Abstract
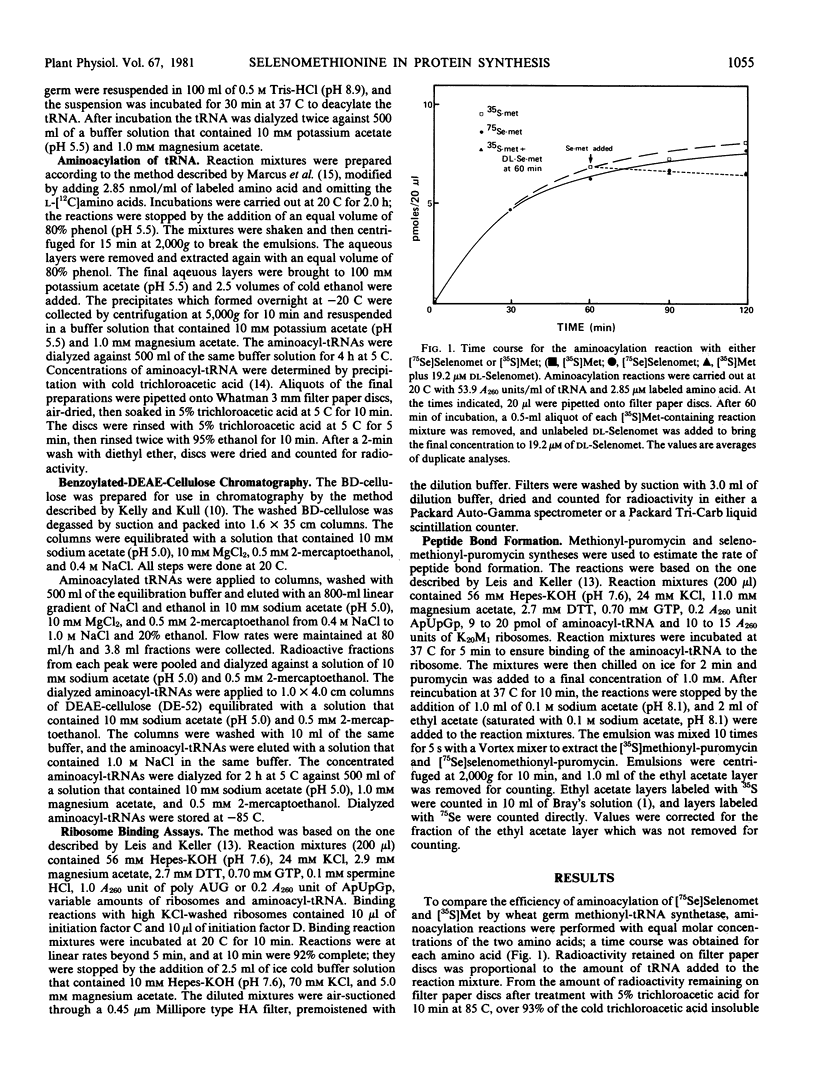
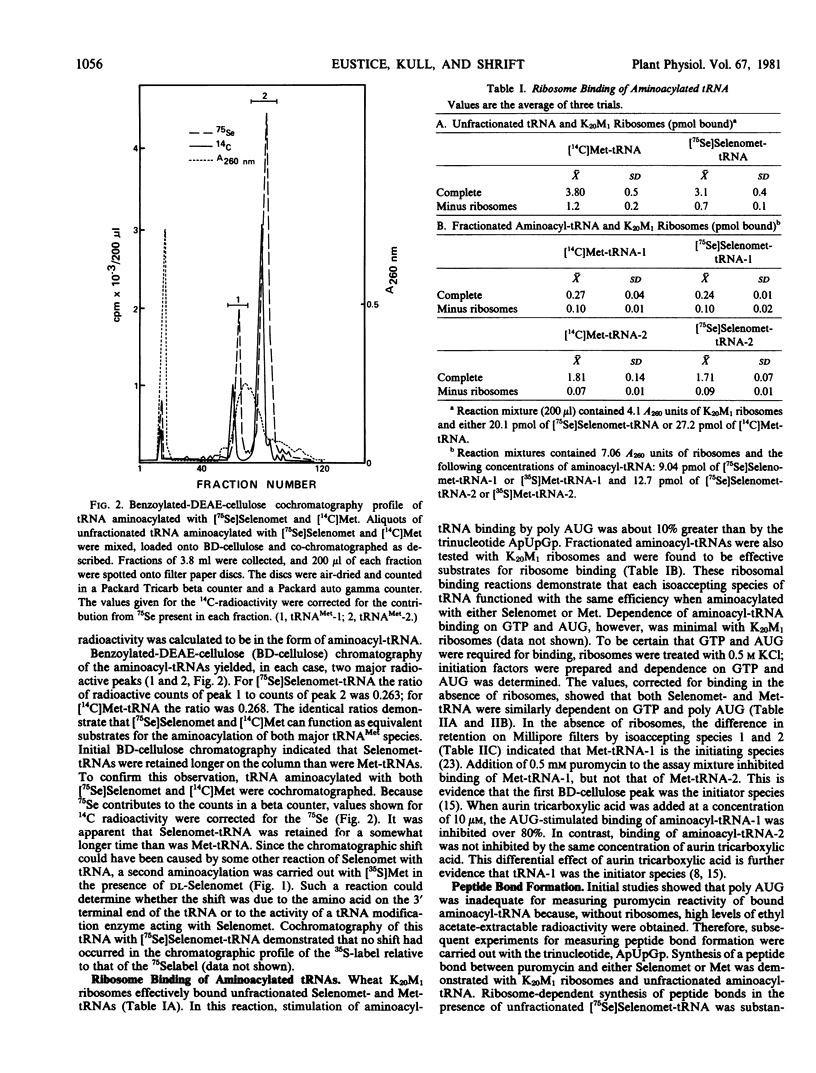
Selenomethionine and methionine were compared as substrates for in vitro aminoacylation, ribosome binding, and peptide bond formation with preparations from wheat germ. Selenomethionine paralleled methionine in all steps of the translation process except peptide bond formation. Peptide bond formation with the initiating species of tRNAMet demonstrated that selenomethionyl-tRNAMet was less effective as a substrate than was methionyl-tRNAfMet. Participation of selenomethionine in the initiation process of translation could be expected to reduce the overall rate of protein synthesis and might aid in explaining selenium toxicity in selenium-sensitive plants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnell J. N. Cysteinyl-tRNA Synthetase from Astragalus Species. Plant Physiol. 1979 Jun;63(6):1095–1097. doi: 10.1104/pp.63.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco C., Busiello V., Di Girolamo M., Cavallini D. Selenaproline and protein synthesis. Biochim Biophys Acta. 1977 Sep 20;478(2):156–166. doi: 10.1016/0005-2787(77)90179-4. [DOI] [PubMed] [Google Scholar]
- Esaki N., Tanaka H., Uemura S., Suzuki T., Soda K. Catalytic action of L-methionine gamma-lyase on selenomethionine and selenols. Biochemistry. 1979 Feb 6;18(3):407–410. doi: 10.1021/bi00570a003. [DOI] [PubMed] [Google Scholar]
- Eustice D. C., Foster I., Kull F. J., Shrift A. In Vitro Incorporation of Selenomethionine into Protein by Vigna radiata Polysomes. Plant Physiol. 1980 Jul;66(1):182–186. doi: 10.1104/pp.66.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. W. Chemical examination of seleniferous cabbage Brassica oleracea capitata. J Agric Food Chem. 1975 Nov-Dec;23(6):1150–1152. doi: 10.1021/jf60202a046. [DOI] [PubMed] [Google Scholar]
- Igarashi S. J., Zmean J. A. Effect of triphenylmethane derivatives on cell-free macromolecular synthesis. II. mRNA-ribosome binding. Can J Biochem. 1975 Feb;53(2):124–127. doi: 10.1139/o75-019. [DOI] [PubMed] [Google Scholar]
- Jenkins K. J., Hidiroglou M. The incorporation of 75-Se-selenite into dystrophogenic pasture grass. The chemical nature of the seleno compounds formed and their availability to young ovine. Can J Biochem. 1967 Jul;45(7):1027–1039. doi: 10.1139/o67-119. [DOI] [PubMed] [Google Scholar]
- Kelly E. P., Kull F. J. Comparison of porcine liver tRNA preparations: purification of tRNA and its separation from RNA-peptidyl complexes. Anal Biochem. 1976 Sep;75(1):9–21. doi: 10.1016/0003-2697(76)90049-x. [DOI] [PubMed] [Google Scholar]
- Konze J. R., Schilling N., Kende H. Enhancement of ethylene formation by selenoamino acids. Plant Physiol. 1978 Sep;62(3):397–401. doi: 10.1104/pp.62.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Keller E. B. Protein chain initiation by methionyl-tRNA. Biochem Biophys Res Commun. 1970 Jul 27;40(2):416–421. doi: 10.1016/0006-291x(70)91025-9. [DOI] [PubMed] [Google Scholar]
- Marcus A., Seal S. N., Weeks D. P. Protein chain initiation in wheat embryo. Methods Enzymol. 1974;30:94–101. doi: 10.1016/0076-6879(74)30013-4. [DOI] [PubMed] [Google Scholar]
- Spremulli L. L., Walthall B. J., Lax S. R., Ravel J. M. Purification and properties of a Met-tRNAf binding factor from wheat germ. Arch Biochem Biophys. 1977 Jan 30;178(2):565–575. doi: 10.1016/0003-9861(77)90227-2. [DOI] [PubMed] [Google Scholar]



