Abstract
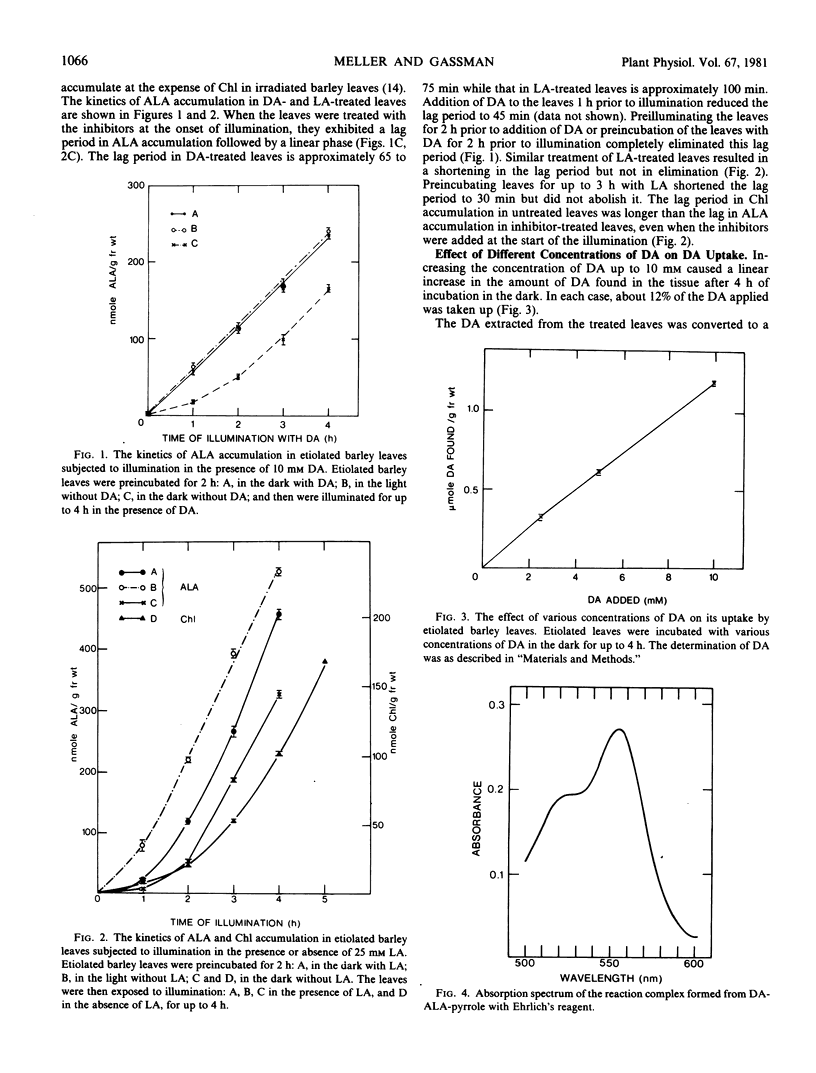
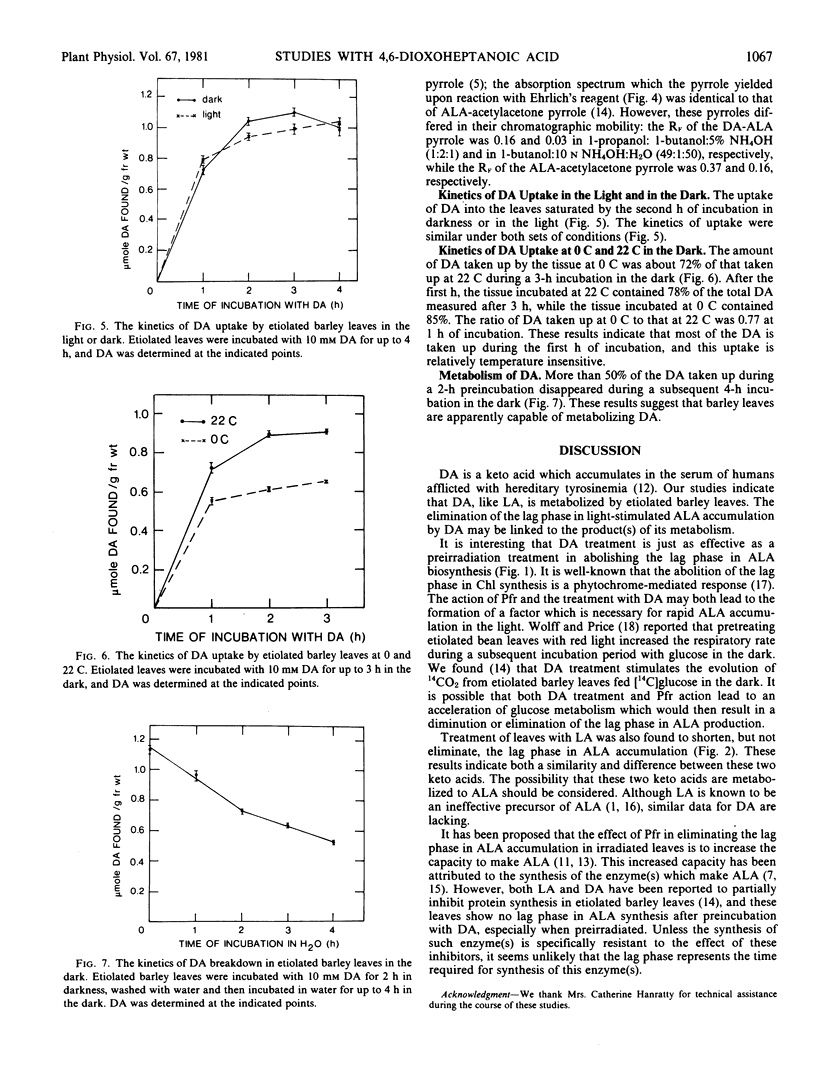
4,6-Dioxoheptanoic acid (DA), an inhibitor of 5-aminolevulinic acid (ALA) dehydratase (EC 4.3.1.24), causes ALA to accumulate at the expense of chlorophyll when applied to greening leaves of Hordeum vulgare L. var. Larker. Preincubating etiolated leaves with DA in darkness eliminates the lag phase in ALA accumulation during a subsequent exposure to illumination. More than 50% of the DA taken up during a 2-hour incubation disappeared during a subsequent 4-hour incubation. These results suggest that barley leaves can metabolize DA, and the products of this metabolism may enhance the capacity for ALA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I. The biosynthesis of delta-aminolevulinic acid in Chlorella. Plant Physiol. 1970 Apr;45(4):504–506. doi: 10.1104/pp.45.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert P. S., Hess R. A., Frykholm B. C., Tschudy D. P. Succinylacetone, a potent inhibitor of heme biosynthesis: effect on cell growth, heme content and delta-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1382–1390. doi: 10.1016/0006-291x(79)91133-1. [DOI] [PubMed] [Google Scholar]
- Gassman M. L. A Reversible Conversion of Phototransformable Protochlorophyll(ide)(656) to Photoinactive Protochlorophyll(ide)(656) by Hydrogen Sulfide in Etiolated Bean Leaves. Plant Physiol. 1973 Jan;51(1):139–145. doi: 10.1104/pp.51.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassman M., Bogorad L. Control of chlorophyll production in rapidly greening bean leaves. Plant Physiol. 1967 Jun;42(6):774–780. doi: 10.1104/pp.42.6.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving E. A., Elliott W. H. A sensitive radiochemical assay method for delta-aminolevulinic acid synthetase. J Biol Chem. 1969 Jan 10;244(1):60–67. [PubMed] [Google Scholar]
- Klein S., Katz E., Neeman E. Induction of delta-Aminolevulinic Acid Formation in Etiolated Maize Leaves Controlled by Two Light Systems. Plant Physiol. 1977 Sep;60(3):335–338. doi: 10.1104/pp.60.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad B., Lindstedt S., Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler K., Granick S. Controls on chlorophyll synthesis in barley. Plant Physiol. 1970 Aug;46(2):240–246. doi: 10.1104/pp.46.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. The effect of sugars on chlorophyll biosynthesis in higher plants. J Biol Chem. 1960 Jun;235:1603–1608. [PubMed] [Google Scholar]


