Abstract
Background
Frailty is a highly prevalent condition in old age leading to vulnerability and greater risk of adverse health outcomes and disability. Detecting and tackling frailty at an early stage can prevent disability. The purpose of this study is to evaluate the effectiveness of a multifactorial intervention program to modify frailty parameters, muscle strength, and physical and cognitive performance in people aged 65 years or more. It also assesses changes from baseline in falls, hospitalizations, nutritional risk, disability, institutionalization, and home-care.
Methods/design
The current study is a randomised single-blind, parallel-group clinical trial, with a one and a half year follow-up, conducted in eight Primary Health Care Centres located in the city of Barcelona. Inclusion criteria are to be aged 65 years or older with positive frailty screening, timed get-up-and-go test between 10 to 30 seconds, and Cognition Mini-Exam (MEC-35) of Lobo greater than or equal to 18. A total of 352 patients have been equally divided into two groups: intervention and control. Sample size calculated to detect a 0.5 unit difference in the Short Physical Performance Battery (Common SD: 1.42, 20% lost to follow-up). In the intervention group three different actions on frailty dimensions: rehabilitative therapy plus intake of hyperproteic nutritional shakes, memory workshop, and medication review are applied to sets of 16 patients. Participants in both intervention and control groups receive recommendations on nutrition, healthy lifestyles, and home risks.
Evaluations are blinded and conducted at 0, 3, and 18 months. Intention to treat analyses will be performed. Multivariate analysis will be carried out to assess time changes of dependent variables.
Discussion
It is expected that this study will provide evidence of the effectiveness of a multidisciplinary intervention on delaying the progression from frailty to disability in the elderly. It will help improve the individual’s quality of life and also reduce the rates of falls, hospital admissions, and institutionalizations, thus making the health care system more efficient. This preventive intervention can be adapted to diverse settings and be routinely included in Primary Care Centres as a Preventive Health Programme.
Trial registration
ClinicalTrials.gov PRS:NCT01969526. Date of registration: 10/21/2013.
Keywords: Frail elderly, Aged, Randomised controlled trial, Exercise, Disability, Primary health care, Treatment outcome
Background
The concept of frailty has long been associated with advancing age although only recently has it been specifically defined as a medical syndrome[1–4]. Disability, comorbidity, malnutrition, biological changes, cognitive impairment, dependency needs, and demands for social services are directly age-related; age, however, as an isolated criterion is not enough to identify vulnerability[5].
Despite considerable discussion, frailty remains to be systematically defined[6]. There is evidence that it increases proportionally to an accumulation of deficits[7]; acute problems -falls, fractures, and infections-, progressive loss of autonomy, and psychosocial limitations all lead to disability and a higher risk of hospitalization, institutionalization, and death[8, 9].
There is no agreement on a valid evaluation model for both research and clinical approaches[10], and some authors differentiate between physical and cognitive frailty[11]. The standard clinical proposal is that which has been presented by Fried et al. who identify someone as having a frail phenotype when three or more of the following components are presented[3]: unintentional weight loss (4.5 kg (=10 lbs) in the past year), self-reported exhaustion (two positive questions of Center for Epidemiologic Studies Depression Scale (CES-D)), weakness, slow walking speed, and low levels of physical activity. Such a definition would be mainly related to physical frailty. For rapid frailty screening of a community-living elderly population these five criteria, however, do not represent a pragmatic approach[10]. Avila-Funes et al. proposed a review to slightly modify Fried’s measurements in order to strengthen the predictive validity of the concept[12], with variable results[13, 14]. In addition, Gill et al. introduced two tests of physical ability strongly associated with disability development and progression: the Rapid-Gait Test and the Stand-Up Test[15]. Other useful batteries of physical performance, such as the Short Physical Performance Battery (SPPB) from Guralnik, can also be found as predictors of old age disability[16, 17].
Sarcopenia is linked to physical frailty and is a key feature of this condition in older people[18]. In fact, a non-negligible proportion of elderly individuals are moderately affected by this it[19]. Sarcopenia is related to loss of muscle mass and muscle strength, strong predictors of adverse health outcomes[20] and death[21, 22].
Epidemiological studies have linked physical frailty and cognitive impairment: frailty increases the risk of cognitive decline and cognitive impairment increases the risk of frailty, therefore, both dimensions would benefit from being addressed[23, 24].
Identifying interventions to prevent or delay the loss of autonomy is currently a public health priority for the successful management of the ageing[25, 26]. Multidimensional home interventions have revealed some benefits, although conclusions are inconsistent and seem to be dependent on factors such as the provider’s experience, access to monitoring, and duration of the follow-up program[15].
A comprehensive geriatric assessment, followed by a multidimensional intervention on disability risk factors -medical, functional, psychological, and environmental problems- through disease management and health promotion in a low-risk elderly population, succeeds in reducing institutionalization and the risk of falls, delaying disabled functional decline, and improving physical performance. The effects, however, are not statistically significant[27–29].
Strategies involving mass screening in Primary Care to apply preventive approaches based on healthy ageing advice, long-term exercise programs, assistance devices including home telecare kits[30], and environmental modifications can reduce falls[31]. Nevertheless, when considered separately, these methods have no impact on reducing disability[32].
Exercise programs improve strength, aerobic capacity, balance, and function[33, 34], but these benefits depend on long-term adherence, extended training, and exercise-related behaviours acquired in early life. The most promising strategies to increase physical activity in the elderly are those which provide appropriate written advice and generate feelings of fun and satisfaction[35].
Recent surveys have put forward new strategies for the management of sarcopenia to slow down the decline of muscle features: resistance training in combination with adequate protein and energy intake and, additionally, treatment of vitamin D deficiency[36, 37].
Nutritional interventions alone show weak correlation with health improvement in the vulnerable, elderly population. However, dietary advice in association with protein supplementation intake seems to have some effects on sarcopenia, inducing muscle hypertrophy, accelerating weight gain in undernourished older people[38], and reducing fractures[39]. There is a lack of evidence, however, concerning its effects on mortality and hospital admission rates[40].
Findings from cognitive training studies show positive effects. Memory training can aid maintaining long-term improvement in performance[41, 42].
Exercise also leads to enhanced cognitive functioning and psychological well-being in frail, older adults[43]. Aerobic exercise has shown effects on some measures of cognitive function, without consistency for all values[44].
There are few randomized, controlled trials concluding that cognitive interventions, plus complementary physical exercise, can produce significant global improvements in cognitive function, and quality of life, and delay the onset of disability[45].
What about medication use in frail, older adults? The rates of adverse drug events are higher in the elderly population, as many of them have comorbidities, multiple drug prescriptions, and deteriorated physical and cognitive impairment[46]. In the previous decade, deprescribing, based on clinical and ethical criteria, has been defended as an option for managing chronic conditions, avoiding adverse effects, and improving patient outcomes. Polypharmacy has been independently associated with an increase of mortality in the elderly[47], indeed, several multifaceted interventional studies have demonstrated that medication review has a positive effect on reducing mortality, hospital admissions and falls, and enhances quality of life[48].
Such a wide range of interrelated factors gives weight to our proposal to conduct a multifactorial intervention aimed at non-disabled, i.e. frail, elderly individuals. Our objective is to focus on this population whose health status still permits some positive modifications in the inevitable evolution from frailty to dependence so that by preventing home confinement or institutionalization, older people can stay active and live by themselves in the community.
Study aim
This is a research protocol for a randomized, controlled trial aimed at assessing the effectiveness of a multifactorial intervention program to modify parameters of frailty, muscle strength, and physical and cognitive performance in elderly people living in the community. The intervention includes various professional disciplines and is based on physical activity, diet supplementation, memory workshops, and medication review.
Secondary aims include evaluating changes in rates of falls fractures, hospital admissions, inclusions in home care programs, and institutionalizations.
Methods/design
Study design
The study design is a single-blind, parallel-group, pragmatic, randomised, clinical trial with one year and a half follow-up.
Changes from baseline measurements (month 0) in the parameters of frailty, muscle strength, and physical and cognitive performance are compared between the intervention (IG) and control group (CG) at the end of the intervention (month 3). An 18 month follow-up after randomization will be established in order to determine whether intervention effects can be sustained. The 18 month changes in rates of falls, fractures, hospital admissions, inclusions in home care programs, institutionalizations and vital status will be analysed.
The CONSORT Statement extensions for trials of non-pharmacological interventions and pragmatic intervention trials were used to design the study and will be used to report it.
Sample size calculation
Sample size has been calculated to detect minimal significant effects on the variable of physical performance (SPPB): Accepting an alpha risk of 0.05 and a beta risk of 0.20 in a bilateral contrast, 318 individuals are required in order to detect a difference equal to or greater than 0.5 units in the SPPB[49, 50]. The common standard deviation has been taken to be 1.42. A drop-out rate of 20% is anticipated. Finally, 352 subjects have been included (n = 176 IG and n = 176 CG).
Ethical aspects
Written informed consent has been obtained from all recruited subjects. Objectives, tests and other details about methodology and interventions were explained orally and in writing. The trial was approved by the Ethics Committee of the IDIAP Jordi Gol (code number P12/047) on June 1st, 2012. Funding from the Carlos III Health Institute was granted on December 20, 2012 (project code PI12/01503).
Participants and recruitment
From February 2013 to January 2014, 370 individuals aged 65 years and over were recruited from 8 Primary Healthcare Centres (PHCC) located in two different districts of Barcelona. A total population of 33,857 aged 65 years and over live in the reference area.
Subjects were recruited by referral from the PHCC where the opportunity to participate in the study was offered on a regular daily basis to all patients meeting preliminary frailty criteria (Barber Questionnaire[51]). Eligibility was then verified with an assessment by a Case Management Nurse (CMN) through a personal interview. Participants meeting at least 3 Fried modified frailty criteria were included whilst those individuals with very slow or rapid gait speed, or cognitive impairment based on MEC-35 of Lobo[52], were excluded.
Inclusion and exclusion criteria are shown in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| • 65 years or older | • Medical conditions such as the presence of: unstable angina, uncontrolled congestive heart failure, unstable arrhythmia, COPD stage III or IV which contraindicate following a program of physical activity |
| • Resident in Barcelona, community-dwelling | |
| • Assigned to one of the 8 PHCC | |
| • Can attend on-site the consultation room at the PHCC | |
| • Will stay in the reference area a minimum of one year and a half | • Home Care Program or institutionalization at baseline. Planned admission to nursing home |
| • Frailty inclusion criteria: | • Participation in other physical activity program |
| • score of 1 point or above in the Barber Questionnaire | • Has been operated on hip and/or knee the last 6 month (walking independently with technical assistance is not a contraindication) |
| • Fried modified frailty criteria: 3 or more | |
| • Gait time between 10 to 30 seconds in the Timed Get Up and Go test | • Suffering a non-controlled neoplastic disease, terminal or severe disabling illness |
| • MEC-35 of Lobo ≥18 points (no severe cognitive impairment) | • Cannot understand Spanish |
| • Capable of consent. Agreement to participate in the study |
The flow-chart of the trial according to CONSORT 2010 is visualized in Figure 1.
Figure 1.
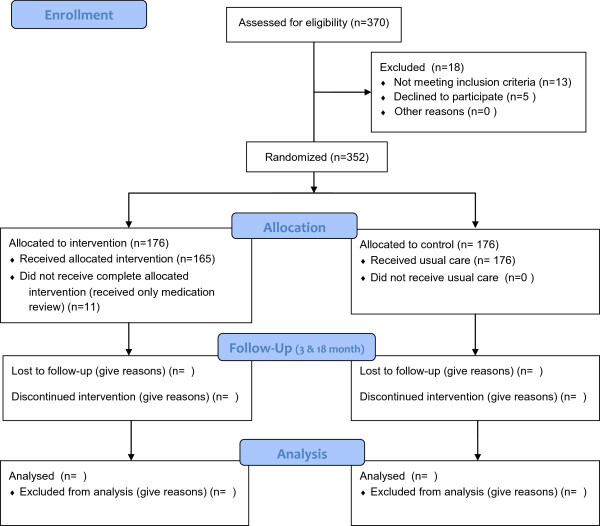
Study flow diagram.
Eligible patients who agreed to participate in the study were invited to sign the informed consent. Baseline variable collection was carried out by the CMN. Patients were then randomly assigned to the intervention and control groups. The computer-assisted simple randomization process was performed not by the recruiters but by an independent researcher. Random allocation sequence was implemented using sequentially numbered containers. Sequence was concealed until the interventions were assigned. Baseline and outcome measurements were blinded to group assignment. Follow-up evaluations are conducted by blind trained clinical researchers.
Measures
Both cohorts receive identical baseline and follow-up evaluations. Table 2 shows the different time points when variables are measured.
Table 2.
Measurements at several time points
| Selection | Baseline | After intervention | 18 months | ||||
|---|---|---|---|---|---|---|---|
| All | IG | CG | IG | CG | IG | CG | |
| FRAILTY MEASURES | |||||||
| Barber Questionnaire | x | ||||||
| Fried modified criteria | x | x | x | ||||
| SPPB | x | x | x | x | x | x | |
| FRT | x | x | x | x | x | x | |
| Unipodal Station | x | x | x | x | x | x | |
| Strength of upper extremities | x | x | x | x | x | x | |
| Strength of lower extremities | x | x | x | x | x | x | |
| FUNCTIONAL ASSESSMENT | |||||||
| Lawton & Brody Scale | x | x | x | x | |||
| Barthel Index | x | x | x | x | |||
| NUTRITION | |||||||
| MNA | x | x | x | x | |||
| COGNITIVE EVALUATION | |||||||
| MEC-35 of Lobo | x | ||||||
| Short and Medium-Term Verbal Memory | x | x | x | x | x | x | |
| Animal Naming Test | x | x | x | x | x | x | |
| Evocation of words | x | x | x | x | x | x | |
| Designation of famous people names | x | x | x | x | x | x | |
| Verbal designation of images | x | x | x | x | x | x | |
| Verbal abstraction of word pairs | x | x | x | x | x | x | |
| DRUGS and PRESCRIPTION | |||||||
| Total number of drugs | x | x | x | x | x | x | |
| Psychotropic Medication presence | x | x | x | x | x | x | |
| Withdrawal of drugs | x | x | x | x | |||
| OTHER VARIABLES | |||||||
| Comorbidities | x | x | x | x | |||
| Biological measurements | x | x | x | x | |||
| Analytical parameters | x | x | x | x | |||
| Sphincter incontinence | x | x | x | x | |||
| Visual impairment | x | x | x | x | |||
| Auditive impairment | x | x | x | x | |||
| Technical support aids | x | x | x | x | |||
| Quality of Life: SF12 | x | x | x | x | |||
| ADVERSE OUTCOMES | |||||||
| Falls | x | x | x | x | |||
| Fractures | x | x | x | x | |||
| Hospital admissions | x | x | x | x | |||
| Home care inclusions | x | x | |||||
| Institutionalizations | x | x | |||||
| Death | x | x | |||||
- Fried modified criteria for frailty [3] (three or more of the following criteria have to be present):
- Unintentional weight loss (3 kg in past 6 months)
- Self-reported exhaustion (2 questions from CES-D scale)
- Weakness (5 chair stand-up test, unable)
- Slow walking speed (more than 10 seconds) evaluated by Timed -up-and-Go test (TGUGT). This is a reliable test for quantifying functional mobility (lower extremities function) and for measuring balance (fall risk). The person may wear their usual footwear and can use any assistive device normally employed. The TGUGT is conducted using a chair with arms, and a seat height of 46 cm, placed upon a flat surface with a line marking the 3m turning point. Subjects are instructed on the word ‘go’, to get up and walk as quickly and as safely as possible to cross the line marked on the path, turn around, walk back to the chair and sit down again. The activity will be timed from the subject’s back leaving the back of the chair to the return of the subject to this same position.
- Low physical activity measured by the IPAQ Questionnaire[53].
- Physical performance:
-
2.1Short Physical Performance Battery.The short physical performance battery (SPPB) is a simple standardised objective assessment tool of lower limb function[16] that tests standing balance, ability to repeatedly stand from a sitting position, and habitual gait speed. Each component is scored between 0–4 (total score 0–12) with higher scores indicating better functioning. In community-dwelling older adults, lower SPPB scores predict greater risk of mortality, nursing home admission, hospitalization, and incidence of disability. The SPPB consists of:
-
2.1.1)Balance testParticipants are asked to hold three increasingly challenging standing positions for 10 seconds each: (1) a side-by-side position, (2) semi-tandem position (the heel of one foot beside the big toe of the other foot), (3) tandem position (the heel of one foot in front of and touching the toes of the other foot).
-
2.1.2)Repeated chair stands testThis is performed using a straight-backed chair, placed with its back against a wall. Participants are first asked to stand from a sitting position without using their arms. If they are able to perform the task, they are then asked to stand up and sit down five times, as quickly as possible, with arms folded across their chest. The time to complete five stands is recorded and used for future analyses.
-
2.1.3)Gait speed (8 meters walk)2.1.3)
-
2.2Functional Reach Test (FRT).This is a valuable test used to measure standing balance and stretching, detecting balance impairment over time. It can predict the risk of falling. After the examiner explains and shows the FRT, each subject performs 2 trial tests. Functional reach is measured by using a levelled yardstick attached to the wall at the height of the subject’s right acromion. To measure the subject’s reaching distance, an examiner stands 0,5 m away from the measuring tape and records the end reach position, checking that the initial position is correct: subjects stand comfortably with feet approximately shoulder-width apart, just before a line marked on the floor at the same level as the measuring tape beginning (0cm); participants then extend the right arm parallel to the yardstick and, without touching the wall, place the third metacarpal along the measuring tape and have to reach as far forward as they can without losing their balance (end position). Subjects are allowed to balance on their toes; however, touching the wall, stepping while reaching forward, or holding onto their clothing with the left hand invalidate the trial. If invalidated, the trial is repeated with a maximum of 2 tests more to achieve 2 valid trials. All subjects are protected during the test. The best result from the two attempts is recorded.
-
2.3Unipodal stationThe patient is placed in a standing position, arms crossed over the chest, with one leg used for support in an extended position, and the other slightly bent at the knee (there can be no contact between the two legs). Once placed in the correct position (eyes open), the chronometer is activated and then stopped when either the patient moves the foot used as a base or when 30 seconds have passed; two attempts with the same foot are made and the best result recorded.
-
2.1
- Muscle strength.
-
3.1Evaluation of upper extremities strength is assessed through the measurement of force with a handgrip dynamometer. Grip strength is assessed using a portable hand dynamometer (JAMAR®0-90 kg, coding 506320). The participants are seated with their shoulder in a neutral position and their elbow flexed at 90°. Three attempts are performed alternately in each hand; the mean of the three measures is recorded.
-
3.2Evaluation of lower extremities strength is assessed through the bilateral measurement of the quadriceps muscle force using a digital dynamometer (Chronojump 1.4.5-1.4.6 Boscosystem® Encoder).
-
3.1
- Activities of Daily Living (ADL) assessment:
-
4.1Lawton & Brody Instrumental Activities of Daily Living scale[54].This is an instrument assessing independent living skills which are considered more complex than the basic activities of daily living. The instrument is most useful for identifying how a person is functioning at the present and for observing improvement or deterioration over time. There are 8 domains of function measured with the Lawton & Brody scale. Women have been traditionally assessed in all 8 areas of function whilst men have not been asked about the domains of food preparation, housekeeping, and laundering. Individuals are scored according to their highest level of functioning in that category. A summary score ranges from 0 (low function, dependent) to 8 (high function, independent).
-
4.2Barthel Index of Basic Activities of Daily Living[55].First developed in 1965, it measures functional disability by quantifying patient performance in 10 activities of daily life. These activities can be grouped according to self-care (feeding, grooming, bathing, dressing, bowel and bladder care, and toilet use) and mobility (ambulation, transfers, and stair climbing). 5-point increments are used in scoring, with a maximal score of 100 indicating that a patient is fully independent in physical functioning, and a lowest score of 0 representing a totally dependent bed-ridden state.
-
4.1
-
Nutritional Assessment:
Mini Nutritional Assessment MNA®[56]. The MNA consists of four parts: anthropometric measurements, general status, diet information, and subjective assessment. A score of less than 17 points (out of a maximum of 30) is regarded as an indication of malnutrition, 17–23.5 points indicate a risk of malnutrition and >23.5 points indicate that the person is well nourished.
-
Neuropsychologist Performance:
The first neuro-psychometric instrument developed in Spain to measure semi-quantitatively cognitive status in clinical neurology was the Barcelona Test (BT). A shortened version of the BT, named Barcelona Test Review[57], is used for neuropsychological area evaluation in our participants and it takes only 20–30 minutes to administer. Tests applied are:-
6.1.Short and Medium-Term Verbal Memory is the capacity to hold a small amount of information in the mind in an active, readily available state for a short period of time (seconds) and medium-term period of time (minutes). For the condition referred to as short term, subjects will be instructed to listen to a little story text, 21-pieced-sentences (elements), read by a blind evaluator; once finished, the patient will be asked to repeat the general content and as many details as he or she can remember; the duration of short-term memory is believed to be in the order of seconds. Then, the participant will be submitted to other cognitive trials to divert attention. After 25 minutes, the subject is directly asked for the story again to evaluate medium-term verbal memory. A commonly cited capacity of short and medium-term is 7±2 elements.
-
6.2.Animal Naming Test consists of asking the patient to name as many animals as possible in one minute. A blind evaluator must write down the answers, so they can be checked for duplicate responses (repeated words invalidate one of them). The goal of this test is to score at least 14.
-
6.3.Evocation of words beginning with one explicit letter, similar to the previous test, in this case the participant will tell the examiner as many words beginning with “p” as possible in three minutes (repeated words invalidate one of them). All kinds of words are allowed, except plurals or the masculine and feminine of the same word, conjugating verbs, and diminutives. The goal of this test is to score at least 27.
-
6.4.Designation of famous people names, the identification of 30 famous faces and their corresponding name permits an examination of the semantic brain area and can identify a possible clinical syndrome of prosopagnosia. Evaluated by “success”, “failure” or “tip of the tongue (TOT) phenomenon”, goal is 23 success, 3 failure, 4 TOT.
-
6.5.Verbal designation of images. Fourteen pictures of different objects or animals are presented to participants and they have to identify each name as quickly as they can: if subjects guess the name between 0–3 seconds this signifies 3 points, between 3–10 seconds, 2 points, and if takes 10–30 seconds it represents 1 point. If the patient does not recognize the picture-name, it is equivalent to 0 points. Goal of the test is to score 41 points.
-
6.6.Verbal abstraction of word pairs, also called “Similarities – Abstraction”, explores patients’ concept formation ability, as the participant must “extract” the common abstract element that links the two words featured. Through this test the ability to discriminate “concrete thinking “from” abstract thought” can be evaluated. The goal of this test is to score at least 5 of 6 pair of words.
-
6.1.
- Medication
-
7.1.Number of prescribed drugs.
-
7.2.Number of prescribed benzodiazepines.
-
7.3.Presence of antidepressants (yes/no).
-
7.4.Withdrawal of drugs (yes/no).
-
7.5.Number of drugs retired at the closing date of the study.
-
7.1.
Quality of life. 12-Item Short-Form Health Survey (SF-12) [58].
Adverse Outcomes: Falls, fractures, hospital admissions, institutionalization, inclusion in a Home-Care Program, or death.
Independent variables
Age. Gender. Marital status. Cohabitation. Education Level. Socioeconomic status. Existence of elevator in the building. Provision of regular company.
Co-morbidities assessed in the clinical record: osteoarthritis, fractures in the last 5 years (hip fracture specified), presence of prosthetic joints, vision impairment, hearing impairment, cardiovascular diseases (hypertension, stroke, ischemic heart disease, arrhythmia, congestive heart failure, intermittent claudication, chronic venous insufficiency), pulmonary diseases (Chronic Obstructive Pulmonary Disease (COPD), asthma), endocrinology diseases (diabetes, dyslipidemia, obesity, hypothyroidism, hyperthyroidsim), hematological (anemia), neurologic (Parkinson’s disease), psychiatric (anxiety, depression), chronic kidney disease.
Comorbidity measured with Charlson Index [59].
Biological variables: weight, height, body mass index, waist circumference, blood pressure.
Analytical variables: hemoglobin, serum lipid profile, serum protein, serum albumin, glomerular filtration rate, plasma creatinine, glycated hemoglobin, ferritin, iron, vitamin D, vitamin B12, folic acid.
Incontinence (urinary, fecal, both).
Urinary catheter (yes/no).
Wearing a diaper (yes/no).
Usual sensation of light-headedness.
Smoking (non-smoker, ex-smoker, current smoker).
Devices for mobility (cane, walker).
Falls, fractures, and hospitalizations in the previous year.
Intervention
The intervention consists of a triple disability preventive therapy, consecutively applied to each subject in the intervention group, in groups of 16 participants (see Table 3):
Table 3.
Description of Interventions
| 3.1 Description of the rehabilitation therapy | |||
|---|---|---|---|
| Basic exercise | Alternative exercise | Muscle group | |
| ▪ Chest Press against elastic resistance - sitting on a chair | ▪ Chest Press against the wall | ▪ Pectoral muscles | |
| ▪ Reverse Butterfly against elastic resistance - sitting on a chair | ▪ Upper back muscles | ||
| ▪ Arm press against elastic resistance - sitting on a chair | ▪ Arm press against elastic resistance - standing position | ▪ Muscles of the arms, and shoulders | |
| ▪ Stand up with palms on thighs - sitting on a chair | ▪ Stand up using hand weighs - sitting on a chair | ▪ Quadricep, hamstring, and gluteal muscles | |
| ▪ Lift the legs with hands on hips - sitting on a chair | ▪ Hip flexor muscles | ||
| ▪ Hip abduction/adduction - sitting on a chair | ▪ Hip abduction/adduction - standing position | ▪ Abductor/Adductor muscles | |
| ▪ Knee flexion - sitting on a chair | ▪ Knee flexion - standing position | ▪ Hamstring muscles | |
| ▪ Knee extension - sitting on a chair | ▪Quadricep muscles | ||
| ▪ Heel raises - sitting on a chair | ▪ Heel raises - standing position | ▪ Gastrocnemius and soleus muscles | |
| 3.2 Description of the memory workshops | |||
| Memory | Language | Sensory activation | Reasoning and calculation |
| Short and Long-Term Visual Memory | Evocation of words beginning with different letters | Series of logical visual pattern recognition | Gnosia and praxia different developing techniques (reproduction of pragmatic models) |
| Short and Long-Term Written Memory | Crosswords | Marking edge of silhouettes | Letters and numbers matching through Maze Paths |
| Short and Long-Term Oral Memory | Completeness of unfinished sentences | Coloring components of Hidden Figures Test | Executive functions enhancing: abstract concepts of similar but different objects |
| Short and Long-Term Musical Memory | Oral communication with clue words | Spot the differences between two pictures | Identification of the inappropriate word in a pool of words |
| Working memory: identification of hidden figures test | Word search | Picture copies execution | Reading and exclusion of senseless sentences |
| Memorize an image and draw it from memory | Synonyms and antonyms | Objects, materials and sounds recognition with closed eyes | Filling the gap |
| True/False sentences | Matching words and their meaning | Group interaction by singing and musical performances | Numerical skills practice: operations and mental agility |
| Logos recognition | Visual-verb generation task: denomination of images, objects, parts of the human body | Famous faces recognition | |
| Geographical memory practice | Rearrange letters to form a word and rearrange words to form grammatical sentences | ||
| 3.3 Description of the polymedication review | |||
| Who does the intervention? | What are the objectives and criteria? | How is the intervention performed? | |
| 2 doctors from the Project Group. | To reduce drug prescription of polymedicated patients* if possible, following : | A personalized e-mail is sent to each GP responsible for the patient participating in the intervention group throughout the first week of patient inclusion. | |
| -Stopp criteria, | |||
| Depending on the baseline drug prescription at the beginning of the study. | Every e-mail considers the individual profile of the patient referred and tries to adapt the general criteria to each particular case. | ||
| The GP who regularly attends the intervention patient performs both reduction and re-education of unnecessary drugs. This approach should be done in a maximum of 3 clinical interviews specifically designed for this subject. | |||
| E-mail content suggests the most recommended changes but the final decision corresponds to the discretion of the physician responsible for the patient. | |||
*Polymedicated Patient: one that takes more than five drugs daily and continuously for a period not less than six months.
Rehabilitation therapy plus the posterior intake of 1 hyperproteic nutritional shake which is then taken daily for 1 month. All patients in the intervention group perform the aerobics exercise plan in the primary care centre, 60-minute session twice a week on non-consecutive days for 6 weeks (12 sessions of 60 minutes each). Subjects must incorporate a progressive increase in the intensity of the exercise in each session. One session a week is dedicated to work with balance and the other to strength training. Both balance and strength are based on functional exercises. All sessions begin with a warm up for 5 minutes, and end by cooling off for another 5 minutes with relaxing exercises. The sessions are conducted under the supervision of a specialist in physical activity. A hyperproteic nutritional shake is provided at the end of each session, and the amount of shakes needed for one month’s consumption post-physical therapy is assigned. The safety of the exercise program is measured by reviewing the record sheet for each patient in the training program, ascertaining cardiovascular decompensation and musculoskeletal injuries.
Memory workshops. Two speech therapists from the rehabilitation unit conduct 12 sessions of practical exercises (written, oral, corporal, and musical) in groups of 16 participants. Each of the 12 sessions lasts 90 minutes and is conducted twice a week. Each person in the intervention group has their own material to work short and long-term memory, with exercises for the identification of figures and images, evocation of words, true or false sentences, crosswords, completion of unfinished sentences, and other language exercises such as synonyms and antonyms.
Medication Review. Reduction of potentially inappropriate medications, especially in polymedicated patients, after review from general practitioners. A patient is considered to be polymedicated when taking more than five medications daily and continuously for a period not less than six months. Medication review follows the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) criteria [60]. In addition to the review of medication, verbal guidance on each of the drugs consumed is also provided. After an e-mail sent with the changes suggested by two doctors from the Project Group, this intervention is carried out by every patient’s general practitioner, during the first month of the intervention, in a maximum of 3 clinical interviews for that purpose. It especially focuses on reducing the consumption of benzodiazepines or other psychotropic drugs.
The intervention group also receives two group sessions regarding dietary advice, lifestyles, and home hazards.
Control group
Subjects in the control group continue with their daily activities and receive regular monitoring and treatment of their diseases by their general practitioners. They are also invited to two group sessions regarding dietary advice, lifestyles, and home hazards.
Statistics
Intention to treat analyses will be performed. Baseline characteristics will be compared between groups by independent t tests and Chi-square tests. Outcome variables will be calculated for each individual and time point (difference between the result of SPPB, muscle strength, and other frailty variables in each time point and the initial value), and 95% confidence intervals for the differences between groups will be calculated. Data will be analysed using repeated measures analysis of variance (ANOVA) consisting of intervention and control groups and time (baseline, post-intervention, follow-up).
Also, for longitudinal adverse outcome measures (disability, home care inclusion, institutionalization or death), survival analyses using Cox’s regression models will be applied. The statistical significance level will be set at p <0.05.
Discussion
Our study is addressed at evaluating the effectiveness of a multifactorial intervention to improve frailty parameters and prevent disability in patients 65 years or older. Improvements in physical performance, muscle strength, nutritional status, and cognitive performance are expected, as well as a reduction in the incidence of new complications such as falls, fractures, hospital admissions, and worsening of ADL scales, all of which are related to the appearance of disability[33, 34]. Tackling frailty in a multifaceted manner will also diminish adverse outcomes such as inclusion in a home-care program, institutionalization or death.
In the field of preventive geriatrics, studies have shown that exercise training has clinical benefits inducing positive physiologic changes in muscle and function while multi-nutrient supplementation alone, without concomitant exercise, does not reduce muscle weakness or physical frailty[61].
The innovation of our study lies in regard to the follow-up and evaluation of a multifaceted strategy focused on different risk factors: physical decline, cognitive impairment, nutritional status, and polypharmacy. Previous series have provided a certain degree of evidence about improvement with these interventions on only an individual basis.
The greatest limitation of this study could proceed from the lack of agreement in the scientific community with respect to the definition of frailty and the most suitable measurements to gauge it. Including non- frail subjects (non-homogeneous risk state) could affect generalizability. The initial inclusion criterion, the low specificity Barber Questionnaire, has been included in this study because it was the first frailty test to be used in our clinical records. It has been also complemented, however, with other inclusion criteria such as the TGUGT. Participants scoring lower than 10 seconds or higher than 30 are excluded as they are considered either too frail or not frail enough to benefit from the intervention. The exclusion of more severely affected frail patients, because of their poor physical or cognitive condition, may limit external validity. Nevertheless, the random distribution of our patients to both groups guarantees comparability. Also, additional information about potential confounders (comorbidity, sensory impairment, and social risk) and the use of other parameters and tests of frailty are expected to solve the possible selection bias and help to further characterize the study population. Losses to follow-up are minimized through contacting the participants by telephone.
If evidence of a multi-strategy composed of physical exercise and a cognitive workshop, along with nutritional support and medication review, is achieved as an effective approach, a future implementation should be considered as a Frail-Community Prevention Program for the elderly to prevent or delay disability.
Acknowledgments
The authors gratefully acknowledge to all the professionals participating in the “Fragility Study” and all those who were involved and are no longer with us, Guillem Cifré. Special thanks to the staff and participants of the eight Primary Healthcare Centres of the Institut Català de la Salut, all them located in Barcelona. We would like to thank the Primary Heathcare Research Institution IDIAP Jordi Gol for it collaboration. We also thank Xavier de Blas for his infinite patience and support about the Chronojump Encoder.
The authors owe much gratitude to the special contribution of the following collaborators, without whom this project would not have been possible: Maria-Milagros Guerrero, Sonia Martínez, Carmen López, Maria José Riazuelo, Neu Aizcorbe, Laura-Isabel Pérez and Mario Martin (Raval Sud Primary Health Centre), Patricia Furió (Casc Antic Primary Health Centre), Ana Muñoz (Poble Sec and Universitat Primary Health Centres), M Teresa Isidro (Avinguda Roma Primary Health Centre), Nuria Lladró (Sant Antoni Primary Health Centre), Hèlia Marta Cebrian (Gotic Primary Health Centre), Agnés Salvador, David Garcia, Lucía López, Francesc Torres, Núria Bernaus, Ferran Povedano (Raval Nord Primary Health Centre) and Núria Duch (Manso Primary Health Centre).
We also thank Nestlé Health Science for supplying nutritional shakes.
Sources of funding
A major funding was received from the Carlos III Health Institute of the Ministry of Health of Spain (FIS:PI12/01503) for the main development of the trial protocol. Also from the Fundació Mutuam Conviure, Becas Esteve de Innovación en Salud 2013 and the VIII Primary Health Care Research Award from Regió Sanitària de Barcelona. Dr Romera has a PhD research grant from IDIAP Jordi Gol.
Abbreviations
- MEC-35
Cognition Mini-Exam of Lobo
- SD
Standard deviation
- CES-D
Center for Epidemiologic Studies Depression Scale
- SPPB
Short Physical Performance Battery
- IG
Intervention group
- CG
Control group
- CONSORT
Consolidated standards of reporting trials
- PHCC
Primary Health Care Centre
- CMN
Case Management Nurse
- TGUGT
Timed get up and go test
- FRT
Functional reach test
- ADL
Activities of daily living
- MNA
Mini Nutritional Assessment
- BT
Barcelona Test
- SF-12
12-Item Short-Form Health Survey
- COPD
Chronic obstructive pulmonary disease
- STOPP
Screening Tool of Older Persons’ potentially inappropriate Prescriptions.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conception of the idea for the study: AR, JMS, LR and FO. Development of the protocol, organization and funding: LR, JMS and FO. All authors contributed to the study design and development of the trial protocol. Writing of the manuscript: LR, FO, JMS, GF. All the authors read the draft critically to make contributions and approved the final manuscript.
Contributor Information
Laura Romera, Email: lromera.bcn.ics@gencat.cat.
Francesc Orfila, Email: forfila.bcn.ics@gencat.cat.
Josep Maria Segura, Email: jmsegura.bcn.ics@gencat.cat.
Anna Ramirez, Email: anaramirez.bcn.ics@gencat.cat.
Mercedes Möller, Email: mmoller.bcn.ics@gencat.cat.
Maria Lluïsa Fabra, Email: mllfabra.bcn.ics@gencat.cat.
Santiago Lancho, Email: slancho.bcn.ics@gencat.cat.
Núria Bastida, Email: nbastida.bcn.ics@gencat.cat.
Gonçal Foz, Email: gfozg.bcn.ics@gencat.cat.
Maria Assumpta Fabregat, Email: mafabregat.bcn.ics@gencat.cat.
Núria Martí, Email: nmarti.bcn.ics@gencat.cat.
Montserrat Cullell, Email: mcullell.bcn.ics@gencat.cat.
Dolors Martinez, Email: dolorsmartinez.bcn.ics@gencat.cat.
Maria Giné, Email: mariagg@blanquerna.url.edu.
Anna Bistuer, Email: anabisla@gmail.com.
Patricia Cendrós, Email: patriciadocent@gmail.com.
Elena Pérez, Email: eperezr@gencat.cat.
References
- 1.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: an emerging research and clinical paradigm-issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.M255. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 4.Rockwood K. Frailty and its definition: a worthy challenge. J Am Geriatr Soc. 2005;53:1069–1070. doi: 10.1111/j.1532-5415.2005.53312.x. [DOI] [PubMed] [Google Scholar]
- 5.Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty [review]. In steering comm, can Initiat frailty and aging. Aging Clin Exp Res. 2003;15(3 Suppl):1–29. [PubMed] [Google Scholar]
- 6.Sternberg SA, Schwartz AW, Karunananthan S, Bergman H, Clarfield M. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59:2129–2138. doi: 10.1111/j.1532-5415.2011.03597.x. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 8.Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991;39:46–52. doi: 10.1111/j.1532-5415.1991.tb05905.x. [DOI] [PubMed] [Google Scholar]
- 9.Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123:1457–1460. doi: 10.1016/S0047-6374(02)00082-9. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 11.Auyeung TW1, Lee JS, Kwok T, Woo J. Physical frailty predicts future cognitive decline - a four-year prospective study in 2737 cognitively normal older adults. J Nutr Health Aging. 2011;15:690–694. doi: 10.1007/s12603-011-0110-9. [DOI] [PubMed] [Google Scholar]
- 12.Ávila-Funes JA, Helmer C, Amieva H, Barberger-Gateau P, Le Goff M, Ritchie K, Portet F, Carrière I, Tavernier B, Gutiérrez-Robledo LM, Dartigues JF. Frailty among community-dwelling elderly people in France: the three-city study. J Gerontol A Biol Sci Med Sci. 2008;63:1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jürschik P, Nunin C, Botigué T, Escobar MA, Lavedán A, Viladrosa M. Prevalence of frailty and factors associated with frailty in the elderly population of Lleida, Spain: the FRALLE survey. Arch Gerontol Geriatr. 2012;55:625–631. doi: 10.1016/j.archger.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen J, Neyens JC, Van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community-dwelling elderly people using physical frailty indicators: a systematic review. BMC Geriatr. 2001;11:33. doi: 10.1186/1471-2318-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A Short Physical Performance Battery assessing lower extremity function: Associated with self-reported disability and prediction of mortality and nursing home admission. J Gerontol, Ser A, Med Sci Biol Sci. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 17.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 18.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83:1173. doi: 10.1016/S0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 20.Abellan Van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 21.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferruci L, Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment. A review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. doi: 10.1016/j.arr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Kelaiditi E, Cesari M, Canevelli M, van Kan GA, Ousset PJ, Gillette-Guyonnet S, Ritz P, Duveau F, Soto ME, Provencher V, Nourhashemi F, Salvà A, Robert P, Andrieu S, Rolland Y, Touchon J, Fitten JL, Vellas B, IANA/IAGG Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17:726–734. doi: 10.1007/s12603-013-0367-2. [DOI] [PubMed] [Google Scholar]
- 25.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8:1–17. [PubMed] [Google Scholar]
- 26.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. The Interventions on Frailty Working Group. Designing Randomized, Controlled Trials Aimed at Preventing or Delaying Functional Decline and Disability in Frail, Older Persons: A Consensus Report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 27.Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, Koch ML, Trainor K, Horwitz RI. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 28.Gates S, Fisher JD, Cooke MW, Carter YH, Lamb SE. Multifactorial assessment and targeted intervention for preventing falls and injuries among older people in community and emergency care settings: systematic review and meta-analysis. Br Med J. 2008;336:130–133. doi: 10.1136/bmj.39412.525243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Cameron ID. Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trial. BMC Med. 2012;10:120. doi: 10.1186/1741-7015-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlow J. Building an evidence base for successful telecare implementation-updated report of the Evidence Working Group of the Telecare Policy Collaborative. London: Care Service Improvement Partnership. Health and Social Care. Change Agent Team. Department of Health; 2006. [Google Scholar]
- 31.Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH. Cochrane Database Syst Rev. 2009. Interventions for preventing falls in older people living in the community. [DOI] [PubMed] [Google Scholar]
- 32.Frost H, Haw S, Frank J. Interventions in community settings that prevent or delay disablement in later life: An overview of the evidence. Qual Ageing Older Adults. 2012;13:212–230. doi: 10.1108/14717791211264241. [DOI] [Google Scholar]
- 33.Giné-Garriga M, Guerra M, Pagès E, Manini TD, Jiménez R, Unnihan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18:401–424. doi: 10.1123/japa.18.4.401. [DOI] [PubMed] [Google Scholar]
- 34.Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81:960–965. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- 35.Thurston M, Green K. Adherence to exercise in later life: how can exercise on prescription programmes be made more effective? Health Promot Int. 2004;19:379–387. doi: 10.1093/heapro/dah311. [DOI] [PubMed] [Google Scholar]
- 36.Rolland Y, Dupuy C, Abellan van Kan G, Gillette S, Vellas B. Treatment strategies for sarcopenia and frailty. Med Clin N Am. 2011;95:427–438. doi: 10.1016/j.mcna.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, Doehner W, Fearon KC, Ferruci L, Hellerstein MK, Kalantar-Zadeh K, Lochs H, MacDonald N, Mulligan K, Muscaritoli M, Ponikowski P, Posthauer ME, Rossi F, Schambelan M, Schols AM, Schuster MW, Anker SD, Society for Sarcopenia, Cachexia, and Wasting Desease Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11:391. doi: 10.1016/j.jamda.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans WJ. Protein nutrition, exercise and aging. J Am Coll Nutr. 2004;23(6 Suppl):601–609. doi: 10.1080/07315724.2004.10719430. [DOI] [PubMed] [Google Scholar]
- 39.Zoltick ES, Sahni S, McLean RR, Quach L, Casey VA, Hannan MT. Dietary protein intake and subsequent falls in older men and women: the framingham study. J Nutr Health Aging. 2011;15:147–152. doi: 10.1007/s12603-011-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, de Groot LC. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:720–726. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 41.McDougall G, Becker H, Pituch K, Acee T, Vaughan P, Delville C. The SeniorWise study: Improving everyday memory in older adults. Arch Psychiatr Nurs. 2010;24:291–306. doi: 10.1016/j.apnu.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebok GW, Langbaum JB, Jones RN, Gross AL, Parisi JM, Spira AP, Kueider AM, Petras H, Brandt J. Memory training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25(Suppl 8):21–42. doi: 10.1177/0898264312461937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langlois F, Vu TT, Chassé K, Dupuis G, Kergoat MJ, Bherer L. Benefits of physical exercise training on cognition and quality of life in frail older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:400–404. doi: 10.1093/geronb/gbs069. [DOI] [PubMed] [Google Scholar]
- 44.Baker L, Frank L, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Fishel MA, Watson GS, Cholerton BA, Duncan GE, Mehta PD, Craft S. Effects of Aerobic Exercise on Mild Cognitive Impairment. A controlled trial. Arch Neurol. 2010;67:71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes DE, Santos-Modesitt W, Poelke G, Kramer AF, Castro C, Middleton LE, Yaffe K. The Mental Activity and eXercise (MAX) Trial: A randomized controlled trial to enhance cognitive function in older adults. JAMA Intern Med. 2013;173:797–804. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiatti C, Bustacchini S, Furneri G, Mantovani L, Cristiani M, Misuraca C, Lattanzio F. The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people: a systematic review. Drug Saf. 2012;35(Suppl 1):73–87. doi: 10.1007/BF03319105. [DOI] [PubMed] [Google Scholar]
- 47.Jyrkkä J, Enlund H, Korhonen MJ, Sulkava R, Hartikainen S. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging. 2009;26:1039–1048. doi: 10.2165/11319530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Clyne B, Bradley MC, Smith SM, Hughes CM, Motterlini N, Clear D, McDonnell R, Williams D, Fahey T, OPTI-SCRIPT Study Team Effectiveness of medicines review with web-based pharmaceutical treatment algorithms in reducing potentially inappropriate prescribing in older people in primary care: a cluster randomized trial (OPTI-SCRIPT study protocol) Trials. 2013;14:72. doi: 10.1186/1745-6215-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 50.Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barber JH, Wallis JB, McKecting E. A postal screening questionnaire in preventive geriatric care. J Coll Gen Pract. 1980;30:49–51. [PMC free article] [PubMed] [Google Scholar]
- 52.Lobo A, Saz P, Marcos G, Día JL, de la Cámara C, Ventura T, Morales Asín F, Fernando Pascual L, Montañés JA, Aznar S. Revalidation and standardization of the cognition mini-exam (first Spanish version of the Mini-Mental Status Examination) in the general geriatric population. Med Clin (Barc) 1999;112:767–774. [PubMed] [Google Scholar]
- 53.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1389. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 54.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. doi: 10.1093/geront/9.3_Part_1.179. [DOI] [PubMed] [Google Scholar]
- 55.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965;4:61–65. [PubMed] [Google Scholar]
- 56.Guigoz Y, Vellas B, Garry P. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54(Suppl 2):59–65. doi: 10.1111/j.1753-4887.1996.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 57.Peña-Casanova J, Guardia J, Bertran-Serra I, Manero RM, Jarne A. Shortened version of the Barcelona test (I): subtest and normal profiles. Neurologia. 1997;12:99–111. [PubMed] [Google Scholar]
- 58.Ware JE, Kosinski M, Keller SD. A 12-Item Short Form Health Survey. Construction of scales and preliminaty tests of reliability and Validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher P, Ryan C, Byrne S, Kennedy J, O´Mahony D. STOPP (Screening Tool of Older Persons’ Prescriptions) and START (Screening Tool to Alert Doctors to Right Treatment): consensus validation. Int J Clin Pharm Ther. 2008;46:72–83. doi: 10.5414/CPP46072. [DOI] [PubMed] [Google Scholar]
- 61.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2318/14/125/prepub


