Abstract
Nucleotide-sugar transporters (NSTs) transport activated sugars (e.g. UDP-GlcNAc) from the cytosol to the lumen of the endoplasmic reticulum or Golgi apparatus where they are used to make glycoproteins and glycolipids. UDP-Glc is an important component of the N-glycan-dependent quality control (QC) system for protein folding. Because Entamoeba has this QC system while Giardia does not, we hypothesized that transfected Giardia might be used to identify the UDP-Glc transporter of Entamoeba. Here we show Giardia membranes transport UDP-GlcNAc and have apyrases, which hydrolyze nucleoside-diphosphates to make the antiporter nucleoside-monophosphate. The only NST of Giardia (GlNst), which we could identify, transports UDP-GlcNAc in transfected Saccharomyces and is present in perinuclear and peripheral vesicles and increases in expression during encystation. Entamoeba membranes transport three nucleotide-sugars (UDP-Gal, UDP-GlcNAc, and UDP-Glc), and Entamoeba has three NSTs, one of which has been shown previously to transport UDP-Gal (EhNst1). Here we show recombinant EhNst2 transports UDP-Glc in transfected Giardia, while recombinant EhNst3 transports UDP-GlcNAc in transfected Saccharomyces. In summary, all three NSTs of Entamoeba and the single NST of Giardia have been molecularly characterized, and transfected Giardia provide a new system for testing heterologous UDP-Glc transporters.
Keywords: Giardia, Entamoeba, Nucleotide sugar transporter, Apyrase, transfection, N-glycan-dependent quality control of protein folding
1. Introduction
Nucleotide-sugar transporters (NSTs) transport nucleotide-diphosphate-sugars (e.g. UDP-Glc, -GlcNAc, and –Gal; GDP-Man and -Fuc) and CMP-sialic acid from the cytosol to lumen of the endoplasmic reticulum (ER) or Golgi apparatus [1, 2]. In the ER and Golgi, nucleotide-sugars provide substrates to make O-linked and O-phosphodiester-linked glycans, complex N-glycans, and glycolipids. In addition, UDP-Glc is the substrate for the UDP-Glc: glycoprotein glucosyltransferase (UGGT), which glucosylates N-glycans of misfolded proteins that are then bound and refolded by calreticulin (CRT) and/or calnexin (CNX) in association with a protein disulfide isomerase [3, 4]. While all the other components of the N-glycan-dependent quality control (QC) system for protein folding have been molecularly characterized, the UDP-Glc transporter has not.
Fungi and metazoa contain multiple NSTs, which allow each organism to transport more than one nucleotide-sugar. Although all NSTs have a similar structure, which includes approximately ten transmembrane helices (TMHs), phylogenetic methods identify three different families of NSTs [5, 6]. The specific nucleotide-sugar(s) transported by the NST cannot be predicted by the NST family, and individual organisms may have redundant NSTs that transfer the same nucleotide-sugar [1, 2]. The specificity of numerous NSTs of metazoa, fungi, and protists (e.g. UDP-Gal, GDP-Fuc, and CMP-sialic acid) has been determined by isolating membranes from transfected Saccharomyces cerevisiae, which have endogenous NSTs for UDP-Glc and GDP-Man [1, 2]. Because of the high endogenous transport of UDP-Glc by Saccharomyces membranes, this system has not been used to characterize exogenous UDP-Glc transporters.
In the lumen of the endoplasmic reticulum, sugars are transferred to glycoproteins or glycolipids, and nucleoside-diphosphates are released [1]. Nucleoside-diphosphates are hydrolyzed to nucleoside-monophosphates by NDPases that may be specific for a particular nucleotide (e.g. UDPase) or by apyrases, which degrade multiple nucleoside-diphosphates and nucleoside-triphosphates [7, 8]. NSTs are antiporters that transport a specific nucleoside-monophosphate out of the lumen of the endoplasmic reticulum or Golgi apparatus and into the cytosol at the same time that the nucleotide-sugar is moving in the opposite direction [1].
Knowledge of the NSTs of a given organism is important for two reasons. First, while dolichol-phosphate-mannose can also be used to make O-glycans in some organisms [9], the repertoire of sugar modifications to glycoproteins is otherwise limited by the set of nucleotide-sugars transported [1, 2]. For example, because metazoa in general have more NSTs than fungi, metazoa are able to make glycoproteins with a greater variety of sugars than fungi. Second, mutations in NSTs result in human leucocyte adhesion deficiency syndrome II (GDP-Fuc), vertebral malformations in cows (UDP-GlcNAc), nicked wings and short legs in fruit flies (multiple UDP-sugars), and squashed vulvas in nematodes (UDP-Gal, -GalNAc, and –glucuronic acid) [2]. A GDP-Man- transporter is necessary for synthesis of lipophosphoglycans in Leishmania donovani [10], while two UDP-Gal transporters are involved in lipophosphoglycan and proteophosphoglycan synthesis in Leishmania major [11]. UDP-Gal and UDP-Glc are both used by Entamoeba for synthesis of proteophosphoglycans [12] and complex N-glycans (our unpublished data (i)).
The senior author and others recently identified a UDP-Gal transporter (EhNst1) and apyrase activity in Entamoeba histolytica, the cause of amebic dysentery [13]. Entamoeba membranes also have UDP-Glc transport activity and N-glycan-dependent QC system for protein folding [3, 4]. In contrast, Giardia lamblia, an important cause of diarrhea, makes a very short N-glycan and is missing the N-glycan-dependent QC system for protein folding [3, 14]. Because UDP-Glc transport has been associated with UGGT activity but not with other glycosylation reactions [4], we hypothesized that Giardia might lack UDP-Glc transport activity. Therefore transfected Giardia might be used to determine which of two other predicted Entamoeba NSTs (EhNst2 or EhNst3) transports UDP-Glc [13, 15].
The present study began with the observation that membranes of Giardia lamblia transport a single nucleotide-sugar (UDP-GlcNAc) (3.1) and the Giardia genome predicts a single NST (GlNst), which is localized to perinuclear and peripheral vesicles and is increased during encystation (3.2). Phylogenetic methods showed that Giardia and all other eukaryotes examined have a family 2 NST (3.3). Transfected Saccharomyces were used to characterize GlNst and one of the two uncharacterized Entamoeba NSTs (EhNst3) (3.4 and 3.5) [13]. The final uncharacterized Entamoeba NST (EhNst2), which encodes a putative UDP-Glc transporter, was characterized in transfected Giardia that do not transport UDP-Glc (3.6). The importance of these results to the glycobiology of Giardia and Entamoeba and to characterization of other eukaryotic NSTs is discussed in 4.1 to 4.3.
2. Materials and Methods
2.1. Radioactive substrates
The following radioactive substrates were from American Radiolabeled Chemicals Inc (St. Louis, MO): UDP-[3H]Glc (60 Ci/mmol), UDP-[14C]GlcNAc (55 mCi/mmol), UDP-[3H]GalNAc (20 Ci/mmol),), GDP-[3H]mannose (20 mCi/mmol), GDP-[14C]fucose (200 mCi/mmol), CMP-[3H]sialic acid (20 Ci/mmol), UDP-[3H]Gal (20 Ci/mmol) and UDP-[14C]GlcA (300 mCi/mmol).
2.2. Cell culture and vesicle isolation from Giardia
Giardia lamblia strain MR4 or WB1267 trophozoites were grown in TYI-S-33 supplemented with 10% bovine serum (Biosource) and bovine bile (Sigma-Aldrich) at 37°C. Log-phase Giardia from 100 ml of culture (~107 parasites) were chilled on ice for 20 min, collected by centrifugation, and washed twice in PBS. Giardia were resuspended in 0.5 ml of hypotonic lysis buffer (10 mM Hepes-KOH pH 7.2, 10 mM MgCl2, 25 mM KCl, and complete protease inhibitor mixture (Sigma)). After 10 min on ice, the cells were homogenized with 40 strokes in a Dounce homogenizer. The homogenate was diluted with an equal volume of the lysis buffer containing 0.5 M sucrose and further homogenized with another 40 strokes. The homogenate was centrifuged at 2000g for 5 min to remove any unbroken cells. The suspension was centrifuged 8 min at 5000g to obtain a first low-speed membrane fraction, which also included nuclei. The supernatant was centrifuged for 45 min at 125,000g to obtain a second high-speed membrane fraction. Pellets were resuspended in lysis buffer containing 0.5 M sucrose and 1X complete protease inhibitor mixture at a concentration of 0.5 mg/ml. The vesicle fraction was either used immediately or frozen at −80°C in small aliquots.
2.3. Tests of the NST activities of Giardia and Entamoeba membranes
NST activity of Giardia membranes was assayed in two ways [13]. In order to rapidly screen a panel of radiolabeled nucleotide-sugars, an assay that measures transport and transfer was performed. In this assay, 25–50 μl of Giardia membranes, which contained ~50 μg of protein as measured by BCA protein assay kit (Pierce), were incubated in 200 μl reaction mixture containing 500 mM sucrose, 30 mM triethanoloamine, pH 7.2, 5 mM MgCl2, 5 mM MnCl2, and the radioactive nucleotide to be tested. After 30 min at 37oC, the reaction was stopped by adding 200 μl of ice-cold 10% perchloric acid and 20 μl of a 10 mM concentration of cold nucleotide-sugar. The precipitate after 30 min was recovered by centrifugation at 16,000g for 30 min, washed, neutralized, and counted with a scintillation counter after addition of scintillation fluid. Negative controls included membranes incubated for 30 min at 4°C and membranes incubated at 37°C in the presence of 0.1% Triton X-100, which permeabilizes vesicles.
For assaying transport of nucleotide-sugars, Giardia vesicles were incubated with varying concentrations of the radioactive nucleotide sugars under the same conditions for the transport and transfer assay, but the reaction was carried out for five min rather than 30 min at 37°C, and the reaction was stopped with 3 ml of ice cold stop solution (1 M sucrose and 1 mM EDTA) [16]. Vesicles were separated from incubation medium by centrifugation at 125,000g for 30 min, and the pelleted vesicles were washed 4 times in the stop solution before resuspension in 4% perchloric acid. The acid-soluble radioactivity, which represents nucleotide-sugar that has been transported but not yet transferred to acid-insoluble counts, was counted with the scintillation counter. Negative controls included vesicles incubated at 4°C and vesicles incubated in the presence of EDTA.
Encysting Giardia use cytosolic UDP-GalNAc to make an acid-precipitable, β-1,3-linked GalNAc polymer, which is associated with the cyst wall [17]. In vitro synthesis of the GalNAc polymer was distinguished from UDP-GalNAc transport and transfer in the following ways. Membranes were isolated as described above from Giardia induced to encyst for 12–24 h using published protocols [18]. Transport of radiolabeled UDP-GalNAc, which measures soluble rather than insoluble counts associated with membranes, was measured first, because GalNAc polymer synthesis only forms insoluble products [17]. Second, transported and transferred GalNAc, which consists of precipitated material, were treated with strong acid that degrades and releases radioactivity from the GalNAc polymer [17] but does not release counts from GalNAc attached to glycoproteins. Conversely, transported and transferred GalNAc was treated with strong base, which releases counts from glycoproteins but does not degrade the GalNAc polymer.
The HM1:IMSS strain of Entamoeba histolytica was grown axenically in TYI-S-33 supplemented with 10% bovine serum (Biosource) at 37°C. Entamoeba membranes were prepared exactly as described in ref. 13, and transport and transfer assays were performed as described above for Giardia.
2.4. NDPase/Apyrase assay
The assay was essentially as described previously [19]. Reaction mixtures in a final volume of 100 μl contained 10 μg of Giardia membrane protein and 200 mM imidazole, pH 7.5; either 2 mM CaCl2 or EDTA; either 2 mM nucleoside diphosphates or nucleoside triphosphates; with or without 0.1% Triton X-100. After incubation at 30°C for 30 min, the reaction was stopped by adding 200 μl of 7.5% SDS. Inorganic phosphate released was determined by adding 700 μl of Ames reagent (0.42% ammonium molybdate in 1 N sulfuric acid:10% ascorbic acid, 6:1), followed by incubation for 20 min at 45°C. Absorbance was measured at 660 nm.
2.5. Bioinformatic methods
The genome of Giardia lamblia has been sequenced to ~12 times redundancy, so that the vast majority of genes have been identified [20]. The predicted proteins of Giardia, which are present at The Giardia Genome Database (www.giardiadb.org), were searched with Psi-Blast and multiple NST genes from Saccharomyces cerevisiae and Homo sapiens [21]. Similar methods were used to search the predicted proteins of representative protists, metazoa, and fungi.
TMHs were predicted using the Phobius combined transmembrane topology and signal peptide predictor [22]. Predicted NST sequences were examined for conserved domains using the CD search at the NCBI [23]. NST sequences were aligned using MUSCLE (Multiple Sequence Comparison by Log-Expectation) [24]. The alignment was manually refined, and gaps were removed using BioEdit. The finished alignment was used to construct the phylogenetic tree using TREE-PUZZLE, a program to reconstruct phylogenetic trees from molecular sequence data by the maximum likelihood method [25].
Localization of GlNst and measurement of its expression during encystation
The GlNst gene was amplified using primers CCATGGATGAACGAGACTGTCCGTTTC (sense with Nco1 site) and GCGGCCGCTTACAGATCCTCTTCTGAGATGAGTTTTTTATTCTTACTGCGG (anti-sense primer with a myc tag and Not1 site) was cloned into the pGFP-BglII vector, from which the GFP sequence had been removed (a gift from Heidi Elmendorf) [15]. This vector, which contains selectable markers for ampicillin in E. coli and puromycin in Giardia, places the GlNst-myc gene between 5’ and 3’ untranslated regions of Giardia tubulin gene. WB1267 Giardia cells were electroporated with 50 μg plasmid DNA (350V, 750 ohms 1000 μF) in a BTX electroporator [15]. After 24 h, 100 μM puromycin was added for selecting the transfected parasites, which were maintained in 100 μM puromycin. For localization, live Giardia were labeled with succinimidyl ester of Alexafluor (Invitrogen) for 1 h at room temperature in 0.1 M carbonate buffer pH 8. Labeled cells were washed twice in PBS and fixed in 2% paraformaldehyde at 40C for 10 min. Fixed cells were permeabilized in 0.05% Triton X-100 for 2 min and the stained with FITC conjugated anti-myc antibody (Invitrogen) for 1 h at room temperature. DAPI was used to stain the nuclei. Stained cells were washed thoroughly and then put in poly-lysine coated slides for viewing. WB1267 transfected with an empty vector was used as a negative control.
For measurement of GlNst gene expression during encystation, Giardia cells were collected at 0 h, 6 h and 24 h after adding the high bile encystation media. For quantitative RT-PCR, encysting protists were chilled on ice for 20 min and collected by centrifugation (2000g for 10 min). The protist pellet was washed once, and RNA was isolated using Trizol (Invitrogen). Quantitative RT-PCR was performed using the Retroscript kit (Ambion). Primers for GlNst were CGAGACTGTCCGTTTCCTTC (sense) and GCAAGAGCGTGGAAAAACTC (anti-sense). Control β-tubulin primers were TCCCAGATGTTCGACAACAA (sense) and GACTCGGCCTCTGTTGAACTC (anti-sense). Primers for glucose-6-phosphate N-acetyltransferase (GNA), a cytosolic enzyme involved in UDP-GalNAc synthesis that increases during encystation, were CAGCAAATGGAGATCGTGAA (sense) and TTGGGCTCGACAAGAAGAGT (anti-sense) [26]. Primers for a Giardia O-GlcNAc transferase (GlOGT), an enzyme responsible for cytosolic O-glycosylation, were GTGTTCCTGTCGTCGGATTT (sense) and GCTCTTCACGTTTGCAATGA (anti-sense) [27] (our unpublished data (iii)). Quantitative RT-PCR was performed on Stratagene Mx 4000, using the Brilliant SYBR Green QPCR Master Mix (Stratagene). The concentration of double stranded DNA formed over time (Ct) was determined from log phase of the reactions.
2.6. Cloning and expression of GlNst and EhNst3 in yeast
The GlNst gene was amplified from the total genomic DNA of Giardia (sense primer = GAATTCATGAACG AGACTGTCCGTTTC and anti-sense primer = AAGCTTATCTTGTTTTTTATTCTTACTGCGG), and the PCR product was cloned into pYES2.1/V5-His-TOPO vector (Invitrogen). This vector makes polyHis-tagged proteins, which are under the Saccharomyces Gal1 promoter. The GlNst sequence within the vector was confirmed by sequencing. The GlNst construct was transfected into Saccharomyces cerevisiae strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0). For expression, yeast cells with the GlNst plasmid and controls with an empty vector were induced with 2% Gal for 16 h at 300C. Expression of GlNst was checked on Western blots of lysed Saccharomyces, using a horse-radish peroxidase-labeled antibody to the polyHis epitope-tag. The similar methods were used to express in Saccharomyces the EhNst3 gene (sense primer = CATGGATGACTATATCAACTATTCAATCT and anti-sense = GCGGCCGCTTAGCCTTTATCATCATCATCTTTATAATCGCTAGCATTATTCTTCTTAG, which was amplified with RT-PCR from the mRNA of Entamoeba, because the EhNst3 gene contains a short intron. The EhNst3 was expressed in a chs1/chs3 double mutant in a BY4741 background to reduce noise in the UDP-GlcNAc transport assay [28].
2.7. Isolation of vesicles from yeast and measurement of NST activity
Yeast transfectants expressing GlNst or EhNst3 were centrifuged and converted to spheroplasts by incubating for 30 min at 37°C using 1 mg of Zymolase 100T (Seikagaku America, Rockville, MD) per gram of cells in spheroplast buffer (1.4 M sorbitol, 20mM NaN3, 48 mM 2-mercaptoethanol, 50 mM potassium phosphate buffer, pH 7.5). Cells were broken by resuspending the pellet in 1.5 volumes of membrane buffer (0.8 M sorbitol, 1 mM EDTA, protease inhibitor cocktail (Sigma), and 10 mM triethanolamine/acetic acid, pH 7.2) followed by drawing the cells rapidly several times into a narrow bore serological pipette. The suspension was centrifuged successively for 10 min at 2000g and 8 min at 5000g to remove the nuclei. The supernatant was centrifuged again for 45 min at 125,000g to obtain the pellet fraction enriched in endoplasmic reticulum- and Golgi apparatus-derived vesicles.
The transport assays to measure the activity of the Giardia and Entamoeba NSTs in Saccharomyces vesicles with various radiolabeled nucleotide-sugars were performed as described above for Giardia membranes. Transport activity was defined as total radioactive solutes in the vesicle pellet after incubation at 30°C minus the control incubated at 0°C.
2.8. Expression of Entamoeba Nst2 gene in Giardia
An uncharacterized Entamoeba NST gene (EhNst2) [13] was isolated from genomic DNA with the PCR (sense primer = CCATGGATGAACCAAGCAATTTTAAGTATCATC and anti-sense primer with FLAG tag = GCGGCCGCCTAGCCTTTATCATCATCATCTTTATAATCGCTAGCATTTTCAAGGTCAT) and ligated into the pGFP-BglII vector, from which the GFP sequence had been removed (see above) [15]. WB1267 strain Giardia were transfected, and membranes were isolated for transport activities with radiolabeled nucleotide-sugars, as described above.
3. Results
3.1. Giardia vesicles transport UDP-GlcNAc and contain an apyrase that makes the antiporter UMP
The goal of these experiments was to determine which nucleotide sugars are transported by membranous vesicles prepared from Giardia trophozoites. These vesicles transported and transferred to acid-insoluble counts UDP-GlcNAc but did not transport and transfer UDP-Glc, -Gal, -GalNAc, or -glucuronate; GDP-Man and –Fuc; and CMP-sialic acid (Fig. 1A). Transport only of UDP-GlcNAc was saturable with a Km of 1.9 μM (Fig. 1B). UDP-GlcNAc was transferred to a small extent to glycoproteins but primarily to glycolipids, which we are presently in the process of characterizing.
Fig. 1.
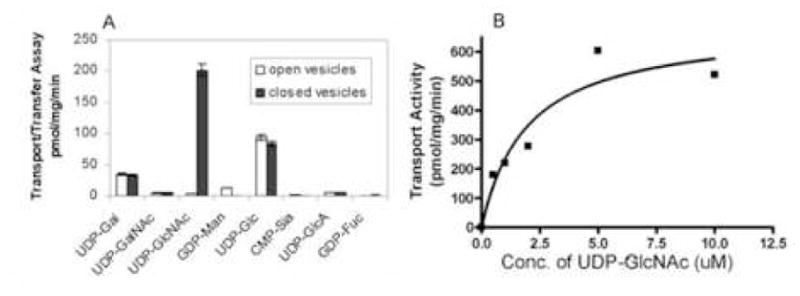
Transport of UDP-GlcNAc by Giardia vesicles. A. Transport and transfer of various nucleotide sugars by Giardia membranes. Shaded bars indicate sealed vesicles, while unshaded bars indicate vesicles permeabilized with 0.1% Triton X-100 (negative control). B. Nucleotide-sugar transport by Giardia membranes with changing concentrations of UDP-GlcNAc. The Km of transport is 1.9 μM.
UDP-GlcNAc transport was associated with intact membranous vesicles that were pelleted with nuclei at 5000g. These vesicles also contain the oligosaccharyltransferase activity (addition of N-glycans to the nascent peptide) of Giardia (our unpublished data (ii)). In contrast, there was little UDP-GlcNAc transport in Giardia vesicles that were pelleted at 125,000g after removal of nuclei. There was also almost no UDP-GlcNAc transport by Giardia vesicles, which had been permeabilized with detergent. This negative control and the demonstration that the apyrase is released with detergent (next paragraph) show that the Giardia vesicles are intact and not inside out.
NSTs, which transport nucleotide sugars from the cytosol to the lumen of the endoplasmic reticulum or Golgi apparatus, are antiporters for the nucleoside-monophosphates [1]. The nucleoside-monophosphate may be made by an NDPase and/or by an apyrase, which hydrolyzes both nucleoside-diphosphates and nucleoside-triphosphates [7, 8, 29]. Giardia vesicles showed NDPase and/or apyrase activity, which was more prominent in vesicles permeabilized with 0.1% Triton X-100 than in untreated vesicles (negative control) (Fig. 2).
Fig. 2.
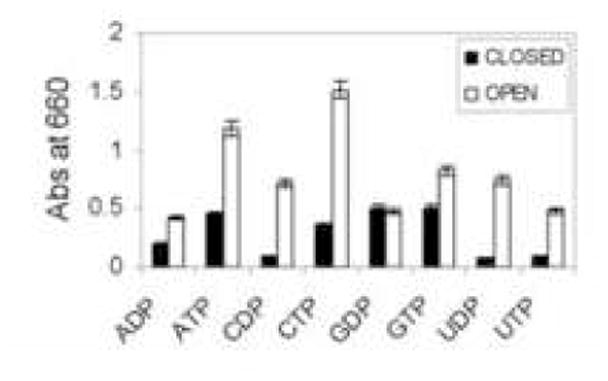
NDPase/apyrase activity of Giardia vesicles. Shaded bars indicate sealed vesicles, while unshaded bars indicate vesicles permeabilized with 0.1% Triton X-100. While nucleotide-sugar transport and transfer is increased in closed vesicles (Fig. 1A), NDPase/apyrase activity, which is normally present in the lumen of vesicles or on the cell surface, is increased in open vesicles.
In addition, we found evidence for cytosolic O-GlcNAc transferase (OGT), which is a glycosyl transferase that catalyses the transfer of a single GlcNAc to the Ser or Thr of nucleocytoplasmic proteins [27]. In unpublished data (iii), we have demonstrated the activity of a single Giardia OGT (GlOGT), which resembles those of higher eukaryotes, in transfected Saccharomyces.
We looked for, but did not find, evidence of UDP-GalNAc transport in either trophozoites or encysting Giardia (see Supplemental Fig. 1). Instead encysting Giardia have a GalNAc polymer synthase, which uses cytosolic UDP-GalNAc to make the β-1,3-linked GalNAc polymer that is the major structural carbohydrate of the cyst wall [17]. In the presence of radiolabeled UDP-GalNAc, membranes from encysting Giardia but not from trophozoites make the GalNAc polymer, which is degraded in acid but is resistant to base. In contrast, GalNAc, if it were transported by an NST and transferred to glycoproteins, would be resistant to acid and released by base.
In addition, all other well characterized sugar homopolymers (chitin, cellulose, and β-1,3-glucan) are made by a membrane-spanning synthases, which uses cytosolic nucleotide-sugars to make a polymer that is threaded through a membrane pore onto the surface of the organism [30, 31]. Therefore no nucleotide-sugar transport is involved in synthesis of chitin, and as shown in Figs. 6 and 7 (below), Saccharomyces has no endogenous UDP-GlcNAc transporter. By homology to other sugar homopolymer synthases and because we were unable to identify any UDP-GalNAc transport in encysting Giardia, we think it is very unlikely that synthesis of the GalNAc polymer by Giardia involves a UDP-GlcNAc transporter.
Fig. 6.
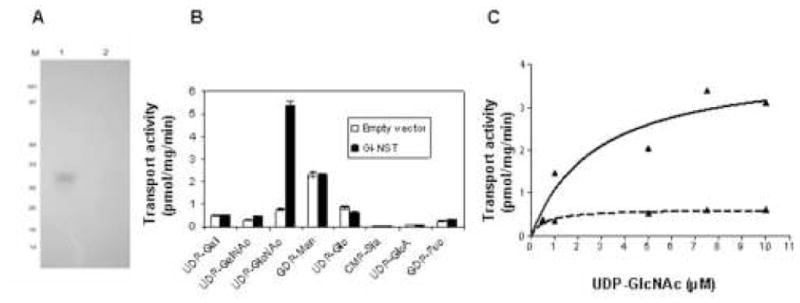
Expression of Giardia Nst in Saccharomyces. A. Western blot with anti-His antibodies of Saccharomyces transfected with GlNst with a C-terminal polyHis tag (lane 1) and Saccharomyces transfected with an empty vector (lane 2). B. Transport activity of closed vesicles of Saccharomyces transfected with a vector containing GlNst (black bars) or an empty vector (white bars). While transport of GDP-Man and UDP-Glc were the same, UDP-GlcNAc transport was dramatically increased in fungi transfected with GlNst. C. Plot of nucleotide-sugar transport by Saccharomyces transfected with GlNst with changing concentrations of UDP-GlcNAc (solid line). The Km of UDP-GlcNAc transport is 2.7 μM. In contrast, there was minimal or no transport of UDP-GlcNAc in Saccharomyces transfected with an empty vector (dotted line).
Fig. 7.
Expression of Entamoeba Nst3 in Saccharomyces. A. Transport and transfer of various nucleotide sugars by Entamoeba membranes. Shaded bars indicate sealed vesicles, while unshaded bars indicate vesicles permeabilized with 0.1% Triton X-100 (negative control). Transport and transfer of UDP-Glc and UDP-Gal by Entamoeba membranes were previously shown in ref. 10, while transport and transfer of UDP-GlcNAc is new. B. Transport of UDP-GlcNAc by closed vesicles of Saccharomyces transfected with a vector containing EhNst3 (black bars) was dramatically increased versus transport by control vesicles from yeast transfected with an empty vector (white bars). No UDP-GlcNAc transport was observed in open vesicles (negative control).
3.2. The Giardia nucleotide-sugar transporter (GlNst) is present in perinuclear and peripheral vesicles and shows increased expression during encystation
A single putative nucleotide-sugar transporter (GlNst) (NCBI gene id: 29248511), which is 387-aa long and included nine putative TMHs, was identified from the Giardia whole genome sequences (Supplemental Fig. 2) [20–23]. While no other putative NSTs could be identified from the predicted proteins of Giardia despite exhaustive searching with metazoan and fungal Nsts, we cannot rule out the possibility that an NST, which has a unique structure and transports untested nucleotide-sugars, is present in Giardia. Eight of the predicted TMHs of GlNst align with those of other eukaryotic NSTs (e.g. Hut1 of Saccharomyces). GlNst also contains a conserved transporter domain (pfam08449.1), which is present in transporters of UDP-GlcNAc [23]. GlNst has a C-terminal sequence (KKQD), which resembles endoplasmic reticulum-retention signals in transmembrane proteins of other eukaryotes.
Myc-tagged GlNst is present in the perinuclear region and peripheral vesicles of transfected Giardia (Fig. 3) [15]. This distribution of myc-tagged GlNst is similar to that of wheat germ agglutinin, which binds to short N-glycans of Giardia [14] (and our unpublished data (iv and vi)). The perinuclear region is where the ER marker BiP has been localized on numerous occasions by others [32]. Multiple lysosomal enzymes have been localized to peripheral vesicles [33].
Fig. 3.
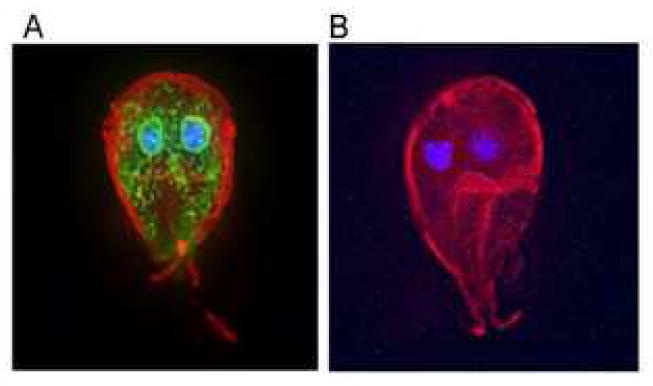
Myc-tagged GlNst is present in the perinuclear region and peripheral vesicles of transfected Giardia. A. GlNst is visualized with FITC-anti-myc antibodies (green) in permeabilized Giardia, the surface of which is labeled red with Alexafluor and the nucleus of which is labeled blue with DAPI. B. Negative control, which is transfected with an empty vector shows no binding with the anti-myc antibody.
Quantitative RT-PCR showed that mRNAs of GlNst are increased during encystation of Giardia (Fig. 4). Similarly, mRNAs of the cytosolic OGT and GNA, an enzyme involved in the synthesis of precursors for the GalNAc polymer, are increased during encystation [26, 27]. In contrast, tubulin mRNAs remain constant during encystation. Because the targets for UDP-GlcNAc transported by GlNst and the targets of the cytosolic OGT have not been characterized, the significance of the increases in mRNAs during encystation is not clear. In unpublished work (iv and v), we have found that there are dramatic differences in the WGA-affinity purified glycoproteins of Giardia during encystation.
Fig. 4.
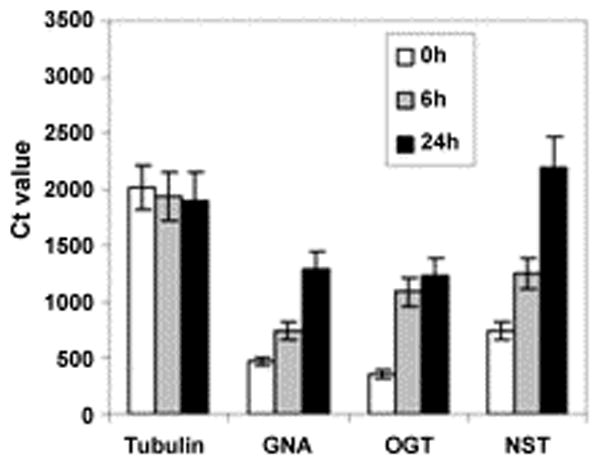
Quantitative RT-PCR, where Ct is the concentration of double stranded DNA produced by samples run in parallel, shows that mRNAs of GlNst are increased during encystation of Giardia. Trophozoites were induced to encyst for 6 h or 24 h and mRNAs for GlNst (putative UDP-GlcNAc transporter), GlOST (putative cytosolic O-GlcNAc transferase [27]), GNA (glucose-6-phosphate N-acetyltransferase, which is increased during encystation [26]), and tubulin were measured quantitatively.
3.3. Phylogenetic reconstructions show Giardia and all other eukaryotes have family 2 nucleotide-sugar transporters
Phylogenetic reconstructions have separated eukaryotic NSTs into three families [5, 6]. Here phylogenetic methods were used to characterize GlNst and predicted NSTs of other protists, the genomes of which have been extensively sequenced (Fig. 5 and Supplemental Table 1) [5, 6, 21, 24, 25]. As expected, the eukaryotic NSTs separate into three families, which are well-supported by bootstrap values. In contrast, most of the NSTs within a particular family collapse into a single node, consistent with the idea that the NSTs are ancient and so have lost much of their phylogenetic information.
Fig. 5.
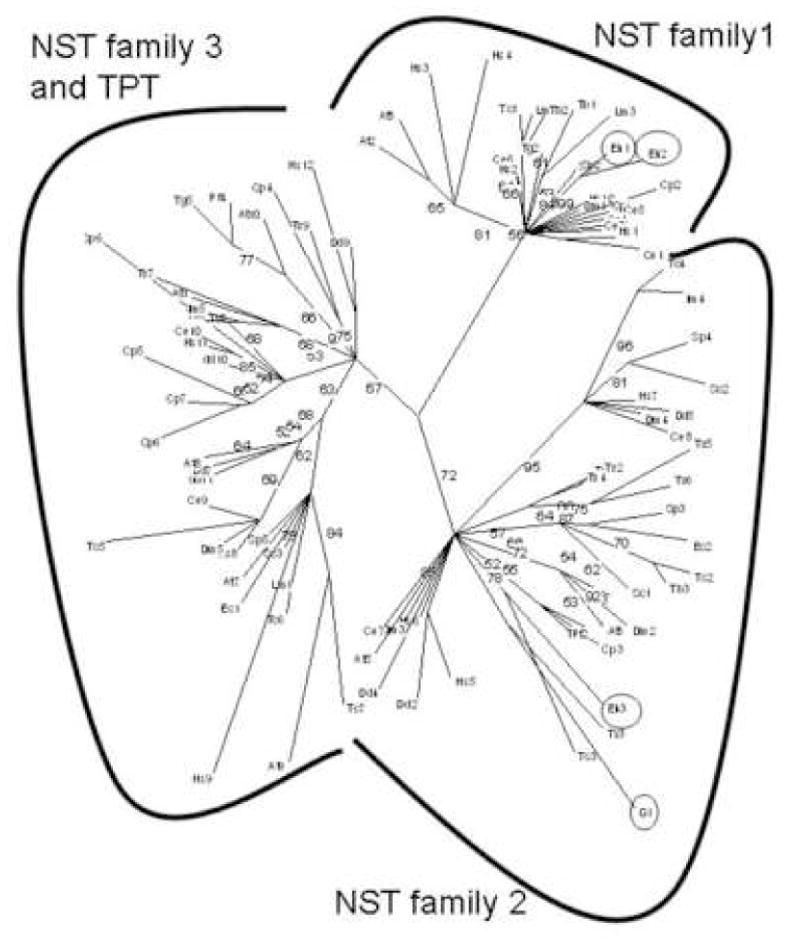
Phylogenetic tree of protist, fungal, and metazoan NSTs constructed by maximum likelihood method (21, 22). Protists include Giardia lamblia (Gl), Entamoeba histolytica (Eh), Trichomonas vaginalis (Tv), Trypanosoma cruzi (Tc), Trypanosoma brucei (Tb), Leishmania major (Lm), Plasmodium falciparum (Pf), Toxoplasma gondii (Tg), Cryptosporidium parvum (CP), and Dictyostelium discoideum (Dd). Fungi include Saccharomyces cerevisiae (Sc), Schizosaccharomyces pombe (Sp), and Encephalitizoon cuniculi (Ec), while metazoans include Homo sapiens (Hs), Caenorhabditis elegans (Ce), and Drosophila melanogaster (Dm). Arabidopsis thaliana (At) is the only plant. NCBI gene id numbers for each NST are listed in Supplemental Table 1. Giardia and Entamoeba NSTs are circled. Lengths of branches are proportional to differences between sequences, while numbers at nodes refer to bootstrap values for 100 trees. These bootstraps strongly support three distinct families of NSTs (3, 4).
Giardia NST is present in the NST family 2 (Fig. 5 and Supplemental Table 1) [5, 6]. Entamoeba has two NST family 1 genes (EhNst1 and EhNst2) and one NST family 2 gene (EhNst3) [13]. A single, uncharacterized NST of Plasmodium falciparum, is also present in the NST 2 family clade. Indeed, all eukaryotes examined have one or more predicted NSTs in family 2. It is likely that at least some of the present diversity of NST genes derives from secondary loss, because similar organisms have NSTs of different families. For example, Toxoplasma gondii has NSTs from three families, while Plasmodium has only an NST from family 2. Dictyostelium discoideum has NSTs from three families, while Entamoeba has NSTs from two families. The present diversity of N-glycan precursors among extant eukaryotes also appears to have been due to secondary loss of sets of glycosyltransferase genes [14].
3.4. Saccharomyces vesicles expressing Giardia Nst transport UDP-GlcNAc
Vesicles prepared from Saccharomyces transport GDP-Man and UDP-Glc but fail to transport other nucleotide sugars [1]. It is possible then to overexpress in Saccharomyces NSTs from other eukaryotes and determine what nucleotide-sugars other than GDP-Man and UDP-Glc are transported. For example, we recently showed that the Entamoeba Nst1 transports UDP-Gal [13]. Here GlNst with a His-tag was overexpressed in Saccharomyces and identified by Western blotting (Fig. 6A). Vesicles from Saccharomyces expressing GlNst showed a marked increase in transport of UDP-GlcNAc, with no changes in transport of other nucleotide-sugars (Fig. 6B). Transport of UDP-GlcNAc was saturable with a Km of transport was 2.7 μM (Fig. 6C). In contrast, there was no increase in UDP-GlcNAc transport by vesicles of Saccharomyces transfected with an empty vector (negative control). These results, which show that GlNst is a UDP-GlcNAc transporter, are consistent with the finding that Giardia membranes transport UDP-GlcNAc (Fig. 1) and the Giardia genome appears to contain just one NST gene (GlNst) (Fig. 5).
3.4. Saccharomyces vesicles expressing Entamoeba Nst3 transport UDP-GlcNAc
Although Entamoeba has three putative NST genes, Entamoeba membrane vesicles that exclude nuclei only show transport for UDP-Gal and UDP-Glc [13]. Here we found that Entamoeba vesicles that included nuclei also transported UDP-GlcNAc (Fig. 7A). While UDP-GlcNAc transport by Entamoeba membranes was less than transport of UDP-Gal and UDP-Glc, the negative control with permeabilized vesicles was at background for UDP-GlcNAc. Consistent with these observations, one of the two uncharacterized Entamoeba NST genes (EhNst3; NCBI gene id: 67477389) transported UDP-GlcNAc when expressed in transfected Saccharomyces (Fig. 7B). Because the UDP-GlcNAc activity of recombinant EhNst3 was not as great as that of GlNst, EhNst3 was expressed in the chs1/chs3 mutant of Saccharomyces, which has an extremely low background of UDP-GlcNAc transport in the absence of a foreign UDP-GlcNAc transporter [28].
3.5. Giardia vesicles expressing Entamoeba Nst2 transport UDP-Glc
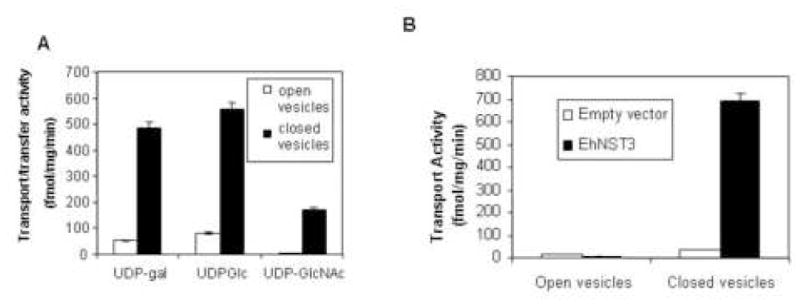
The idea here was to determine whether Giardia might be used to show the activity of a heterologously expressed transporter of UDP-Glc. Here the last uncharacterized NST of Entamoeba (EhNst2; NCBI gene id: 67478973) [13] was overexpressed in transfected Giardia under a Giardia tubulin promoter (Fig. 8A) [15]. Vesicles from Giardia expressing EhNst2 showed a marked increase in transport of UDP-Glc, with no changes in transport of other relevant nucleotide-sugars (UDP-GlcNAc and UDP-Gal) (Fig. 8B). Transport of UDP-Glc was saturable with a Km of 1.6 μM (Fig. 8C). These results show EhNst2 is a UDP-Glc transporter.
Fig. 8.
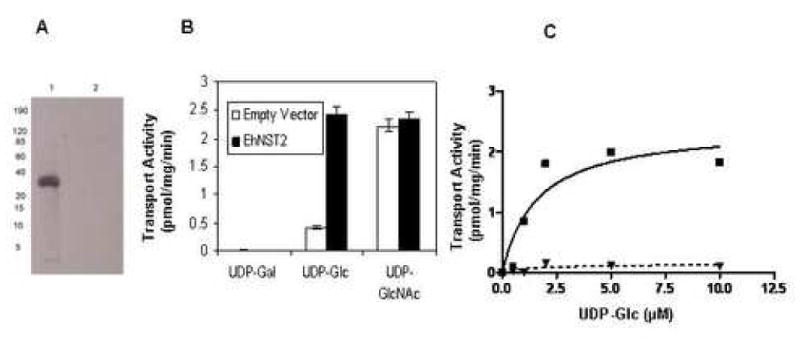
Expression of Entamoeba Nst2 in Giardia. A. Western blot with anti-FLAG antibodies to proteins of Giardia transfected with a vector expressing Entamoeba Nst2 with a C-terminal FLAG tag (lane 1) or Giardia transfected with an empty vector (lane 2). B. Transport activities of closed vesicles of Giardia transfected with Entamoeba Nst2 (black bars) or Giardia transfected with an empty vector (white bars). While transport of UDP-GlcNAc and UDP-Gal were the same, UDP-Glc transport was dramatically increased in Giardia transfected with EhNst2. C. Plot of nucleotide-sugar transport by Giardia transfected with EhNst2 with changing concentrations of UDP-Glc (solid line). The calculated Km of transport was 1.6 μM. In contrast, there was minimal or no transport of UDP-Glc in Giardia transfected with an empty vector (dotted line).
4. Discussion
4.1. The importance of GlcNAc in Giardia
With regards to nucleotide sugar transport, Giardia appears to be the simplest eukaryote yet described [1, 2, 5, 6]. Giardia vesicles only transport UDP-GlcNAc, and the single NST predicted from the Giardia genome transports UDP-GlcNAc in transfected Saccharomyces. The present results mirror our recent discovery that Giardia has the simplest N-glycans yet described, in which one or two GlcNAc are added to Asn rather than 14 sugars in higher eukaryotes [14]. N-glycans with two GlcNAc are recognized by the plant lectin wheat germ agglutinin, which we and others have used to localize glycoproteins in trophozoites and encysting Giardia [34] (and our unpublished data (vi)). Giardia also has a cytosolic O-GlcNAc transferase (OGT) that catalyses the transfer of a single GlcNAc to the Ser or Thr of nucleocytoplasmic proteins [27] (and our unpublished data (iii)).
4.2. A complete set of NSTs identified in Entamoeba
Entamoeba membranes transfer three nucleotide-sugars, and the Entamoeba genome predicts three NST genes. The results here and in ref. 13 pair each Entamoeba NST gene with its transporter: EhNst1 transports UDP-Gal, EhNst2 transports UDP-Glc, and EhNst3 transports UDP-GlcNAc. As far as we know, this is the first time that all of the NST activities of a particular organism have been linked with the specific genes that encode the transporters (Supplemental Table 1) [1, 2, 5, 6]. This is likely because 1) UDP-Glc transporters have not previously been molecularly characterized (see next section). 2) Metazoa, fungi, and plants have numerous NST genes, each of which may transport more than one nucleotide-sugar and may be redundant with other genes [1, 2]. 3) With the exception of Leishmania [10, 11], protist NST genes, which are likely simpler than those of higher eukaryotes, have not been characterized. While UDP-Glc is involved in N-glycan-associated quality control of protein folding in Entamoeba [3, 4], UDP-Gal and UDP-Glc are both involved in synthesis of proteophoglycans and complex N-glycans [12] (and our unpublished data (i)).
4.3. Transfected Giardia provide a novel system for characterizing UDP-Glc transporters
While it has been asserted on multiple occasions that Giardia is a relatively simple protist [20, 35], this is the first time that our lab (and to our knowledge any other lab) has used transfected Giardia to test the function of a heterologous gene (EhNst2). Because Giardia membranes only transport UDP-GlcNAc, transfected Giardia are an excellent system for testing transporters for UDP-Glc and GDP-Man, which cannot easily be tested in Saccharomyces [1, 2]. This is particularly important for UDP-Glc transporters, which are present in all eukaryotes that have N-glycan-dependent quality control of protein folding [3, 4].
Supplementary Material
Acknowledgments
This work was supported by NIH grants AI048082 (to JS) and GM31318 (to PWR). Thanks to Heidi Elmendorf of Georgetown University for the Giardia expression vector.
Abbreviations
- NST
nucleotide-sugar transporter
- NDPase
nucleoside-diphosphatase
- OGT
O-GlcNAc transferase
- TMH
transmembrane helix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Revised references
- 1.Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Ann Rev Biochem. 1998;67:71–98. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Caffaro CE, Hirschberg CB. Nucleotide sugar transporters of the Golgi apparatus: from basic science to diseases. Acc Chem Res. 2006;39:805–812. doi: 10.1021/ar0400239. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Vishwanath P, Cui J, Kelleher DJ, Gilmore R, Robbins PW, Samuelson J. The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation. Proc Natl Acad Sci USA. 2007 doi: 10.1073/pnas.0704862104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trombetta ES, Parodi AJ. Quality control and protein folding in the secretory pathway. Annu Rev Cell Dev Biol. 2003;19:649–776. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Duncker I, Mollicone R, Codogno P, Oriol R. The nucleotide-sugar transporter family: a phylogenetic approach. Biochimie. 2003;85:245–60. doi: 10.1016/s0300-9084(03)00046-4. [DOI] [PubMed] [Google Scholar]
- 6.Jack DL, Yang NM, Saier MH., Jr The drug/metabolite transporter superfamily. Eur J Biochem/FEBS. 2001;268:3620–3639. doi: 10.1046/j.1432-1327.2001.02265.x. [DOI] [PubMed] [Google Scholar]
- 7.Gao XD, Kaigorodov V, Jigami Y. YND1, a homologue of GDA1, encodes membrane-bound apyrase required for Golgi N- and O-glycosylation in Saccharomyces cerevisiae. J Biol Chem. 1999;274:21450–21456. doi: 10.1074/jbc.274.30.21450. [DOI] [PubMed] [Google Scholar]
- 8.Plesner L. Ecto-ATPases: identities and functions. Int Rev Cytol. 1995;158:141–214. doi: 10.1016/s0074-7696(08)62487-0. [DOI] [PubMed] [Google Scholar]
- 9.Strahl-Bolsinger S, Gentzsch M, Tanner W. Protein O-mannosylation. Biochim Biophys Acta. 1999;1426:297–307. doi: 10.1016/s0304-4165(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 10.Ma D, Russell DG, Beverley SM, Turco SJ. Golgi GDP-mannose uptake requires Leishmania LPG2. A member of a eukaryotic family of putative nucleotide-sugar transporters. J Biol Chem. 1997;272:3799–3805. [PubMed] [Google Scholar]
- 11.Capul AA, Barron T, Dobson DE, Turco SJ, Beverley SM. Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J Biol Chem. 2007;282:14006–14017. doi: 10.1074/jbc.M610869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moody-Haupt S, Patterson JH, Mirelman D, McConville MJ. The major surface antigens of Entamoeba histolytica trophozoites are GPI-anchored proteophosphoglycans. J Mol Biol. 2000;297:409–420. doi: 10.1006/jmbi.2000.3577. [DOI] [PubMed] [Google Scholar]
- 13.Bredeston LM, Caffaro CE, Samuelson J, Hirschberg CB. Golgi and endoplasmic reticulum functions take place in different subcellular compartments of Entamoeba histolytica. J Biol Chem. 2005;280:32168–32176. doi: 10.1074/jbc.M507035200. [DOI] [PubMed] [Google Scholar]
- 14.Samuelson J, Banerjee S, Magnelli P, Cui J, Kelleher DJ, Gilmore R, Robbins PW. The diversity of dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc Natl Acad Sci U S A. 2005;102:1548–1553. doi: 10.1073/pnas.0409460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmendorf HG, Singer SM, Pierce J, Cowan J, Nash TE. Initiator and upstream elements in the alpha2-tubulin promoter of Giardia lamblia. Mol Biochem Parasitol. 2001;113:157–169. doi: 10.1016/s0166-6851(01)00211-0. [DOI] [PubMed] [Google Scholar]
- 16.Perez M, Hirschberg CB. Transport of sugar nucleotides into the lumen of vesicles derived from rat liver rough endoplasmic reticulum and Golgi apparatus. Methods Enzymol. 1987;138:709–715. doi: 10.1016/0076-6879(87)38061-9. [DOI] [PubMed] [Google Scholar]
- 17.Gerwig GJ, van Kuik JA, Leeflang BR, Kamerling JP, Vliegenthart JF, Karr CD, Jarroll EL. The Giardia intestinalis filamentous cyst wall contains a novel beta (1-3)-N-acetyl-D-galactosamine polymer: a structural and conformational study. Glycobiology. 2002;12:499–505. doi: 10.1093/glycob/cwf059. [DOI] [PubMed] [Google Scholar]
- 18.Gillin FD, Reiner DS, Boucher SE. Small-intestinal factors promote encystation of Giardia lamblia in vitro. Infect Immun. 1988;56:705–707. doi: 10.1128/iai.56.3.705-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez M, Hirschberg CB. Topography of glycosylation reactions in the rough endoplasmic reticulum membrane. J Biol Chem. 1986;261:6822–6830. [PubMed] [Google Scholar]
- 20.Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, Davids BJ, Dawson SC, Elmendorf HG, Hehl AB, Holder ME, Huse SM, Kim UU, Lasek-Nesselquist E, Manning G, Nigam A, Nixon JE, Palm D, Passamaneck NE, Prabhu A, Reich CI, Reiner DS, Samuelson J, Svard SG, Sogin ML. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science. 2007;317:1921–1926. doi: 10.1126/science.1143837. [DOI] [PubMed] [Google Scholar]
- 21.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Käll L, Krogh A, Sonnhammer ELL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Marchler-Bauer A, Anderson JB, et al. CDD: a conserved domain database for protein classification. Nucleic Acids Res. 2005;33:D192–196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt HA, Strimmer K, Vingron M, von Haeseler A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- 26.Lopez AB, Sener K, Jarroll EL, van Keulen H. Transcription regulation is demonstrated for five key enzymes in Giardia intestinalis cyst wall polysaccharide biosynthesis. Mol Biochem Parasitol. 2003;128:51–57. doi: 10.1016/s0166-6851(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 27.Slawson C, Housley MP, Hart GW. O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks. J Cell Biochem. 2006;97:71–83. doi: 10.1002/jcb.20676. [DOI] [PubMed] [Google Scholar]
- 28.Lucero HA, Kuranda MJ, Bulik DA. A nonradioactive, high throughput assay for chitin synthase activity. Anal Biochem. 2002;305:97–105. doi: 10.1006/abio.2002.5594. [DOI] [PubMed] [Google Scholar]
- 29.Guranowski A. Specific and nonspecific enzymes involved in the catabolism of mononucleoside and dinucleoside polyphosphates. Pharmacol Ther. 2000;87:117–139. doi: 10.1016/s0163-7258(00)00046-2. [DOI] [PubMed] [Google Scholar]
- 30.Latgé JP. The cell wall: a carbohydrate armour for the fungal cell. Mol Microbiol. 2007;66:279–290. doi: 10.1111/j.1365-2958.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- 31.Joshi CP, Mansfield SD. The cellulose paradox--simple molecule, complex biosynthesis. Curr Opin Plant Biol. 2007;10:220–226. doi: 10.1016/j.pbi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Soltys BJ, Falah M, Gupta RS. Identification of endoplasmic reticulum in the primitive eukaryote Giardia lamblia using cryoelectron microscopy and antibody to Bip. J Cell Sci. 1996;109:1909–1917. doi: 10.1242/jcs.109.7.1909. [DOI] [PubMed] [Google Scholar]
- 33.Touz MC, Kulakova L, Nash TE. Adaptor protein complex 1 mediates the transport of lysosomal proteins from a Golgi-like organelle to peripheral vacuoles in the primitive eukaryote Giardia lamblia. Mol Biol Cell. 2004;15:3053–3060. doi: 10.1091/mbc.E03-10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortega-Barria E, Ward HD, Evans JE, Pereira ME. N-acetyl-D-glucosamine is present in cysts and trophozoites of Giardia lamblia and serves as receptor for wheat germ agglutinin. Mol Biochem Parasitol. 1990;43:151–165. doi: 10.1016/0166-6851(90)90141-8. [DOI] [PubMed] [Google Scholar]
- 35.Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


