Abstract
Cholesteryl hemisuccinate has been incorporated into pea chloroplast thylakoids to investigate the relationship between fluidity and functioning of this membrane system. Levels of sterol which increased the apparent viscosity of the membrane, estimated by fluorescence polarization measurements using the lipophilic probe, 1,6-diphenyl-1,3,5 hexatriene, affected several photosynthetic processes. A decrease in fluidity was accompanied by an inhibition of dark limiting steps associated with electron transfer between photosystems two and one (PSII and PSI) as observed by the oxidation of the primary acceptor of PSII and by electron flow to ferricyanide. Also, treatment with cholesteryl hemisuccinate inhibited the saltinduced rise in chlorophyll fluorescence and changed the ionic conductivity of the membrane as judged by measurements of the decay of the lightinduced proton gradient. The results are discussed in terms of the effect of fluidity changes on the lateral diffusion of plastoquinone and chlorophyll protein complexes in the lipid matrix of the membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Cox R. P. The reduction of artificial electron acceptors at sub-zero temperatures by chloroplasts suspended in fluid media. Biochim Biophys Acta. 1975 Jun 17;387(3):588–598. doi: 10.1016/0005-2728(75)90096-1. [DOI] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Hildenbrand K., Nicolau C. Nanosecond fluorescence anisotropy decays of 1,6-diphenyl-1,3,5-hexatriene in membranes. Biochim Biophys Acta. 1979 Jun 2;553(3):365–377. doi: 10.1016/0005-2736(79)90292-x. [DOI] [PubMed] [Google Scholar]
- Itoh S., Nishimura M. [pH dependent changes in the reactivity of the primary electron acceptor of system II in spinach chloroplasts to external oxidant and reductant]. Biochim Biophys Acta. 1977 Jun 9;460(3):381–392. doi: 10.1016/0005-2728(77)90079-2. [DOI] [PubMed] [Google Scholar]
- Jursinic P., Govindjee Thermoluminescence and temperature effects on delayed light emission (corrected for changes in quantum yield of fluorescence) in DCMU-treated algae. Photochem Photobiol. 1972 Apr;15(4):331–348. doi: 10.1111/j.1751-1097.1972.tb06244.x. [DOI] [PubMed] [Google Scholar]
- Kraayenhof R., Katan M. B., Grunwald T. The effect of temperature on energy-linked functions in chloroplasts. FEBS Lett. 1971 Nov 15;19(1):5–10. doi: 10.1016/0014-5793(71)80592-6. [DOI] [PubMed] [Google Scholar]
- Madden T. D., Chapman D., Quinn P. J. Cholesterol modulates activity of calcium-dependent ATPase of the sarcoplasmic reticulum. Nature. 1979 Jun 7;279(5713):538–541. doi: 10.1038/279538a0. [DOI] [PubMed] [Google Scholar]
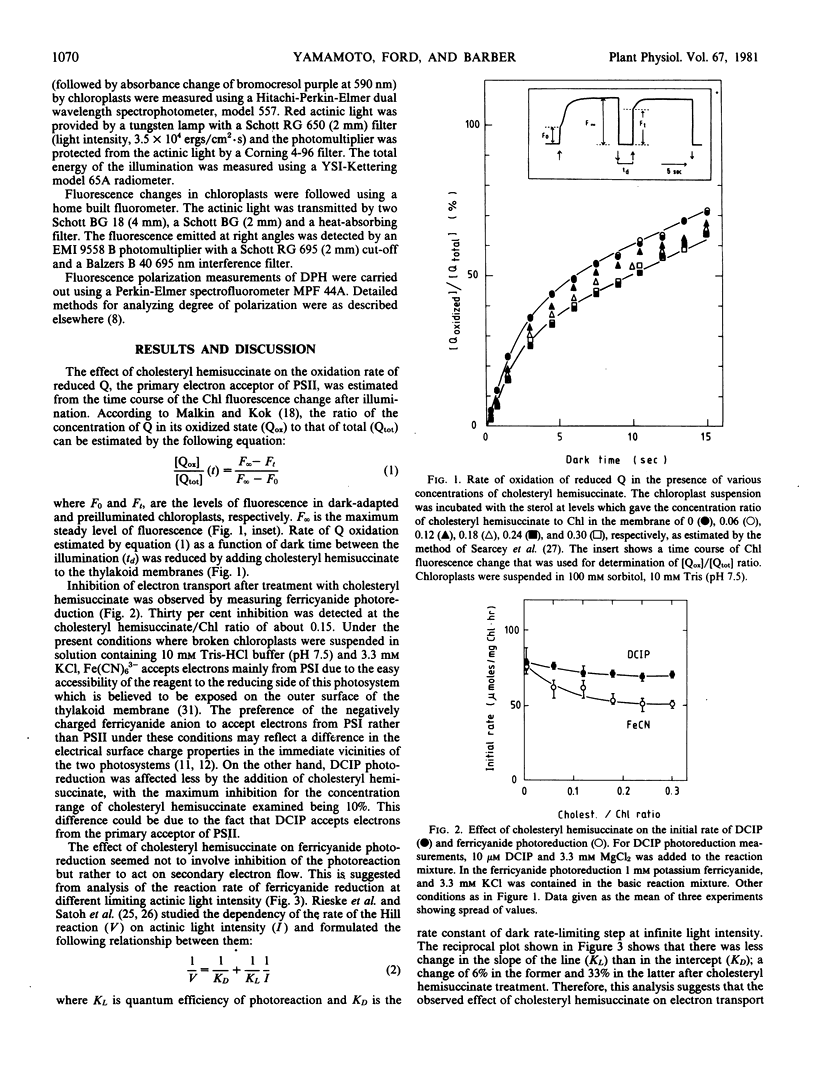
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- McEvoy F. A., Lynn W. S. The effects of temperature on photophosphorylation and on the two forms of cytochrome 559 in subchloroplast particles. Arch Biochem Biophys. 1972 Jun;150(2):632–635. doi: 10.1016/0003-9861(72)90083-5. [DOI] [PubMed] [Google Scholar]
- Murata N. Relationships between the Transition of the Physical Phase of Membrane Lipids and Photosynthetic Parameters in Anacystis nidulans and Lettuce and Spinach Chloroplasts. Plant Physiol. 1975 Oct;56(4):508–517. doi: 10.1104/pp.56.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Nolan W. G., Smillie R. M. Multi-temperature effects on Hill reaction activity of barley chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):461–475. doi: 10.1016/0005-2728(76)90034-7. [DOI] [PubMed] [Google Scholar]
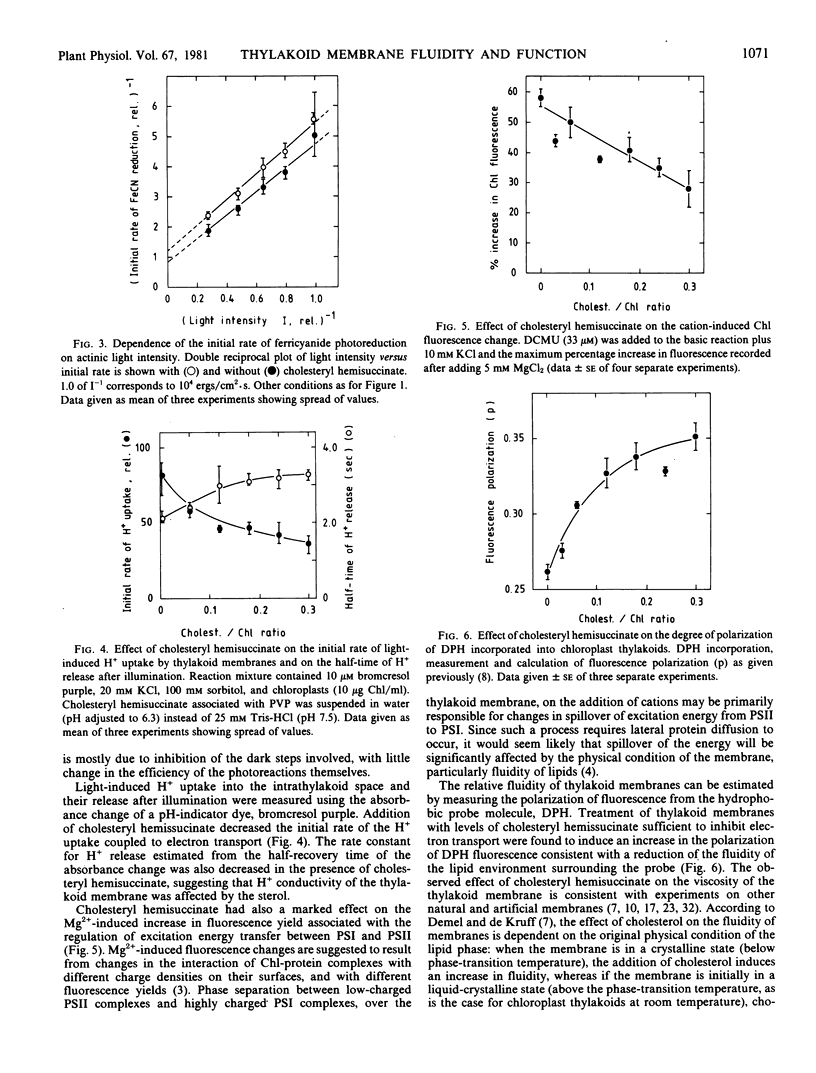
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Williams W. P. Plant lipids and their role in membrane function. Prog Biophys Mol Biol. 1978;34(2):109–173. doi: 10.1016/0079-6107(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Rieske J. S., Lumry R., Spikes J. D. The Mechanism of the Photochemical Activity of Isolated Chloroplasts. III. Dependence of Velocity on Light Intensity. Plant Physiol. 1959 May;34(3):293–300. doi: 10.1104/pp.34.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARCY R. L., BERGQUIST L. M., JUNG R. C. Rapid ultramicro estimation of serum total cholesterol. J Lipid Res. 1960 Jul;1:349–351. [PubMed] [Google Scholar]
- Shinitzky M., Skornick Y., Haran-Ghera N. Effective tumor immunization induced by cells of elevated membrane-lipid microviscosity. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5313–5316. doi: 10.1073/pnas.76.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneyour A., Raison J. K., Smillie R. M. The effect of temperature of the rate of photosynthetic electron transfer in chloroplasts of chilling-sensitive and chilling-resistant plants. Biochim Biophys Acta. 1973 Jan 18;292(1):152–161. doi: 10.1016/0005-2728(73)90259-4. [DOI] [PubMed] [Google Scholar]
- Telfer A., Barber J., Jagendorf A. T. Electrostatic control of chloroplast coupling factor binding to thylakoid membranes as indicated by cation effects of electron transport and reconstitution of photophosphorylation. Biochim Biophys Acta. 1980 Jul 8;591(2):331–345. doi: 10.1016/0005-2728(80)90164-4. [DOI] [PubMed] [Google Scholar]
- van Hoeven R. P., van Blitterswijk W. J., Emmelot P. Fluorescence polarization measurements on normal and tumour cells and their corresponding plasma membranes. Biochim Biophys Acta. 1979 Feb 20;551(1):44–54. doi: 10.1016/0005-2736(79)90351-1. [DOI] [PubMed] [Google Scholar]


