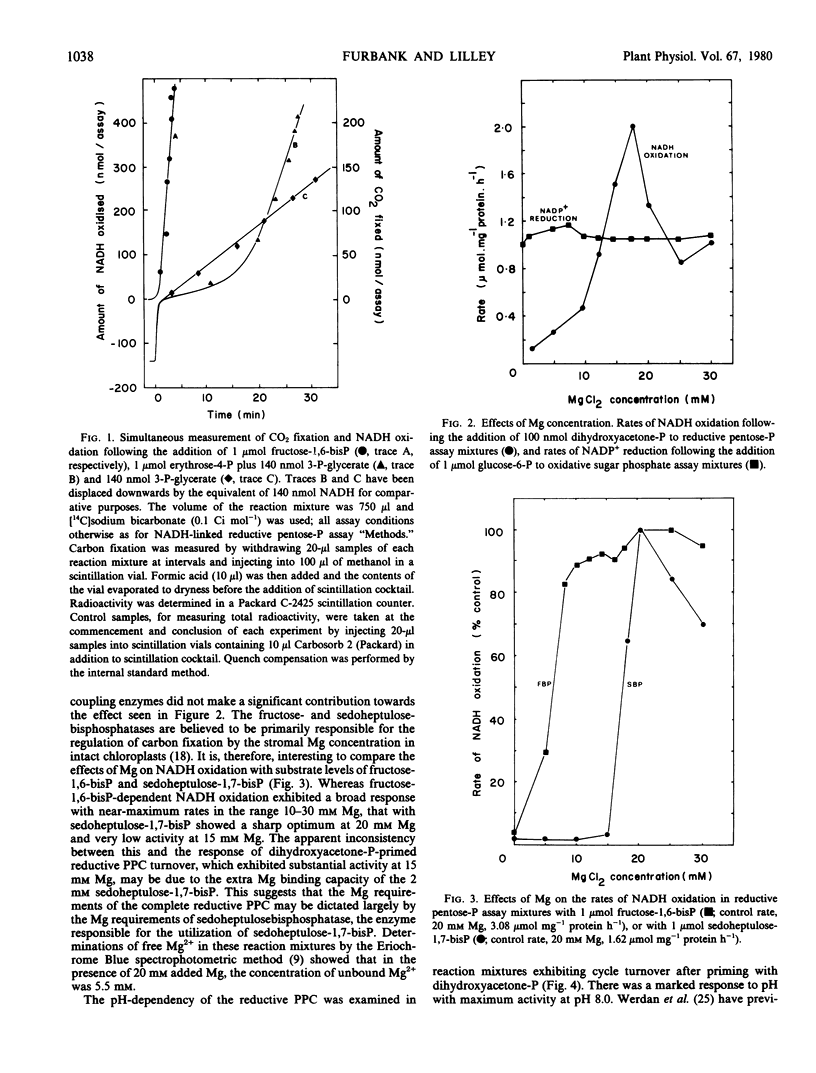
When provided with ATP, an enzymic ATP-regenerating system and reduced pyridine nucleotide, substantial rates of CO2 fixation and pyridine nucleotide oxidation were observed following the addition of millimolar concentrations of reductive pentose phosphate cycle intermediates. The reduced pyridine nucleotide requirement could be met either by NADPH, or by NADH plus the added enzymes NAD+-glyceraldehyde phosphate dehydrogenase and phosphoglycerate kinase. When the assay system was primed with small amounts of reductive pentose phosphate cycle intermediates, lower rates of pyridine nucleotide oxidation were observed, but turnover of the complete cycle was demonstrated. Autocatalytic effects were not evident. The optimum pH and Mg concentrations for cycle turnover were similar to those believed to exist in the stroma of intact chloroplasts in the light.
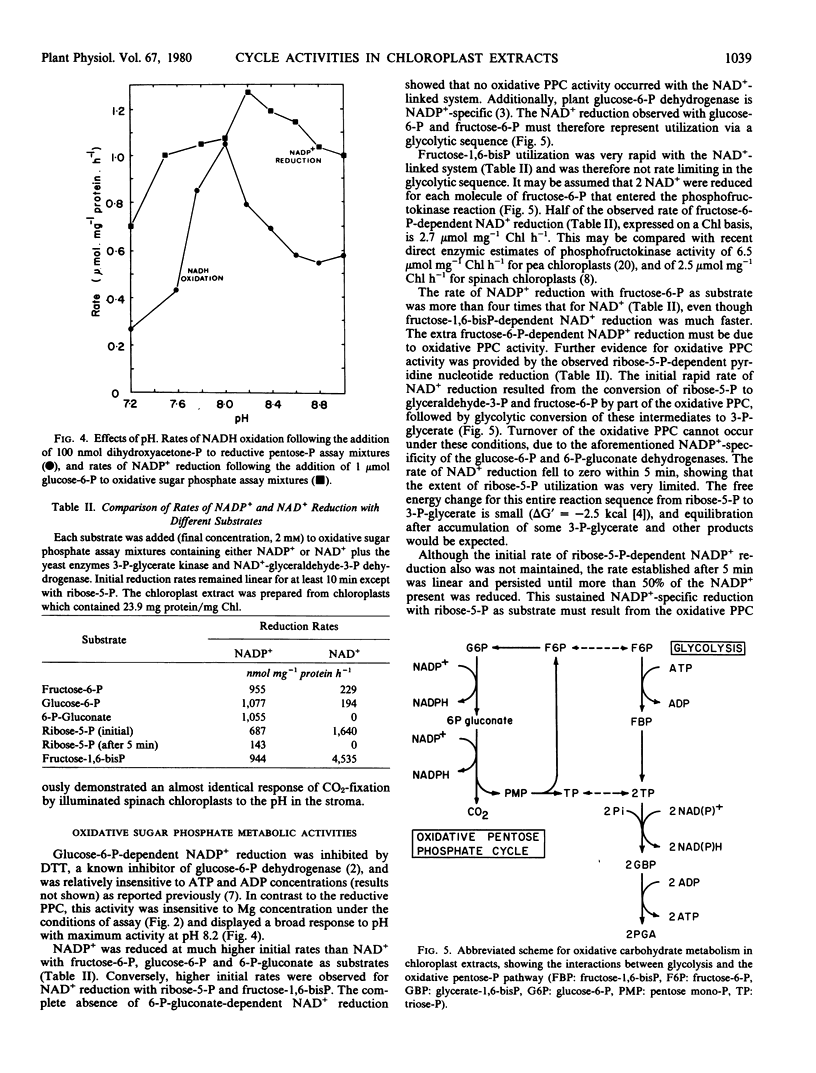
Oxidative carbohydrate metabolism was studied by supplying oxidized pyridine nucleotide and measuring its rate of reduction in the presence of sugar phosphates. Glycolytic activity, estimated as the rate of fructose-6-phosphate entry to the phosphofructokinase reaction was 2.7 micromoles per milligram chlorophyll per hour when fructose-6-phosphate was provided as substrate. Evidence based on glucose-6-phosphate and ribose-5-phosphate-dependent NADP+ reduction showed that the oxidative pentose phosphate cycle was also active. Apparent oxidative pentose phosphate cycle turnover in the presence of ribose-5-phosphate, estimated as the rate of glucose-6-phosphate entry to the glucose-6-phosphate dehydrogenase reaction, was 1.7 micromoles per milligram chlorophyll per hour.
It was concluded that under the defined conditions, reductive pentose phosphate cycle activity could be measured without interference from oxidative carbohydrate metabolism in this experimental system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., BANDURSKI R. S., GREINER C. M., JANG R. The metabolism of hexose and pentose phosphates in higher plants. J Biol Chem. 1953 Jun;202(2):619–634. [PubMed] [Google Scholar]
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassham J. A., Krause G. H. Free energy changes and metabolic regulation in steady-state photosynthetic carbon reduction. Biochim Biophys Acta. 1969 Oct 21;189(2):207–221. doi: 10.1016/0005-2728(69)90048-6. [DOI] [PubMed] [Google Scholar]
- Furbank R. T., Lilley R. M. Effects of inorganic phosphate on the photosynthetic carbon reduction cycle in extracts from the stroma of pea chloroplasts. Biochim Biophys Acta. 1980 Aug 5;592(1):65–75. doi: 10.1016/0005-2728(80)90114-0. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Latzko E. Chloroplast phosphofructokinase: I. Proof of phosphofructokinase activity in chloroplasts. Plant Physiol. 1977 Aug;60(2):290–294. doi: 10.1104/pp.60.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G. H. Light-induced movement of magnesium ions in intact chloroplasts. Spectroscopic determination with Eriochrome Blue SE. Biochim Biophys Acta. 1977 Jun 9;460(3):500–510. doi: 10.1016/0005-2728(77)90088-3. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Chon C. J., Mosbach A., Heldt H. W. The distribution of metabolites between spinach chloroplasts and medium during photosynthesis in vitro. Biochim Biophys Acta. 1977 May 11;460(2):259–272. doi: 10.1016/0005-2728(77)90212-2. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. An improved spectrophotometric assay for ribulosebisphosphate carboxylase. Biochim Biophys Acta. 1974 Jul 17;358(1):226–229. doi: 10.1016/0005-2744(74)90274-5. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, Chon C. J., Mosbach A., Heldt H. W. Fructose-and sedoheptulosebisphosphatase. The sites of a possible control of CO2 fixation by lightdependent changes of the stromal Mg2+ concentration. Biochim Biophys Acta. 1977 Aug 10;461(2):313–325. doi: 10.1016/0005-2728(77)90181-5. [DOI] [PubMed] [Google Scholar]
- Slabas A. R., Walker D. A. Localization of inhibition by adenosine diphosphate of phosphoglycerate-dependent oxygen evolution in a reconstituted chloroplast system. Biochem J. 1976 Jan 15;154(1):185–192. doi: 10.1042/bj1540185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Rees T. A. Carbohydrate breakdown by chloroplasts of Pisum sativum. Biochim Biophys Acta. 1980 Jan 17;627(2):131–143. doi: 10.1016/0304-4165(80)90315-3. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Lilley R. M. Autocatalysis in a reconstituted chloroplast system. Plant Physiol. 1974 Dec;54(6):950–952. doi: 10.1104/pp.54.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Slabas A. R. Stepwise generation of the natural oxidant in a reconstituted chloroplast system. Plant Physiol. 1976 Feb;57(2):203–208. doi: 10.1104/pp.57.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


